Abstract
This article reviews the contribution made by functional electron microscopy towards identifying and understanding the reactions of plant roots and shoots to anaerobic stress. Topics examined include: (1) unexpected hypersensitivity, rather than hyper‐resistance, to anoxia of root tips of flooding‐tolerant plants; (2) protective, rather than damaging, effects of a stimulated energy metabolism (glycolysis and fermentation) under anaerobic conditions; (3) the concept of two main strategies of plant adaptation to anaerobic environments, namely avoidance of anaerobiosis on the whole plant level, termed ‘apparent’ tolerance, and metabolic adaptation at the cellular and molecular levels, termed ‘true’ tolerance; (4) the importance of protein synthesis during hypoxia and anoxia for enhanced energy production and metabolic adaptation; (5) a general adaptive syndrome in plants to stress at the ultrastructural level and a possible molecular mechanism for its realization under anoxia; (6) the physiological role of anaerobically synthesized lipids and nitrate as alternative electron acceptors in an oxygen‐free medium; and (7) the selection of cell lines derived from callus cultures that possess enhanced tolerance to anoxia and can regenerate whole plants with improved tolerance of soil waterlogging.
Keywords: Key words: Review, anaerobiosis, mitochondrial ultrastructure, glycolysis, lipid metabolism, general adaptation syndrome, nitrate reduction, cell culture selection.
INTRODUCTION
In recent decades, progress in understanding plant life under oxygen deficiency has been based principally on the adoption of modern physical and chemical methods of analysis, and on conceptualized interpretations of physiological and biochemical findings. This can be exemplified by contributions made using electron microscopy (EM) to study fine structure of plant cells responding to stress from oxygen shortage. As described below, results first obtained in EM studies promoted further research and development of the problems using physiological and biochemical approaches. In contrast to cytologists interested in a static state of cell ultrastructure, physiologists and biochemists have used electron microscopy to reveal dynamic rearrangements of cellular membranes provoked by physiological experiments. Such rearrangements have often be related directly to adaptive or degenerative changes induced by stress. We define such an approach as functional electron microscopy.
We have employed electron microscopy for almost 30 years to study plant anaerobiosis and to elucidate the specific features of plant function and metabolism under oxygen deficiency. Some our findings will be used to illustrate the contribution of functional electron microscopy to the study of plant anaerobiosis taking into account complimentary electron microscopic studies by other research groups (Andreeva and Grineva, 1970; Ueda and Tsuji, 1971; Öpik, 1973; Oliveira, 1977; Morisset, 1978, 1983; Campbell and Drew, 1983; Aldrich et al., 1985; Davies et al., 1985; Webb and Jackson, 1986; Kennedy et al., 1991).
It should be noted that hypoxia and anoxia are extremely stressful conditions since higher plants are essentially aerobic organisms. Consequently, all plant cell organelles, including endoplasmic reticulum, polysomes and cytoplasm undergo marked rearrangements under oxygen deficiency. The rearrangements in mitochondrial membranes are especially informative. Mitochondria are primary oxygen consumers and the major energy source of aerobic organisms. Consequently, they suffer from oxygen deficiency before other cell organelles. Mitochondria may also have other key roles. For example, in experiments in which maize cell suspension cultures were transferred from an aerobic to anaerobic medium, Subbaiah et al. (1998) demonstrated that Ca2+ ions released rapidly from mitochondria into the cytosol participate in signalling O2 deprivation leading to anaerobic gene expression. In addition, mitochondria undergo characteristic destructive or adaptive rearrangements under anaerobic stress. These changes hold the key to understanding features of cell adaptation or damage under oxygen deficiency. Accordingly, our EM studies have concentrated on assessing mitochondrial status.
Experimental material selected and presented below is grouped into two categories. The first includes results of EM studies dealing with principal problems of plant life under hypoxia and anoxia, i.e. main strategies of adaptation. The results of these studies have been supported subsequently in numerous physiological and biochemical studies conducted by others. Therefore, it is unlikely that conclusions drawn from these studies are controversial. The second category includes findings of more recent studies in which the functional electron microscopic approach has also played an important role. However, these results have not yet received wide recognition and remain to be rigorously tested. This comment refers specifically to demonstrations of a general adaptation syndrome in plants under anoxia, a physiological role of anaerobic synthesis of lipids as alternative electron acceptors in oxygen‐free medium and the in vitro selection of anoxia‐resistant cell lines holding promise for regeneration of whole plants with superior tolerance to soil waterlogging.
DESTRUCTIVE AND ADAPTIVE REARRANGEMENTS OF MITOCHONDRIAL ULTRASTRUCTURE UNDER ANAEROBIC STRESS
Destructive, degradative and adaptive rearrangements take place in mitochondria membranes in plant organs after transfer from aerobic to anaerobic environments (Figs 1B and C and 2A–D). Mitochondria of aerobic cells (Fig. 1A) are round or oval, contain several cristae and have a dense matrix. After transfer to an anaerobic environment, oxidative phosphorylation is arrested almost immediately, followed by mitochondrial swelling, a decrease in the number of cristae and gradual clarification of the mitochondrial matrix (Fig. 1B). If anaerobic incubation is continued for 5–6 h, mitochondrial swelling increases further. However, despite these considerable destructive changes mitochondria do not lose their capacity for oxidative phosphorylation. This is seen by a remarkable capacity to increase ATP levels and the ATP/ADP ratio immediately after transfer back to an aerobic environment (Andreev et al., 1991). The longer the roots are deprived of oxygen, the longer the duration of subsequent aeration needed to restore initial ATP levels. Ultrastructural changes of anaerobic mitochondria are also reversible. Soon after cell aeration recommences, the fine structure of mitochondria reverts back to that of initial aerobic mitochondria. However, if anaerobic incubation is prolonged further (e.g. 10–15 h in pumpkin roots), irreversible degradation of mitochondrial ultrastructure takes place that cannot be rescued by re‐aeration (Fig. 1C).
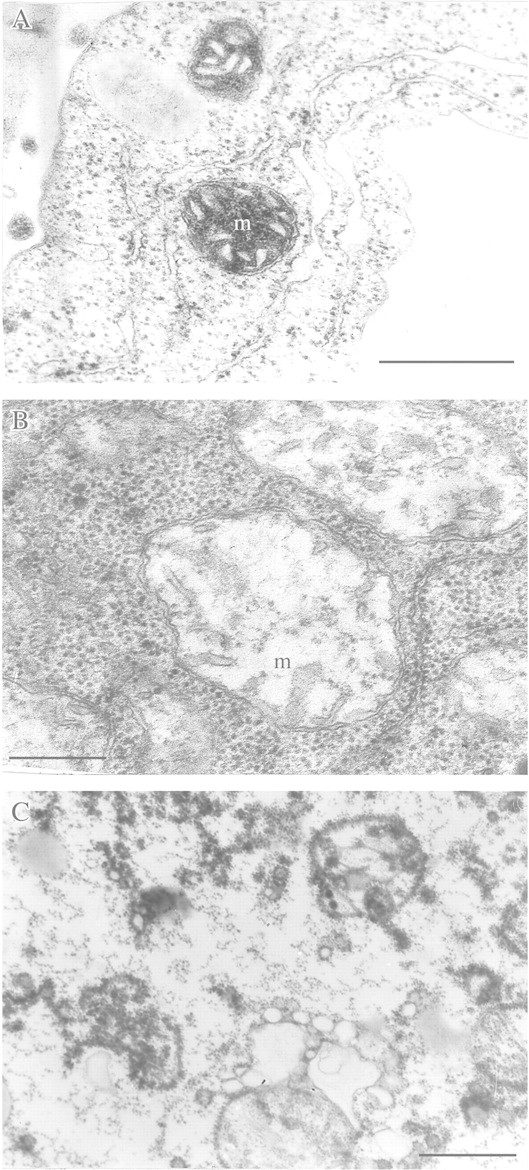
Fig. 1. Destructive and degradative rearrangement of mitochondrial ultrastructure under anaerobic stress. A, Mitochondrial ultrastructure of a detached root Cucurbita pepo under aerobic conditions (control). B, Reversible destruction after 6 h anaerobic incubation. C, Irreversible degradation after 15 h anaerobic incubation. m, Mitochondrion. Bar = 0·5 µm.
