Abstract
Submergence tolerance of 13 doubled haploid lines of rice and their parents (submergence tolerant FR13A and submergence intolerant CT6241) was assessed using 2‐week‐old seedlings. Plants were scored for leaf senescence and percentage of seedlings that survived up to 15 d submergence, followed by a 12 d recovery period. Seven lines proved to be submergence tolerant, and six relatively intolerant. In all lines, activity of pyruvate decarboxylase (PDC), extracted from the apical 3–5 cm of root axes, decreased by 46–96 % and 38–76 %, respectively, during 5 or 10 d submergence under natural day/night conditions, compared with pre‐submergence values (100 %). However, when the enzyme was extracted at night, submergence increased PDC activity of all rice lines (approx. 112 % on average), compared with pre‐submergence values (100 %). The stimulating effect of the dark period on PDC activity was reproduced and amplified by submerging rice seedlings for up to 5 d in continuous darkness in water containing sub‐ambient concentrations of oxygen (2·3 mg l–1). Such increased PDC activity was also observed in seedlings exposed to anoxia for 6 h (approx. 6–175 % higher than pre‐submergence values). Irrespective of tolerance class, submergence decreased soluble protein concentrations under all conditions and sampling times. No positive correlation was found between PDC activity and tolerance of the various rice lines to submergence. However, PDC activity was slightly higher in submergence intolerant lines, compared with tolerant lines, under both dark submergence and anoxia. Such differences in PDC activity between the two groups of rice lines were not observed when they were submerged under the natural diurnal cycle. Increased PDC activity in roots at night demonstrated a probable incidence of tissue hypoxia or anoxia during submergence during each dark period.
Keywords: Key words: Doubled haploid rice lines, fermentation, oxygen deficiency, rice (Oryza sativa L.), pyruvate decarboxylase, roots, submergence tolerance.
INTRODUCTION
Rice (Oryza sativa L.) is the staple food for billions of people. The success of the crop can be partially explained by its ability to grow in diverse tropical environments, including the extensive and complex rainfed lowlands of south and south‐east Asia. Such lowlands occupy 25 % of the world’s rice‐producing lands. In these areas, rice plants can become completely submerged for varying periods when rivers overflow their banks; this is extremely damaging to the plants especially when they are small. The extent of injury and subsequent survival of submergence are influenced by the depth of the floodwater, the concentration of oxygen, carbon dioxide and ethylene dissolved in the water, pH, and the degree of turbidity (Ito et al., 1999).
During submergence, plants are sometimes exposed to a reduction in oxygen supply because of the slow diffusion rate of oxygen in water and its limited solubility (Armstrong, 1979). Turbid floodwater can become anaerobic, especially during the night (Setter et al., 1987), and plants react to an absence of oxygen by switching from an oxidative to a solely substrate‐level phosphorylation of ADP to ATP; the latter reactions predominantly involve glycolysis and fermentation. The overall yield of ATP produced during fermentation is only two molecules of ATP per glucose molecule while 32 molecules of ATP are produced during oxidative phosphorylation. Ethanol has been shown by a number of research workers to be the major product of fermentation in rice seedlings (Raymond et al., 1985; ap Rees et al., 1987; Johnson et al., 1989; Menegus et al., 1993; Ricard et al., 1994). The dominance of alcoholic fermentation is further supported by studies of the two enzyme‐mediated steps in fermentation, involving pyruvate decarboxylase (PDC) and alcohol dehydrogenase (ADH). Rapid increases in the activities of both PDC and ADH were observed in rice (John and Greenway, 1976), germinating seeds of rice (Rivoal et al., 1989) and 3‐ to 4‐d‐old rice roots (Reggiani et al., 1986) when oxygen supply was severely restricted. An increase in the activity of PDC, in the absence of oxygen, was also reported in wheat (Waters et al., 1991) and maize root tips (Drew et al., 1994; Sachs et al., 1996). Biochemical studies have indicated that ADH does not limit fermentation. Regulation of the fermentation pathway is more likely to be achieved by the fine control of PDC activity, due to its low maximum catalytic activity (Morell et al., 1990; Drew, 1997). Also, Quimio et al. (2000) demonstrated that the over‐expression of PDC in transgenic rice promoted high rates of ethanolic fermentation, which were correlated with higher PDC activities and enhanced submergence tolerance. Accordingly, we examined the extent to which submergence stress affected the activity of this key enzyme, in the belief that the control of the fermentation pathway would be critical for submergence tolerance. This point was examined further by comparing doubled haploid lines of rice, taken from a population of over 100 such lines displaying a full range of submergence tolerance, and also the parental lines. Each doubled haploid line contains a unique random combination of the parental genomes and its homozygosity gives rise to genetically identical seeds when selfed. Hence, these doubled haploid lines are a useful resource for elucidating the physiological and genetical bases of submergence tolerance. The lines selected for study were based on tolerance tests conducted under indoor and outdoor conditions at Faizabad (India), Prachinburi (Thailand), Bristol (UK) and in our own laboratory. Activity of PDC was determined in apical root sections, 3–5 cm long, since these were the parts of the plants most likely to experience oxygen deficiency by virtue of their distance from oxygen‐generating photosynthetic shoots, their high tissue density, and fast rates of respiration (Vartapetian and Jackson, 1997).
MATERIALS AND METHODS
Germination of seeds and growth of seedlings
Seeds of the F1 generation of the doubled haploid lines and their parental lines were supplied by Dr S. Sarkarung of the International Rice Research Institute, Bangkok, Thailand. They were produced from anthers of plants of the F1 generation, derived from a cross between submergence‐tolerant FRI3A and a submergence‐intolerant upland rice CT6241. All seeds were surface‐sterilized with 1 % Clorox™ for 5 min, rinsed, and soaked overnight in distilled water at room temperature (22 °C); they were then incubated for 3 d on a piece of filter paper in 9‐cm‐diameter Petri dishes in the dark at 32 °C. Germinated seeds with healthy coleoptiles were transferred to cotton wool‐lined plastic growth trays (approx. 100 coleoptiles per tray) and allowed to grow further. Coleoptiles of the different rice lines were transferred to different trays, with one rice line growing in each tray. For each line, at least three trays of seedlings were raised; each tray of seedlings was divided into ten to 13 groups of plants (six to ten seedlings per group). Roots of the seedlings were always immersed in a complete nutrient solution (initially 20 % Hoagland solution with an additional 0·03 mm Fe‐EDTA). These seedlings were grown at 26 °C with 50 µmol quanta m–2 s–1 provided by Philips TL daylight fluorescent tubes. Seedlings were raised under this low photosynthetic photon flux density (PPFD) to ensure low carbohydrate reserves and thus high sensitivity of the seedlings to submergence. The photoperiod was 12 h and the nutrient solution was changed once every 5 d. Seedlings were used when they had developed three leaves (about 14 d after germination).
