Abstract
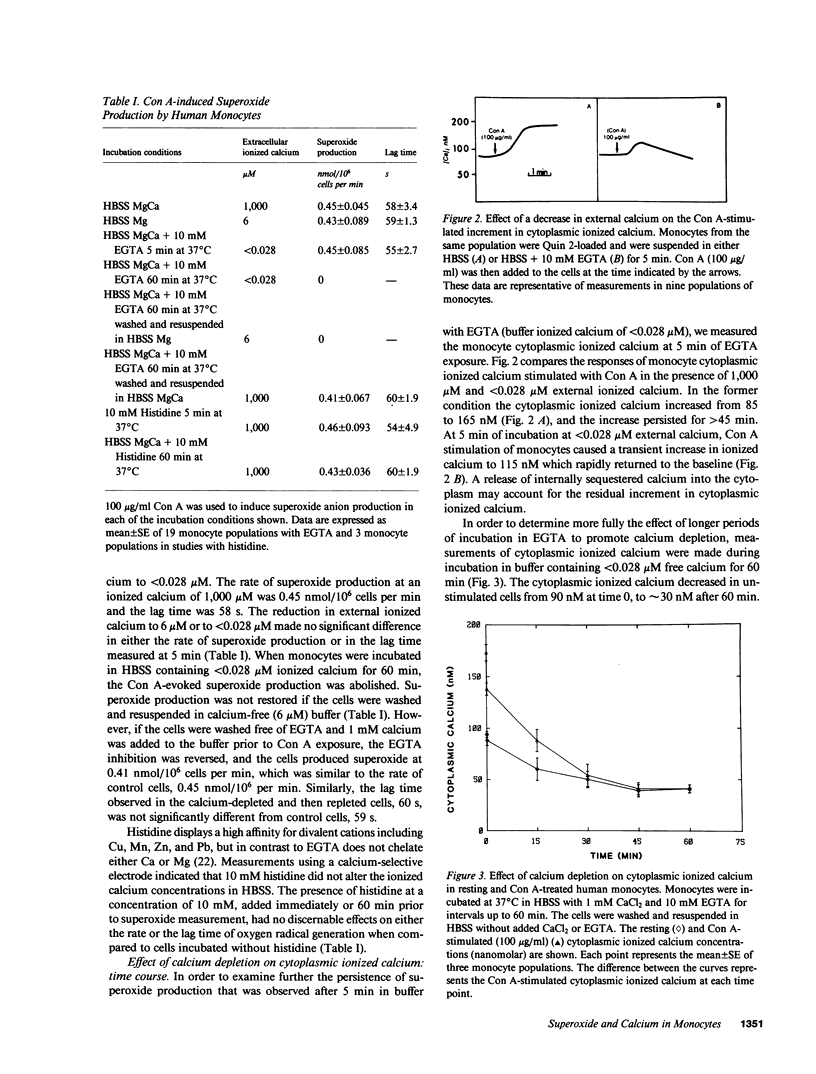
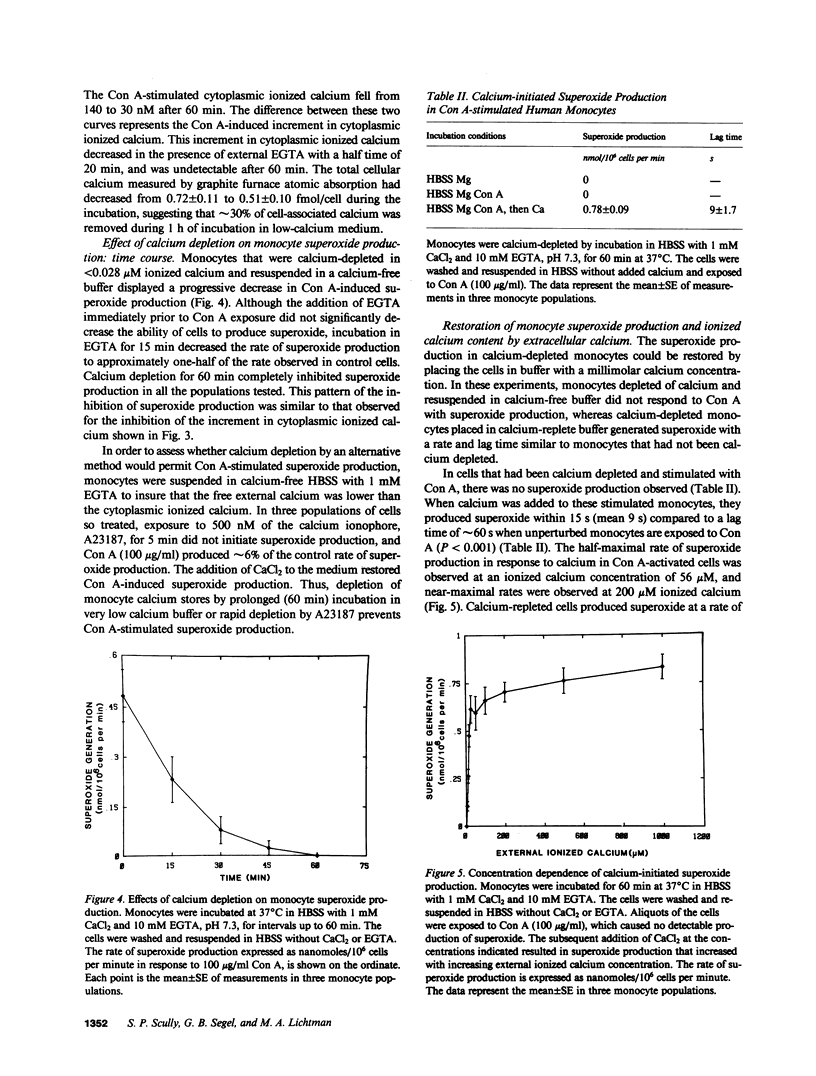
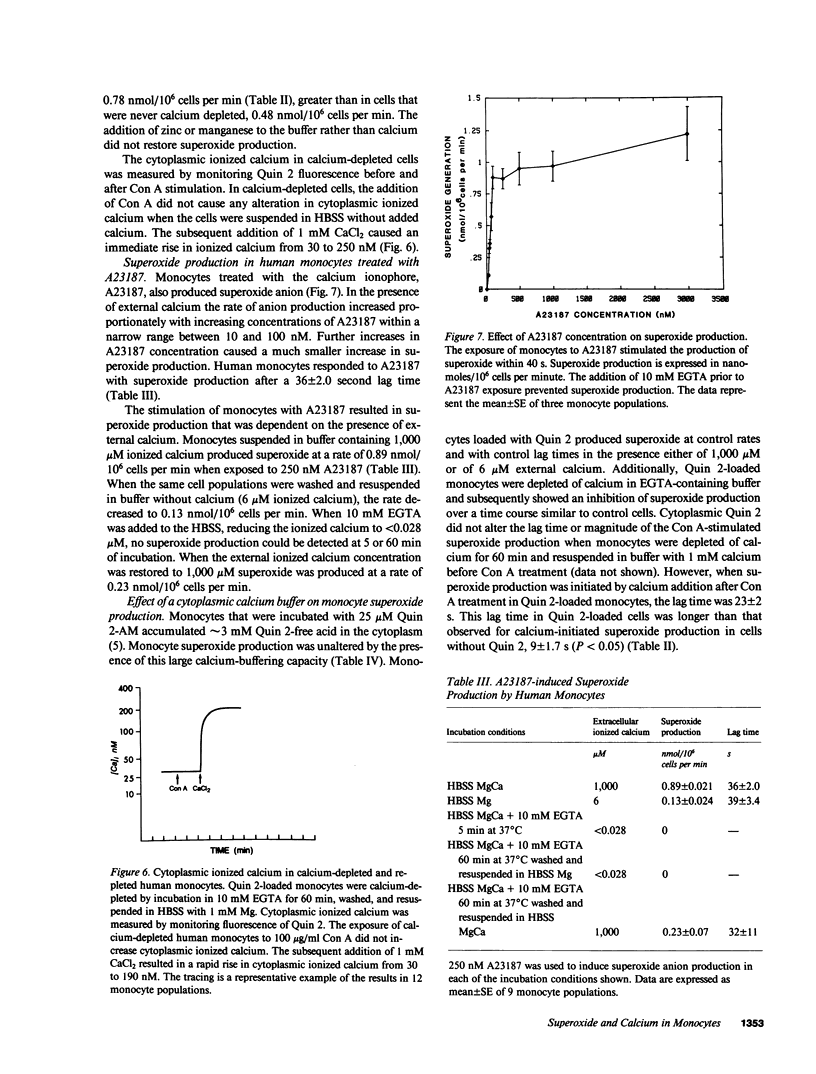
Calcium has been proposed as an intracellular second messenger for activation of secretion, phagocytosis, and the oxidative burst of neutrophils. We have examined the role of calcium in human monocyte activation. Concanavalin A (Con A)-stimulated monocytes displayed an increment in cytoplasmic ionized calcium at 31 +/- 6 s and the onset of superoxide production at 61 +/- 9 s. The increase in cytoplasmic calcium invariably preceded the onset of superoxide production. If the external calcium concentration was reduced to less than 28 nM by the addition of 10 mM EGTA, superoxide production was not diminished at 5 min; however, superoxide production decreased thereafter. The Con A-evoked increment in cytoplasmic ionized calcium was blunted upon the addition of EGTA and decreased further with time. Both the production of superoxide and the Con A-evoked increment in cytoplasmic ionized calcium displayed a 50% inhibition after 15 min of calcium depletion and were completely inhibited after 60 min. Total cell calcium fell from 0.7 to 0.5 fmol/cell, and the basal level of ionized calcium fell from 83 to 30 nM after 60 min. Histidine, a strong chelator of divalent cations other than calcium and magnesium, had no effect on monocyte superoxide production or on ionized calcium concentrations, indicating that EGTA inhibition was due to cell calcium depletion. In calcium-depleted cells, Con A did not evoke superoxide production until calcium was restored to the incubation medium. The restoration of calcium to Con A-treated, calcium-depleted monocytes permitted a rapid rise in the cytoplasmic ionized calcium, and the production of superoxide within 9 s. These data suggest that an increase in ionized cytoplasmic calcium is necessary for the activation of monocyte superoxide production by Con A. The rise in ionized calcium in response to Con A results, in part, from an internal redistribution of calcium, which is sufficient to permit superoxide generation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M. The respiratory burst of phagocytes. J Clin Invest. 