Ultrastructural rearrangements in mitochondria can differ in cells of species tolerant and intolerant to anoxia. For example, the ultrastructure of mitochondria in coleoptile cells of anaerobically germinating seeds of rice, a highly flood‐tolerant species, is not substantially different from that of mitochondria of aerobic coleoptiles (Vartapetian et al., 1978a). A similar picture is obtained with anoxia‐tolerant Echinochloa species (Kennedy et al., 1991). Alternatively, non‐destructive rearrangements of mitochondria membranes can take place, as shown for roots of anoxia‐intolerant Cucurbita pepo (Fig. 2A and C) provided they are fed with exogenous glucose during anaerobic incubation. Under these circumstances, mitochondria become elongated or take the form of mitochondrial reticulum (Vartapetian et al., 1977). Transfer of aerobically germinated rice seedlings to anoxia (Fig. 2B) results in the appearance of mitochondria with stacks of parallel cristae and a dense matrix (Vartapetian et al., 1974a; Morisset, 1978). Sometimes, dumb‐bell (Fig. 2D) or ring‐shaped mitochondria occur (Vartapetian et al., 1978a; Vartapetian and Polyakova, 1999) but with no evidence of associated membrane degeneration.
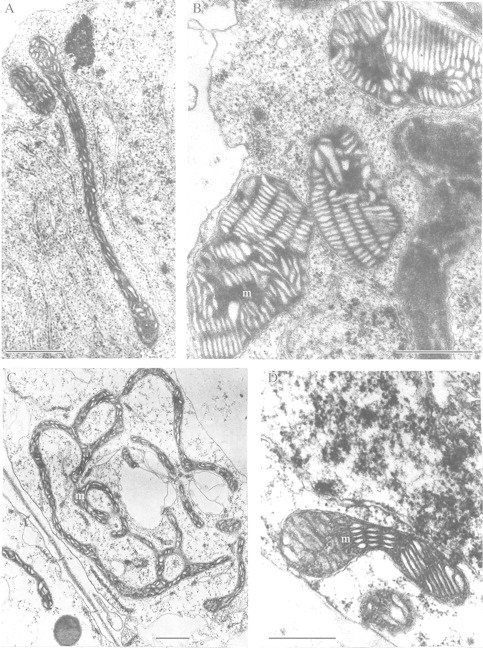
Fig. 2. Adaptive rearrangements of plant mitochondrial ultrastructure under anaerobic stress. A, Elongated mitochondria after 24 h anaerobic incubation of detached root of Cucurbita pepo in the presence of exogenous glucose (3 %). B, Mitochondria of rice coleoptile developing stacks of parallel cristae with a dense matrix after 5 d anaerobic incubation. C, Mitochondria taking the form of mitochondrial reticulum after 72 h anaerobic incubation of detached root of C. pepo in the presence of exogenous glucose (3 %). D, Dumb‐bell shaped mitochondria in detached coleoptile of O. sativa after 48 h anaerobic incubation in the presence of exogenous KNO3 (10 mm). m, Mitochondrion. Bars = 0·5 µm.
HYPERSENSITIVITY OF MITOCHONDRIAL ULTRASTRUCTURE IN ROOT CELLS OF FLOODING‐TOLERANT PLANTS, OR THE STRATEGY OF APPARENT TOLERANCE
In contrast to coleoptiles of rice seedlings, which are highly tolerant of anoxia, electron microscopy of detached root tips of mature rice (Oryza sativa), cultivated on flooded anaerobic soil, demonstrated unexpectedly high sensitivity of mitochondria ultrastructure to anoxia. Cells of rice roots not only appeared to be less resistant than the coleoptile but, paradoxically, they were even more anoxia‐sensitive than roots of non‐tolerant plants (Vartapetian et al., 1970, 1978a; Vartapetian, 1973). For example, mitochondria in roots of pumpkin (Cucurbita pepo, a flooding‐sensitive species) degraded irreversibly after 6–10 h of anaerobic incubation, whereas in roots of rice (a flooding‐tolerant plant), mitochondrial destruction was observed after only 3–5 h of anaerobic treatment at 20 °C. Anaerobic incubation of rice roots at 32 and 42 °C resulted in marked mitochondrial destruction after only 60 and 30 min, respectively (Vartapetian et al., 1972). Under aerobic conditions no destructive changes in ultrastructure of rice mitochondria were observed, even at 42 °C. The results of this investigation with rice, confirmed later in experiments with other wild wetland species (Vartapetian and Andreeva, 1986), gave grounds for the conclusion that the tolerance of roots of whole plants to flooding is the consequence of apparent tolerance to anoxia but not of true tolerance. Thus, the ability of flooding‐tolerant species to inhabit anaerobic soils is not a consequence of high resistance of root cells to oxygen shortage as has been suggested (Soldatenkov and Chirkova, 1963; McManmon and Crawford, 1971; Crawford, 1978) and widely accepted (Marshall et al., 1973; Francis et al., 1974; Chirkova, 1978; Larcher, 1980; Moore, 1982). Instead flooding tolerance of these plants is explained by their capacity to avoid root anaerobiosis due to oxygen transport from aerated above‐ground organs. This conclusion has been confirmed in physiological experiments (Webb and Armstrong, 1983) and biochemical studies (Ap Rees and Wilson, 1984; Ap Rees et al., 1987). For example, tests of the capacity of pea, rice and pumpkin roots for post‐anaerobic growth demonstrated that rice roots were more rather than less damaged by anoxia than pea or pumpkin roots (Webb and Armstrong, 1983). A similar situation was found when protein synthesis was studied in roots of flooding‐tolerant Glyceria maxima and non‐tolerant pea, Pisum sativum (Ap Rees and Wilson, 1984; Ap Rees et al., 1987). Roots of G. maxima were the more sensitive to anoxia. In addition, comparative biochemical studies did not reveal marked differences in root metabolism of tolerant and intolerant plants (Smith and Ap Rees, 1979; Davies, 1980). Direct measurements of molecular oxygen translocated from above‐ground organs into roots of tolerant and non‐tolerant species demonstrated that in contrast to non‐tolerant species, flooding‐tolerant species transported sufficient oxygen internally to support aerobic metabolism (Vartapetian et al., 1970, 1974b, 1978a; Armstrong, 1978, 1979; Armstrong et al., 1994). Any oxygen lost from the root axis by radial diffusion also has the benefit of oxidizing chemically reduced toxic substances, such as Fe2+ and Mn3+, in the flooded soil to less soluble and less toxic forms (Ponnamperuma, 1984; Gambrell et al., 1991). This oxygen may also support nitrifying bacteria that convert ammonia to nitrate (Blom et al., 1994). Oxygen transport from above‐ground parts to roots of wetland plants is facilitated by aerenchyma. Aerenchyma is also the pathway for reverse diffusion of reduced volatile compounds accumulated in roots, including CO2, ethylene, organic acids and CH4 (Neue et al., 1990). Methane generated in anaerobic soils of rice fields and diffusing out into the atmosphere via aerenchyma is a major source of this greenhouse gas, believed to increase global warming. The function of aerenchyma, the mechanism of its formation in roots of flooding‐tolerant and intolerant species, and the role of ethylene and Ca2+ as triggers of aerenchyma formation have been reviewed thoroughly (Jackson, 1991; Armstrong et al., 1994; Voesenek and Blom, 1996; Drew, 1997; Jackson and Armstrong, 1999).