Screening for submergence tolerance
Seedlings with three leaves were completely submerged in tap water in plastic tanks (80 cm tall with a volume of 80 1) under natural day/night (12 h day/l2 h night) and ambient atmospheric conditions in the teaching garden of the Department of Biological Sciences, National University of Singapore. Average shoot height of these seedlings was 15–20 cm. The sides of these tanks were opaque to light. Tap water was allowed to stand in the tanks overnight prior to submergence. To prevent the breeding of mosquitoes, each tank was covered with a piece of green plastic mesh. The resultant PPFD in air at mid‐tank level was 150–200 µmol m–2 s–1. Ambient PPFD levels outside the tanks could reach 1800 µmol m–2 s–1 at midday. The temperature of the water, at the level of the leaves, was 31 ± 5 °C. The concentration of oxygen in the water was measured at various times with an oxygen meter (AQUALYTIC, Nue‐Isenburg, Germany). After 5, 10 and 15 d of submergence, the seedlings were taken out of the water and allowed to recover in air for 12 d before the percentage of surviving seedlings was recorded. The submergence tolerance score of the seedlings (in terms of leaf senescence) was also determined on a per tray basis immediately after desubmergence: a score of 1 was recorded when almost no dead leaves were found in the tray; 3 when up to 50 % of the leaves were dead; 5 when 50–70 % of the leaves had died; 7 when most leaves were dead; and 9 when all leaves had died.
Submergence of rice seedlings in total darkness and in anoxia
Rice seedlings were raised and submerged as described above, but they were submerged in total darkness for 1, 3 and 5 d. To exclude light, the plastic tanks were covered with two layers each of aluminium foil and black plastic. For anoxia studies, the rice seedlings were transferred to 2 l Erlenmeyer flasks, submerged under pre‐adaptive, near‐anaerobic and then anaerobic conditions. Each flask was wrapped with two layers of aluminium foil, filled with tap water, sealed tightly with two clear plastic bags and covered with two layers of aluminium foil and left for 24 h. By this time, oxygen concentration in the flasks had decreased to 2·3 mg l–1 on average. All flasks with 24 h dark‐submerged seedlings were flushed with nitrogen gas at 5 l h–1 to remove all traces of oxygen, and then continuously at 2 l h–1 to maintain anaerobic conditions.
Extraction and assay of pyruvate decarboxylase
After various periods of submergence, seedlings were transferred to beakers of water, covered with aluminium foil, and taken to the laboratory where they were rinsed free of any algal deposit. To extract PDC, apical 3·0–5·0‐cm‐long sections of roots (0·2–0·5 g f. wt) were excised from several seedlings and ground at 4 °C with 3 ml extraction buffer [50 mm MES‐NaOH (pH 6·0), 2·0 mm DTT, 2·5 mm MgCl2, 0·50 mm TPP, 1·0 mm EDTA, 1·0 mm NaCl] using a pestle and mortar. The brie was centrifuged at 20 000 g at 4 °C for 30 min and the supernatant collected was used for the assay of PDC activity. The crude enzyme extract (570 µl), in a final volume of 3 ml, was first incubated in the assay medium for 15–20 min at 25 °C to develop maximum activity. The assay medium contained 80 mm MES‐NaOH (pH 6·0), 1·0 mm NaCl, 0·50 mm TPP, 1·0 mm MgCl2, 10·0 mm DTT, 0·17 mm NADH and ten units of yeast ADH (Börhringer, Mannheim, Germany). Sodium oxamate (10 mm) was also added to the assay mixture to inhibit lactate dehydrogenase. The reaction was started with 20 mm pyruvate (Na salt), and the activity of PDC was estimated in terms of NADH oxidation at 340 nm (Morrell et al., 1990). The concentrations of total soluble proteins in the crude enzyme extracts were estimated using the Bio‐Rad protein assay kit (Bio‐Rad, Hercules, CA, USA) at 595 nm.
To validate the PDC assay further, PDC was extracted from hypoxically grown coleoptiles of lines FR13A, CT6241, 380 and 406, and their activities compared with those of rice coleoptiles reported by Gibbs et al. (2000). Our values of PDC activity were 418, 331, 486 and 607 nmol min–1 g–1 f. wt, or 378, 648, 738 and 562 nmol min–1 mg–1 protein, for FR13A, CT6241, 380 and 406, respectively. These enzyme activities were similar to those reported by Gibbs et al. (2000). The methods of PDC extraction and assay were further authenticated by recovery experiments of known amounts of yeast PDC (Sigma Co., St Louis, MO, USA) added to the extraction buffer prior to extraction of the enzyme, to assess the loss of enzyme activity during the experimental procedure. The recovery of pure yeast PDC averaged 80 % in three separate assays, indicating that extraction losses were modest.
Experimental design
All experiments were conducted at least twice. For each experiment, at least 150 seedlings of each rice line were submerged or retained as controls. Submergence tolerance was determined as the average percentage survival of three different trays of seedlings (80–100 seedlings per tray), submerged in three different tanks, after 5–15 d of submergence. For the determination of PDC activity, ten to 12 groups (each group including six to ten plants) of seedlings of each rice line were randomly collected from three different trays and the roots were divided into two or three groups. For the anoxia experiment, 60–80 plants were collected from three different flasks and divided into three equal groups for the extraction of PDC. The activities of PDC are presented as means ± s.e. of three tissue samples.
Statistical analysis
Differences between treatments for each rice line were compared by ANOVA using Fisher’s least significant difference (LSD) (P < 0·05), as this provided a good test for determining whether means were significantly different. The overall means of PDC activity and the overall percentages of increase in PDC activity of the tolerant and intolerant lines were compared using Students’ t‐test (P < 0·05).
RESULTS
All experiments were replicated at least twice in this study.
Submergence screening test for doubled haploid lines
Submergence tolerance, in terms of leaf senescence scores and percentage of seedlings that could survive 5–15 d of submergence, assessed after 12 d of recovery, are shown in Table 1 for 13 doubled haploid lines and their parents, FR13A and CT6241. Seven doubled haploid lines (331, 332, 336, 367, 380, 381 and 502) proved to be similar to FR13A in their tolerance to 5, 10 or 15 d of complete submergence under natural day/night conditions. In contrast, the remaining six doubled haploid lines (334, 337, 384, 401, 402 and 406) were as intolerant to submergence as CT6241 (Table 1). All seedlings of FR13A and the submergence‐tolerant lines remained green (leaf senescence score = 1) and healthy after 10 d of submergence. In contrast, more than 75 and 100 % of leaves of CT6241 and the submergence‐intolerant lines were dead after 10 and 15 d of submergence, respectively (Table 1).
Table 1.
Percentage survival (%) and leaf senescence score (LS) of rice seedlings after 5 (5DS), 10 (10DS) and 15 d (15DS) of submergence under natural day/night conditions
| Rice lines | |||||||||||||||
| FR 13A | 331 | 332 | 336 | 367 | 380 | 381 | 502 | CT 6241 | 334 | 337 | 384 | 401 | 402 | 406 | |
| 5DS | |||||||||||||||
| % | 100 ± 0 | 97 ± 3 | 100 ± 0 | 100 ± 0 | 97 ± 3 | 97 ± 2 | 100 ± 0 | 96 ± 3 | 78 ± 6 | 97 ± 2 | 75 ± 3 | 75 ± 4 | 83 ± 8 | 84 ± 5 | 80 ± 8 |
| LS | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 3 | 1 | 5 | 3 | 1 | 1 | 1 |
| 10DS | |||||||||||||||
| % | 93 ± 4 | 97 ± 6 | 97 ± 2 | 100 ± 0 | 89 ± 5 | 94 ± 3 | 90 ± 3 | 99 ± 1 | 4 ± 3 | 7 ± 3 | 0 ± 0 | 4 ± 3 | 4 ± 2 | 2 ± 2 | 0 ± 0 |
| LS | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | 7 | 9 | 7 | 7 | 7 | 9 |
| 15DS | |||||||||||||||
| % | 10 ± 4 | 42 ± 8 | 37 ± 9 | 78 ± 4 | 58 ± 8 | 6 ± 2 | 11 ± 4 | 86 ± 4 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| LS | 7 | 5 | 5 | 1 | 3 | 7 | 7 | 1 | 9 | 9 | 9 | 9 | 9 | 9 | 9 |
Values are means ± s.e.
