Abstract
Variation in the onset of summer dormancy and flowering capacity of 16 populations of Poa bulbosa, collected along a steep north–south aridity gradient in Israel (810–110 mm rain year–1), was studied under controlled conditions in a phytotron (16 h daylength, 22/16 °C day/night) and under natural conditions in a garden experiment in a net‐house. Plant age at the onset of dormancy varied markedly amongst populations (7–16 weeks under controlled conditions) and was positively correlated with mean annual precipitation at the site of origin of the population, i.e. dormancy was earlier as aridity increased. Flowering capacity in the different populations was negatively correlated with rainfall in the original habitat and, consequently, also with the age at onset of dormancy, i.e. the lower the mean annual precipitation, the earlier the onset of dormancy and the higher the proportion of flowering plants and panicles per plant. Differences in xeromorphic leaf traits were also observed among populations from locations differing in aridity. Plants from the more arid sites (110–310 mm year–1) generally had greyish and curved leaves, whereas plants from more humid sites (500–810 mm year–1) tended to have green and straight leaves. Thus, plants with curved and/or greyish leaves generally had a higher flowering capacity and entered dormancy earlier than plants with straight and/or green leaves. The significance of the association among these traits for the adaptation of P. bulbosa to increasing aridity is discussed.
Key words: Aridity, ecotypes, flowering, leaf traits, Poa bulbosa L., summer dormancy
INTRODUCTION
Perennial plants that inhabit regions with seasonal environments must adapt their life cycle to the changing conditions posed by the alternating favourable and constraining seasons. Induction of plant dormancy, either by external factors (Noodén and Weber, 1978; Lang et al., 1987) or by developmentally programmed endogenous processes (Le Nard and De Hertogh, 1993; Naor and Kigel, 2002), minimizes the risk of death during subsequent stressful periods. Within this context dormancy represents a form of stress evasion since active growth and reproduction are suppressed in dormant plants, rendering them more tolerant of extreme environmental conditions (Koller, 1969; Hoffman and Parsons, 1993). Furthermore, as a result of adaptive selection, variation in the timing of onset, and duration, of dormancy should be correlated with changes in environmental conditions that occur along climatic gradients. Indeed, latitudinal and altitudinal clines in the onset and duration of the dormant phase have been reported for locally adapted populations or ecotypes of winter‐dormant woody and herbaceous perennial species (Noodén and Weber, 1978; Myking and Heide, 1995; Clapham et al., 1998).
In regions with a Mediterranean climate, where a prolonged hot and dry summer follows a mild, wet winter, many perennial plants and geophytes enter a phase of summer dormancy in the spring, before the environmental conditions become too stressful (Laude, 1953; Dafni et al., 1981; Ofir and Kerem, 1982; Ofir and Dorenfeld, 1992; Volaire, 1995; Rundel, 1996; Parsons, 2000). Summer dormancy may last several months. Growth is resumed in the autumn, after the first effective rains, when the availability of soil moisture increases and temperatures become more moderate. Growth continues during winter and early spring. In this case, imposition of summer dormancy allows plants to escape from prolonged drought stress, and increases their chances of survival, future growth and reproduction (Pate and Dixon, 1982).
In some perennial grasses the resting phase during the summer is enforced by drought, and growth is resumed quickly if water is provided during the dry season (Laude, 1953; Oram, 1983; Volaire, 1995; Pugnaire et al., 1996); this is known as enforced dormancy (Lang et al., 1987). In other grasses and geophytes, summer dormancy is induced by the interaction of specific environmental factors prevailing during late winter and early spring, such as lengthening days, increasing temperature and drought (Ofir and Kerem, 1982; Ben‐Hod et al., 1988; Brewster, 1990). After the onset of dormancy, growth does not occur even under favourable conditions until dormancy is relaxed; this is true dormancy. In these species the rate of relaxation of dormancy is affected by environmental conditions, mainly by high temperature during the summer (Ofir, 1986). As dormancy is lost, growth will take place under a progressively wider range of climatic conditions (mainly temperature), and during this period plants are in a relative or conditional dormancy (Vegis, 1964).
Intraspecific variation ranging between enforced and true summer dormancy (i.e. the ability to grow during the summer after occasional summer rains or under irrigation) has been reported for perennial grasses collected along wide climatic gradients [Phalaris tuberosa L. (syn. aquatica) L., Sankary et al., 1969; Oram, 1983; Dactylis glomerata L., Volaire, 1995]. In geophytes, information on whether variation in dormancy is genetically or environmentally determined is scarce (Rees, 1992; Parsons, 2000). Induction of true summer dormancy in geophytes may require threshold levels of specific inductive environmental factors, such as daylength (Ofir and Kigel, 1999), temperature (Ben‐Hod et al., 1988) and drought (Vaughton and Ramsey, 2001). Thus, it can be argued that in habitats with interannual variation in summer conditions, in which threshold levels of the limiting environmental factor may occur quite late in the season or are not met every year, induction of summer dormancy can be facultative for some or all genotypes of a given population, allowing a longer growth period if conditions are favourable (Vaughton and Ramsey, 2001). In contrast, in habitats in which the constraining summer conditions are highly predictable and occur every year, obligate and more synchronous summer dormancy should occur in the population. Furthermore, earlier and less variable onset of dormancy should characterize populations along gradients of increasing aridity since this reduces the risk of death due to the increased variability in temporal distribution of rainfall events.
The onset of summer dormancy was studied in populations of the winter‐growing geophytic grass Poa bulbosa L., sampled along a steep gradient of decreasing rainfall (810 to 110 mm year–1), across a relatively narrow transect (approx. 200 km) between the Mediterranean and desert regions in Israel. Differences in altitude and latitude along this transect are small. It was therefore assumed that along the transect rainfall was the main climatic factor affecting plant growth, since differences in temperature and daylength during late winter and spring are relatively small. Variation in the timing of onset of dormancy along an aridity gradient, in which the amount and predictability of rainfall changes drastically, is crucial for survival of this species, particularly since it commonly grows in poor and shallow soils (Ofir and Dorenfeld, 1992).
Populations of P. bulbosa differ in the extent of flowering (Youngner, 1960; Heyn, 1962). In some populations flowering is widespread, whereas in others it is absent or rare, and reproduction is solely or mainly vegetative, i.e. by tillering and development of basal tiller bulbs. The development of small vegetative propagules with bulbils within the florets (inflorescence proliferation) is also common (Heyn, 1962; Davis, 1985). Since tillering ceases as plants become dormant, variation in the time of the onset of dormancy may also impinge on the flowering potential as well as on the balance between seed and asexual (vegetative and/or proliferation) reproduction in the different populations. In addition to this variation in reproduction pattern, populations of P. bulbosa from different habitats also vary in the shape (curved vs. straight) and colour (greyish vs. green) of their leaves (Feinbrun‐Dothan, 1986). Such variation in foliar type may represent various degrees of xeromorphic adaptation to the conditions prevailing in habitats differing in aridity (Ehleringer, 1980).
The main goals of the present study on P. bulbosa were to examine populations along an aridity gradient and explore (1) relationships between the timing of the onset of summer dormancy during the growth cycle and mean rainfall at the site of the origin of the population; (2) the relationship between onset of dormancy and flowering capacity; and (3) the association among onset of dormancy, flowering and leaf traits in relation to adaptation to increasing aridity.