THE ROLE OF ENERGY METABOLISM (GLYCOLYSIS) IN PLANT RESISTANCE TO ANOXIA, OR THE STRATEGY OF TRUE TOLERANCE
Hyper‐resistance of mitochondrial ultrastructure in coleoptile cells of anaerobically germinating rice seeds
EM studies have helped understand the mechanism of true tolerance, i.e. metabolic adaptation of plants to anaerobic stress. As with apparent tolerance, rice was used in the first experiments. However, instead of roots, coleoptiles were examined since they can elongate to a limited extent in the complete absence of oxygen, even in a vacuum (Vartapetian et al., 1978a). Previous EM studies showed that coleoptiles germinating under anaerobic conditions contained structurally intact mitochondria that are potentially active (Ueda and Tsuji, 1971; Vartapetian et al., 1971; Öpik, 1973; Costes and Vartapetian, 1978). Mitochondria of coleoptiles after 5–6 d of anaerobic germination of seeds possess a full range of electron‐transporting enzymes, including cytochrome‐oxidase and cytochromes b and c, and are capable of oxidative phosphorylation when exposed to molecular oxygen (Costes and Vartapetian, 1978; Vartapetian et al., 1978a; Shibasaka and Tsuji, 1991). Coleoptiles of aerobically germinating rice seeds are also highly resistant to anoxia, with their mitochondria remaining intact during several days of subsequent anoxia (Vartapetian et al., 1974a; Couée et al., 1992). However, EM studies have shown that the high anoxia‐resistance of mitochondrial ultrastructure in coleoptiles germinated under both aerobic and anaerobic conditions is lost when coleoptiles are detached from the seedling (Vartapetian et al., 1976, 1978a): anaerobic incubation of detached coleoptiles results in complete degradation of mitochondrial membranes within 48 h (Fig. 3B). The resistance of mitochondrial ultrastructure in detached coleoptiles to anoxia can be restored by submerging coleoptiles in glucose solution during anaerobic incubation to stimulate glycolysis and fermentation (Vartapetian et al., 1976) (Fig. 3C). Thus, anaerobic mitochondria are responsive to external hexose and, in intact seedlings, are dependent on such supply from the seed. It is clear that sufficient glycolytic ATP can be generated anaerobically by the coleoptile to maintain mitochondrial ultrastructure. This ATP is of cytoplasmic origin and is apparently utilized by mitochondria to maintain structural integrity and potential functional activity under complete arrest of electron transport in the mitochondrial respiratory chain. In contrast, in yeast (Saccharomyces cerevisiae), the structural and functional integrity of mitochondria could be preserved only by electron transport and oxidative phosphorylation (i.e. by ATP production) within mitochondria (Luzikov et al., 1973; Luzikov, 1985). A similar but erroneous conclusion was also made in respect of mitochondria of higher plants (Zalenskii, 1977). The conclusion concerning utilization of extra‐mitochondrial ATP of glycolytic origin by mitochondria is made on the basis of EM studies of both rice coleoptiles and pumpkin roots (Vartapetian et al., 1977). It has been supported in biochemical studies showing that mitochondria isolated from rice seedlings after anaerobic incubation utilize exogenous ATP in mitochondrial protein synthesis (Couée et al., 1992).
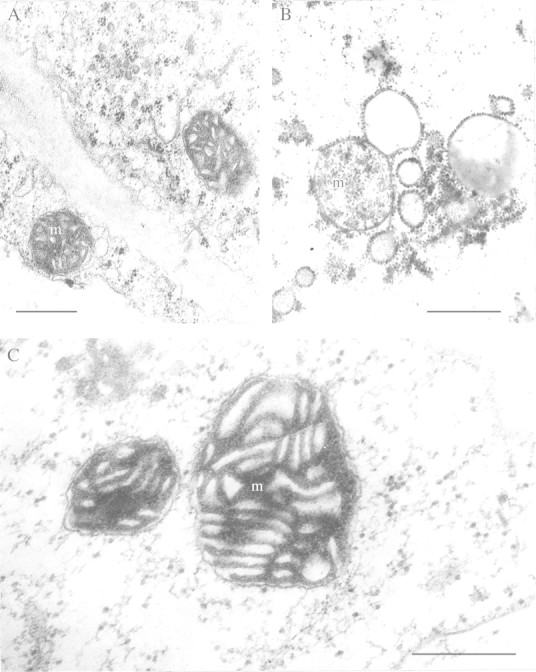
Fig. 3. Degradative and adaptive rearrangements of mitochondrial ultrastructure of detached rice coleoptiles with and without glucose feeding under anoxia. A, After 6 d anaerobic seed germination (control). B, After 48 h anaerobic incubation of detached coleoptile without glucose feeding. C, After 5 d anaerobic incubation of detached coleoptile with glucose feeding (0·5 %). m, Mitochondrion. Bars = 0·5 µm.
Experiments prolonging the life of detached rice coleoptiles (Vartapetian et al., 1976) and detached pumpkin roots (Vartapetian et al., 1977) by exogenous sugar in the absence of oxygen argue against the notion that damage and death under anoxia is the outcome of self‐poisoning by the extra ethanol delivered from the fast fermentation (McManmon and Crawford, 1971; Crawford, 1978). In most experiments, stimulation of glycolysis and fermentation by feeding with exogenous sugar has a protective rather than damaging effect on survival (Vartapetian, 1993).
A further important contribution of the experiments on feeding with exogenous glucose was to demonstrate the key importance of energy metabolism (glycolysis) in tolerance and adaptation of plant cells to anaerobic stress. These results emphasize the particular importance of anaerobic mobilization and utilization of seed reserves in ensuring rice coleoptile resistance to anoxia. It can be concluded that the remarkable capacity of rice coleoptiles to grow without oxygen and to preserve undamaged mitochondrial ultrastructure and function is not due to resistance of the organelles themselves, but to the ability of rice seedlings to transport organic substances a considerable distance even under conditions of anoxia, and to mobilize storage substances provided by the endosperm in the absence of oxygen (Vartapetian et al., 1976). Data obtained in EM investigations that indicate the importance of energy metabolism and glycolytic substrate provision in plant adaptation to anaerobic stress are supported by a number of physiological, biochemical and molecular biological studies. These are discussed briefly below.
Protective role of exogenous sugars under anoxia in non‐tolerant plants
The protective effect of glucose‐stimulated glycolysis leading to increased resistance of rice coleoptile cell ultrastructure to anoxia also applies to roots of species that are not tolerant to flooding. For example, incubation of pumpkin (Cucurbita pepo) roots for 10–15 h in an oxygen‐free medium (Vartapetian et al., 1977) resulted in marked destruction of mitochondrial ultrastructure (Fig. 4B). However, no evidence of mitochondrial destruction was observed even after 72 h without oxygen when roots were fed exogenous glucose (Fig. 4C), although mitochondrial morphology altered markedly to give unusual elongated or ramified forms. Stimulation of energy metabolism in cells of maize root tips fed with exogenous glucose was demonstrated by accumulation of the final products of anaerobic respiration and by the elevated level of cell energy charge (Saglio et al., 1980). Webb and Armstrong (1983) demonstrated a protective role of exogenous glucose under anoxia on rice, pea and pumpkin roots using the roots’ capacity for post‐anaerobic growth as a criterion. In the absence of exogenous glucose, roots of rice, pea and pumpkin lost the capacity for post‐anaerobic growth after 4, 6 or 12 h of anoxia, respectively, while glucose feeding resulted in post‐anaerobic growth even after 4, 19 or 25 h anoxia. Increased cell tolerance to hypoxia and anoxia by glucose feeding has also been demonstrated in experiments with roots of other flooding‐sensitive plants (Waters et al., 1991; Hole et al., 1992; Zhang and Greenway, 1994). In particular, experiments by Waters et al. (1991) demonstrated that glucose feeding of anaerobic wheat roots increased the capacity for post‐anaerobic growth from 10 to 30 h.
