Abstract
Fungicides can be detrimental to flower development, pollen function and fruit set in a number of crops. Almond is a self‐incompatible nut crop that has a fruit set of only approx. 30 % of the total number of flowers. Thus, interference of pollination and fertilization by fungicide sprays is of concern, and identification of chemicals having the least detrimental effects would be desirable. The objective of this study was to evaluate the effect of fungicide sprays on stigma morphology in almond using a laboratory spray apparatus that simulated field applications. Four fungicides (azoxystrobin, myclobutanil, iprodione and cyprodinil) were applied, and fresh, unfixed stigmatic surfaces were observed using a scanning electron microscope at 4 and 24 h after spraying. Increased exudate accumulation was induced by azoxystrobin at both time periods, and localized damage and collapse of stigmatic cells were observed after 24 h. Damaged stigmatic papillae exhibited wrinkling, surface distortion or collapse. Likewise, myclobutanil caused significant damage to and collapse of papillae; these were more extensive at later observations. Iprodione had no effect on exudate accumulation but caused marked and severe collapse of stigmatic papillae which was pronounced at 24 h. Cyprodinil promoted a copious increase in exudate secretion and caused the most severe collapse of stigmatic cells of all the fungicides evaluated. Damage was somewhat localized at 4 h but more global at 24 h. This study has verified that certain fungicide sprays have direct detrimental effects on stigma morphology and enhance exudate production in almond flowers.
Key words: Almond, collapse, exudate, fungicide, pesticide, Prunus dulcis, scanning electron microscopy, stigmatic papillae
INTRODUCTION
Adequate pollination and fertilization are critical in agricultural crops where fruit or seed is the final product, and have been identified as factors limiting yield and crop quality (e.g. fruit size, shape, sugar content and storage ability) in numerous economically important crops including apple (Malus × domestica Bokh.), kiwifruit [Actinidia deliciosa (A. Chev.) C.F. Liang et A.R. Ferguson], cacao (Theobroma cacao L.) and melon (Cucumis melon L.) (Costa et al., 1993; Dag and Eisikowitch, 1995; Falque et al., 1995; Volz et al., 1996; Gonzalez et al., 1998). Critical events associated with pollination in higher plants include the development of receptive stigmas and functional pollen, pollen transfer and attachment, pollen hydration and activation, pollen germination and tube growth (Johri, 1984; Raghavan, 1997). These events are necessary for fruit set to proceed.
Almond [Prunus dulcis (Mill.) D. A. Webb] is an important nut crop that blooms relatively early in the spring (February in California, USA). The crop is self‐incompatible and requires cross‐pollination. Pollination can be limiting in certain production areas owing, in part, to cool, damp weather conditions during the blooming period which may limit insect activity. It has been reported that the percentage of fruit set in commercial orchards is commonly only 30 % (Gary et al., 1976). Almond is heavily sprayed during the bloom period for blossom blight caused by Monilinia fructicola and/or M. laxa. Interference with pollen germination and function by fungicide sprays during the pollination period would be of particular concern in almond production.
The stigma is the receptive surface for pollen. It can provide nutrients to the pollen and direct pollen tube growth. The stigmatic surface must have the correct physiological condition for the pollen, i.e. a balanced osmolarity and sufficient water supply (Johri, 1984). Any damage to the stigmatic surface by fungicide sprays may potentially cause the pollination process to fail. A number of studies have reported detrimental effects of fungicide sprays on fruit set and/or yield in crops such as apple (Hutcheon et al., 1986), cranberry (Vaccinium macrocarpon Ait.) (Shawa et al., 1966; Bristow and Shawa, 1981; Ozgen and Palta, 2001), raspberry (Rubus idaeus L.) (Redalen, 1980), strawberry (Fragaria × ananassa Duch.) (Eaton and Chen, 1969; Kovach et al., 2000) and pecan (Carya illinoensis Wangenh C. Koch) (Wetzstein, 1990; He and Wetzstein, 1994). However, studies specifically evaluating the effects of fungicides on stigma morphology are extremely limited for fruit crops and absent in almond.
