Abstract
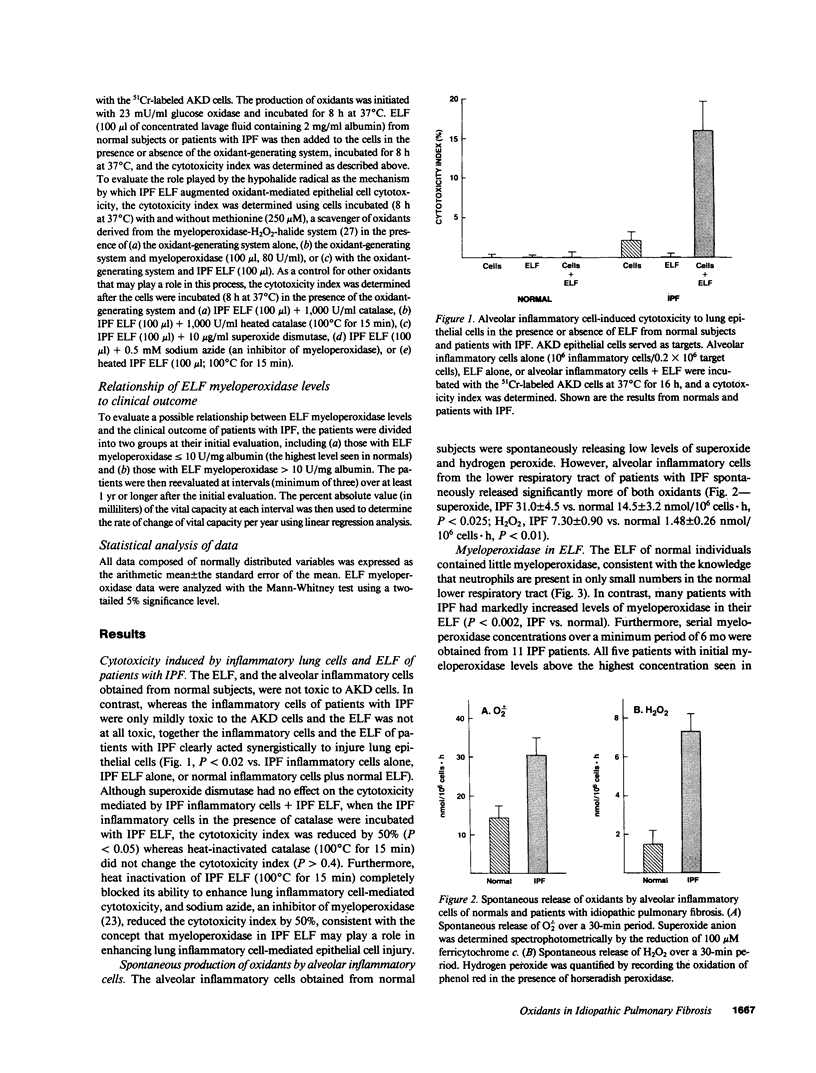
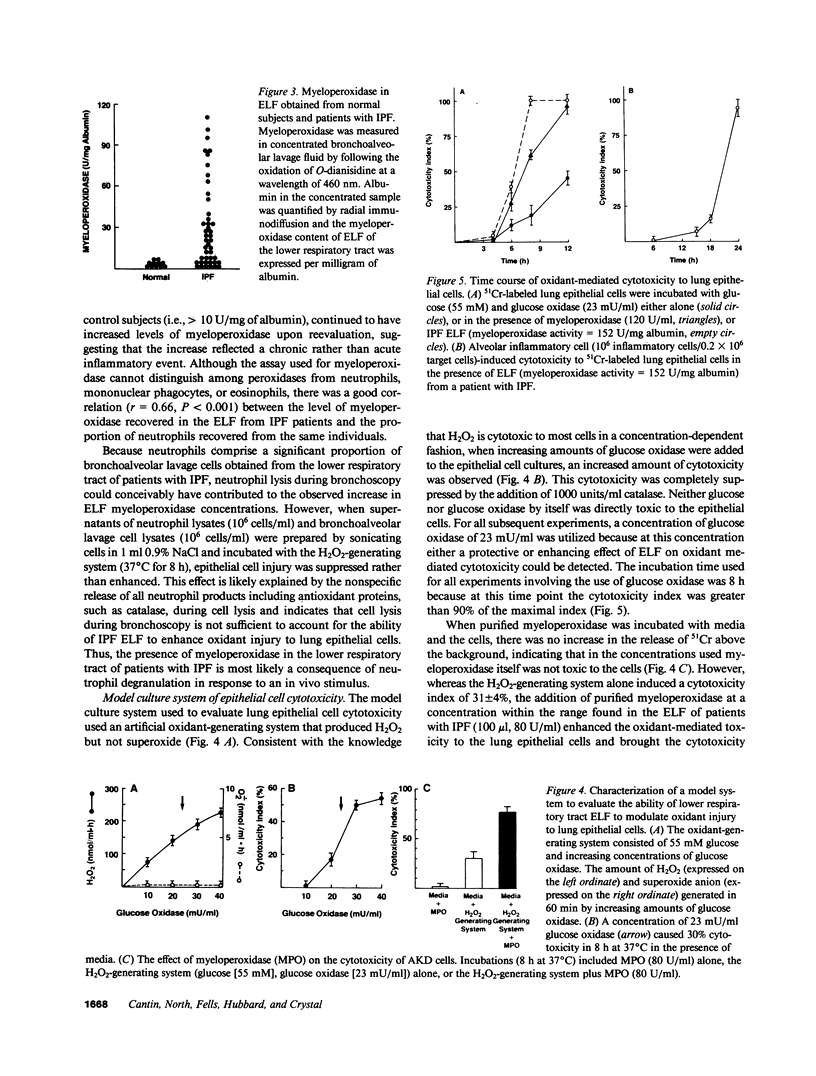
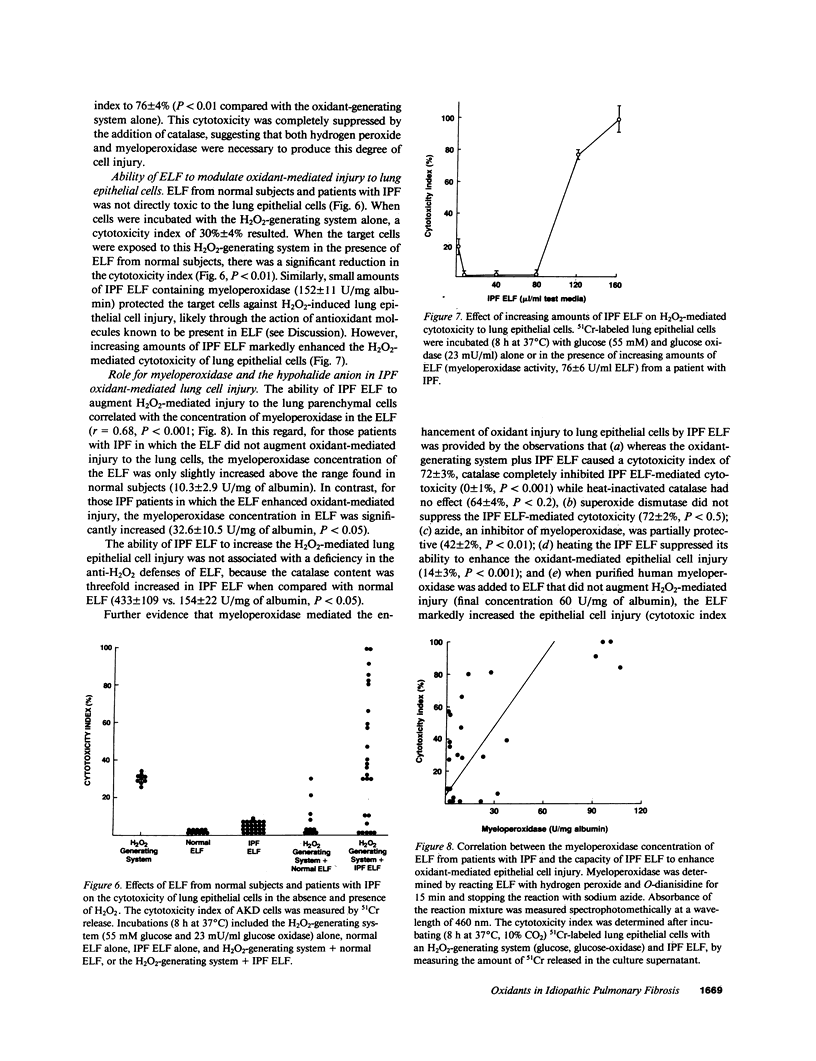
Lung inflammatory cells of patients with idiopathic pulmonary fibrosis (IPF) were evaluated for their ability to injure 51Cr-labeled AKD alveolar epithelial cells in the presence and absence of IPF alveolar epithelial lining fluid (ELF). The IPF cells were spontaneously releasing exaggerated amounts of superoxide (O.2) and hydrogen peroxide (H2O2) compared with normal (P less than 0.02). Cytotoxicity of the AKD cells was markedly increased when the IPF inflammatory cells were incubated with autologous ELF (P less than 0.02). The majority of IPF patients had ELF myeloperoxidase levels above normal (P less than 0.002). Incubation of IPF ELF with AKD cells in the presence of H2O2 caused increased cellular injury (P less than 0.01 compared with control), which was suppressed by methionine, a myeloperoxidase system scavenger. IPF patients with high concentrations of ELF myeloperoxidase deteriorated more rapidly than those with low ELF myeloperoxidase (P less than 0.05). Thus, IPF is characterized by an increased spontaneous production of oxidants by lung inflammatory cells, the presence of high concentrations of myeloperoxidase in the ELF of the lower respiratory tract, and a synergistic cytotoxic effect of alveolar inflammatory cells and ELF on lung epithelial cells, suggesting oxidants may play a role in causing the epithelial cell injury of this disorder.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Albrich J. M., McCarthy C. A., Hurst J. K. Biological reactivity of hypochlorous acid: implications for microbicidal mechanisms of leukocyte myeloperoxidase. Proc Natl Acad Sci U S A. 1981 Jan;78(1):210–214. doi: 10.1073/pnas.78.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (second of two parts). N Engl J Med. 1978 Mar 30;298(13):721–725. doi: 10.1056/NEJM197803302981305. [DOI] [PubMed] [Google Scholar]
- Badwey J. A., Karnovsky M. L. Active oxygen species and the functions of phagocytic leukocytes. Annu Rev Biochem. 1980;49:695–726. doi: 10.1146/annurev.bi.49.070180.003403. [DOI] [PubMed] [Google Scholar]
- Baugh R. J., Travis J. Human leukocyte granule elastase: rapid isolation and characterization. Biochemistry. 1976 Feb 24;15(4):836–841. doi: 10.1021/bi00649a017. [DOI] [PubMed] [Google Scholar]
- Clark R. A., Klebanoff S. J., Einstein A. B., Fefer A. Peroxidase-H2O2-halide system: Cytotoxic effect on mammalian tumor cells. Blood. 1975 Feb;45(2):161–170. [PubMed] [Google Scholar]