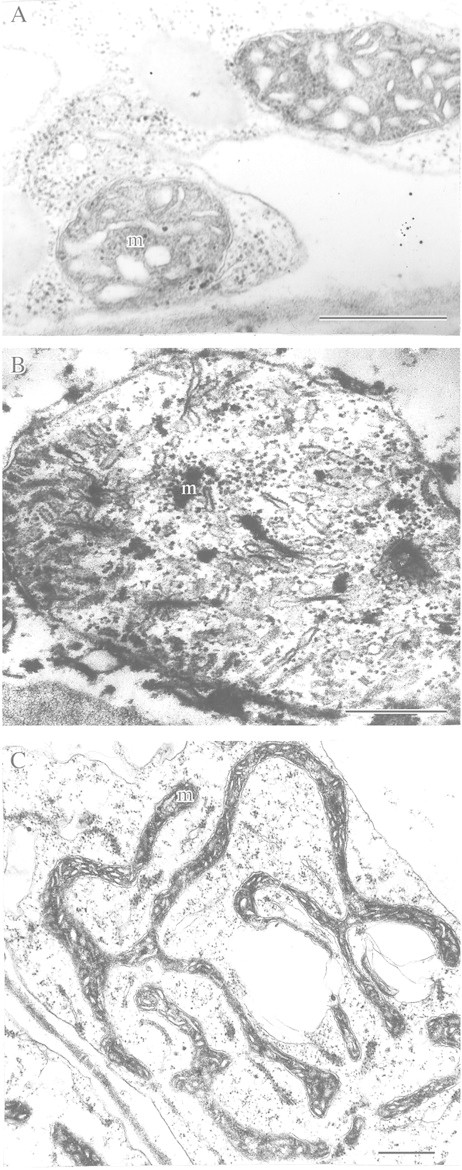
Fig. 4. Destructive and adaptive rearrangements of mitochondrial ultrastructure of detached pumpkin roots with and without glucose feeding under anoxia. A, Under aerobic conditions (control). B, After 10 h anaerobic incubation without glucose feeding. C, After 72 h anaerobic incubation with glucose feeding (3 %). m, Mitochondrion. Bar = 0·5 µm.
Induction of anaerobic protein synthesis as the underlying mechanism of adaptation to hypoxia and anoxia
Demonstrations of anaerobic protein synthesis in higher plants gave further grounds for the assertion that cell energy metabolism is of primary importance in plant adaptation to anaerobic stress. Specific proteins emerging under anaerobic conditions were first reported in experiments on rice seedlings (Maslova et al., 1975). Seven anaerobic proteins appeared when 6‐d‐old air‐grown rice seedlings were transferred to an anaerobic environment. These proteins disappeared when the seedlings were returned to air. It was shown that synthesis of anaerobic proteins took place simultaneously with a promotion of alcohol dehydrogenase activity. Expression of anaerobic proteins in tolerant and non‐tolerant plants has since been studied in several laboratories. For example, using tips of maize roots incubated anaerobically, Sachs et al. (1980) showed that synthesis of the proteins typical for aerobic conditions ceased, with a concurrent induction of synthesis of about 20 anaerobic stress proteins. Expression of synthesis of anaerobic proteins has also been shown under field conditions in rhizomes of Acorus calamus during long‐term winter anaerobiosis (Bucher et al., 1996). Anaerobic proteins appear to be mainly enzymes participating in glycolysis and fermentation, or the enzymes responsible for mobilization of carbohydrates, hexokinases, α‐amylase and sucrose synthase (Sachs, 1993; Sachs et al., 1996; Perata et al., 1997; Saglio et al., 1999).
Several studies have dealt with plant acclimation to anoxia through responses to previous hypoxic treatment. The phenomenon of acclimation to anoxia was originally demonstrated by Andrews and Pomeroy (1981, 1983). In their studies, pre‐treatment of winter wheat by flooding at low temperature (2 °C) increased plant resistance and survival under subsequent anoxia imposed by a gas‐impermeable ice crust. The pre‐treatment increased activity of certain enzymes participating in anaerobic respiration, namely, pyruvate decarboxylase and alcohol dehydrogenase. There was also an acceleration of fermentation and a rise in cell energy status (Andrews, 1997). Saglio et al. (1988) incubated maize root tips for 18 h at low partial oxygen pressure (2–4 kPa) at 20–25 °C and also observed a considerable increase in root resistance to subsequent anoxic treatment. For example, after hypoxic pre‐treatment, maize roots tolerated anoxic exposure for 22 h, whereas in the absence of such pre‐treatment they died after only 7 h of anoxia. Johnson et al. (1989) showed that maize roots retained their capacity for growth even after 90 h anoxia if they first underwent hypoxic pre‐treatment. Without hypoxic pre‐incubation, these roots lost their growth potential after only 24 h anoxia. It is significant that roots given hypoxic pre‐treatment show a higher activity of several glycolytic and fermentative enzymes, faster and more prolonged ethanol production, an increased ATP concentration in their tissues, and a higher level of energy charge and ATP/ADP ratio (Saglio et al., 1988, 1999; Johnson et al., 1989; Hole et al., 1992). Even in roots of wheat seedlings which are highly sensitive to oxygen deficiency (Vartapetian and Zakhmilova, 1990), hypoxic pre‐treatment of tips of either detached roots or roots of intact seedlings markedly increased both the activity of enzymes involved in alcoholic fermentation and the rate of anaerobic respiration, as well as root resistance to anaerobic stress (Waters et al., 1991). It should be mentioned that increased resistance of hypoxically pre‐incubated roots was most pronounced if they were also fed with exogenous sugars. Waters et al. (1991) concluded that ‘high rates of alcoholic fermentation are one important aspect of tolerance of plant tissues to anoxia’. It has been suggested that the induction of synthesis of anaerobic proteins takes place during hypoxic pre‐treatment, and that hypoxia rather than sudden anoxia that increases transcription and activity of glycolytic and fermentation enzymes and improves tolerance to anoxia (Saglio et al., 1999). It seems that substantial protein synthesis occurs under anoxia only if roots have been hypoxically pre‐treated. It has been shown in rice and maize seedlings that the induction of synthesis of the enzymes participating in glycolysis and fermentation during hypoxic treatment is accompanied by induction of the synthesis of sucrose synthase (Saglio et al., 1999). Sucrose synthase is of great importance in the tolerance of rice and maize roots to anoxia because it is this enzyme rather than invertase that is responsible for introducing sucrose into anaerobic metabolism (Guglielminetti et al., 1995; Perata et al., 1997; Ricard et al., 1998). Thus, data obtained by different research groups in experiments on hypoxic pre‐treatment of roots also indicate the key role of energy metabolism in root resistance to hypoxia and anoxia. The results support the view that increased resistance of plants arises from an adequate supply of substrate and increased synthesis of anaerobic proteins. Many of these proteins are enzymes that participate in glycolysis and alcoholic fermentation or in other aspects of carbohydrate metabolism. Xia et al. (1995) and Rawyler et al. (1999) have proposed that there is a threshold for ATP production below which hypoxic enhancement of anoxia tolerance is not realized.
Synthesis of anaerobic proteins can be expected to facilitate substrate phosphorylation through mobilization of carbohydrate reserves and intensification of glycolysis and fermentation when the main mechanism of energy supply of the aerobic cell (oxidative phosphorylation) is arrested due to a deficiency or absence of molecular oxygen. This fact should probably be considered as an adaptive property of the plant cell that developed via natural or artificial selection, resulting in enhanced energy metabolism under hypoxia and anoxia. These observations also suggest that cell energy metabolism is of great adaptive importance for plant resistance to low‐oxygen stress.