MATERIALS AND METHODS
Plant material
Poa bulbosa L. is a small geophytic, perennial grass with a distinct summer dormancy (Burns, 1946; Ofir and Kerem, 1982). It grows on shallow, poor soils and withstands intensive grazing, though its value as a pasture grass is limited due to its low production capacity and short growing season. The plants grow in dense, usually well‐defined, small clumps that probably represent separate clones within the population. It is widely distributed in the Mediterranean and central Asia phytogeographic regions, and has become naturalized throughout temperate and sub‐tropical regions of America, particularly in regions with a Mediterranean type of climate, as in California and central Chile. In Israel, three species (P. bulbosa L., P. sinaica Steud. and P. eigii Feinbr.) are recognized as being part of an old evolutionary complex, i.e. P. bulbosa, that may reproduce sexually, apomictically and vegetatively by means of seeds, basal tiller bulbs and dispersal bulbils formed on proliferating panicles (Kennedy, 1929; Youngner, 1960; Heyn, 1962; Davis, 1985). Seed set is rare even if non‐proliferating panicles are produced (Heyn, 1962).
Poa bulbosa becomes dormant quite early in the growth season, well before the end of the rainy period. Leaf production ceases, leaves senesce and desiccate, and small, dormant true bulbs are produced by vegetative tillers at the soil surface. These are clear morphological and developmental indications of the imposition of summer dormancy. In the coastal plain of Israel, leaf senescence and bulbing were observed by February (Ofir and Dorenfeld, 1992), and leaves desiccated during March (Ofir and Kerem, 1982). Summer dormancy is induced by relatively short ‘long days’ (critical photoperiod of 11–12 h; Ofir and Kigel, 1999). High temperatures enhance the induction by long days (Ofir and Kerem, 1982). Preliminary observations indicate that drought, even under short days, could impose dormancy. Dormancy relaxation was hastened by high temperatures (30–40 °C) under dry conditions (dry storage), as experienced during summer. Bulb sprouting occurred in wet conditions and was improved by low temperatures (10 vs. 20 °C), as experienced in the autumn (Ofir, 1986). In the field, bulbs sprout after the first rains in the autumn, and plants may flower by the end of winter (March).
Sampling of populations
Sixteen populations of P. bulbosa were sampled along a north–south aridity (rainfall) gradient in Israel (approx. 200 km), during April 2000 (Table 1). The latitude range across the collecting sites was 31°15′ to 33°03′, and altitude varied between 50 and 600 m a.s.l. Mean daily temperature in spring (February–April), when the onset of dormancy was observed in the field, ranged between 17·2 and 17·9 °C (Bitan and Rubin, 1991). Six to 15 clumps of P. bulbosa per population were lifted from the field and transplanted to a net‐house, affording protection against bird attack, at the Faculty of Agriculture in Rehovot. Each clump was considered a ‘parent plant’.
Table 1.
Description of locations where P. bulbosa populations were sampled
| Population code | Location | Soil | Annual rainfall (mm year–1) | No. of parent plants |
| 8 | Road Arad to Dead Sea, N.F.S. | Stony loess* | 110 | 11 |
| 10 | Mitspe Jericho, E.F.S. | Rendzinic desert lithosol | 120 | 15 |
| 5 | Tel Arad, N.F.S. | Stony loess* | 180 | 8 |
| 4 | Har Amasa, Plain | Loessial alluvium | 220 | 7 |
| 11 | Maale Edumim, E.F.S. | Terra rossa | 250 | 15 |
| 1 | Har Amasa, N.F.S. and hill‐top, rock crevices | Rendzina | 276 | 12 |
| 2 | Har Amasa, foothill | Rendzina | 276 | 9 |
| 3 | Har Amasa, plain | Stony loess* | 276 | 11 |
| 17a | Lehavim, N.F.S. | Stony loess* | 307 | 6 |
| 17b | Lehavim, wadi | Loess* | 307 | 7 |
| 17c | Lehavim, S.F.S. | Stony loess* | 307 | 6 |
| 15 | Nahshon, N.F.S. | Rendzina | 514 | 7 |
| 13 | Karei Deshe, meadow | Basaltic | 520 | 7 |
| 18 | Tirat Shalom, N.F.S. and hill‐top | Calcareous sandstone | 530 | 5 |
| 12 | Park Rabin, hill‐top, rock crevices | Terra rossa | 620 | 7 |
| 14 | Montfort, exposed terraces | Terra rossa | 810 | 9 |
N.F.S., E.F.S., S.F.S, North‐, east‐ and south‐facing slope, respectively.
Wadi, Dry river terraces.
* Loess‐derived soil (Serozem).
Net‐house experiment: natural conditions
Parent plants (6–15 per population) were grown in 4 l pots filled with loess soil. They were kept continuously outdoors in the net‐house under natural conditions and were exposed to natural rainfall. Pots were marked to identify the source population and parent plants. During the growth season (November–March), plants were irrigated occasionally to prevent extreme drought stress. Leaf colour and shape, the proportions of flowering plants and of plants with proliferated panicles, the number of panicles per plant and the proportion of dormant plants were recorded in February and March 2002, approx. 2 years after transplanting from the field, to avoid short‐term carry‐over effects from previous field conditions in the different locations where populations were sampled. Basal tiller bulbs produced by these parent plants were collected separately and planted under controlled conditions (in a phytotron). Plants that sprouted from these bulbs were termed ‘daughter plants’ and were used in the phytotron experiment described below.
Phytotron experiment: controlled conditions
The experiment under controlled daylength and temperature was carried out in the phytotron of the Faculty of Agriculture, Rehovot, in glass‐covered growth rooms transmitting approx. 80 % of outside solar radiation. Plants were grown at 22/16 °C (day/night temperatures controlled within ± 0·5 °C) and 65/75 % relative humidity. Day temperature and humidity were maintained between 0700 and 1600 h. Changes between day and night temperatures were gradual, spanning 3 h. The thermoperiod chosen was favourable for vegetative development of non‐vernalized plants (Ofir and Kigel, 1999). The photoperiod was 16 h [long days (LD) 0400–2000 h], attained by extending the natural daylength with the use of supplementary lighting (3–5 µmol m–2 s–1 photosynthetically active radiation at plant level) using 75 W incandescent tungsten lamps (LM960 Osram GmbH, München, Germany). This daylength was chosen since it is highly inductive for dormancy and is much longer than the critical daylength of 11–12 h necessary for the induction of dormancy at 22/17 °C (Ofir and Kigel, 1999). Plants were grown in 0·8 l drained pots, in a substrate of vermiculite and volcanic tuff‐gravel (1 : 1 v/v), and irrigated alternately with tap water and 50 % Hoagland’s nutrient solution once every 2 d.
Dormant bulbs for use in the phytotron experiment were collected from parent plants in the net‐house on 20 May 2001 (6–15 parent plants per population). Bulbs were stored dry at 40 °C to eliminate dormancy and facilitate simultaneous sprouting (0fir, 1986). Bulbs were planted on 10 July 2001 in 1 l drained plastic pots (30 per pot) containing wet vermiculite and volcanic tuff gravel, and kept for 8–13 d at 10 °C to enhance sprouting. After sprouting, ‘daughter plants’ were transferred to the phytotron (22/16 °C, LD) and transplanted to 0·8 l drained pots, with four daughter plants per pot, and four to five pots per parental plant. Each pot was marked to identify the parent plant and source population. Some of the plants died during the experiment, leaving 4–16 daughter plants per parental plant. Plant traits recorded in the phytotron were time course of onset of dormancy, flowering and leaf traits.