All plants were submerged at the three‐leaf stage and measurements were made after 12 d of recovery from submergence in air.
Higher leaf senescence scores indicate greater leaf senescence or necrosis.
Activity of PDC in seedlings submerged under natural light conditions
The activity of PDC from root tissues of the parental lines and three submergence‐tolerant and intolerant doubled haploid lines was assayed in the photoperiod just prior to submergence (0900–1100 h). Activities ranged from 49·7 ± 3·3 to 82·1 ± 5·1 nmol mg–1 protein min–1 (Fig. 1). Compared with these values, submergence for 5 or 10 d decreased PDC activity in many lines tested. After 5 d of submergence, PDC activities of FRI3A and CT6241 were, respectively, 13 and 25 % lower than those before submergence; they became, respectively, 52 and 62 % lower after 10 d submergence. With the exception of tolerant lines 367 and 381, which changed little, PDC activity of seedlings of the other tolerant lines (FR13A and 380) decreased by 13 and 54 %, respectively, after 5 d of submergence (Fig. 1). Prolonging submergence to 10 d reduced root PDC activity of submergence‐tolerant seedlings by a further 29 % (in 367), 18 % (in 380), 43 % (in 381) and 47 % (in FR13A). In submergence‐intolerant lines, root PDC activity decreased by a further 26 % (in 384), 42 % (in 401), 34 % (in 406) and 49 % (in CT6241) after 10 d of submergence. The overall decrease in PDC activity after 10 d of submergence was 34·3 ± 6·7 % in tolerant lines and 38·0 ± 5·0 % in intolerant lines. As the two overall means were not statistically different, PDC activity of rice seedlings of the two tolerance classes did not differ markedly. Concentrations of total soluble proteins extractable from root tissues of all seedlings decreased with 5 d of submergence and decreased further when the duration of submergence was extended to 10 d (Fig. 1, insert). Thus, total soluble proteins of the submergence‐tolerant lines decreased by 25–48 % and 39–72 % after 5 and 10 d of submergence, respectively. The corresponding decreases were 43–49 % and 61–79 % in submergence‐intolerant lines. Once again, there was no clear difference between the two tolerance groups. The oxygen concentration of the floodwater varied between 8·0 and 8·4 mg l–1 during the day (dissolved oxygen concentration in equilibrium with air at 30 °C is 7·57 mg l–1) and the pH of the water was in the range of 6·8–7·1. Oxygen concentration of the floodwater fell to approx. 6·5 mg l–1 during the night.
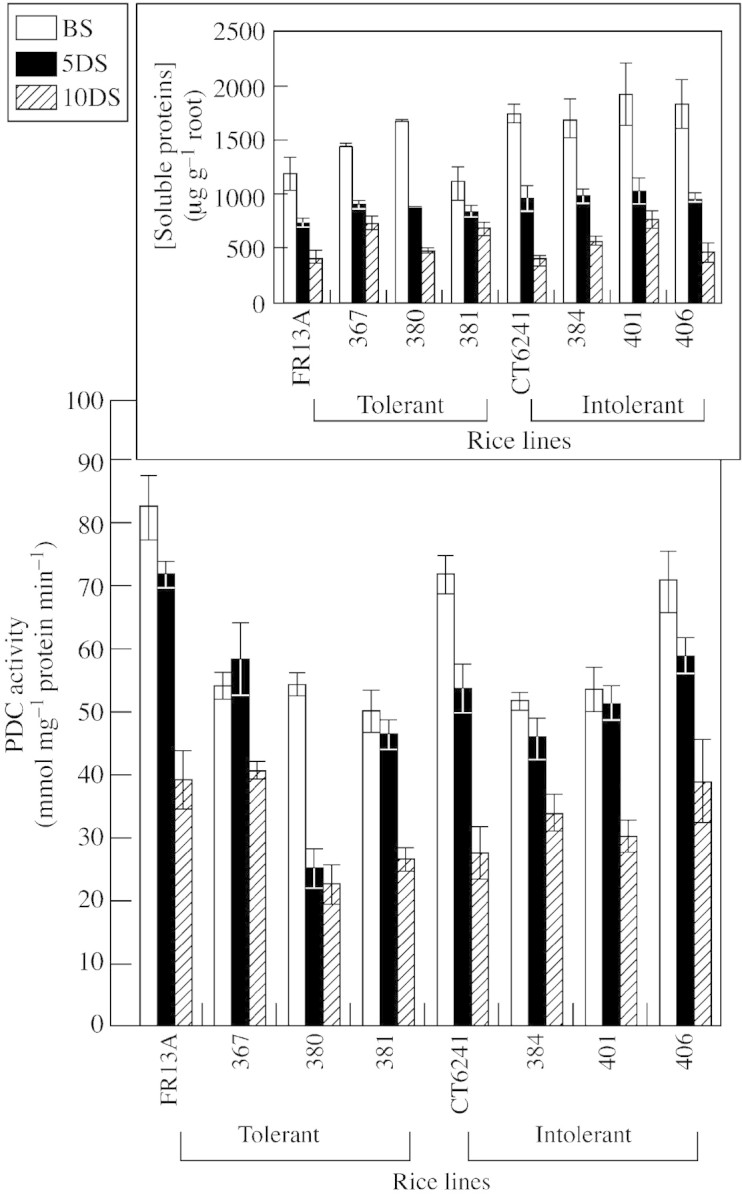
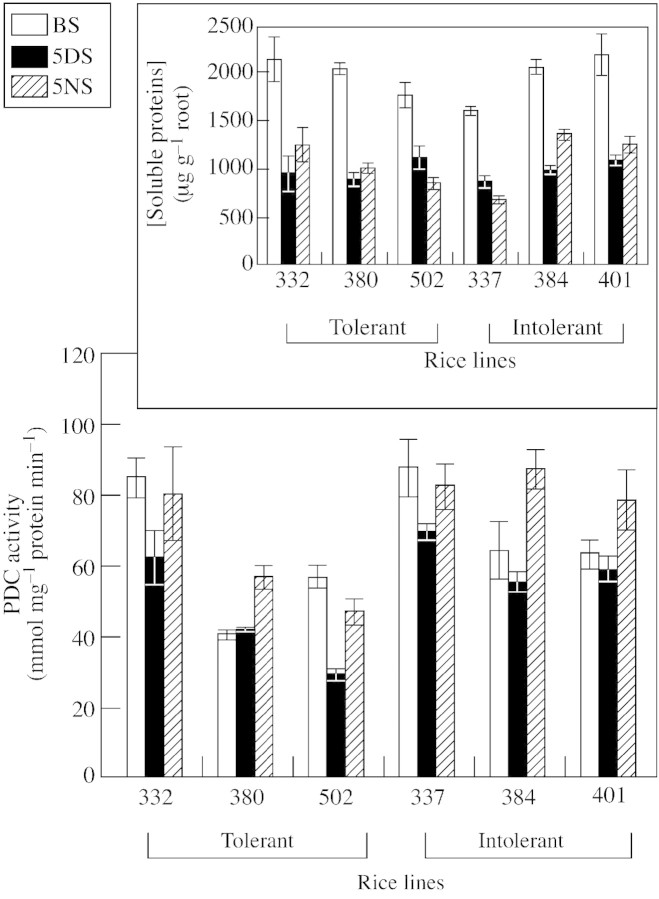
Fig. 1. Changes in the activity of pyruvate decarboxylase (nmol mg–1 protein min–1) in apical root sections of submergence‐tolerant and intolerant lines of rice seedlings before submergence (BS), and after 5 (5DS) and 10 d (10DS) of submergence. Insert shows changes in the concentrations of total soluble proteins. Vertical bars represent s.e. Treatments started at the three‐leaf stage.