- Clark R. A., Klebanoff S. J. Myeloperoxidase--H2O2--halide system: cytotoxic effect on human blood leukocytes. Blood. 1977 Jul;50(1):65–70. [PubMed] [Google Scholar]
- Clark R. A., Klebanoff S. J. Neutrophil-mediated tumor cell cytotoxicity: role of the peroxidase system. J Exp Med. 1975 Jun 1;141(6):1442–1447. doi: 10.1084/jem.141.6.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. A., Klebanoff S. J. Role of the myeloperoxidase-H2O2-halide system in concanavalin A-induced tumor cell killing by human neutrophils. J Immunol. 1979 Jun;122(6):2605–2610. [PubMed] [Google Scholar]
- Clark R. A., Szot S. The myeloperoxidase-hydrogen peroxide-halide system as effector of neutrophil-mediated tumor cell cytotoxicity. J Immunol. 1981 Apr;126(4):1295–1301. [PubMed] [Google Scholar]
- Coalson J. J. The ultrastructure of human fibrosing alveolitis. Virchows Arch A Pathol Anat Histol. 1982;395(2):181–199. doi: 10.1007/BF00429611. [DOI] [PubMed] [Google Scholar]
- Crystal R. G., Fulmer J. D., Baum B. J., Bernardo J., Bradley K. H., Bruel S. D., Elson N. A., Fells G. A., Ferrans V. J., Gadek J. E. Cells, collagen and idiopathic pulmonary fibrosis. Lung. 1978;155(3):199–224. doi: 10.1007/BF02730694. [DOI] [PubMed] [Google Scholar]
- Crystal R. G., Fulmer J. D., Roberts W. C., Moss M. L., Line B. R., Reynolds H. Y. Idiopathic pulmonary fibrosis. Clinical, histologic, radiographic, physiologic, scintigraphic, cytologic, and biochemical aspects. Ann Intern Med. 1976 Dec;85(6):769–788. doi: 10.7326/0003-4819-85-6-769. [DOI] [PubMed] [Google Scholar]
- Daniele R. P., Henson P. M., Fantone J. C., 3rd, Ward P. A., Dreisin R. B. Immune complex injury of the lung. Symposium held at the 74th annual meeting of the American Thoracic Society, Las Vegas, Nevada, May 1979. Am Rev Respir Dis. 1981 Dec;124(6):738–755. [PubMed] [Google Scholar]
- Davis W. B., Fells G. A., Sun X. H., Gadek J. E., Venet A., Crystal R. G. Eosinophil-mediated injury to lung parenchymal cells and interstitial matrix. A possible role for eosinophils in chronic inflammatory disorders of the lower respiratory tract. J Clin Invest. 1984 Jul;74(1):269–278. doi: 10.1172/JCI111411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dechatelet L. R., Migler R. A., Shirley P. S., Bass D. A., McCall C. E. Enzymes of oxidative metabolism in the human eosinophil. Proc Soc Exp Biol Med. 1978 Sep;158(4):537–541. doi: 10.3181/00379727-158-40241. [DOI] [PubMed] [Google Scholar]