Genetic transformation technology has been used to stimulate plant anaerobic energy metabolism and tolerance to anoxia. Tadege et al. (1998) incorporated a bacterial gene into tobacco that encoded pyruvate decarboxylase, the key pacemaker enzyme in ethanolic fermentation. Transgenic plants showed faster rates of ethanolic fermentation. However, this resulted in earlier depletion of substrate reserves and the outcome was decreased rather than increased tolerance of oxygen shortage. Although this result was contrary to that expected, the result demonstrated the feasibility of increasing fermentation rates by genetic engineering. The experiment also demonstrated the critical importance of carbohydrate reserves as fuel for glycolysis and fermentation.
Blocking of anaerobic protein synthesis destabilizes cell ultrastructure and decreases plant tolerance under anoxia
The importance of anaerobic stress proteins for plant resistance to anoxia and hypoxia was demonstrated in experiments that blocked the synthesis of these proteins. Inhibition of synthesis using cycloheximide (Fig. 5) destabilizes mitochondrial fine structure in rice coleoptiles and essentially decreases tolerance to anoxia even in the presence of exogenous glucose (Vartapetian and Polyakova, 1994). Similar observations were made by Subbaiah et al. (1994) who inhibited anaerobic protein synthesis in maize root tips using Ruthenium Red acting on transcription. In this case tolerance to anoxia was reduced from 72 to only 2 h.
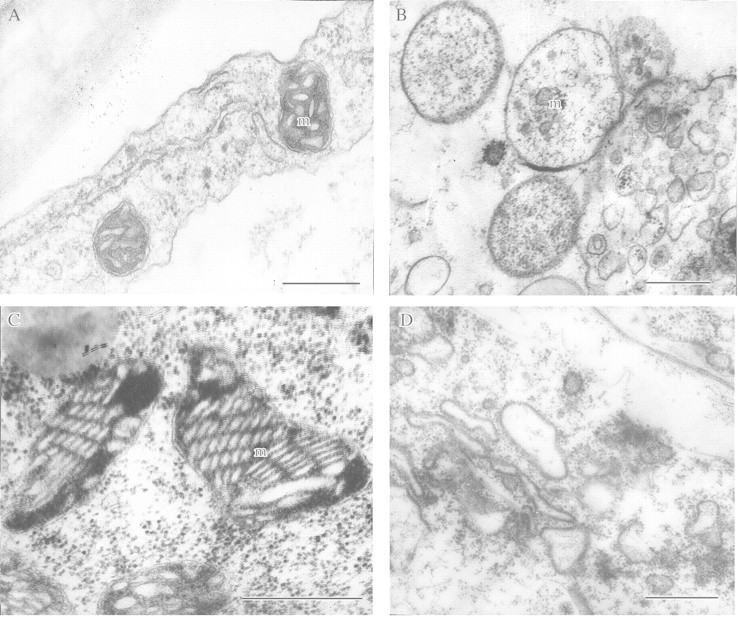
Fig. 5. Inhibition of anaerobic protein synthesis in detached rice coleoptile cells has destabilized mitochondrial fine structure. A, Under aerobic conditions (control). B, After 48 h anaerobic incubation without exogenous glucose. C, After 72 h anaerobic incubation in the presence of exogenous glucose (2 %). D, After anaerobic incubation in the presence of cycloheximide (10–5 m) and glucose (2 %). m, Mitochondrion. Bars = 0·5 µm.
The role of carbohydrate reserves in the maintenance of glycolysis and tolerance to anoxia
The importance of polymeric carbohydrate reserves mobilized by tolerant plant species under anaerobiosis was inferred from EM observations discussed above (Vartapetian et al., 1976, 1978a), and highlighted in biochemical studies on both anaerobically germinating rice seeds (Perata et al., 1992, 1993, 1997) and rhizomes of wetland plants (Brändle, 1991; Hanhijarvi and Fagerstedt, 1995; Crawford and Brändle, 1996). The enzymes responsible for mobilization of endosperm starch reserves in anaerobically germinating rice seeds have been identified and synthesis demonstrated under anoxia. The products of starch degradation are presumed to be transported from endosperm to growing coleoptile, and utilized there in glycolysis and fermentation. High tolerance of germinating rice seeds is also associated with induction of sucrose synthase and hexokinases (Guglielminetti et al., 1995). In contrast to rice, anaerobically imbibing wheat seeds fail to form enzymes responsible for starch mobilization, causing the seeds to perish (Perata et al., 1992). However, when anaerobically imbibing wheat seeds are fed with exogenous glucose, limited anoxic germination is then possible.
The role of starch reserves in tolerance to anaerobiosis has also been demonstrated in experiments on rhizomes of some wetland species (Henzi and Brändle, 1993; Hanhijarvi and Fagerstedt, 1995; Crawford and Brändle, 1996). Rhizomes of species that accumulate a large quantity of starch before winter anaerobiosis were able to withstand prolonged anoxia lasting several months and were able to support slowly growing shoots even under anoxia. Rhizomes that do not accumulate large starch reserves before anaerobiosis are not capable of withstanding long‐term anaerobiosis. It was demonstrated that the unusually large Pasteur effect and anoxia tolerance of the aquatic monocot, Potamogeton pectinatus, which grows rapidly in oxygen‐free medium, were supported by sucrose derived from the hydrolysis of starch reserves when anaerobic (Summers et al., 2000). This was sufficient to support a strongly accelerated rate of stem elongation induced by the complete absence of oxygen over periods of up to 14 d.
VISUALIZATION OF THE GENERAL ADAPTIVE SYNDROME AND MECHANISM FOR ITS REALIZATION IN PLANTS UNDER ANAEROBIC STRESS
As discussed previously, anaerobic stress is associated with abnormal swelling of mitochondria which becomes more pronounced as the duration of anaerobic incubation increases. It finally results in irreversible degradation of mitochondria and other organelles. The initial stages of membrane destruction are reversible since the mitochondrial ultrastructure of plants transferred from anaerobic conditions into a normal aerobic environment soon becomes normal again and oxidative phosphorylation recommences. However, more detailed EM investigations on wheat and maize seedlings have revealed a pattern of rearrangements of the mitochondrial membranes that was overlooked in earlier experiments (Vartapetian, 1991). After only 30 min anoxia, a distinct swelling of mitochondria was observed which increased further when the stress was prolonged to 60–90 min (Fig. 6B). Surprisingly, further continuous anaerobiosis did not result in the progressive destruction of the mitochondrial membranes as expected on the basis of previous experiments involving extended periods of anaerobic exposure. On the contrary, after 3–5 h of anaerobiosis, complete (but temporary) restoration of the intact mitochondrial ultrastructure was seen (Fig. 6C). This intact mitochondrial ultrastructure was retained for several hours more. Further anaerobiosis then caused a new progressive wave of mitochondrial destruction, and by 24–48 h of anaerobiosis, irreversible degradation of mitochondria took place.