Activity of PDC during day and night in plants submerged for 5 d
To determine the effects of light and darkness on root PDC activity, seedlings of six selected doubled haploid lines were completely submerged for 5 d under natural day/night conditions. This duration of submergence was chosen because submerged seedlings showed no visible signs of serious damage at this time (Table 1). Seedlings were harvested in the morning (0900–1100 h) and the following night (2000–2200 h). As expected, PDC activity of roots collected in the morning (0900–1100 h), after submergence, was usually less than that of roots harvested before submergence (Fig. 2). However, activities of PDC determined in seedlings collected at night were higher than those observed in the morning (Fig. 2). The effect of darkness was strong enough to overcome almost totally the inhibiting influence of submergence on PDC activity in line 502 (tolerant), to overcome completely the inhibition in lines 332 (tolerant) and 337 (intolerant), and to stimulate activity in lines 384 and 401 (both intolerant). After 5 d of submergence, the overall increase in PDC activity during the night, compared with daytime activity, was 41·8 ± 9·6 % in tolerant lines and 36·6 ± 11·5 % in intolerant lines (no statistical difference). Concentrations of extractable soluble proteins in roots of all seedlings decreased with submergence, and, in contrast to PDC activity, were not affected by harvesting in the light or dark period (Fig. 2, insert) Thus, the effect of darkness on PDC was not a result of the change in total protein level.
Fig. 2. Changes in the activity of pyruvate decarboxylase (nmol mg–1 protein min–1) in the apical root sections of submergence‐tolerant and intolerant lines during submergence under natural day/night conditions. BS, Before submergence; 5DS, plants harvested after 5 d of submergence during the day; 5NS, plants harvested after 5 d of submergence during the night. Insert shows changes in the concentrations of proteins of the corresponding rice lines. Vertical bars represent s.e. Treatments started at the three‐leaf stage.
Activity of PDC after 1, 3 or 5 d of submergence in total darkness
Four tolerant lines (336, 380, 381, 502), four intolerant lines (334, 337, 384, 401) and the two parental lines (FR 13A and CT6241) were tested for responses to submergence in total darkness. The doubled haploid lines 333, 364 and 395 were also included in this test. The survival percentages of these lines, after 10 d of submergence under normal day/night conditions, were 56, 0, and 0 %, respectively. Doubled haploid line 341, known to be tolerant of submergence under local natural day/night conditions in India (B. B. Singh et al., unpubl. res.), was also tested. Apical root sections were excised in the morning (0900–1100 h) for PDC extraction, just prior to submergence. This was 2–4 h after the end of the natural dark period. After 1 or 3 d of submergence in total darkness, seedlings of tolerant lines remained healthy and green while those of intolerant lines appeared weak and yellow (equivalent to a leaf senescence score of 5).
Submergence of seedlings in total darkness for 1 d increased root PDC activity in all lines (Table 2). Overall, PDC activity increased by 67·7 ± 13·2 % in the submergence‐tolerant lines and 94·0 ± 2·5 % in intolerant lines; the overall increases in PDC activity were significantly different (P < 0·05). Prolonging dark submergence for a further 2 d increased PDC activity further except in lines 336 (tolerant), and CT6241 and lines 334 and 337 (intolerant); the 3 d values of PDC activity for all lines remained substantially above pre‐submergence levels (Table 2). Overall PDC activity in 3 d dark‐submerged seedlings increased by 88·4 ± 18·5 % for tolerant lines and by 95·7 ± 10·2 % for intolerant lines; differences between tolerant and intolerant lines were not significant. Con centrations of total extractable soluble proteins from the roots of all seedlings decreased with increasing duration of dark submergence (Table 3). During this period, oxygen con centration of the floodwater decreased from 8·0–8·4 mg l–1 to an average of 6·1 and 5·2 mg l–1 after submerging the seedlings in total darkness for 1 and 3 d, respectively. The activity of PDC was measured again after 5 d of submergence in the dark. At this time, the tolerant lines still retained healthy‐looking green leaves, whereas leaves of intolerant lines were dead. With the exception of the intolerant parental line (CT6241), the roots of which were flaccid and probably dead, the activity of PDC increased further in both tolerant and intolerant lines after 5 d of dark submergence (Table 2). Although such increases were greater in some lines than in others, the overall increase in PDC activity of the 5 d dark‐submerged tolerant and intolerant lines was not significantly different from that of 3 d dark‐submerged seedlings. Over this same period, the concentrations of soluble proteins in all seedlings decreased further (Table 3) while the concentration of oxygen in the water declined from 8·0–8·4 mg l–1 to 4·4 mg l–1 over the whole 5 d period.
Table 2.
Changes in activity of pyruvate decarboxylase (nmol mg–1 protein min–1) in roots of submergence‐tolerant and intolerant rice lines during submergence in darkness
| Rice lines | BS | 1DD | 3DD | 5DD |
| Tolerant lines | ||||
| FR13A | 49·3 ± 1·0a | 93·5 ± 2·7b | 101·6 ± 8·8b | 112·8 ± 4·8c |
| 33 | 56·2 ± 2·3a | 70·2 ± 4·5b | 77·2 ± 3·4b | – |
| 336 | 65·1 ± 3·4a | 132·3 ± 3·5b | 111·9 ± 9·0b | 210·9 ± 20·4c |
| 341 | 66·6 ± 3·0a | 90·1 ± 10·8b | 103·3 ± 7·5b | – |
| 380 | 52·4 ± 3·9a | 81·2 ± 4·9b | 113·9 ± 18·0c | 155·2 ± 5·3d |
| 381 | 42·2 ± 3·1a | 90·6 ± 3·3b | 117·6 ± 5·8c | – |
| 502 | 48·8 ± 2·1a | 73·8 ± 6·3b | 75·1 ± 3·0b | 165·4 ± 19·1c |
| Intolerant lines | ||||
| CT6241 | 46·4 ± 1·4a | 112·2 ± 13·6c | 94·3 ± 6·1b | 80·4 ± 5·9b |
| 334 | 59·7 ± 4·8a | 137·4 ± 4·5b | 120·2 ± 5·6b | 191·7 ± 18·6c |
| 337 | 45·4 ± 2·7a | 93·9 ± 7·7b | 62·8 ± 5·9a | 131·7 ± 22·3c |
| 364 | 56·3 ± 6·4a | 97·2 ± 6·1b | 118·9 ± 4·2c | – |
| 384 | 44·9 ± 1·7a | 82·6 ± 7·4b | 99·4 ± 3·8c | 184·1 ± 10·4d |
| 395 | 56·0 ± 2·0a | 93·3 ± 4·4b | 108·5 ± 1·9c | – |
| 401 | 71·3 ± 0·0a | 110·4± 5·4b | 144·1 ± 11·5c | 175·4 ± 14·1c |
These values were compared with those of the respective rice line before submergence.