- Dreisin R. B., Schwarz M. I., Theofilopoulos A. N., Stanford R. E. Circulating immune complexes in the idiopathic interstitial pneumonias. N Engl J Med. 1978 Feb 16;298(7):353–357. doi: 10.1056/NEJM197802162980701. [DOI] [PubMed] [Google Scholar]
- Fulmer J. D., Roberts W. C., von Gal E. R., Crystal R. G. Morphologic-physiologic correlates of the severity of fibrosis and degree of cellularity in idiopathic pulmonary fibrosis. J Clin Invest. 1979 Apr;63(4):665–676. doi: 10.1172/JCI109349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulmer J. D., Roberts W. C., von Gal E. R., Grystal R. G. Small airways in idiopathic pulmonary fibrosis. Comparison of morphologic and physiologic observations. J Clin Invest. 1977 Sep;60(3):595–610. doi: 10.1172/JCI108811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadek J. E., Kelman J. A., Fells G., Weinberger S. E., Horwitz A. L., Reynolds H. Y., Fulmer J. D., Crystal R. G. Collagenase in the lower respiratory tract of patients with idiopathic pulmonary fibrosis. N Engl J Med. 1979 Oct 4;301(14):737–742. doi: 10.1056/NEJM197910043011401. [DOI] [PubMed] [Google Scholar]
- Goldstein I. M., Roos D., Kaplan H. B., Weissmann G. Complement and immunoglobulins stimulate superoxide production by human leukocytes independently of phagocytosis. J Clin Invest. 1975 Nov;56(5):1155–1163. doi: 10.1172/JCI108191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERBERT F. A., NAHMIAS B. B., GAENSLER E. A., MACMAHON H. E. Pathophysiology of interstitial pulmonary fibrosis. Report of 19 cases and follow-up with corticosteroids. Arch Intern Med. 1962 Nov;110:628–648. doi: 10.1001/archinte.1962.03620230074012. [DOI] [PubMed] [Google Scholar]
- Haslam P. L., Turton C. W., Heard B., Lukoszek A., Collins J. V., Salsbury A. J., Turner-Warwick M. Bronchoalveolar lavage in pulmonary fibrosis: comparison of cells obtained with lung biopsy and clinical features. Thorax. 1980 Jan;35(1):9–18. doi: 10.1136/thx.35.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson P. M., Johnson H. B., Spiegelberg H. L. The release of granule enzymes from human neutrophils stimulated by aggregated immunoglobulins of different classes and subclasses. J Immunol. 1972 Dec;109(6):1182–1192. [PubMed] [Google Scholar]
- Henson P. M. The immunologic release of constituents from neutrophil leukocytes. I. The role of antibody and complement on nonphagocytosable surfaces or phagocytosable particles. J Immunol. 1971 Dec;107(6):1535–1546. [PubMed] [Google Scholar]