The objective of this study was to evaluate the effect of fungicide sprays on stigma morphology in almond at the ultrastructural level. Fungicide sprays were applied in the laboratory to detached flowers using a mechanized spray apparatus to simulate field application conditions. Stigmatic surfaces were observed in a fresh, living state to avoid loss of stigmatic exudate associated with fixation and critical point drying.
MATERIALS AND METHODS
Plant material
Almond (Prunus dulcis ‘Nonpareil’) budwood was collected from a commercial almond orchard (Paramount Farming Co., Bakersfield, CA, USA), packed in coolers, and air‐shipped for next‐day delivery. Upon delivery, shoots were recut and stored with their bases in water in a cold room at 7 °C. Buds were forced as needed and flowers were emasculated before anther dehiscence to remove sources of pollen contamination. Just prior to spraying, individual flowers were selected and removed with approx. 1 cm of the shoot still attached, then transferred into wells of tissue culture plates (Costar Tissue Culture Clusters 12; Costar, Cambridge, MA, USA) containing tap water. Flowers that showed normal stigma development and similar stigma orientations and style lengths were chosen with the aid of a dissecting microscope. Flowers were carefully selected so that they were all at the open petal stage of development within a day after anthesis.
Chemicals and spray application
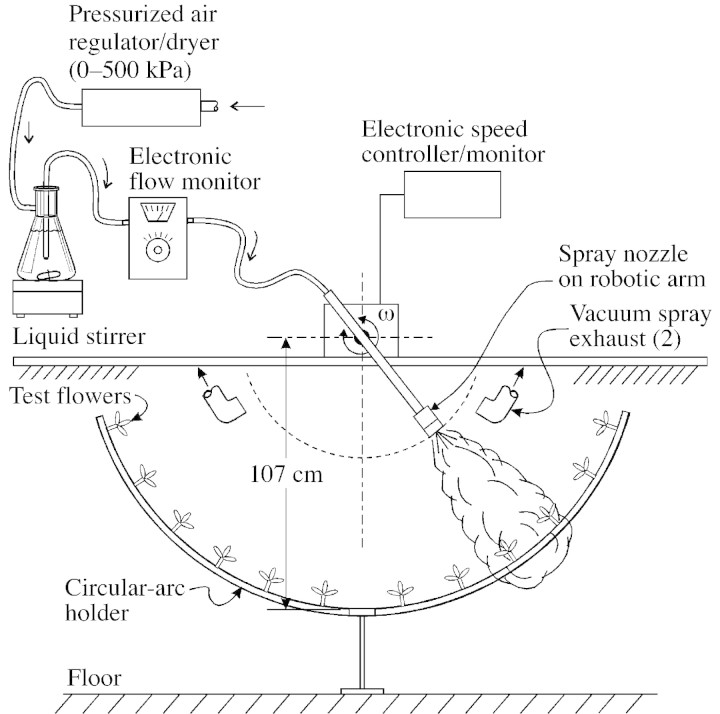
Four fungicides that are widely applied during the bloom season to prevent blossom blight were tested: (1) azoxystrobin (Zeneca Agric. Products, Wilmington, DL, USA); (2) myclobutanil (Rohm and Haas Co., Philadelphia, PA, USA); (3) iprodione (Aventis CS, Research Triangle Park, NC, USA); and (4) cyprodinil (Novartis Crop Protection, Inc., Greensboro, NC, USA). The formulated product name and classification of each fungicide are shown in Table 1. A water spray served as control. Just prior to spraying, eight flowers were attached (via a spring‐clip to their woody stem) to a circular‐arc holder with stigmas oriented towards the oncoming spray. Fungicide or water was then applied using a laboratory spray apparatus designed to simulate field application conditions (Fig. 1). The spray apparatus was set to provide application conditions equivalent to a spray volume of 100 US gal acre–1 (935 l ha–1), 2 mph (3·2 km h–1) tractor ground speed, tree spacing of 24 ft (7·3 m) between rows, and an application rate of 0·263 gal min–1 (16·6 ml s–1) per nozzle (using TeeJet 8003; Spraying Systems Co., Wheaton, IL, USA) at 40 p.s.i. (276 kPa) for dual‐pass by targets. Immediately after spraying, flowers were transferred back into the culture plates and kept under light conditions at 24 °C. In a separate experiment, Rhodamine B stain solution (0·1 % w/v) was applied using the same conditions to evaluate the spray patterns.