1984 Mar;73(3):599–601. doi: 10.1172/JCI111249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Dawson R. M., Downes C. P., Heslop J. P., Irvine R. F. Changes in the levels of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides. Biochem J. 1983 May 15;212(2):473–482. doi: 10.1042/bj2120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess G. M., Godfrey P. P., McKinney J. S., Berridge M. J., Irvine R. F., Putney J. W., Jr The second messenger linking receptor activation to internal Ca release in liver. Nature. 1984 May 3;309(5963):63–66. doi: 10.1038/309063a0. [DOI] [PubMed] [Google Scholar]
- Burgess G. M., McKinney J. S., Irvine R. F., Berridge M. J., Hoyle P. C., Putney J. W., Jr Inositol 1,4,5-trisphosphate may be a signal for f-Met-Leu-Phe-induced intracellular Ca mobilisation in human leucocytes (HL-60 cells). FEBS Lett. 1984 Oct 15;176(1):193–196. doi: 10.1016/0014-5793(84)80939-4. [DOI] [PubMed] [Google Scholar]
- Cohen H. J., Chovaniec M. E. Superoxide production by digitonin-stimulated guinea pig granulocytes. The effects of N-ethyl maleimide, divalent cations; and glycolytic and mitochondrial inhibitors on the activation of the superoxide generating system. J Clin Invest. 1978 Apr;61(4):1088–1096. doi: 10.1172/JCI109008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen H. J., Whitin J. C., Chovaniec M. E., Tape E. H., Simons E. R. Is activation of the granulocyte by concanavalin-A a reversible process? Blood. 1984 Jan;63(1):114–120. [PubMed] [Google Scholar]
- Dewald B., Payne T. G., Baggiolini M. Activation of NADPH oxidase of human neutrophils. Potentiation of chemotactic peptide by a diacylglycerol. Biochem Biophys Res Commun. 1984 Nov 30;125(1):367–373. doi: 10.1016/s0006-291x(84)80377-0. [DOI] [PubMed] [Google Scholar]
- Fujita I., Irita K., Takeshige K., Minakami S. Diacylglycerol, 1-oleoyl-2-acetyl-glycerol, stimulates superoxide-generation from human neutrophils. Biochem Biophys Res Commun. 1984 Apr 30;120(2):318–324. doi: 10.1016/0006-291x(84)91256-7. [DOI] [PubMed] [Google Scholar]
- Gallo R. L., Wersto R. P., Notter R. H., Finkelstein J. N. Lysophosphatidylcholine cell depolarization: increased membrane permeability for use in the determination of cell membrane potentials. Arch Biochem Biophys. 1984 Dec;235(2):544–554. doi: 10.1016/0003-9861(84)90228-5. [DOI] [PubMed] [Google Scholar]
- Hallett M. B., Campbell A. K. Direct measurement of intracellular free Ca2+ in rat peritoneal macrophages: correlation with oxygen-radical production. Immunology. 1983 Nov;50(3):487–495. [PMC free article] [PubMed] [Google Scholar]