- Hoidal J. R., Fox R. B., LeMarbe P. A., Perri R., Repine J. E. Altered oxidative metabolic responses in vitro of alveolar macrophages from asymptomatic cigarette smokers. Am Rev Respir Dis. 1981 Jan;123(1):85–89. doi: 10.1164/arrd.1981.123.1.85. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W., Gadek J. E., Kawanami O., Ferrans V. J., Crystal R. G. Inflammatory and immune processes in the human lung in health and disease: evaluation by bronchoalveolar lavage. Am J Pathol. 1979 Oct;97(1):149–206. [PMC free article] [PubMed] [Google Scholar]
- Hunninghake G. W., Gadek J. E., Lawley T. J., Crystal R. G. Mechanisms of neutrophil accumulation in the lungs of patients with idiopathic pulmonary fibrosis. J Clin Invest. 1981 Jul;68(1):259–269. doi: 10.1172/JCI110242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. J., Fantone J. C., 3rd, Kaplan J., Ward P. A. In vivo damage of rat lungs by oxygen metabolites. J Clin Invest. 1981 Apr;67(4):983–993. doi: 10.1172/JCI110149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. J., Ward P. A. Acute and progressive lung injury after contact with phorbol myristate acetate. Am J Pathol. 1982 Apr;107(1):29–35. [PMC free article] [PubMed] [Google Scholar]
- Johnson K. J., Ward P. A. Role of oxygen metabolites in immune complex injury of lung. J Immunol. 1981 Jun;126(6):2365–2369. [PubMed] [Google Scholar]
- Johnston R. B., Jr, Godzik C. A., Cohn Z. A. Increased superoxide anion production by immunologically activated and chemically elicited macrophages. J Exp Med. 1978 Jul 1;148(1):115–127. doi: 10.1084/jem.148.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanofsky J. R., Wright J., Miles-Richardson G. E., Tauber A. I. Biochemical requirements for singlet oxygen production by purified human myeloperoxidase. J Clin Invest. 1984 Oct;74(4):1489–1495. doi: 10.1172/JCI111562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawanami O., Ferrans V. J., Crystal R. G. Structure of alveolar epithelial cells in patients with fibrotic lung disorders. Lab Invest. 1982 Jan;46(1):39–53. [PubMed] [Google Scholar]
- Klebanoff S. J., Clark R. A. Hemolysis and iodination of erythrocyte components by a myeloperoxidase-mediated system. Blood. 1975 May;45(5):699–707. [PubMed] [Google Scholar]
- Klebanoff S. J., Hamon C. B. Role of myeloperoxidase-mediated antimicrobial systems in intact leukocytes. J Reticuloendothel Soc. 1972 Aug;12(2):170–196. [PubMed] [Google Scholar]