BS, before submergence; 1DD, 1 d submerged in darkness; 3DD, 3 d submerged in darkness; 5DD, 5 d submerged in darkness.
Values are means ± s.e.
Treatments started when the plants were at the three‐leaf stage.
Fisher’s least significant difference procedure was used to test the effects of various treatments on PDC activity.
Values in the same row followed by the same superscript do not differ significantly at P < 0·05.
Table 3.
Changes in concentrations of total soluble proteins of roots (mg g–1 root tissue) of seedlings of submergence‐tolerant and intolerant rice lines during submergence in darkness
| Rice lines | BS | 1DD | 3DD | 5DD |
| Tolerant lines | ||||
| FR13A | 1·34 ± 0·08 | 1·19 ± 0·19 | 0·99 ± 0·13 | 1·02 ± 0·04 |
| 333 | 1·86 ± 0·03 | 1·64 ± 0·14 | 1·33 ± 0·07 | – |
| 336 | 1·87 ± 0·13 | 1·57 ± 0·16 | 1·42 ± 0·25 | 1·25 ± 0·08 |
| 341 | 1·58 ± 0·04 | 1·45 ± 0·04 | 1·05 ± 0·09 | – |
| 380 | 1·84 ± 0·16 | 1·37 ± 0·14 | 1·15 ± 0·09 | 0·84 ± 0·10 |
| 381 | 2·09 ± 0·23 | 1·86 ± 0·09 | 1·40 ± 0·12 | – |
| 502 | 1·56 ± 0·13 | 1·77± 0·12 | 1·31 ± 0·13 | 0·46 ± 0·03 |
| Intolerant lines | ||||
| CT6241 | 2·06 ± 0·12 | 1·68 ± 0·07 | 1·40 ± 0·05 | 0·73 ± 0·08 |
| 334 | 2·01± 0·14 | 1·72 ± 0·09 | 1·32 ± 0·15 | 1·42 ± 0·19 |
| 337 | 1·64 ± 0. 24 | 1·62 ± 0·12 | 1·21 ± 0·12 | 0·60 ± 0·04 |
| 364 | 2·09 ± 0·11 | 1·30 ± 0·19 | 1·21 ± 0·19 | – |
| 384 | 2·50 ± 0·19 | 1·82 ± 0·04 | 1·12 ± 0·08 | 0·68 ± 54·1 |
| 395 | 1·51 ± 0·14 | 1·55 ± 0·17 | 0·88 ± 0·08 | – |
| 401 | 1·89 ± 0·15 | 2·00 ± 0·25 | 1·57 ± 0·13 | 1·40 ± 0·17 |
BS, before submergence; 1DD, 1 d submergence in darkness; 3DD, 3 d submergence in darkness.
Values are means ± s.e.
Treatments started when the plants were at the three‐leaf stage.
Activity of PDC after a near‐anaerobic treatment followed by anaerobic submergence
To determine the effect of anaerobic conditions on root PDC activity, seedlings of both parental lines and selected doubled haploid lines were pre‐adapted to anaerobiosis by submerging them in a 2 l flask in total darkness for 1 d at 2·3 mg l–1 oxygen. The plants were then exposed to a strictly anaerobic environment for either 6 or 24 h. The activity of PDC of all lines increased substantially after the 24 h pre‐treatment in near‐anaerobic conditions (Table 4). Such an increase was less than 40 % in line 502 (tolerant), while increases of up to 171 % were observed in other lines (Table 4). These increases developed despite a considerable loss of soluble proteins that was similar in extent in almost all lines (30–50 %). Overall, PDC activity increased by 71·4 ± 17·0 % in submergence‐tolerant lines and by 103·2 ± 19·1 % in intolerant lines. The overall mean PDC activity of the submergence‐tolerant lines was statistically different from that of the intolerant rice lines (P < 0·05), and PDC activity was higher in intolerant lines. Transferring all these plants to anaerobic conditions for 6 h raised the PDC activity further in intolerant lines 337, 384 and 401, and also in the tolerant FR13A and line 502. PDC activity was 133·5 ± 13·3 % above non‐submerged values in intolerant lines (excluding line 364, the plants of which were almost dead); PDC activity was 111·0 ± 14·1 % above non‐submerged values in tolerant lines. Extending the anaerobic treatment to 24 h depressed PDC activity in all rice lines, although this effect was much more pronounced in the submergence‐intolerant lines (Table 4). In one submergence intolerant line (401), 24 h of anaerobic treatment reduced the enzyme activity to far below the pre‐submergence value. Such reduction in PDC activity was associated with flaccid roots and the apparent death of the roots. Overall, after 24 h of anaerobic treatment, PDC activity decreased by 56·5 ± 10·8 and 28·4 ± 4·4 %, respectively, in intolerant and tolerant lines, compared with that observed after 6 h of anaerobic treatment. Dark‐submergence of all seedlings in partial oxygen deficiency for 1 d and subsequent exposure to anaerobic conditions markedly reduced total extractable proteins (Table 5).
Table 4.
The effects of anaerobic conditions on root pyruvate decarboxylase activity (nmol mg–1 protein min–1) of submergence‐tolerant and intolerant rice lines
| Rice lines | BS | 1DD | 6HA | 1DA |
| Tolerant lines | ||||
| FR13A | 45·9 ± 1·6a | 89·5 ± 11·3b | 114·7 ± 1·8c | 90·8 ± 9·6b |
| 331 | 40·7 ± 3·3a | 66·3 ± 19·6b | 72·2 ± 9·8b | 44·5 ± 6·3a |
| 332 | 84·7 ± 5·6a | 126·2 ± 21·4b | 154·3 ± 26. 5b | 131·4 ± 8·8b |
| 380 | 43·5 ± 2·0a | 97·0 ±14·7c | 100·9 ± 8·2c | 68·1 ± 8·4b |
| 502 | 56·4 ± 3·2a | 71·6 ± 10·2a | 120·7 ± 21·2b | 78·6 ± 1·6a |
| Intolerant lines | ||||
| CT6241 | 34·7 ± 2·2a | 94·0 ± 11·1c | 68·6 ± 5·0b | 44·9 ± 4·6a |
| 337 | 87·2 ± 8·3a | 139·6 ± 2·5c | 200·0 ± 16·8d | 108·2 ± 6·8b |
| 364 | 67·2 ± 3·9a | 140·1 ± 5·7c | 71·1 ± 1·2a | 88·3 ± 1·6b |
| 384 | 74·0 ± 6·2a | 130·5 ± 9·8b | 184·0 ± 22·7c | 74·2 ± 12·5a |
| 401 | 62·9 ± 3·9b | 125·7 ± 11·1c | 161·8 ± 12·8d | 24·8 ± 2·0a |
Seedlings were pre‐adapted to a near‐anaerobic condition (dissolved oxygen concentration = 2·3 mg l–1) before being subjected to anaerobic conditions.
BS, before submergence; 1DD, 1 d in darkness; 6HA, 6 h in anaerobic condition; 1DA, 24 h in anaerobic condition.
Values are means ± s.e.
Treatments started when the plants were at the three‐leaf stage.
Fisher’s least significant difference procedure was used to test the effects of various treatments on PDC activity.
Values in the same row followed by the same superscript do not differ significantly at P < 0·05.
Table 5.