Table 1.
Fungicides applied to almond flowers and rates of application
| Active ingredient | Formulated product name | Class | Field rate per acre | Laboratory equivalent |
| Azoxystrobin | Abound | Strobilurin | 12·8 oz | 1 ml l–1 |
| Myclobutanil | Rally | Conazole | 6·0 oz | 0·45 g l–1 |
| Iprodione | Rovral | Iprodione | 1·0 lb | 1·2 g l–1 |
| Cyprodinil | Vangard | Pyrimidine | 5 oz | 0·37 g l–1 |
Spray solutions were calculated based on spray application volumes of 100 gallon per acre (935 l ha–1).
Fig. 1. Laboratory apparatus used to simulate field fungicide applications. A conventional hydraulic‐atomizing nozzle at operational pressure commonly used for fungicide applications provided the appropriate droplet‐size spectrum, volumetric flow rate and active ingredient concentration for each fungicide. An electronically controlled robotic arm swept the spray nozzle at controlled speed past test flowers positioned around a circular‐arc holder.
Experimental design
A factorial design was used, evaluating different spray compounds and time periods after spraying. The design was: two time periods × five spray compounds (including water control) × eight flowers. Five stigmas were used in observations of fresh flowers without fixation; three stigmas were fixed, coated and observed as described below.
SEM observations
Flowers were sampled 4 or 24 h after spraying. Spray treatments were scheduled and staggered to allow adequate sampling and observation time. Five pistils were dissected from flowers and the stigmas and styles were mounted on aluminium stubs using carbon paste. Each sample was observed immediately using a JSM‐5800 scanning electron microscope (JEOL, Tokyo, Japan) at 5 kV, and images captured digitally. Three other flowers from each treatment were dissected and fixed with 2 % glutaldehyde in 0·1 m cacodylate buffer, pH 7·2. The tissues were dehydrated in a graded ethanol series, then critical‐point dried using a Samdri 780‐A Critical Point Drier. The tissue was mounted in carbon paste on an aluminium stub and coated with gold using an SPI module sputter coater. Observations were conducted at 20 kV.
RESULTS
Fixed and critical point dried tissue samples failed to preserve stigmatic structure effectively as compared with observations of fresh, living samples. Considerable amounts of stigmatic exudate were retained, but losses during fixation and/or critical point drying were evident when fresh and fixed tissues were compared. Surface secretions in critical point dried tissues appeared as dried residues that were often irregular, plate‐like or granular. This was in contrast to the fluid‐like secretions observed in fresh tissue samples. Observations of fresh tissues were deemed more informative and accurate. Thus, the data summarized in Table 2 are based on fresh tissue observations.
Table 2.
Effects of spray treatments on the morphology of almond stigmatic surfaces
| Time after | Characteristic features* | ||
| Fungicide | spraying (h) | Stigma papillae | Exudate production |
| Water control | 4 | No damage, cells raised and intact | None‐to‐slight accumulation |
| 24 | No damage, cells raised and intact | Slight‐to‐extensive accumulation | |
| Azoxystrobin | 4 | No damage | Enhanced production, intermediate‐to‐extensive accumulation |
| 24 | Collapsed cells in localized areas | Enhanced production, extensive copious accumulation | |
| Myclobutanil | 4 | Collapsed cells, damage varying in area and location | Slight increase in exudate |
| 24 | Collapsed cells, damage more extensive than at 4 h | Same as control | |
| Iprodione | 4 | Collapsed cells, damage varying in area and location | Same as control |
| 24 | Severe collapse and flattening of cells, damage varying in area and location | Same as control | |
| Cyprodinil | 4 | Severe collapse of cells, damage varying in area and location | Same as control |
| 24 | Severe collapse of cells over extensive regions of the stigma | Enhanced production, copious exudate engulfing papillae | |
* Based on observations of five flowers per treatment. Times of observation were 4 and 24 h after spraying. All flowers within a treatment exhibited the features listed and, when specified, all five flowers had damage or enhanced exudate production greater than that of the controls.