Plant traits
Dormancy.
Plants with mostly green leaves were considered non‐dormant (dormancy degree 0). If approx. half of a plant’s leaves were yellowing or drying, the plant was scored as dormancy degree 1. A plant was considered dormant (dormancy degree 2) when most (>75 %) of its leaves were yellowing or drying and all its tillers had bulbs (Ofir and Dorenfeld, 1992). The number of days from sprouting to full dormancy (degree 2) was taken to be the plant age at onset of dormancy.
Flowering and proliferation.
The proportion of flowering plants and plants with proliferating panicles was recorded.
Leaf characteristics.
Parental plants in the net‐house and corresponding daughter plants grown in the phytotron had leaves of similar shape and colour. Therefore, results involving leaf characteristics were based on phytotron observations. The following leaf traits were recorded: (1) colour: green (gn), greyish (gy), and intermediate (gy‐gn); and (2) shape (degree of leaf curvature): straight (S), curved (C), and intermediate, slightly curved (sC).
Statistical analyses
Pots in both experiments were arranged in a completely randomized design. Statistical analyses were carried out using JMPIN (version 4.04; SAS Institute Inc., Cary, NC, USA). For the phytotron experiment, age at the onset of dormancy of daughter plants, as replicates, was subjected to a nested ANOVA, with parent plants nested within populations. The dependence of age at dormancy on leaf colour or shape (data from the phytotron experiment) was analysed using parent‐plant means as replicates in a one‐way ANOVA. This was also the case for comparisons involving flowering data from the net‐house experiment. Means were compared using the LSMEANS test at a significance level of P = 0·05. Confidence intervals (95 %) were calculated to assess the significance of differences between results that were presented as proportions (%) (Snedecor and Cochran, 1980); confidence intervals that did not overlap were considered significant (P < 0·05). Associations between traits were analysed using contingency tables and Pearson’s χ2‐test (Sokal and Rohlf, 1995).
RESULTS
Plant dormancy
Among populations sampled along the rainfall gradient, differences in plant age at the onset of dormancy in the phytotron under LD (22/16 °C) were highly significant (F = 175·4, d.f. = 15, P < 0·0001). The ANOVA model explained 78 % of the experimental variance in plant age at the onset of dormancy, with the origin of populations and of parental plants accounting for 57 and 43 % of the explained variance, respectively. Plant age at onset of dormancy ranged between 7 weeks in the Mitspe Jericho population (population 10; 120 mm rain year–1 at the site of origin) and 16 weeks in the Tirat Shalom population (population 18; 530 mm rain year–1 at the site of origin). Furthermore, a general trend of earlier onset of plant dormancy with decreasing mean annual rainfall at the site of origin was observed, as shown by the significant linear regression between age at the onset of dormancy and mean annual rainfall at the site of origin (y = 57·7 + 0·07x, r = 0·600, P < 0·02, d.f = 14; Fig. 1A). Similar trends were found in the net‐house experiment. However, since a significant correlation was found between the dormancy score of parental plants as determined in March 2002 in the net‐house experiment, and age at the onset of dormancy in the phytotron experiment (r = –0·535, P = 0·05, d.f. = 12), data from the phytotron experiment only are presented.
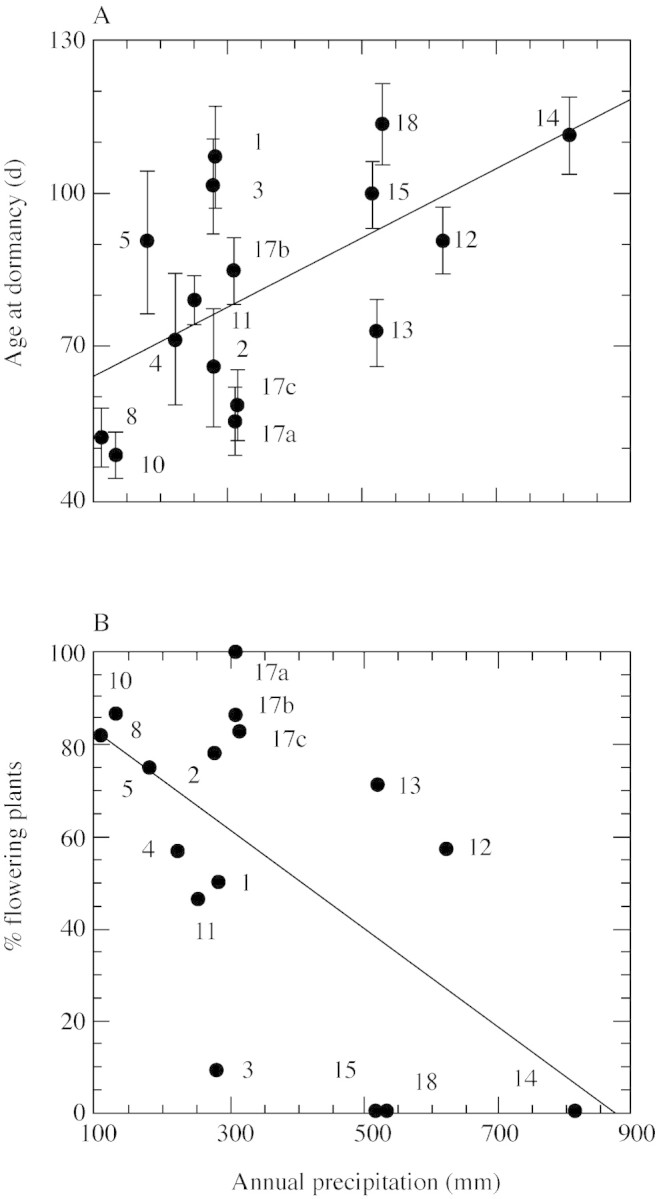
Fig. 1. Relationships between (A) age at onset of dormancy and (B) proportions of flowering plants (%) and aridity (mean annual precipitation) at the site of origin of the populations of P. bulbosa. Data are population means ± s.e. Numbers indicate population codes (see Table 1). A, Age at onset of dormancy (days from sprouting) was determined in daughter plants grown in the phytotron under 16 h days, 22/16 °C (day/night). Regression y = 57·7 + 0·07x, r = 0·600, P < 0·02, d.f = 14. B, Proportions of flowering parental plants were determined under natural conditions (net‐house). Regression y = 92·9 – 0·11x, r = –0·596, P < 0·02, d.f = 14.
Flowering
Proportions of flowering daughter plants in the phytotron were very low (0–14 %) compared with those in the net‐house (up to 100 %). Apparently, the daylength in the phytotron (16 h) was highly inductive for dormancy, and resulted in a marked reduction in flowering due to the early dormancy. Thus, the flowering data of the parental plants in the net‐house only are presented (Table 3). Populations of P. bulbosa differed markedly in the proportion of flowering plants. In some populations no flowering was observed, whereas in others most plants flowered. The incidence of flowering was greater in populations from the more xeric sites. A significant negative, linear regression was found between the percentage of flowering in the different populations and mean annual precipitation at the site of population origin (r = –0·596, P < 0·02, d.f = 14; Fig. 1B). A similar trend was observed for panicles per plant, but the correlation was not significant (r = –0·265, P > 0·1, d.f. = 14).