Changes in the concentrations of total soluble proteins (mg g–1 root tissue) in roots of submergence‐tolerant and intolerant rice lines during submergence in anaerobic conditions
| Rice lines | BS | 1DD | 6HA | 1DA |
| Tolerant lines | ||||
| FR13A | 1·47 ± 0·14 | 1·02 ± 0·05 | 1·10 ± 0·03 | 0·97 ± 0·07 |
| 331 | 1·85 ± 0·09 | 0·95 ± 0·05 | 1·03 ± 0·05 | 0·90 ± 0·10 |
| 332 | 2·12 ± 0·22 | 1·40 ± 0·17 | 0·99 ± 0·12 | 1·43 ± 0·24 |
| 380 | 2·00 ± 0·20 | 1·31 ± 0·06 | 1·36 ± 0·11 | 1·20 ± 0·11 |
| 502 | 1·75 ± 0·13 | 1·03 ± 0·08 | 1·14 ± 0·03 | 1·23 ± 0·07 |
| Intolerant lines | ||||
| CT6241 | 1·62 ± 0·15 | 0·98 ± 0·08 | 1·09 ± 0·09 | 0·67 ± 0·03 |
| 337 | 1·58 ± 0·05 | 1·61 ± 0·07 | 1·10 ± 0·01 | 1·36 ± 0·07 |
| 364 | 2·03 ± 0·03 | 1·29 ± 0·14 | 1·01 ± 0·06 | 0·82 ± 0·05 |
| 384 | 2·32 ± 0·11 | 1·42 ± 0·14 | 0·96 ± 0·13 | 0·66 ± 0·02 |
| 401 | 2·17 ± 0·21 | 1·22 ± 0·07 | 1·23 ± 0·11 | 0·91 ± 0·04 |
BS, Before submergence; 1DD, 1 d in darkness; 6HA, 6 h in anaerobic condition; 1DA, 1 d in anaerobic condition.
Values are means ± s.e.m.
Treatments started when the plants were at the three‐leaf stage.
DISCUSSION
We hypothesized that the various degrees of reduction in PDC activity were the result of differences in submergence tolerance and submergence damage of FR13A (submergence‐tolerant parent), CT6241 (submergence‐intolerant parent) and the doubled haploid rice lines studied. The emphasis on PDC was based on a large amount of literature indicating that this enzyme is a major determinant of the rate of ethanolic fermentation and that its activity is markedly stimulated by a shortage of oxygen. For example, Hossain et al. (1996) reported an induction of PDC 1 mRNA accumulation in rice (cultivar IR 54) seedlings under anoxic conditions. Umeda and Uchimiya (1994) showed that the PDC‐RNA transcript titre of both submergence‐tolerant (FR13A) and intolerant (cultivar IR 42) rice seedlings increased dramatically between 12 and 24 h of complete submergence in dark conditions; it then decreased after 48 h of dark submergence as plant stress became more severe. These authors also reported that during the early stage (0–12 h) of dark submergence, transcript levels of PDC were higher in the submergence‐intolerant IR 42 than in tolerant FR13A; the transcript levels, however, became similar in both lines after a further 12 h of submergence. Their study suggested that the early increase in PDC expression and enhancement of fermentation could depress submergence tolerance of rice plants. However, Gibbs et al. (2000) found no close link between PDC activity in coleoptiles and submergence tolerance in the rice cultivars Calrose and IR 22. Surprisingly, there was also no relationship between the activity of PDC and the rate of ethanol production. Using transgenic rice seedlings obtained from rice cultivar Taipei (309) and over‐expressing PDC, Quimio et al. (2000) demonstrated that increased PDC activity did indeed result in faster ethanolic fermentation and greater tolerance of submergence. Rivoal et al. (1997) also observed a rapid increase in PDC activity in 2‐d‐old rice (cultivar Cigalon) seedlings during the first 24 h of anoxic treatment. There is also evidence that anoxia increases PDC activity in shoots of seedlings in submergence‐tolerant (IR 49839 and FR13A) and intolerant (IR 36 and IR 42) cultivars of rice (Ellis and Setter, 1999). These authors observed that PDC activity was higher in submergence‐tolerant than in submergence‐intolerant lines. However, the number of rice lines compared by these authors was small and the lines came from varied genetic backgrounds. In contrast, we quantified the activity of PDC in root tissues from a much larger number of tolerant and intolerant rice lines with a shared parentage. Moreover, our values of PDC activity were expressed on the basis of soluble protein level (mg of proteins), because anoxia can affect the total soluble protein content in plants—a measure of the size of the total catalytic machinery of the cells. We chose to determine the activity of PDC in roots rather than in whole plants as root tissues are more likely to experience oxygen shortage than the photosynthetic shoot tissues. It was considered important not to use whole plants in our sampling because roots and shoots could differ in their response to anoxia. For example, Cobb and Kennedy (1987) determined the distribution of ADH in both roots and shoots of rice (cultivar S201) and the rice‐mimic, Echinochloa, and in two anoxia‐intolerant plants, maize and pea. They found higher levels of ADH activity in the shoots than in the roots of rice and Echinochloa; however, higher ADH activity was observed in the roots of both maize and pea plants.
Survival and submergence
The survival of complete submergence results from complex metabolic adaptations of plants to several likely deprivations or enrichments of resources. These can include restricted oxygen supply, decreased availability of light and carbon dioxide (Setter et al., 1997) and increased internal accumulation of ethylene (Jackson et al., 1987). Despite these traumas, FRI3A recovered well from 10 d of complete submergence under natural day/night conditions. In contrast, only 7 % of the seedlings of CT6241 survived this treatment. Ten‐day‐old seedlings of FRI3A were also found to be tolerant of submergence for up to 6 d under glasshouse conditions (Jackson et al., 1987; Ellis and Setter, 1999), thus confirming the reputation of FRI3A as a highly submergence‐tolerant cultivar (Mazeredo and Vergara, 1982). Of the 13 doubled haploid lines studied, seven were submergence tolerant and six were not. Most of the leaves of the submergence‐intolerant lines were dead after 10 d of inundation. These data indicated that prolonged submergence possibly resulted in oxygen‐deficient conditions within the plants and caused the leaves to die (Ellis and Setter, 1999). Although submergence proved highly damaging to the intolerant lines, it should be noted that concentrations of dissolved oxygen in the floodwater remained above those expected for water in equilibrium with air during the daytime. Though extensive measurements of dissolved oxygen concentrations in tanks with submerged plants were not made at night, our results indicated that night‐time concentrations of dissolved oxygen fell only to approx. 6·5 mg l–1, close to that in equilibrium with air (7·57 mg l–1).
Activity of PDC in submerged plants during daytime
The activities of PDC extracted from apical root sections were found to be very low in non‐submerged seedlings of both tolerant and intolerant lines. However, these activities were comparable to those reported by John and Greenway (1976) for rice roots (cultivar Calrose) and by Ellis and Setter (1999) for shoots of non‐submerged rice seedlings of tolerant (FR13A and IR49830) and intolerant (IR36 and IR42) varieties.