- Hallett M. B., Campbell A. K. Is intracellular Ca2+ the trigger for oxygen radical production by polymorphonuclear leucocytes? Cell Calcium. 1984 Feb;5(1):1–19. doi: 10.1016/0143-4160(84)90150-7. [DOI] [PubMed] [Google Scholar]
- Holian A., Daniele R. P. The role of calcium in the initiation of superoxide release from alveolar macrophages. J Cell Physiol. 1982 Oct;113(1):87–93. doi: 10.1002/jcp.1041130115. [DOI] [PubMed] [Google Scholar]
- Kitagawa S., Takaku F., Sakamoto S. Evidence that proteases are involved in superoxide production by human polymorphonuclear leukocytes and monocytes. J Clin Invest. 1980 Jan;65(1):74–81. doi: 10.1172/JCI109662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korchak H. M., Rutherford L. E., Weissmann G. Stimulus response coupling in the human neutrophil. I. Kinetic analysis of changes in calcium permeability. J Biol Chem. 1984 Apr 10;259(7):4070–4075. [PubMed] [Google Scholar]
- Korchak H. M., Vienne K., Rutherford L. E., Wilkenfeld C., Finkelstein M. C., Weissmann G. Stimulus response coupling in the human neutrophil. II. Temporal analysis of changes in cytosolic calcium and calcium efflux. J Biol Chem. 1984 Apr 10;259(7):4076–4082. [PubMed] [Google Scholar]
- Lagast H., Lew P. D., Waldvogel F. A. Adenosine triphosphate-dependent calcium pump in the plasma membrane of guinea pig and human neutrophils. J Clin Invest. 1984 Jan;73(1):107–115. doi: 10.1172/JCI111180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew P. D., Stossel T. P. Calcium transport by macrophage plasma membranes. J Biol Chem. 1980 Jun 25;255(12):5841–5846. [PubMed] [Google Scholar]
- Lew P. D., Wollheim C. B., Waldvogel F. A., Pozzan T. Modulation of cytosolic-free calcium transients by changes in intracellular calcium-buffering capacity: correlation with exocytosis and O2-production in human neutrophils. J Cell Biol. 1984 Oct;99(4 Pt 1):1212–1220. doi: 10.1083/jcb.99.4.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman A. H., Segel G. B., Lichtman M. A. An ultrasensitive method for the measurement of human leukocyte calcium: lymphocytes. Clin Chim Acta. 1979 Oct 1;97(2-3):107–121. doi: 10.1016/0009-8981(79)90407-8. [DOI] [PubMed] [Google Scholar]
- Lichtman A. H., Segel G. B., Lichtman M. A. Calcium transport and calcium-ATPase activity in human lymphocyte plasma membrane vesicles. J Biol Chem. 1981 Jun 25;256(12):6148–6154. [PubMed] [Google Scholar]