Table 3.
Leaf colour, leaf shape, age at dormancy (± s.e.) and % flowering of P. bulbosa plants from populations sampled along an aridity gradient
| Leaf colour | Leaf shape | ||||||||
| gy | gy‐gn | gn | C | sC | S | ||||
| Population | Annual rainfall (mm year–1) | Age at dormancy (days) | % Flowering plants | % Parental plants | |||||
| 8 | 110 | 52 ± 5·5 | 82 | 100 | 0 | 0 | 73 | 18 | 9 |
| 10 | 120 | 48 ± 4·3 | 87 | 20 | 80 | 0 | 33 | 53 | 13 |
| 5 | 180 | 90 ± 13·8 | 75 | 62 | 25 | 13 | 75 | 0 | 25 |
| 4 | 220 | 71 ± 12·5 | 57 | 71 | 0 | 9 | 57 | 14 | 29 |
| 11 | 250 | 79 ± 4·4 | 47 | 13 | 47 | 40 | 0 | 27 | 73 |
| 1 | 276 | 107 ± 9·8 | 50 | 67 | 33 | 0 | 67 | 33 | 0 |
| 2 | 276 | 65 ± 11·4 | 78 | 100 | 0 | 0 | 78 | 12 | 0 |
| 3 | 276 | 101 ± 8·9 | 9 | 27 | 18 | 55 | 18 | 18 | 64 |
| 17a | 307 | 55 ± 6·5 | 100 | 83 | 17 | 0 | 100 | 0 | 0 |
| 17c | 307 | 58 ± 6·7 | 83 | 100 | 0 | 0 | 83 | 17 | 0 |
| 17b | 307 | 84 ± 6·4 | 86 | 57 | 43 | 0 | 29 | 29 | 43 |
| 15 | 514 | 100 ± 6·5 | 0 | 29 | 57 | 14 | 29 | 29 | 43 |
| 13 | 520 | 72 ± 6·5 | 71 | 29 | 71 | 0 | 14 | 29 | 57 |
| 18 | 530 | 113 ± 7·6 | 0 | 0 | 0 | 100 | 0 | 0 | 100 |
| 12 | 620 | 90 ± 6·3 | 57 | 0 | 14 | 86 | 0 | 0 | 100 |
| 14 | 810 | 111 ± 7·4 | 0 | 22 | 33 | 44 | 0 | 44 | 56 |
See Table 1 for details of populations and number of plants sampled.
Preliminary field observations suggested that populations exhibiting early dormancy tended to have few flowering plants. Relationships between flowering and dormancy were examined by comparing flowering of parental plants in the net‐house, and plant age at the onset of dormancy as determined for the corresponding daughter plants in the phytotron. Indeed, the percentage of flowering plants and the number of panicles per parent plant were negatively correlated with age at the onset of dormancy in daughter plants (% flowering r = –0·826, P < 0·001, d.f. = 14; Fig. 2A; panicles r = –0·594, P < 0·02, d.f. = 14; Fig. 2B).
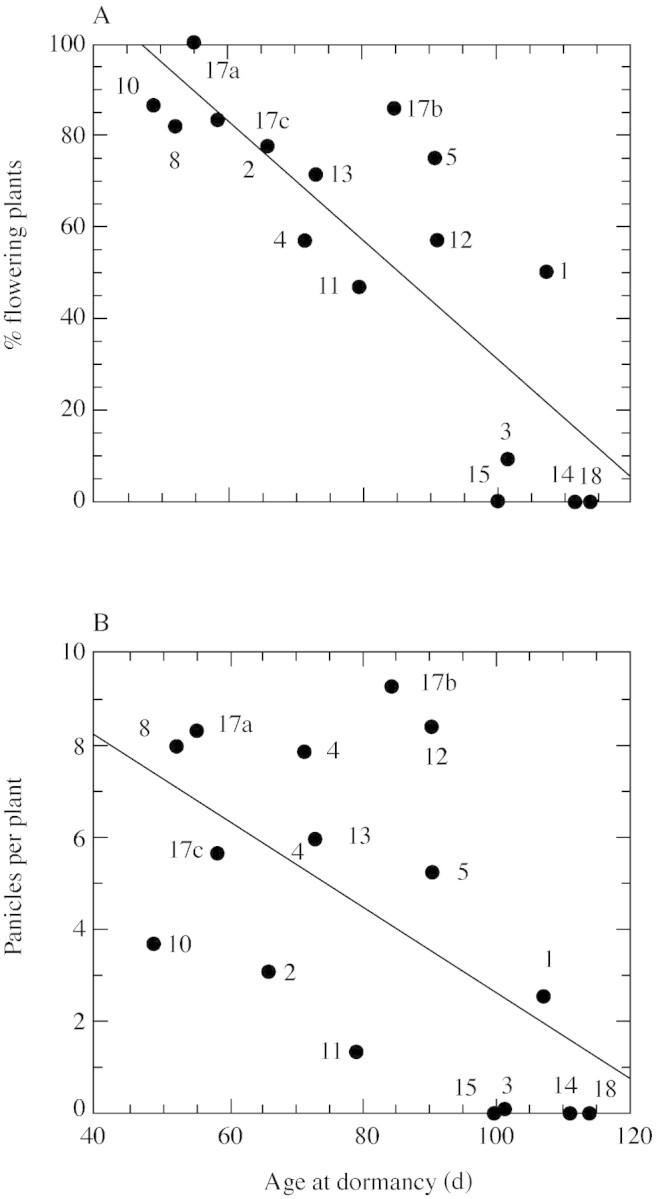
Fig. 2. Relationships between (A) proportions of flowering plants (%) and (B) number of panicles per plant and age at onset of dormancy (days from sprouting) in P. bulbosa plants sampled at locations with different aridities (110–810 mm rain year–1). Flowering was determined in parental plants grown under natural conditions (net‐house). Age at the onset of dormancy was determined in daughter plants grown in the phytotron under 16 h days, 22/16 °C (day/night). A, % flowering y = 162·0 – 1·3x, r = –0·826, d.f. = 14, P < 0·001; B, panicles per plant y = 11·98 – 0·09x, r = –0·594, d.f. = 14, P < 0·02. Numbers indicate population codes (see Table 1).
Inflorescence proliferation (production of propagules in florets) was common in populations 4, 12 and 13 (80, 75 and 100 % of flowering plants, respectively). A small bulb developed at the base of each propagule, in a similar manner to the development of bulbs at the base of dormant tillers. These propagules are capable of growth and contribute to the asexual reproduction and dispersion of the plants. No clear pattern was evident between the potential of the population to produce propagules and mean rainfall at the site of origin of the population. Rainfall at the site of origin ranged from low (population 4, 220 mm year–1) to high (populations 12 and 13, 620 and 520 mm year–1, respectively).