The outstanding result from PDC measurements made on extracts of apical root sections obtained in the mornings (2–4 h after the end of the night) was that submergence for 5 d or 10 d under normal day/night conditions depressed the activity of the enzyme; however, the extent of the effect was not related to submergence tolerance of the rice lines studied. This might have been the outcome of losses of soluble proteins, the extent of which was similar in all lines, and a lack of specific activation of PDC transcription and translation in these rice lines. Pyruvate decarboxylase is known to be activated by oxygen deficiency (Rivoal et al., 1989; Sachs et al., 1996). The data obtained suggested that roots of the rice lines studied were not suffering from oxygen shortage at the time of sampling, as the dissolved oxygen concentration of the floodwater was maintained at about 8 mg l–1 during the daytime; this concentration of oxygen was slightly above that in equilibrium with air (7·57 mg l–1). This enrichment was the likely consequence of photosynthetic evolution of oxygen from the leaf surfaces of the submerged plants and some algae. It was also shown by Rijnders et al. (2000) that in wholly submerged Rumex palustris, photosynthesis was an important source of oxygen and the amount of tissue oxygen was dependent on the duration of submergence and the level of irradiance. However, despite underwater photosynthesis, submergence of the rice lines studied resulted in damage to plant tissues, manifested as foliar and root senescence and large reductions in concentrations of soluble root proteins (Lee and Lin, 1995). Root senescence was visually apparent by the fifth day of submergence in all rice lines. These results indicated that daytime oxygen shortage and the associated activation of PDC, a key regulatory step in the fermentation pathway, were not the cause of the submergence damage and the difference in submergence tolerance between the parental lines and their doubled haploid descendants. Rather, these observations could be attributed to other factors, such as the possibility that submergence damage occurred mostly at night.
Effect of intermittent or continuous darkness on PDC activity in submerged plants
When PDC activity was assayed at night, enzyme activity was much higher than that determined in the extracts of plants sampled in the morning. The overall increase in PDC activity at night, compared with that during the day, was 41·8 ± 9·6 % in tolerant lines and 36·6 ± 11·5 % in intolerant lines (statistically indistinguishable). In general, PDC activity during the dark period was sufficiently high to exceed that measured before submergence. The absence of photosynthesis in the rice seedlings during the night might have resulted in temporarily reduced oxygen levels in plant tissues, thus enhancing PDC activity in the root tissues during the night (Setter et al., 1997). Although PDC activation could be expected to affect the extent of submergence survival by influencing the rate of fermentation, no clear link was found between the amount of night‐time PDC activation underwater and the tolerance of the various lines to submergence.
When the seedlings were submerged in total darkness, they showed more rapid foliar senescence than under the normal day/night cycle. Leaves yellowed within 3–5 d of submergence, possibly as a response to ethylene entrapped within the submerged foliage (Jackson et al., 1987; Voesenek et al., 1997). Submergence in total darkness would not only have inhibited photosynthetic carbon fixation of (mostly) respiratory carbon dioxide, but also halted the release of oxygen from plants and algae. This could explain the lower concentrations of oxygen in the floodwater as the duration of dark submergence increased. After 5 d, dissolved oxygen concentration had already declined to less than half of that dissolved in water equilibrated with air. In line with the known susceptibility of PDC genes and post‐translational processes to enhancement by oxygen shortage (Bucher and Kuhlemeier, 1993), marked increases in the activity of PDC in both FR13A and CT6241 were observed only after 1 d of dark submergence; this increase was higher in CT6241 (approx. 142 %) compared with FR13A (approx. 90 %). The activity of PDC also increased in all doubled haploid lines after 1 d of submergence. When dark submergence was extended to 3 d, PDC activity further increased, or decreased, depending on the line; however, these additional effects were not statistically significant. Overall, the stimulating influence of more than 1 d of dark submergence on PDC activity was slightly greater in the intolerant lines (94·0 ± 12·5 % increase in intolerant lines compared with 67·7 ± 13·2 5 % increase in tolerant lines).
Submerging rice seedlings in total darkness under partial oxygen deficiency for 1 d before exposing them to anoxia was intended to reduce the severity of any damage caused by anoxic shock (Drew, 1997); such treatment of the submergence tolerant and intolerant seedlings resulted in increased PDC activity, supporting the observations of our earlier experiment. PDC activity of the 1 d dark‐submerged submergence tolerant and intolerant plants were, respectively, approx. 130–220 % and 160–270 % higher than that before submergence. However, such increases were not statistically significant in the tolerant line 502 (approx. 27 % increase). In contrast, dark submergence for 1 d significantly increased PDC activity in all submergence‐intolerant lines, including the parent CT6241. Dark submergence‐induced increases in PDC activity were greater in the submergence‐intolerant lines.
After the pre‐anoxic treatment, 6 h submergence in anaerobic water resulted in further increases in PDC activity in submergence‐tolerant FR13A and line 502. However, statistically significant decreases in PDC activity were observed in submergence‐intolerant CT6241 and line 364, both of which showed degenerated and flaccid shoots. In other more robust intolerant lines, statistically significant increases in PDC activity were observed. Submergence of the seedlings in anoxia for a further 18 h reduced PDC activity in all lines. This might be a reflection of the fast‐diminishing reserves of respirable carbohydrates, known to decline quickly in anaerobic conditions (Perata et al., 1996; Sarkar, 1998), and the highly degenerated character of the roots at this time.
CONCLUSIONS
The superior tolerance of FR13A to total submergence for up to 15 d under natural day/night cycles, compared with cultivar CT6241, was further confirmed. Similarly, two groups of doubled haploid lines, derived from a cross between FR13A and CT6241, were found to exhibit contrasting tolerance to submergence. Activity of PDC in apical root sections decreased during submergence in the daytime and increased at night; these changes were associated with substantial losses of soluble proteins. We assume that increased PDC activity is a marker for tissue hypoxia or anoxia. Hence, our results indicated that the roots experienced severe oxygen shortage only at night (under the experimental conditions used here) and that this effect of oxygen deficiency on PDC was rapidly reversed by daytime oxygenation via photosynthesis. There was no link between the scale of these effects and the submergence tolerance of the various rice lines studied. Submergence in continuous darkness for 1 or 3 d in water enhanced root PDC activity more strongly than submergence in a 12 h day/12 h night cycle. A subsequent imposition of anoxia caused a further amplification indicating that PDC activity is indeed a good marker for the extent of tissue oxygen deficiency. The effect was marginally greater in submergence‐intolerant plants suggesting that under conditions of severe oxygen shortage in the field, submergence damage might be exacerbated by faster fermentation in the roots of these seedlings. The observation that root PDC activity of submerged rice was higher at night than during the day indicates a need to check if such day/night changes in PDC activity also occur in the roots and/or shoots of other plant species during submergence. Also, the effect of the natural day/night cycle on PDC activity of submergence tolerant plants, independent of submergence, remains to be elucidated.
Overall, our results indicate that the difference in susceptibility to submergence between FR13A and CT6241 and their progeny was not the outcome of differential susceptibility to oxygen deficiency at the roots. If, however, oxygen shortage was a key component of injury sustained during submergence under natural day/night conditions, the resultant damage was likely to have occurred in roots and at night.
ACKNOWLEDGEMENTS
We thank Dr M. B. Jackson (University of Bristol, UK) and Professor H. Greenway (University of Western Australia, Nedlands, Australia) for helpful comments. This research was fully supported by a grant from the European Union under the INCO‐DC Framework 4 Programme (contract number IC18‐CT96‐0078: ‘Rice for Life: A Physiological Basis for Improving Submergence Tolerance in Rainfed Lowland Rice’).