Morphology of control stigmas
Pistil morphology in almond is characterized by a circular, bilobed stigmatic surface that expands slightly in a fan‐like manner beyond an elongated and cylindrical style (Fig. 2A). Stigmatic surface cells are composed of raised papillae (Fig. 2A and B). In flowers observed 4 h after spraying with water (Table 2), papilla cells were bulbous, elevated and intact. Electron‐dense, raised regions were evident, and visualized as mottled stigmatic exudate. In most cases exudate was uniformly dispersed. However, some flowers exhibited regions on the stigma where slight fluid secretions accumulated in interstices at the base of papillae (Fig. 2C). Stigmas of control flowers observed 24 h after spraying exhibited a range of exudate accumulation from minor (Fig. 2D) to more abundant (Fig. 2E and F). Exudate accumulation (Fig. 2F) occurred particularly at the central region of the stigma.
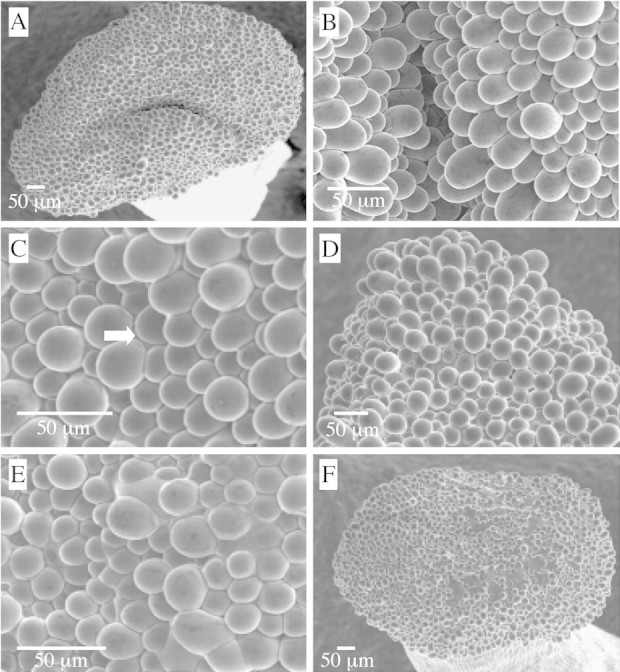
Fig. 2. Stigma morphology of almond flowers 4 (A–C) or 24 h (D–F) after spraying with water. A, Pistil morphology in almond is characterized by a circular, bilobed stigmatic surface that expands slightly in a fan‐like manner beyond an elongated and cylindrical style. B, Stigmatic surface cells are composed of raised papillae. C, Slight exudate secretions accumulated in interstices at the base of papillae; arrow indicates exudate accumulation. D–F, Showing variable exudate production. D, Slight accumulation, similar to that at 4 h. E and F, More extensive exudate accumulation.
Spray‐induced responses
Some of the fungicide spray treatments induced similar morphological responses. Thus, the types of general cytological reactions will be described first (Figs 3 and 4), followed by a characterization of the specific responses observed for each of the fungicide treatments (Table 2).