Leaf characteristics
Besides differences in the onset of dormancy and flowering, populations of P. bulbosa also differed in leaf characteristics. Different combinations of leaf colour and shape were observed (Table 2). Taking all 142 parental plants into account, the largest sub‐groups were those with greyish and curved leaves (gy,C) and those with green and straight leaves (gn,S) (33·8 and 20·4 %, respectively; Fig. 3). However, other combinations, mainly with the intermediate greyish‐green (gy‐gn) or slightly curved (sC) leaf types were also observed. No plant had green, curved leaves, and only one had greyish, straight leaves. The association between the colour and shape traits was significant, as shown by pooling together all parental plants from all populations (χ2 = 27·7, P = 0·0002, n = 142; Fig. 4). Thus, 85 % of plants with curved leaves were of the greyish type and none were of the green type; 56 % of plants with straight leaves were of the green type and only 2 % were of the greyish type (Fig. 4).
Table 2.
Proportion of leaf types in P. bulbosa plants in populations sampled along an aridity gradient
| Leaf colour | ||||
| Greyish | Greyish‐green | Green | Total | |
| Leaf shape | % parental plants | |||
| Curved | 33·8 | 5·6 | 0·0 | 39·4 |
| Slightly curved | 12·7 | 9·9 | 1·4 | 24·0 |
| Straight | 0·7 | 15·5 | 20·4 | 36·6 |
| Total | 47·2 | 31·0 | 21·8 | 100·0 |
Different combinations of leaf colour and shape are presented. Data are for 142 parental plants. The association between leaf shape and leaf colour traits was significant (χ2 = 27·7, P = 0·0002).

Fig. 3. Leaf types in P. bulbosa plants. Plant on right‐hand side is from Tirat Shalom (population 18), showing green and straight leaves; plant on left‐hand side is from Lehavim (population 17) with greyish, curved leaves. Bar = 5 cm.
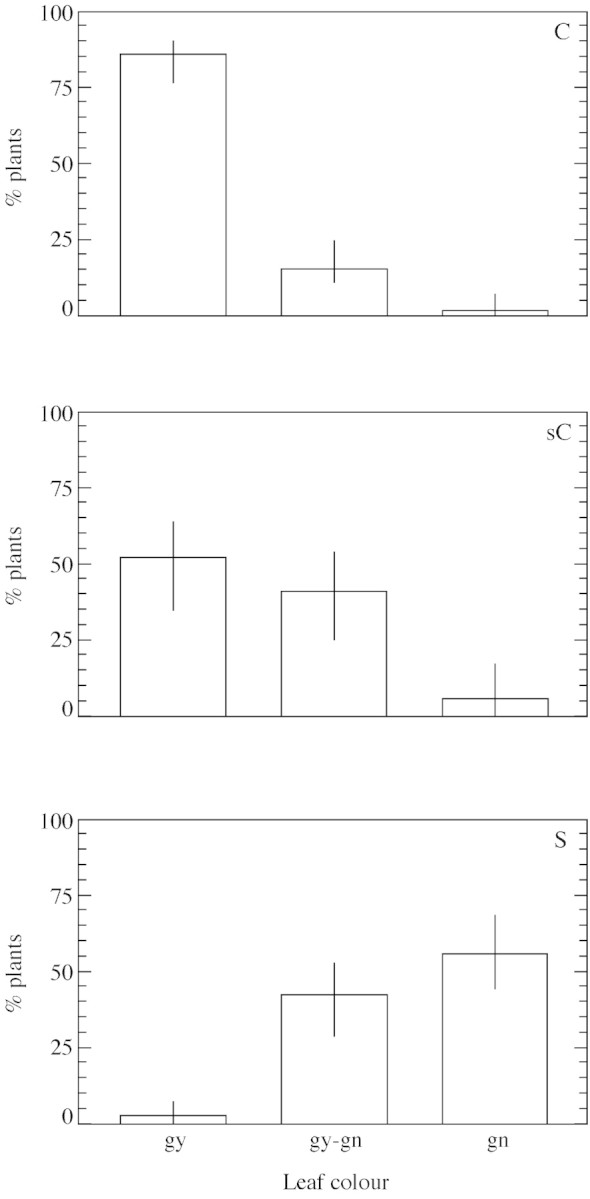
Fig. 4. Proportions (%) of P. bulbosa plants with different leaf colours (gy, greyish; gy‐gn, greyish‐green; gn, green) in each of the sub‐groups with the following leaf shapes: curved (C, 55 plants), slightly curved (sC, 34 plants) and straight (S, 52 plants). Populations were sampled along an aridity gradient of 110–810 mm rain year–1. Bars are 95 % confidence intervals.
Leaf traits, onset of dormancy and flowering
Associations among aridity, leaf traits, dormancy and flowering capacity were analysed by pooling together data for all parent plants. Leaf colour and shape were associated with rainfall at the site of origin (χ2 = 27·7, P = 0·0002, n = 142; Table 3; Fig. 5). In arid habitats (110–310 mm year–1), plants with greyish and curved or slightly curved leaves were more abundant than plants with green and straight leaves, whereas in habitats with high annual precipitation (500–810 mm year–1), plants with green and straight leaves were more typical.
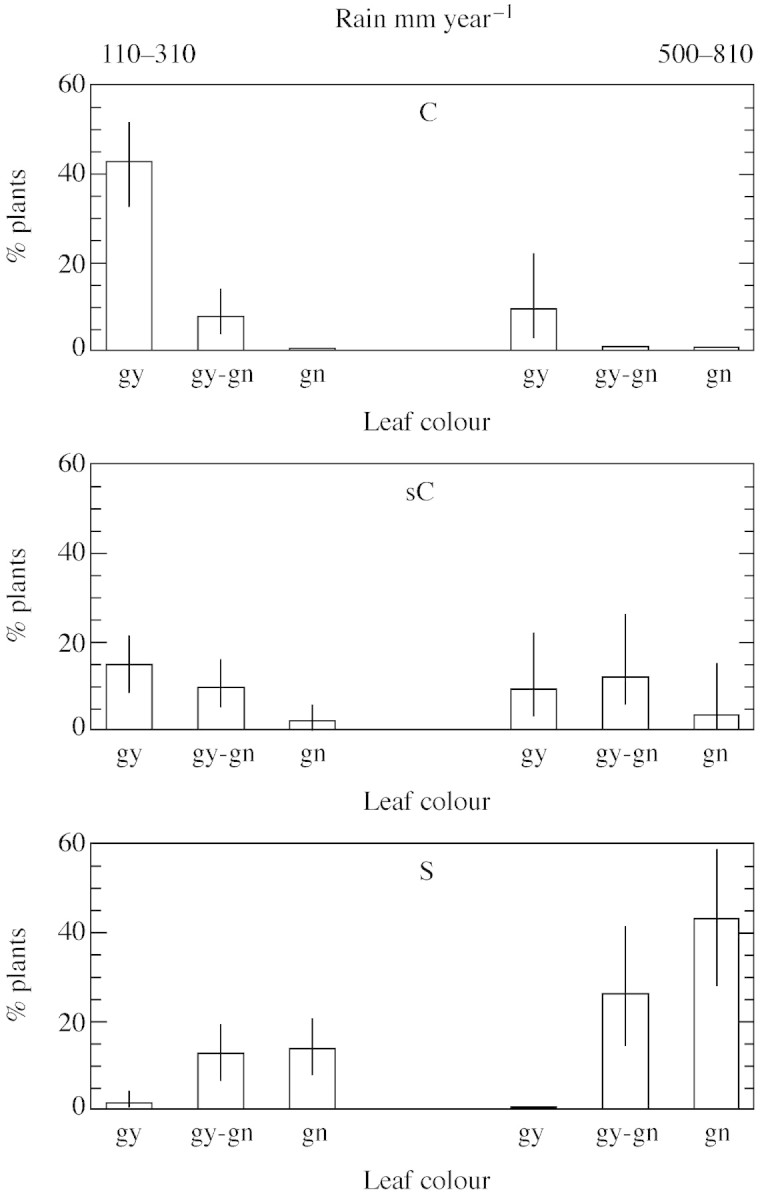
Fig. 5. Proportions (%) of P. bulbosa plants with different leaf shapes (C, curved, sC, slightly curved and S, straight) and leaf colours (gy, greyish; gy‐gn, greyish‐green; gn, green) in populations sampled at arid habitats (110–310 mm rain year–1, n = 107) and at mesic habitats (500–810 mm rain year–1, n = 35). Bars are 95 % confidence intervals. Proportions of plants with different combinations of leaf shape and colour were calculated separately for the arid and mesic populations.