Supplementary Material
Received: 4 September 2000; Returned for revision: 17 October 2000; Accepted: 2 December 2001
References
- ap ReesT, Jenkin LET, Smith AM, Wilson PM.1987. The metabolism of flood tolerant plants. In: Crawford RMM, ed. Plant life in aquatic and amphibious habitats Oxford: Blackwell Scientific Publications, 227–238.
- ArmstrongW.1979. Aeration in higher plants. Advances in Botanical Research 7: 225–332. [Google Scholar]
- BucherM, Kuhlemeier C.1993. Long‐term anoxia tolerance. Multilevel regulation of gene regulation in the amphibious plant Acorus calamus L. Plant Physiology 103: 441–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CobbBG, Kennedy RA.1987. Distribution of alcohol dehydrogenase in roots and shoots of rice (Oryza sativa) and Echinochloa seedlings. Plant Cell and Environment 10: 633–638. [Google Scholar]
- DrewMC.1997. Oxygen deficiency and root metabolism: Injury and acclimation under hypoxia and anoxia. Annual Review of Plant Physiology and Molecular Biology 48: 223–250. [DOI] [PubMed] [Google Scholar]
- DrewMC, Cobb BG, Johnson JR, Andrews D, Morgan PW, Jordan W, He CJ.1994. Metabolic acclimation of root tips to oxygen deficiency. Annals of Botany 74: 281–286. [Google Scholar]
- EllisMH, Setter TL.1999. Hypoxia induced anoxia tolerance in completely submerged rice seedlings. Journal of Plant Physiology 154: 219–230. [Google Scholar]
- GibbsJ, Morrell S, Valdez A, Setter TL, Greenway H.2000. Regulation of alcoholic fermentation in coleoptiles of rice cultivars differing in tolerance of anoxia. Journal of Experimental Botany 51: 785–796. [PubMed] [Google Scholar]
- HossainMA, Huq E, Grover A, Dennis ES, Peacock WJ, Hodges TK.1996. Characterization of pyruvate decarboxylase genes from rice. Plant Molecular Biology 31: 761–770. [DOI] [PubMed] [Google Scholar]
- ItoO, Ella E, Kawano N.1999. Physiological basis of submergence tolerance in rainfed lowland rice ecosystem. Field Crops Research 64: 75–90. [Google Scholar]
- JacksonMB, Waters I, Setter TL, Greenway H.1987. Injury to rice plants caused by complete submergence: a contribution of ethylene (ethane). Journal of Experimental Botany 38: 1826–1838. [Google Scholar]
- JohnCD, Greenway H.1976. Alcoholic fermentation and activity of some enzymes in rice roots under anaerobiosis. Australian Journal of Plant Physiology 3: 325–336. [Google Scholar]
- JohnsonJR, Cobb BG, Drew MC.1989. Hypoxic induction of anoxia tolerance in root tips of Zea mays Plant Physiology 91: 837–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeeTM, Lin VH.1995. Trypsin inhibitor and trypsin‐like protease activity in air‐ or submergence‐grown rice (Oryza sativa L.) coleoptiles. Plant Science 106: 43–54. [Google Scholar]
- MazaredoAM, Vergara BS 1982. Physiological differences in rice varieties tolerant and susceptible to complete submergence. In: Proceedings of the 1981 International Deepwater Rice Workshop Manila: International Rice Research Institute, 327–341.
- MenegusF, Cattaruzza L, Molinari H, Ragg E.1993. Rice and wheat seedlings as plant models of high and low tolerance to anoxia. In: Surviving hypoxia: metabolism of adaptation and control Boca Raton, FL: CRC Press, 53–64.
- MorrellS, Greenway H, Davies DD.1990. Regulation of pyruvate decarboxylase in vitro and in vivo Journal of Experimental Botany 41: 131–139. [Google Scholar]
- PerataP, Guglielminetti L, Alpi A.1996. Anaerobic carbohydrate metabolism in wheat and barley, two anoxia‐intolerant cereal seeds. Journal of Experimental Botany 47: 999–1006. [Google Scholar]
- QuimioCA,Torrizo LB, Setter TL, Ellis M, Grover A, Abrigo EM, Oliva NP, Ella ES, Carpena AL, Ito O, Peacock WJ, Dennis E, Datta SK.2000. Enhancement of submergence tolerance in transgenic rice overproducing pyruvate decarboxylase. Journal of Plant Physiology 156: 516–521. [Google Scholar]
- RaymondP, Al Alni A, Pradet A.1985. ATP production by respiration and fermentation and energy charge during aerobiosis and anaerobiosis in twelve fatty and starchy germinating seeds. Plant Physiology 79: 879–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ReggianiR, Brambilla I, Bertani, A.1986. Effect of exogenous nitrate on anaerobic metabolism in excised rice roots III. Glycolytic intermediates and enzymatic activities. Journal of Experimental Botany 37: 1472–1478. [Google Scholar]
- RicardB, Couee I, Raymond P, Saglio PH, Saint‐Ges V, Pradet A.1994. Plant metabolism under hypoxia and anoxia. Plant Physiology and Biochemistry 32: 1–10. [Google Scholar]
- RijndersJGHM, Armstrong W, Darwent MJ, Blom CWPM, Voesenek LACJ.2000. The role of oxygen in submergence‐induced petiole elongation in Rumex palustris: in situ meas urements of oxygen in petioles of intact plants using micro electrodes. New Phytologist 147: 497–504. [DOI] [PubMed] [Google Scholar]
- RivoalJ, Ricard B, Pradet A.1989. Glycolytic and fermentative enzyme induction during anaerobiosis in rice seedlings. Plant Physiology and Biochemistry 27: 43–52. [Google Scholar]
- RivoalJ, Thind S, Ricard B.1997. Differential induction of pyruvate decarboxylase subunits and transcripts in anoxic rice seedlings. Plant Physiology 114: 1021–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SachsMM, Subbaiah CC, Saab IN.1996. Anaerobic gene expression and flooding tolerance in maize. Journal of Experimental Botany 47: 1–15. [Google Scholar]
- SarkarRK.1998. Saccharide content and growth parameters in relation with flooding tolerance in rice. Biologia Plantarum 40: 597–603. [Google Scholar]
- SetterTL, Ellis M, Laureles EV, Ella ES, Senadhira D, Mishra SB.1997. Physiology and genetics of submergence tolerance in rice. Annals of Botany 79(Suppl. A): 61–71. [Google Scholar]
- SetterTL, Kupkanchanakul K, Behekasut B, Kupkanchanapul T, Weingweera A, Greenway H.1987. Concentrations of CO2 and O2 in floodwater and in internodal lacunae of floating rice growing at 1–2 metre water depths. Plant, Cell and Environment 10: 767–776. [Google Scholar]
- UmedaM, Uchimiya H.1994. Differential transcription levels of genes associated with glycolysis and alcoholic fermentation in rice plants (Oryza sativa L.) under submergence stress. Plant Physiology 106: 1015–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VartapetianBB, Jackson MB.1997. Plant adaptations to anaerobic stress. Annals of Botany 79 (Suppl. A): 3–20. [Google Scholar]
- VoesenekLACJ, Vriezen HW, Smekens MJE, Huitink FHM, Bögemann GM, Blom CWPM.1997. Ethylene sensitivity and response sensor expression in petioles of Rumex species at low oxygen and high carbon dioxide concentrations. Plant Physiology 114: 1501–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WatersI, Morrell S, Greenway H, Colmer TD.1991. The effects of anoxia on wheat seedlings. Journal of Experimental Botany 42: 1437–1447. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.