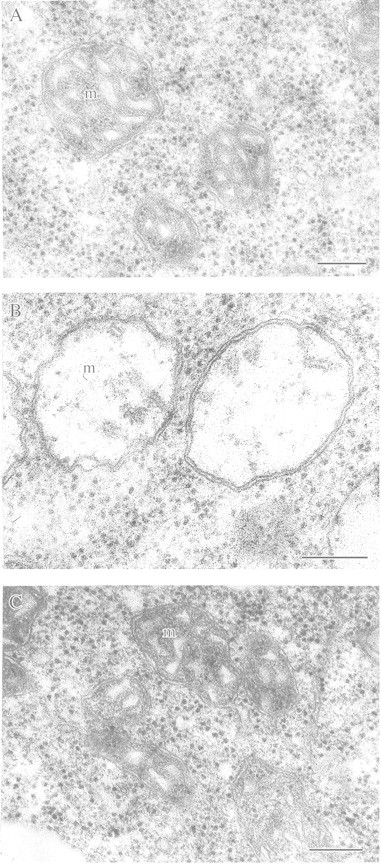
Fig. 6. Reversible destruction and restoration of mitochondrial ultrastructure of wheat seedling leaves during short‐term anoxia. A, Under aerobic conditions (control). B, After 90 min anaerobic incubation (mitochondria are swollen). C, After 3 h of anaerobic incubation (mitochondria are restored to a normal appearance). m, Mitochondria. Bar = 0·5 µm.
To elucidate possible molecular mechanisms of this unexpected phenomenon, anaerobic treatment of wheat leaves was carried out in the presence of exogenous glucose (2 %) to stimulate glycolysis and fermentation. In the presence of exogenous glucose, no evidence of reversible mitochondrial damage was observed after 30, 60 or 90 min of anaerobic incubation (Fig. 7A). These results suggested that mitochondrial damage in the absence of exogenous glucose was likely to have been caused by glycolytic‐substrate starvation and an attendant fall of ATP production during glycolysis. However, these experiments with glucose feeding do not explain how prolonging anaerobic incubation further under a deficiency of glycolytic substrate could restore (albeit temporarily) the ultrastructure of wheat leaf mitochondria. The most probable hypothesis is that synthesis and accumulation of anaerobic proteins, including enzymes participating in glycolysis and fermentation, took place in the first 3–5 h of anaerobic exposure, thus accelerating glycolysis. To examine this hypothesis, anaerobic incubation of wheat leaves was carried out in the presence of cycloheximide (10–5 mm) to inhibit synthesis of anaerobic proteins. This resulted in the expected mitochondrial swelling after 30–90 min anaerobic incubation, but blocked the restoration that normally occurred during 3–5 h of anaerobiosis. Complete destruction of mitochondria in the leaves took place within 6 h of anaerobiosis (Fig. 7B). These observations can be interpreted as demonstrating a key role for energy metabolism in reversible destruction and restoration of mitochondrial ultrastructure under anoxia. Both glucose feeding and synthesis of enzymes of glycolysis and fermentation as well as related enzymes of carbohydrate metabolism by 3–5 h of anaerobiosis are presumed to facilitate the substrate‐enzyme reactivity resulting in increased ATP production and restoration of mitochondrial fine structure. Finally, these experiments may be considered as a demonstration of the general adaptive syndrome in plants. This phenomenon was advanced by the physician Hans Selye more than 50 years ago on the basis of in vivo observations of humans and mammals under stress. It is seen as a non‐specific alarm reaction. In the case of anoxic leaves, this stage corresponds with reversible destruction of the mitochondria ultrastructure. This initial ‘alarm’ reaction is followed by ‘adaptation’ (recovery of the mitochondrial ultrastructure) and finally ‘exhaustion’ (irreversible degradation of mitochondria under prolonged anaerobiosis) according to Selye’s terminology (Selye, 1950).
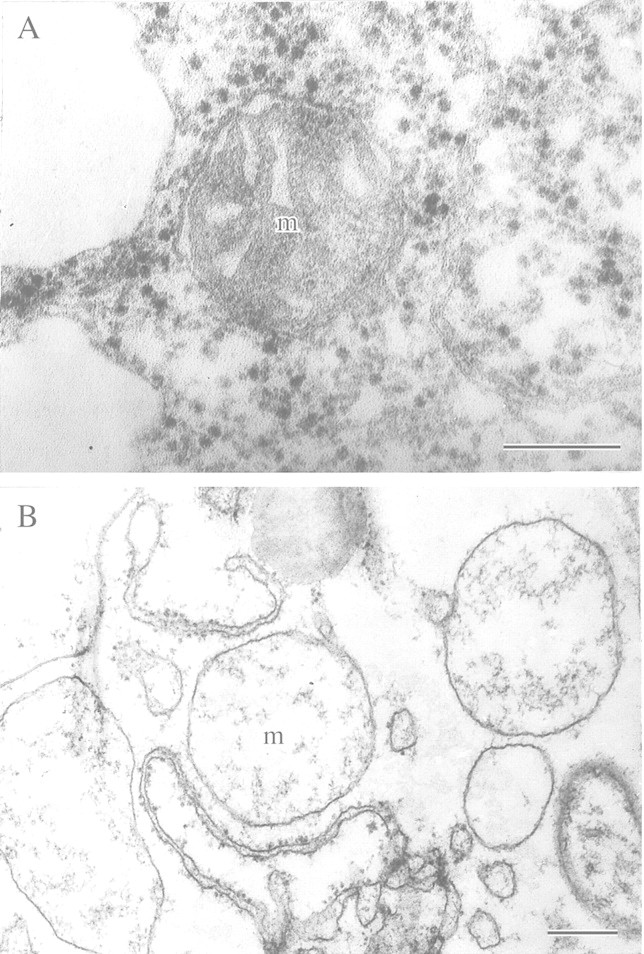
Fig. 7. Mitochondria of wheat seedling leaves after anaerobic incubation in the presence of exogenous glucose or cycloheximide. A, After 90 min of anaerobiosis in the presence of exogenous glucose (2 %). B, After 6 h of anaerobiosis in the presence of cycloheximide (10–5 m). m, Mitochondrion. Bars = 0·5 µm.
PHYSIOLOGICAL ROLE OF NITRATE AS TERMINAL ELECTRON ACCEPTOR UNDER ANAEROBIC STRESS
A possible physiological role for exogenous nitrate under plant hypoxia and anoxia has attracted attention from a fundamental metabolic point of view and, since nitrate is a widely used fertilizer, also from agronomists. Some researchers have considered nitrate reduction to nitrite and ammonia as a compensatory mechanism of NADH and NADPH oxidation in plants under hypoxia and anoxia (Reggiani et al., 1985, 1993; Fan et al., 1988, 1997; Müller et al., 1994; Oberson et al., 1999; Vartapetian and Polyakova, 1999). Other findings are less clear‐cut. For example, the favourable effect of nitrate application on energy metabolism of anoxic maize roots (Reggiani et al., 1985) was not seen by Saglio et al. (1988). Furthermore, a study of the effect of exogenous nitrate on growth and energy metabolism of rice, pea and wheat seedlings under strict anoxia revealed a deteriorative rather than protective effect (Ivanov and Andreev, 1992). On the other hand, experiments using maize roots (Fan et al., 1988) showed decreased accumulation of ethanol under anaerobiosis in the presence of nitrate. On the contrary, in rice and Carex roots, exogenous nitrate stimulated anaerobic respiration (Reggiani et al., 1985; Müller et al., 1994), while in barley roots, anaerobic ethanol fermentation was not affected by increased nitrate reductase activity (Botrel and Kaiser, 1997). In the light of these contradictory results, electron microscopy was used to follow the effect of nitrate on anaerobic tissue. We compared coleoptiles of rice (highly resistant to anoxia) with roots of pea (rapidly damaged by anoxia). These tissues also differed in their ability to metabolize nitrate anaerobically. The rice coleoptile is capable of reducing nitrate by an assimilative pathway, whereas roots of pea can reduce nitrate to nitrite by a dissimilative pathway. The impact of supplying nitrate (10 mm KNO3 compared with 10 mm KCl) was judged in terms of changes in fine structure of mitochondria under anaerobic stress. Detached organs were made anaerobic for 3, 6 or 9 h (root), and 24 or 48 h (coleoptile). Aerobic coleoptile cells contained typical plant mitochondria with numerous cristae and an electron‐dense matrix (Fig. 8A). Transfer to an anaerobic medium for 24 h in the presence of KCl resulted in membrane destruction in 50 % of mitochondria; cristae disappeared and the matrix became transparent (Fig. 8B). Complete mitochondrial degradation took place after 48 h anaerobic incubation (Fig. 8C). However, in the presence of exogenous nitrate (Fig. 8D), marked destructive changes in mitochondrial ultrastructure were delayed until 48 h after the start of anaerobic incubation of coleoptiles, although noticeable non‐pathological changes in ultrastructure were found, such as cristae becoming arranged into parallel rows. A protective effect of exogenous nitrate under anoxia was also found in roots of pea seedlings. In the absence of KNO3, distinctive destruction of the mitochondrial membranes was seen in 50 % of organelles after only 6 h without oxygen. At this time cristae had disappeared and the electron density of the matrix had decreased. After 9 h of anaerobic incubation, complete and irreversible degradation of all mitochondria was observed. However, in the presence of exogenous nitrate, no destructive changes in mitochondrial ultrastructure were observed even after 9 h of anaerobiosis. As in the case of rice coleoptiles, some organelles showed insignificant non‐pathological changes in ultrastructure.