Leaf traits were also related to age at the onset of dormancy (Table 3; Fig. 6A; phytotron data). The dependence of the age at the onset of dormancy on leaf shape or colour was analysed by one‐way ANOVA (shape F = 5·44, P < 0·005, d.f. = 138; colour F = 12·00, P < 0·0001, d.f. = 138). Plants with straight leaves entered dormancy significantly later than plants with curved leaves (P = 0·05). Also, plants with green leaves became dormant significantly later than those with greyish‐green and greyish leaves (gy‐gn and gy; P = 0·05).
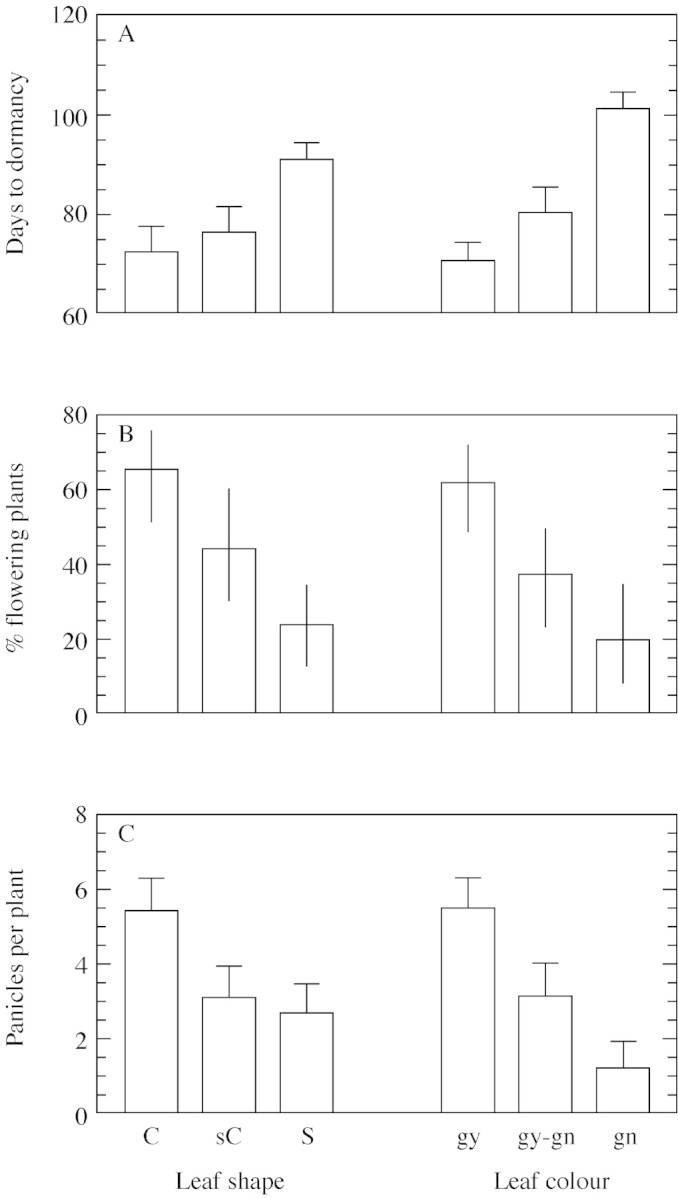
Fig. 6. Age at onset of dormancy (A), % flowering (B) and panicles per plant (C) in P. bulbosa plants with leaves of different shape (C, curved, n = 55; sC, slightly curved, n = 34; S, straight, n = 52) or colour (gy, grey, n = 66; gy‐gn, greyish‐green, n = 44; gn, green, n = 31). Populations were sampled along an aridity gradient of 110–810 mm rain year–1. Data in A and C are means ± s.e.; bars in B are 95 % confidence intervals.
A significant association was also observed between leaf traits and flowering capacity (net‐house data, leaf shape vs. flowering χ2 = 19·4, P < 0·0001; leaf colour vs. flowering χ2 = 17·4, P = 0·0002, n = 142). Significantly higher flowering proportions were observed among plants with curved or greyish leaf types than in plants with straight or green leaves (P = 0·05; Fig. 6B). The dependence of the number of panicles per plant on the shape or colour of leaves was analysed by one‐way ANOVA. Plants with curved leaves had more panicles per plant than plants with straight leaves (F = 3·641, P = 0·03, d.f. = 138). Plants with greyish leaves had more panicles per plant than those with green leaves (F = 6·498, P = 0·002, d.f. = 138; Fig. 6C).
DISCUSSION
The aridity‐gradient analysis suggests that variation in the onset of dormancy in P. bulbosa populations, as measured under controlled conditions, is due to genetic differences among populations, and that plants entered dormancy earlier at more arid sites (Fig. 1A). In P. bulbosa, summer dormancy was induced by long days, and the photoperiodic induction was enhanced by higher temperatures (Ofir and Kerem, 1982) and drought (M. Ofir and J. Kigel, unpubl. res.). Latitudinal variation in dormancy, induced by daylength, can arise through gradual changes in the threshold daylength required for induction, as well as in the responsiveness of plants to the inductive daylength, e.g. changes in minimal number of long days required for induction, or in the duration of the initial non‐responsive vegetative stage (Thomas and Vince‐Prue, 1997). In the phytotron experiment, P. bulbosa plants were grown under a moderate temperature regime of 22/16 °C with continued irrigation. Therefore, the induction of dormancy resulted solely from exposure to the applied 16‐h photoperiod, which was longer than the critical daylength (11–12 h) required for induction in this species (Ofir and Kigel, 1999). Thus, the observed interpopulation variation in the onset of dormancy was due to differences in their responsiveness to inductive daylength. It can be argued that across narrow ranges of latitude in which daylength differences are very small, photoperiodic cues for adaptation to aridity are mainly related to changes in the responsiveness to photoperiod, as in the present study. In contrast, adaptation to aridity across wider ranges of latitude is probably based on differences in the threshold of daylength required for dormancy induction, as well as in responsiveness to inductive daylengths. Genetic variation in the response to photoperiod probably allows P. bulbosa to use the predictable seasonal changes in daylength as cues in its adaptation to increasing aridity in both small and large spatial scales. Moreover, since the frequency of early spells of warm and dry weather is associated with decreasing rainfall along aridity gradients, these climatic factors should enhance the induction of dormancy by daylength, resulting in even earlier onset of dormancy with increasing aridity.