- Klebanoff S. J. Oxygen metabolism and the toxic properties of phagocytes. Ann Intern Med. 1980 Sep;93(3):480–489. doi: 10.7326/0003-4819-93-3-480. [DOI] [PubMed] [Google Scholar]
- Kniazeff A. J., Stoner G. D., Terry L., Wagner R. M., Hoppenstand R. D. Characteristics of epithelial cella cultured from feline lung. Lab Invest. 1976 May;34(5):495–500. [PubMed] [Google Scholar]
- LIEBOW A. A., STEER A., BILLINGSLEY J. G. DESQUAMATIVE INTERSTITIAL PNEUMONIA. Am J Med. 1965 Sep;39:369–404. doi: 10.1016/0002-9343(65)90206-8. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Brukner L. H., Silverstein S. C., Cohn Z. A. Extracellular cytolysis by activated macrophages and granulocytes. I. Pharmacologic triggering of effector cells and the release of hydrogen peroxide. J Exp Med. 1979 Jan 1;149(1):84–99. doi: 10.1084/jem.149.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Silverstein S. C., Brukner L. H., Cohn Z. A. Extracellular cytolysis by activated macrophages and granulocytes. II. Hydrogen peroxide as a mediator of cytotoxicity. J Exp Med. 1979 Jan 1;149(1):100–113. doi: 10.1084/jem.149.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen R. L., Little C. Purification and some properties of myeloperoxidase and eosinophil peroxidase from human blood. Biochem J. 1983 Mar 1;209(3):781–787. doi: 10.1042/bj2090781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pick E., Keisari Y. A simple colorimetric method for the measurement of hydrogen peroxide produced by cells in culture. J Immunol Methods. 1980;38(1-2):161–170. doi: 10.1016/0022-1759(80)90340-3. [DOI] [PubMed] [Google Scholar]
- Pick E., Keisari Y. Superoxide anion and hydrogen peroxide production by chemically elicited peritoneal macrophages--induction by multiple nonphagocytic stimuli. Cell Immunol. 1981 Apr;59(2):301–318. doi: 10.1016/0008-8749(81)90411-1. [DOI] [PubMed] [Google Scholar]
- Reynolds H. Y., Fulmer J. D., Kazmierowski J. A., Roberts W. C., Frank M. M., Crystal R. G. Analysis of cellular and protein content of broncho-alveolar lavage fluid from patients with idiopathic pulmonary fibrosis and chronic hypersensitivity pneumonitis. J Clin Invest. 1977 Jan;59(1):165–175. doi: 10.1172/JCI108615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraufstatter I., Revak S. D., Cochrane C. G. Biochemical factors in pulmonary inflammatory disease. Fed Proc. 1984 Oct;43(13):2807–2810. [PubMed] [Google Scholar]