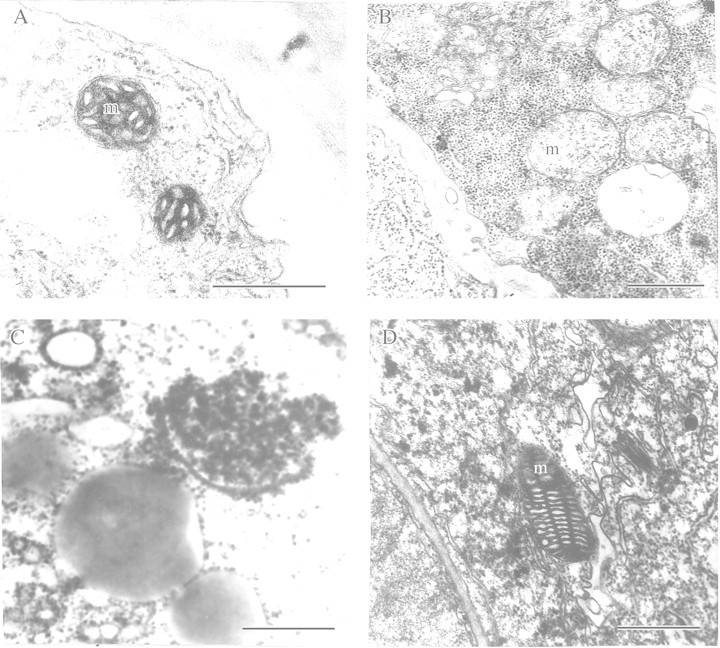
Fig. 8. Ultrastructure of detached rice coleoptile mitochondria after anaerobic incubation in the presence of exogenous KCl (10 mm) or KNO3 (10 mm). A, Before anaerobic incubation (control). B, After 24 h anaerobic incubation in the presence of KCI. C, After 48 h anaerobic incubation in the presence of KCI. D, After 48 h anaerobic incubation in the presence of KNO3. m, Mitochondrion. Bars = 0·5 µm.
The results of these nitrate‐feeding experiments involving anaerobic rice coleoptiles and pea roots contrast with the findings of Ivanov and Andreev (1992). The studies of Ivanov and Andreev (1992) were carried out under similar experimental conditions but demonstrated a clearly negative effect of nitrate on the metabolism of the same plants (rice seedlings and roots of pea and wheat) under strict anoxia. On the other hand, our results agree with the findings of Reggiani et al. (1985, 1993) and Fan et al. (1988, 1997) who demonstrated the assimilative character of nitrate utilization in rice coleoptiles. They also agree with the results of Roberts et al. (1985), Müller et al. (1994) and Fan et al. (1997) who showed a favourable effect of nitrate on energy metabolism and stabilization of the acidity in the cytoplasm of roots under oxygen deficiency.
The protective effect of nitrate under anoxia is probably attributable to nitrate acting as a terminal acceptor of electrons and protons in the absence of molecular oxygen. Theoretically, acceptance of the reductive equivalents should, on the one hand, stimulate energy metabolism and, on the other, prevent deteriorative effects of cytoplasm acidification. The latter is more probable in the case of rice coleoptiles where, under anaerobiosis, nitrate undergoes reduction through an assimilative pathway to NH4+ and amino acids. But in roots, in the absence of oxygen, nitrate is reduced by the dissimilative pathway to nitrite (Lee, 1979; Botrel and Kaiser, 1997). The protective effect of nitrate under anaerobiosis probably results from stimulation of anaerobic energy metabolism due to acceptance of electrons and protons.
THE PHYSIOLOGICAL ROLE OF LIPID METABOLISM UNDER ANAEROBIC STRESS IN SEEDLINGS OF ORYZA SATIVA AND ECHINOCHLOA PHYLLOPOGON
It has been proposed that under anoxia, anaerobically synthesized lipids could play a role as terminal acceptors of respiratory electrons and protons. In addition, unsaturated fatty acids esterified in lipids may also accept protons under anaerobic stress. The role of anaerobically synthesized lipids was studied in seedlings of the rice weed Echinochloa phyllopogon, whose germinating seeds are remarkably resistant to anoxia (Kennedy et al., 1980). Kennedy et al. (1991) found intensive accumulation of lipid bodies (spherozomes) in cells of primary leaves of anaerobically germinating E. phyllopogon. They suggested that de novo‐synthesized lipids served as electron and proton acceptors. This phenomenon was considered as a mechanism of biochemical adaptation of plants tolerant to anoxia (Fox et al., 1994). Indeed, experiments with lipid precursors ([14C]acetate and [3H]glycerol) have demonstrated incorporation of label under anoxia into the molecules of phospho‐, glyco‐ and neutral lipids in shoots of Oryza sativa (Vartapetian et al., 1978b) and Echinochloa phyllopogon (Kennedy et al., 1991). Some authors consider that hydrogenation of unsaturated fatty acids is an adaptive mechanism under anaerobic stress (Zs.‐Nagy and Galli, 1977; Chirkova, 1988). To ascertain the physiological role of lipid synthesis and reduction of unsaturated fatty acids in anaerobically germinating Oryza sativa seeds, ultrastructural and biochemical studies of lipids were carried out during anaerobic germination (primary anaerobiosis) and under conditions of secondary anaerobiosis, i.e. after transfer of detached shoots of aerobically germinated rice seeds into an oxygen‐free medium. In the latter case, anaerobic lipid synthesis was stimulated by feeding tissues with exogenous glucose. There was no evidence that lipid body accumulation in rice seedlings took place under primary or secondary anaerobiosis (Fig. 9A and B). Lipid bodies could be observed only after 48 h anaerobic incubation of detached coleoptiles in the absence of exogenous glucose, when mitochondrial degradation took place along with that of other cell membranes (Fig. 9C). These results have been supported by biochemical studies of the fatty acid composition of lipids during the course of anaerobic germination of rice seedlings (Table 1). No significant effect on the qualitative and quantitative composition of lipid fatty acids was found in the coleoptiles after 5 or 8 d of anaerobic germination. The same was also found in rice shoots subjected to secondary anaerobiosis. We conclude that anaerobic synthesis of lipids is unlikely to act as an alternative mechanism of electron acceptance. The same conclusion is also drawn for hydrogenation of unsaturated fatty acids. If this adaptive mechanism of electron acceptance was realized in rice seedlings under anaerobiosis, then the ratio of unsaturated : saturated fatty acids in the isolated lipids would decrease during anaerobic incubation. As shown in Table 1, no marked decrease in the double bond index took place after 5 or 8 d of anaerobiosis. However, these conclusions do not diminish the physiological significance of anaerobic synthesis of lipids demonstrated in the above‐mentioned experiments involving labelled lipid precursors (Vartapetian et al., 1978b; Kennedy et al., 1991). Anaerobic synthesis of lipids remains an important adaptive mechanism by which a turnover of saturated fatty acids, phospho‐, glyco‐ and neutral lipids is achieved under extreme conditions of oxygen deficiency.