- McPhail L. C., Henson P. M., Johnston R. B., Jr Respiratory burst enzyme in human neutrophils. Evidence for multiple mechanisms of activation. J Clin Invest. 1981 Mar;67(3):710–716. doi: 10.1172/JCI110087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naccache P. H., Showell H. J., Becker E. L., Sha'afi R. I. Transport of sodium, potassium, and calcium across rabbit polymorphonuclear leukocyte membranes. Effect of chemotactic factor. J Cell Biol. 1977 May;73(2):428–444. doi: 10.1083/jcb.73.2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawara M., Takeshige K., Sumimoto H., Yoshitake J., Minakami S. Superoxide release and intracellular free calcium of calcium-depleted human neutrophils stimulated by N-formyl-methionyl-leucyl-phenylalanine. Biochim Biophys Acta. 1984 Sep 14;805(1):97–103. doi: 10.1016/0167-4889(84)90041-7. [DOI] [PubMed] [Google Scholar]
- Onozaki K., Takenawa T., Homma Y., Hashimoto T. The mechanism of macrophage activation induced by Ca2+ ionophore. Cell Immunol. 1983 Feb 1;75(2):242–254. doi: 10.1016/0008-8749(83)90323-4. [DOI] [PubMed] [Google Scholar]
- Penfield A., Dale M. M. Synergism between A23187 and 1-oleoyl-2-acetyl-glycerol in superoxide production by human neutrophils. Biochem Biophys Res Commun. 1984 Nov 30;125(1):332–336. doi: 10.1016/s0006-291x(84)80372-1. [DOI] [PubMed] [Google Scholar]
- Pozzan T., Lew D. P., Wollheim C. B., Tsien R. Y. Is cytosolic ionized calcium regulating neutrophil activation? Science. 1983 Sep 30;221(4618):1413–1415. doi: 10.1126/science.6310757. [DOI] [PubMed] [Google Scholar]
- Rasmussen H., Kojima I., Kojima K., Zawalich W., Apfeldorf W. Calcium as intracellular messenger: sensitivity modulation, C-kinase pathway, and sustained cellular response. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;18:159–193. [PubMed] [Google Scholar]
- Robinson J. M., Badwey J. A., Karnovsky M. L., Karnovsky M. J. Superoxide release by neutrophils: synergistic effects of a phorbol ester and a calcium ionophore. Biochem Biophys Res Commun. 1984 Jul 31;122(2):734–739. doi: 10.1016/s0006-291x(84)80095-9. [DOI] [PubMed] [Google Scholar]
- Scully S. P., Segel G. B., Lichtman M. A. Calcium exchange and ionized cytoplasmic calcium in resting and activated human monocytes. J Clin Invest. 1984 Aug;74(2):589–599. doi: 10.1172/JCI111456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scully S. P., Segel G. B., Lichtman M. A. Plasma membrane vesicles prepared from unadhered monocytes: characterization of calcium transport and the calcium ATPase. Cell Calcium. 1982 Dec;3(6):515–530. doi: 10.1016/0143-4160(82)90042-2. [DOI] [PubMed] [Google Scholar]