- Schraufstätter I. U., Revak S. D., Cochrane C. G. Proteases and oxidants in experimental pulmonary inflammatory injury. J Clin Invest. 1984 Apr;73(4):1175–1184. doi: 10.1172/JCI111303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortland J. R., Darke C. S., Crane W. A. Electron microscopy of desquamative interstitial pneumonia. Thorax. 1969 Mar;24(2):192–208. doi: 10.1136/thx.24.2.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slivka A., LoBuglio A. F., Weiss S. J. A potential role for hypochlorous acid in granulocyte-mediated tumor cell cytotoxicity. Blood. 1980 Feb;55(2):347–350. [PubMed] [Google Scholar]
- Spencer H. Interstitial pneumonia. Annu Rev Med. 1967;18:423–442. doi: 10.1146/annurev.me.18.020167.002231. [DOI] [PubMed] [Google Scholar]
- Tsan M. F., Chen J. W. Oxidation of methionine by human polymorphonuclear leukocytes. J Clin Invest. 1980 May;65(5):1041–1050. doi: 10.1172/JCI109756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkall R. M., Tsan M. F. Oxidation of glutathione by the myeloperoxidase system. J Reticuloendothel Soc. 1982 Apr;31(4):353–360. [PubMed] [Google Scholar]
- Turner-Warwick M. Philip Ellman lecture. Immunological aspects of systemic diseases of the lungs. Proc R Soc Med. 1974 Jun;67(6 Pt 2):541–547. doi: 10.1177/00359157740676P205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdivia E., Hensley G., Leory E. P., Wu J., Jaeschke W. Morphology and pathogenesis of desquamative interstitial pneumonitis. Thorax. 1977 Feb;32(1):7–18. doi: 10.1136/thx.32.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward P. A., Duque R. E., Sulavik M. C., Johnson K. J. In vitro and in vivo stimulation of rat neutrophils and alveolar macrophages by immune complexes. Production of O-2 and H2O2. Am J Pathol. 1983 Mar;110(3):297–309. [PMC free article] [PubMed] [Google Scholar]
- Weiss S. J., Slivka A. Monocyte and granulocyte-mediated tumor cell destruction. A role for the hydrogen peroxide-myeloperoxidase-chloride system. J Clin Invest. 1982 Feb;69(2):255–262. doi: 10.1172/JCI110447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. J., Ward P. A. Immune complex induced generation of oxygen metabolites by human neutrophils. J Immunol. 1982 Jul;129(1):309–313. [PubMed] [Google Scholar]
- Weissmann G., Smolen J. E., Korchak H. M. Release of inflammatory mediators from stimulated neutrophils. N Engl J Med. 1980 Jul 3;303(1):27–34. doi: 10.1056/NEJM198007033030109. [DOI] [PubMed] [Google Scholar]