Variation in the onset of dormancy along climatic gradients has not been studied in other summer‐dormant species. Nevertheless, increasing duration of summer dormancy has been reported for populations of Phalaris tuberosa (syn. aquatica) (Sankary et al., 1969) and Dactylis glomerata (Volaire, 1995) along wide geographic transects of increasing aridity, in which large differences in daylength and temperature occur concomitantly with the variation in rainfall. In Mediterranean genotypes of Phalaris tuberosa (syn. aquatica), the amount of regrowth after summer rainstorms was positively correlated with the mean summer rainfall in the original habitat (Oram, 1983). These trends support the assumption that variation in summer dormancy plays a central role in the adaptation of herbaceous perennials to Mediterranean‐type climates.
Significant variation was observed in the onset of dormancy of P. bulbosa populations collected from sites where amounts of rainfall are similar (Fig. 1A; Table 3). This variation was probably related to heterogeneity in local microhabitats caused by differences in edaphic conditions and topography. The relatively early dormancy in population 13 (520 mm rain year–1 at the site of origin) was probably associated with a faster rate of drying of the basaltic soils present in Karei Deshe, where it was collected, compared with that of calcareous soils in other sites with similar rainfall, e.g. populations 15 (Nahshon) and 18 (Tirat Shalom) with 514 and 530 mm rain year–1, respectively, at the site of origin. Variation in topography, such as slopes vs. valleys, may result in local differences in soil depth and water availability due to run‐off along slopes and accumulation of water in the deeper valley soil. This spatial heterogeneity in resource distribution is more pronounced in semi‐arid regions and may lead to the differentiation of local sub‐populations (Noy‐Meir, 1973). Indeed, this was observed at the Lehavim site (307 mm rain year–1), where sub‐populations from the north‐ and south‐facing slopes (17a and 17c, respectively) showed earlier onset of dormancy and xeromorphic leaf traits (e.g. curved, greyish leaves) compared with a large proportion of the plants in the more mesic valley (17b) which had straight and greyish‐green leaves and a much later onset of dormancy (P < 0·01).
Age at onset of dormancy in daughter plants grown under controlled inductive conditions was negatively correlated with the percentage of flowering parental plants that were collected from the populations along the aridity transect and grown in the net‐house (Fig. 2A). This correlation raises two questions: (1) are the processes of flowering and imposition of dormancy developmentally related; and (2) what is the benefit of flowering for early‐dormant plants in xeric habitats, or lack of flowering in late‐dormant plants in more mesic habitats?
That flowering and imposition of dormancy in P. bulbosa are separate developmental processes is indicated by the fact that even in the flowering plants a relatively low proportion of tillers reached flowering, whereas all tillers became dormant (Ofir and Kerem, 1982), as well as the observation that in many populations a large proportion of plants did not flower but became dormant. In contrast to the lack of association between flowering and dormancy in P. bulbosa, a close relationship was observed between reproductive development and the onset of dormancy in the summer‐dormant geophytic grass Hordeum bulbosum L. (Ofir and Koller, 1972, 1974).
The reproductive benefit of flowering tillers is not clear since, in most populations, seed set was very low (Heyn, 1962; M. Ofir and J. Kigel, unpubl. res.). Flowering tillers do not produce basal bulbs (Ofir and Kerem, 1982) but, in some populations, dispersal bulbils are produced in their panicles. Inflorescence proliferation and apomictic production of dispersal bulbils occurs regularly in the genera Poa, Festuca and Deschampsia (Gustafsson, 1947). In accessions of P. bulbosa from Turkey and Afghanistan grown under controlled conditions, plants flowered under long days, whereas production of bulbils was a labile process, dependent upon the environment, e.g. bulbils developed in place of normal florets under short days and low temperature (Youngner, 1960). In Cynosurus cristatus L., another long‐day plant, bulbils developed under short days (Wycherley, 1953). Thus, it is conceivable that the irregular and sporadic production of seed and bulbils observed in Israeli populations of P. bulbosa in the net‐house was due to inter‐ and intrapopulation variation in response to climatic factors. Nevertheless, the fact that populations from more xeric habitats tend to have a larger fraction of flowering plants suggests that maintaining a certain level of dispersal by production of sexual and/or asexual propagules (i.e. seeds and bulbils) has an advantage under arid conditions. The enhanced flowering capacity of plants in the more arid habitats, which facilitates higher dispersal potential, could have evolved as a compensation for the limited local clonal expansion of clumps due to restricted tillering, resulting from the earlier onset of dormancy. In contrast, production of basal bulbs and gradual expansion of the plant clumps, together with delayed dormancy, are apparently sufficient for clone persistence under mesic conditions. Interannual variation in flowering and in seed vs. bulbil production in the populations along the aridity gradient will be the subject of further study.
A larger proportion of plants with curved and greyish leaves were characteristic of P. bulbosa populations from the more arid sites (Fig. 5). These leaf traits are strongly associated with, and represent adaptations to, the arid environment, since greater light reflectance by greyish leaves and the curved shape reduce heat load (Ehleringer, 1980). Curved and greyish leaves were also associated with early dormancy (Fig. 6A) and higher flowering capacity (Fig. 6B and C). Populations from the most arid locations (populations 8 and 10, where rainfall at the sites of origin was 110 and 120 mm year–1, respectively) were the earliest to become dormant and most of their plants had curved or slightly curved leaves that were greyish or greyish‐green in colour (Table 3). In spite of these general trends of association between leaf traits, dormancy and habitat conditions, the linkage was not strict. For example, in the Har Amassa site (276 mm rain year–1), plants from population 1 entered dormancy significantly later than those of populations 2 and 4 (P < 0·03), whereas leaves were of the arid type in plants from all three populations.
This study shows that P. bulbosa adapts to increasing aridity by a combination of developmental strategies, such as earlier onset of summer dormancy; greater flowering capacity that compensates for the higher risk of death and restricted vegetative growth; increased multiplication and dispersal by seeds or apomictic propagules; and xeromorphic leaves that reduce heat load and water loss. In consequence, the association among these traits is stronger at the extreme range of the aridity gradient. Since these traits are not always associated in the different populations, they may be independently co‐selected during adaptation to aridity. Future work will explore the role of drought stress and ABA in the imposition of dormancy in populations of P. bulbosa along aridity gradients, as well as possible variation in the rate of relaxation of dormancy in the basal bulbs.