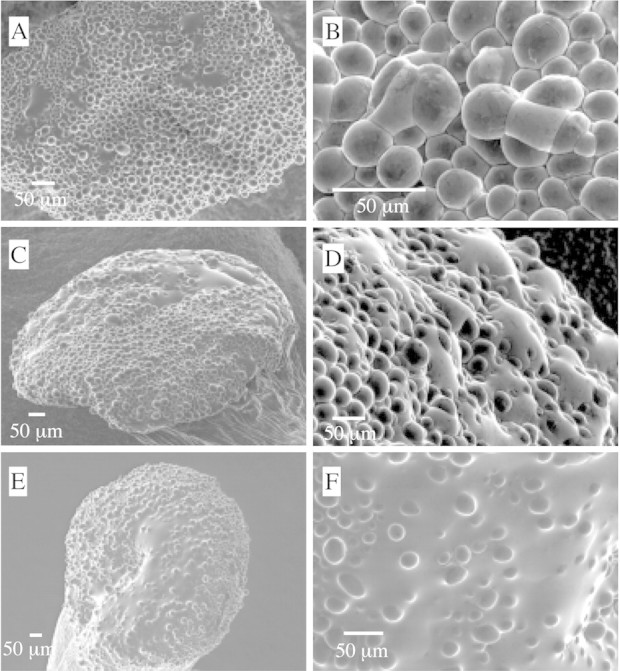
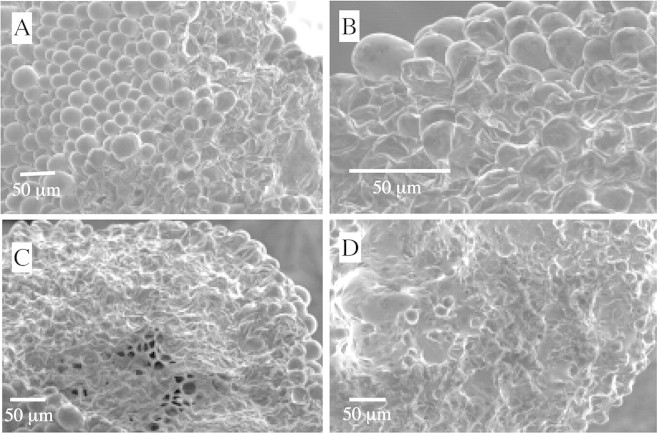
Fig. 3. Increased exudate accumulation induced by some fungicide spray materials. A, Fungicide‐induced accumulation occurred in any region of the stigmatic surface and was not necessarily limited to the depression between lobes. Image taken 4 h after spraying with azoxystrobin. B, Exudate production could be very localized with an accumulation observed between as few as four adjacent cells; neighbouring cells exhibited no or little accumulation. Image taken 4 h after spraying with azoxystrobin. C, Considerable exudate accumulation occurred in any region of the stigmatic surface. Image taken 4 h after spraying with azoxystrobin. D, Accumulation of fluid was observed bridging between cells. Image taken 4 h after spraying with azoxystrobin. E, Substantial and copious secretions inundated the whole stigmatic surface. Image taken 24 h after spraying with cyprodinil. F, Stigmatic papillae completely submerged or only apical regions exposed. Image taken 24 h after spraying with cyprodinil.
Fig. 4. Fungicide spray causes stigmatic papillae collapse. A, Localized fungicide‐spray damage to stigmatic papillae, with severely collapsed papillae occurring adjacent to normal, undamaged areas. Image taken 4 h after spraying with myclobutanil. B, Damaged papillae cells exhibit wrinkling and distortion of cell surfaces. Image taken 4 h after spraying with cyprodinil. C, Extensive damage with collapsed areas encompassing one‐third or more of the stigmatic surface. Papillae concave or totally flattened. Image taken 24 h after spraying with cyprodinil. D, Enhanced stigmatic exudate and cell damage can occur simultaneously. Image taken 24 h after spraying with iprodione.
Increased exudate accumulation was induced by some fungicide sprays and varied in extent (Fig. 3A–F). Exudate production could be very localized (Fig. 3B) with an accumulation between as few as four adjacent cells; neighbouring cells could exhibit no or little accumulation. Unlike controls where the greatest accumulation of stigmatic secretions occurred in the depression between lobes, fungicide‐induced accumulation occurred in all regions of the stigmatic surface (Fig. 3A, C and E). Accumulation of fluid was observed between cells (Fig. 3D). Some fungicide sprays induced substantial and copious secretions which inundated the whole stigmatic surface in some cases (Fig. 3E). Stigmatic papillae could be completely submerged or have only apical regions exposed (Fig. 3F).