- Simchowitz L., Spilberg I. Generation of superoxide radicals by human peripheral neutrophils activated by chemotactic factor. Evidence for the role of calcium. J Lab Clin Med. 1979 Apr;93(4):583–593. [PubMed] [Google Scholar]
- Smolen J. E., Korchak H. M., Weissmann G. The roles of extracellular and intracellular calcium in lysosomal enzyme release and superoxide anion generation by human neutrophils. Biochim Biophys Acta. 1981 Nov 5;677(3-4):512–520. doi: 10.1016/0304-4165(81)90267-1. [DOI] [PubMed] [Google Scholar]
- Stickle D. F., Daniele R. P., Holian A. Cytosolic calcium, calcium fluxes, and regulation of alveolar macrophage superoxide anion production. J Cell Physiol. 1984 Dec;121(3):458–466. doi: 10.1002/jcp.1041210303. [DOI] [PubMed] [Google Scholar]
- Takeshige K., Matsumoto T., Nabi Z. F., Minakami S. Release of the membrane-calcium and its relation to the superoxide formation by polymorphonuclear leukocytes. Adv Exp Med Biol. 1982;141:453–461. doi: 10.1007/978-1-4684-8088-7_43. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. Calcium homeostasis in intact lymphocytes: cytoplasmic free calcium monitored with a new, intracellularly trapped fluorescent indicator. J Cell Biol. 1982 Aug;94(2):325–334. doi: 10.1083/jcb.94.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpi M., Naccache P. H., Sha'afi R. I. Calcium transport in inside-out membrane vesicles prepared from rabbit neutrophils. J Biol Chem. 1983 Apr 10;258(7):4153–4158. [PubMed] [Google Scholar]
- White J. R., Naccache P. H., Molski T. F., Borgeat P., Sha'afi R. I. Direct demonstration of increased intracellular concentration of free calcium in rabbit and human neutrophils following stimulation by chemotactic factor. Biochem Biophys Res Commun. 1983 May 31;113(1):44–50. doi: 10.1016/0006-291x(83)90429-1. [DOI] [PubMed] [Google Scholar]
- Whitin J. C., Chapman C. E., Simons E. R., Chovaniec M. E., Cohen H. J. Correlation between membrane potential changes and superoxide production in human granulocytes stimulated by phorbol myristate acetate. Evidence for defective activation in chronic granulomatous disease. J Biol Chem. 1980 Mar 10;255(5):1874–1878. [PubMed] [Google Scholar]