Supplementary Material
Received: 30 September 2002; Returned for revision: 15 October 2002; Accepted: 6 November 2002
References
- Ben‐HodG, Kigel J, Steinitz B.1988. Dormancy and flowering in Anemone coronaria L. as affected by photoperiod and temperature. Annals of Botany 61: 623–633. [Google Scholar]
- BitanA, Rubin S.1991. Climatic atlas of Israel for physical and environmental planning and design. Beit Dagan: Israel Meteorological Service. [Google Scholar]
- BrewsterJL.1990. Physiology of crop growth and bulbing. In: Rabinowitch HD, Brewster JL, eds. Onions and allied crops, Vol. 1 Boca Raton: CRC Press, 53–88. [Google Scholar]
- BurnsW.1946. Corm and bulb formation in plants, with special reference to the Gramineae. Transactions of the Botanical Society of Edinburgh 34: 316–347. [Google Scholar]
- ClaphamDH, Dormling I, Ekberg I, Eriksson G, Qamaruddin M, Vince‐Prue D.1998. Latitudinal cline of requirement for far‐red light for the photoperiodic control of bud set and extension growth in Picea abies (Norway spruce). Physiologia Plantarum 102: 71–78. [DOI] [PubMed] [Google Scholar]
- DafniA, Cohen D, Noy‐Meir I.1981. Life cycle variation in geophytes. Annals of the Missouri Botanical Garden 68: 652–660. [Google Scholar]
- DavisPH.1985. Flora of Turkey Vol. 9. Edinburgh: Edinburgh University Press. [Google Scholar]
- EhleringerJR.1980. Leaf morphology and reflectance in relation to water and temperature stress. In: Turner NC, Kramer PJ, eds. Adaptation of plants to water and high temperature stress New York: Wiley, 295–308. [Google Scholar]
- Feinbrun‐DothanN.1986. Flora Palaestina. Vol. 4. Jerusalem: Israel Academy of Sciences and Humanities. [Google Scholar]
- GustafssonA.1947. Apomixis in higher plants. Acta University of Lund 2: 1–370. [Google Scholar]
- HeynCC.1962. Studies of bulbous Poa in Palestine. 1. The agamic complex of Poa bulbosa Bulletin of the Research Council of Israel 11D: 117–126. [Google Scholar]
- HoffmanAA, Parsons PA.1993. Evolutionary genetics and environmental stress. Oxford: Oxford University Press. [Google Scholar]
- KennedyPB.1929. Proliferation in Poa bulbosa Journal of the American Society of Agronomy 21: 80–91. [Google Scholar]
- KollerD.1969. The physiology of dormancy and survival of plants in desert environments. Symposium of the Society of Experimental Biology 23: 449–469. [PubMed] [Google Scholar]
- LangGA, Early JD, Martin GC, Darnell RL.1987. Endo‐, para‐, and eco‐dormancy: physiological terminology and classification for dormancy research. HortScience 22: 371–377. [Google Scholar]
- LaudeHM.1953. The nature of summer dormancy in perennial grasses. Botanical Gazette 114: 284–292. [Google Scholar]
- Le NardM, De Hertogh A.1993. Bulb development and flowering. In: De Hertogh A, Le Nard M, eds. The physiology of flower bulbs Amsterdam: Elsevier Science, 29–43. [Google Scholar]
- MykingT, Heide OM.1995. Dormancy release and chilling requirement of buds of latitudinal ecotypes of Betula pubescens Tree Physiology 15: 697–704. [DOI] [PubMed] [Google Scholar]
- NaorV, Kigel J.2002. Temperature affects plant development, flowering and tuber dormancy in calla lily (Zantedeschia). Journal of Horticultural Science and Biotechnology 77: 170–176. [Google Scholar]
- NoodénLD, Weber JA.1978. Environmental and hormonal control of dormancy in terminal buds of plants. In: Clutter ME, ed. Dormancy and developmental arrest New York: Academic Press, 222–268. [Google Scholar]
- Noy‐MeirI.1973. Desert ecosystems: environment and producers. Annual Review of Ecology and Systematics 4: 25–52. [Google Scholar]
- OfirM.1986. Seasonal changes in the response to temperature of summer‐dormant Poa bulbosa L. bulbs. Annals of Botany 58: 81–89. [Google Scholar]
- OfirM, Dorenfeld Y.1992. Induction of summer dormancy in Poa bulbosa L. under natural environment and subsequent controlled photo‐thermal conditions. Israel Journal of Botany 41: 265–277. [Google Scholar]
- OfirM, Kerem D.1982. The effects of temperature and photoperiod on the onset of summer‐dormancy in Poa bulbosa L. Annals of Botany 50: 259–264. [Google Scholar]
- OfirM, Kigel J.1999. Photothermal control of the imposition of summer‐dormancy in Poa bulbosa, a perennial grass geophyte. Physiologia Plantarum 105: 633–640. [Google Scholar]
- OfirM, Koller D.1972. A kinetic analysis of the relationships between flowering and initiation of the dormant state in Hordeum bulbosum L.—a perennial grass. Israel Journal of Botany 21: 21–34. [Google Scholar]
- OfirM, Koller D.1974. Relationship between thermoinduction and photoinduction of flowering and dormancy in Hordeum bulbosum L. a perennial grass. Australian Journal of Plant Physiology 1: 259–270. [Google Scholar]
- OramRN.1983. Ecotypic differentiation for dormancy levels in over summering buds of Phalaris aquatica.Botanical Gazette 144: 544–551. [Google Scholar]
- ParsonsRF.2000. Monocotyledonous geophytes: comparison of California with Victoria, Australia. Australian Journal of Botany 48: 39–43. [Google Scholar]
- PateJS, Dixon KW.1982. Tuberous, cormous and bulbous plants. Perth: University of Western Australia Press. [Google Scholar]
- PugnaireFI, Haase P, Incoll LD, Clark SC.1996. Response of the tussock grass Stipa tenacissima to watering in a semi‐arid environment. Functional Ecology 102: 265–274. [Google Scholar]
- ReesAR.1992. Ornamental bulbs, corms and tubers. Wallingford: CAB International. [Google Scholar]
- RundelPW 1996. Monocotyledonous geophytes in the California flora. Madrono 43: 355–368. [Google Scholar]
- SankaryMN, Laude HM, Love RM, Fox RE.1969. Variation in summer‐dormancy among collections of Phalaris tuberosa at Davis, California. Journal of the British Grassland Society 24: 134–137. [Google Scholar]
- SnedecorGW, Cochran WG.1980. Statistical methods. 7th edn. Ames: Iowa State University Press. [Google Scholar]
- SokalRR, Rohlf FJ.1995. Biometry. 3rd edn. New York: Freeman and Co. [Google Scholar]
- ThomasB, Vince‐Prue D.1997. Photoperiodism in plants. 2nd edn. San Diego: Academic Press. [Google Scholar]
- VaughtonG, Ramsey M.2001. Variation in summer dormancy in the lilioid geophyte Burchardia umbellate (Colchicaceae). American Journal of Botany 88: 1223–1229. [PubMed] [Google Scholar]
- VegisA.1964. Dormancy in higher plants. Annual Review of Plant Physiology 15: 185–224. [Google Scholar]
- VolaireF.1995. Growth, carbohydrate reserves and drought survival strategies of contrasting Dactylis glomerata populations in a Mediterranean environment. Journal of Applied Ecology 32: 56–66. [Google Scholar]
- WycherleyPR.1953. Proliferation of spikelets in British grasses. Watsonia 3: 41–56. [Google Scholar]
- YoungnerVB.1960. Environmental control of initiation of the inflorescence, reproductive structures, and proliferations in Poa bulbosa American Journal of Botany 47: 735–757. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