Damage and collapse of stigmatic cells were also observed following application of some fungicide sprays (Fig. 4A–D). Damaged areas could be localized (Fig. 4A), where severely collapsed papillae occurred adjacent to normal, undamaged areas. Damage could also be extensive, with collapsed areas encompassing one‐third or more of the stigmatic surface (Fig. 4C). Damaged areas ranged from papillae cells exhibiting wrinkling and distortion of cell surfaces (Fig. 4B), to those that were concave or totally flattened (Fig. 4C). In addition to cases where enhanced stigmatic exudate and cell damage were observed independently, some fungicides induced a simultaneous occurrence (Fig. 4D): copious exudate production was associated with collapse and/or inversion of exposed papilla tips.
The specific effects of sprays on almond stigmas are summarized in Table 2. Increased exudate accumulation was induced by azoxystrobin at both 4 and 24 h after spraying. Exudate production ranged from that occurring at very localized regions to very expansive regions that could inundate the entire stigmatic surface. Exudate accumulation was more extensive and copious 24 h after spraying compared with that 4 h after spraying.
Although stigmatic papillae were generally intact 4 h after spraying with azoxystrobin, damage and collapse were observed 24 h after fungicide application. Damaged areas were generally localized, with severely collapsed papillae occurring adjacent to undamaged areas. Damage included wrinkling, distortion of cell surfaces or concave stigmatic papillae. Some stigmas showed both damage and exudate accumulation.
Myclobutanil caused significant damage to and collapse of stigmatic papillae. Damaged areas exhibited wrinkling, distortion of cell surfaces or concave stigmatic papillae as in the case of azoxystrobin. Damaged regions varied in area and location, and ranged from localized to extensive, with collapsed papillae sometimes encompassing one‐third or more of the stigmatic surface. In some samples, exudate production was associated with collapse and/or inversion of exposed papilla tips. More extensive collapse was observed at 24 h vs. 4 h. Compared with controls, a slight increase in exudate accumulation was noted 4 h after spraying. Exudate accumulation at 24 h was similar to that of controls at the same time.
Iprodione did not affect exudate accumulation, but caused marked and severe collapse of stigmatic papillae. As with other fungicides, damaged areas ranged from localized to extensive and varied in severity. More severe injury occurred at 24 h. Stigmatic cell collapse was observed with and without exudate accumulation.
Cyprodinil promoted a copious increase in exudate secretion that was evident after 24 h. Commonly, stigmatic papillae were totally submerged or had only apical regions exposed. Cyprodinil also caused severe collapse of stigmatic cells. Of all the fungicides evaluated, cyprodinil caused the most severe damage. Damage was somewhat localized at 4 h, but was more global at 24 h, often covering extensive regions.
Spraying with Rhodamine B dye
Spray applications of Rhodamine B verified that all stigmas received sprays. Stigmatic areas exhibited some variation in dye deposition.
DISCUSSION
This study has verified that fungicide sprays applied to flowers during anthesis can have a direct effect on stigma morphology and exudate production. All of the fungicides evaluated induced changes in stigmatic surfaces. Common responses were collapse of surface cells and enhanced production of exudate. The current study was conducted under controlled laboratory conditions using an apparatus that closely simulates field spray conditions. Sprays were directly targeted onto stigmatic surfaces of flowers at the same developmental stage. An additional feature of this study was that SEM observations directly assessed spray effects on the stigmatic surface, i.e. ultrastructural examinations were of living tissues, eliminating artefacts induced by fixation and critical point drying.
A marked collapse of stigmatic papillae was observed following application of some fungicides. This loss of cellular integrity could degrade the function of stigmatic cells and cause a loss of surface available to support pollen capture, hydration and germination. Deposition of large numbers of pollen grains on the stigma can be beneficial to fruit set in crops. Dennis (1979) found that a minimum of 50 pollen grains per flower is required for consistent fruit set in ‘Delicious’ apple even though fruits generally contain only ten ovules. Deposition of fewer pollen grains resulted in poorer germination and slower tube growth. In the current study, the collapse of stigmatic papillae might result in smaller numbers of pollen grains germinating. Therefore, although certain undamaged areas might still be receptive to pollen germination, fruit set could be detrimentally impacted.
Pollen activation and hydration is mediated by the uptake of water by colloidal imbibition and endosmosis. This hydrodynamical process strongly depends on the condition of the cytoplasm of the vegetative cell and the thickness of the intine because of its imbibition capacity (Johri, 1984). Wetzstein (1990) evaluated the effects of pesticidal sprays on the stigma in pecan and found that some fungicides were detrimental to pollen function. Benomyl applied in combination with triphenyltin hydroxide caused severe inhibition of pollen–stigma interactions, with pollen grains failing to hydrate. Although pollination was not performed in the current study, fungicides may hinder pollen hydration, especially in the collapsed areas without exudate, thereby reducing pollen germination on the stigma.
The stigmatic surface is a critical component in post‐pollination responses and plays a crucial role in pollen capture, adherence and germination. Any damage to the stigmatic surface by fungicide sprays could potentially affect stigma receptivity, decrease the effective pollination period (EPP), and thereby detrimentally impact fertilization. The EPP, i.e. the period when the embryo sac remains functional for fertilization minus the time required for pollen to reach the egg apparatus (Williams, 1969), is a very important factor for successful fertilization. It can be limited by the time period in which the stigma remains receptive. In kiwifruit, stigma receptivity was the main factor responsible for a short EPP (Gonzalez et al., 1995). In apricot (Prunus armeniaca L.), a close relative of almond, the short duration of stigma receptivity was the factor limiting EPP (Egea and Burgos, 1992). Stigma receptivity is considered by many as fundamental in explaining fruit yield differences (Egea et al., 1991). In almond, the highest fruit set was obtained in pollinations of newly opened flowers and it decreased significantly at progressively later stages (Vezvaei and Jackson, 1995).
Enhancement of exudate production was particularly marked in flowers sprayed with azoxystrobin and to a lesser extent with cyprodinil. Increased exudate production is commonly associated with flower development. Control flowers in the current study exhibited some increases in exudate accumulation at later observation times. However, fungicide‐induced increases were clearly greater than those induced by spraying with water. Copious exudate formation is characteristic of receptive flowers in some species; this is not the case in almond. In association with flower development studies with almond, we have evaluated stigmatic surface changes at different stages. Stigmatic papillae were intact with minimal exudate production at anthesis when petals were fully open (data not shown). Cell collapse and copious exudate did not appear until petal fall and flower senescence. This indicates that the exudate formation caused by fungicide sprays may be the signature of senescence. Further studies are required to determine whether fungicide‐induced exudates inhibit, or even prompt, pollen germination and tube growth. It is also possible that this is a senescence or stress response which may decrease the period of stigma receptivity or EPP, and thereby detrimentally impact fertilization.
Spray deposition patterns of Rhodamine B showed dye deposition was variable among stigmas. This could explain why damage observed after spraying varied in area and location. Given that under orchard conditions flowers are oriented in many directions whereas the sprays were targeted directly to stigmas in the current study, further field study is warranted. In addition, this study found that extraction of stigmatic exudate occurred during fixation and/or critical point drying, as evidenced by comparison with fresh tissue (data not shown). The dried surface secretions in fixed and critical point dried tissues sharply contrasted with the fluid‐like secretions observed in fresh tissue samples. Likewise, Cresti et al. (1982) observed that part of the stigmatic exudate in citrus (Citrus limon L.) stigmas was removed as a result of fixation and critical point drying. This emphasizes the need to consider sample preparation methods carefully and be aware of the potential introduction of artefacts in ultrastructural studies.
Supplementary Material
Received: 28 May 2002; Returned for revision: 22 July 2002; Accepted: 28 October 2002 Published electronically: 19 December 2002
References
- BristowPR, Shawa AY.1981. The influence of fungicides on pollen germination and yield of cranberry. Journal of American Society for Horticultural Science 106: 290–292. [Google Scholar]
- CostaG, Testolin R, Vizzotto G.1993. Kiwifruit pollination: an unbiased estimate of wind and bee contribution. New Zealand Journal of Crop and Horticultural Science 21: 189–195. [Google Scholar]
- CrestiM, Ciampolini F, Van Went JL, Wilms HJ.1982. Ultrastructure and histochemistry of Citrus limon (L.) stigma. Planta 156: 1–9. [DOI] [PubMed] [Google Scholar]
- 4.DagA, Eisikowitch D.1995. The influence of hive location on honeybee foraging activity and fruit set in melons grown in plastic greenhouse. Apidologie 26: 511–519. [Google Scholar]
- DennisFG Jr. 1979. Factors affecting yield in apple with emphasis on ‘Delicious’. Hortcultural Reviews 1: 395–422. [Google Scholar]
- 6.EatonGW, Chen LI.1969. The effect of Captan on strawberry pollen germination. Journal of American Society for Horticultural Science 94: 558–560. [Google Scholar]
- EgeaJ, Burgos L.1992. Effective pollination period as related to stigma receptivity in apricot. Scientia Horticulturae 52: 77–83. [Google Scholar]
- EgeaJ, Burgos L, Garcia JE, Egea L.1991. Stigma receptivity and style performance in several apricot cultivars. Journal of Horticultural Science 66: 19–25. [Google Scholar]
- FalqueM, Vincent A, Vaissiere BE, Eskes AB.1995. Effect of pollination intensity on fruit and seed set in cacao (Theobroma cacao L.). Sexual Plant Reproduction 8: 354–360. [Google Scholar]
- GaryNE, Whitherell PC, Martson JM.1976. The inter‐ and intra‐orchard distribution of honeybees during almond pollination. Journal of Apicultural Research 15: 43–50. [Google Scholar]
- GonzalezMV, Coque M, Herrero M.1995. Stigmatic receptivity limits the effective pollination period in kiwifruit. Journal of American Society for Horticultural Science 120: 199–202. [Google Scholar]
- 12.GonzalezMV, Coque M, Herrero M.1998. Influence of pollination systems on fruit set and fruit quality in kiwifruit (Actinidia deliciosa). Annals of Applied Biology 132: 349–355. [Google Scholar]
- HeY, Wetzstein HY.1994. Pollen degeneration and retarded leaf development from fungicidal sprays applied during microspore development and shoot expansion. Journal of Horticultural Science 69: 975–983. [Google Scholar]
- HutcheonJA, Coyle J, Holdgate ME, Byrde RJW.1986. Effects of fungicides on long‐term cropping and fruit quality of apple. Plant Pathology 35: 249–353. [Google Scholar]
- JohriBM.1984. Embryology of angiosperms. Berlin: Springer‐Verlag. [Google Scholar]
- KovachJ, Petzoldt R, Harman GE.2000. Use of honey bees and bumble bees to disseminate Trichoderma harzianum 1295‐22 to strawberries for Botrytis control. Biological Control 18: 235–242. [Google Scholar]
- OzgenM, Palta JP.2001. Use of Lysophoshatidylethanolamine (LPE), a natural lipid, to mitigate undesirable effects of a fungicide (Bravo) on cranberries. HortScience 36: 579. [Google Scholar]
- RaghavanV.1997. Molecular embryology of flowering plants. Cambridge: Cambridge University Press. [Google Scholar]
- RedalenG.1980. Effects of fungicides on pollen germination and fruit set in raspberries. Gartenbauwissenschaft 45: 248–251. [Google Scholar]
- ShawaAY, Doughty CC, Johnson F.1966. Effect of fungicides on McFarlin cranberry pollen germination and fruit set. Proceedings of American Society for Horticultural Science 89: 255–258. [Google Scholar]
- VezvaeiA, Jackson JF.1995. Effect of pollen parent and stages of flower development on almond nut production. Australian Journal of Experimental Agriculture 35: 109–113. [Google Scholar]
- VolzRK, Tustin DS, Ferguson IB.1996. Pollination effects on fruit mineral composition, seeds and cropping characteristics of ‘Braeburn’ apple trees. Scientia Horticulturae 66: 169–180. [Google Scholar]
- WetzsteinHY.1990. Stigmatic surface degeneration and inhibition of pollen germination with selected pesticidal sprays during receptivity in pecan. Journal of American Society for Horticultural Science 115: 656–661. [Google Scholar]
- WilliamsRR.1969. Factors affecting pollination in fruit trees. In: Luckwill LC, Cutting CV, eds. Physiology of tree crops London: Academic Press, 193–207. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.