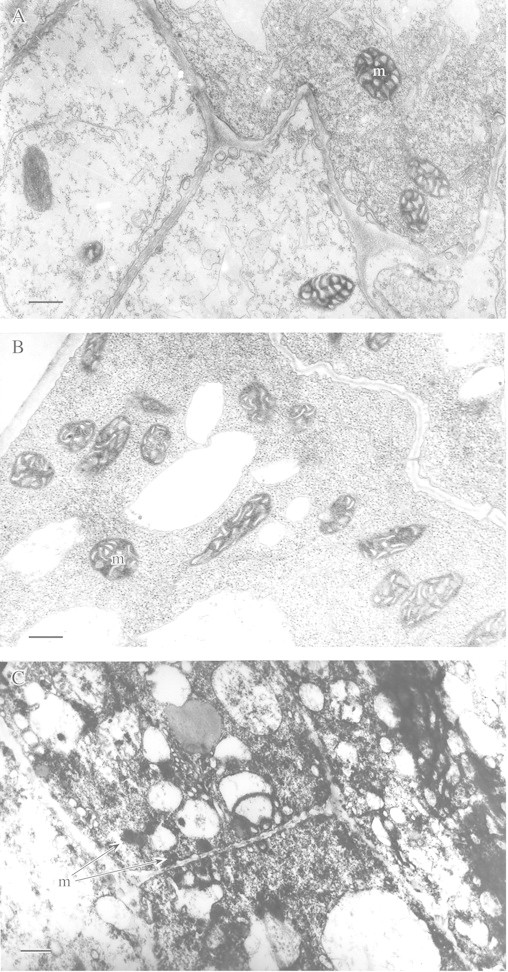
Fig. 9. Ultrastructure of coleoptile cells after anaerobic germination of rice seeds (A, B) and after anaerobic incubation of rice detached coleoptiles (C). Five‐day old (A) and 8‐d‐old (B) coleoptiles; cytoplasm without lipid bodies. C, After 48 h incubation; lipid bodies in collapsed cells are indicated by arrows. m, Mitochondrion. Bars = 0·5 µm.
Table 1.
Fatty acid (FA) composition (µg FA per coleoptile × 100) of rice coleoptiles after 5 and 8 d of anaerobic germination (primary anoxia)
| Coleoptile age | C 16:0 | C 16:1 | C 18:0 | C 18:1 | C 18:2 | C 18:3 | Sum satur* | Sum unsatur† | DBI‡ |
| 5 d | 177·0 ± 14·3 | 4·50·± 0·5 | 15·5 ± 2·5 | 182·0 ± 28·0 | 417·0 ± 46·0 | 31·0·± 4·5 | 192·5 ± 14·5 | 634·5 ± 54·5 | 1·34 |
| 8 d | 172·0 ± 21·9 | 4·0 ± 0·7 | 18·0 ± 2·9 | 195·0 ± 13·3 | 429·0 ± 22·0 | 31·0 ± 4·3 | 190·0 ± 22·1 | 659·0 ± 26·1 | 1·35 |
Seeds were imbibed and germinated anaerobically in a special vacuum apparatus (Vartapetian and Polyakova, 1994) after evacuation of air by oil vacuum pump.
Experiments were run in triplicate, each replicate comprising 100–120 coleoptiles.
Data represent mean ± s.d. of three replicates.
* Total sum of saturated FA; † total sum of unsaturated FA; ‡ double bond index: the sum of weight per cent of each acid multiplied by the number of double bonds it contains per molecule and divided by 100.
SELECTION OF IN VITRO CELL LINES AND REGENERATED PLANTS TOLERANT OF ANAEROBIC STRESS
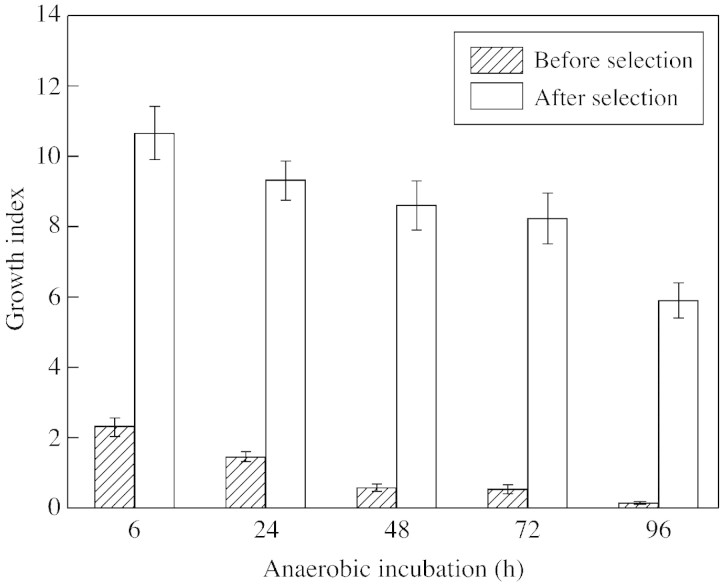
The creation of plants with higher resistance to oxygen deficiency is an important goal of applied research in the field of plant anaerobiosis. Recent attempts in this direction include transferring a bacterial pyruvate decarboxylase gene into tobacco to enhance anaerobic energy metabolism (glycolysis and fermentation) and thus tolerance to oxygen deficiency (Tadege et al., 1998). Another approach to creating more tolerant plants is in vitro selection of anoxia‐resistant cell lines and regenerating tolerant whole plants from them. We have attempted this using sugar cane (Saccharum officinarum) and wheat (Triticum aestivum). Callus obtained from the meristem of sugar cane and from wheat embryos was first grown aerobically on Murashigi and Skoog medium before being incubated anaerobically in the dark in the presence or absence of exogenous sugar. Resistance of callus to anoxia was monitored by electron microscopy using mitochondrial structure as a marker. Post‐anaerobic mitotic and growth activity of the cells were also assessed. In addition, the capacity of selected cells to produce regenerated plants tolerant to soil flooding was studied. As expected, EM studies showed a marked difference in mitochondrial ultrastructure in callus cells incubated anaerobically in the presence or absence of exogenous sugar. When glucose was present in the growth medium, no significant pathological change in mitochondrial ultrastructure of callus cells was observed even after 96 h of anaerobic incubation (Fig. 10B). In contrast, 48 h (S. officinarum) or 32 h (T. aestivum) of anaerobic incubation in glucose‐free medium caused 90 % of mitochondria to show typical signs of membrane degeneration (Fig. 10C). A test for post‐anaerobic mitotic and growth activity demonstrated that after 48 h without oxygen, 12 % of S. officinarum cells retained growth activity and were capable of mitosis. When anaerobiosis was extended to 72–96 h, almost all cells were killed. Accordingly, callus of S. officinarum that survived 48 h anoxia without exogenous sugar was used for further selection of tolerant cells. After three consecutive selection cycles at 48, 72 and 96 h of anaerobic incubation, selected surviving cells were capable of growing again when placed in air (Figs 11 and 12). Whole plants regenerated from the tolerant callus cells after 48 h of anaerobic incubation were tested for tolerance to soil flooding. Preliminary data demonstrated that plants regenerated from the selected cells were more resistant to soil flooding compared with initial plants used for callus cell preparation. However, these promising results now require thorough confirmation and checking to determine whether tolerance can pass through meiosis and can be inherited through sexual reproduction.
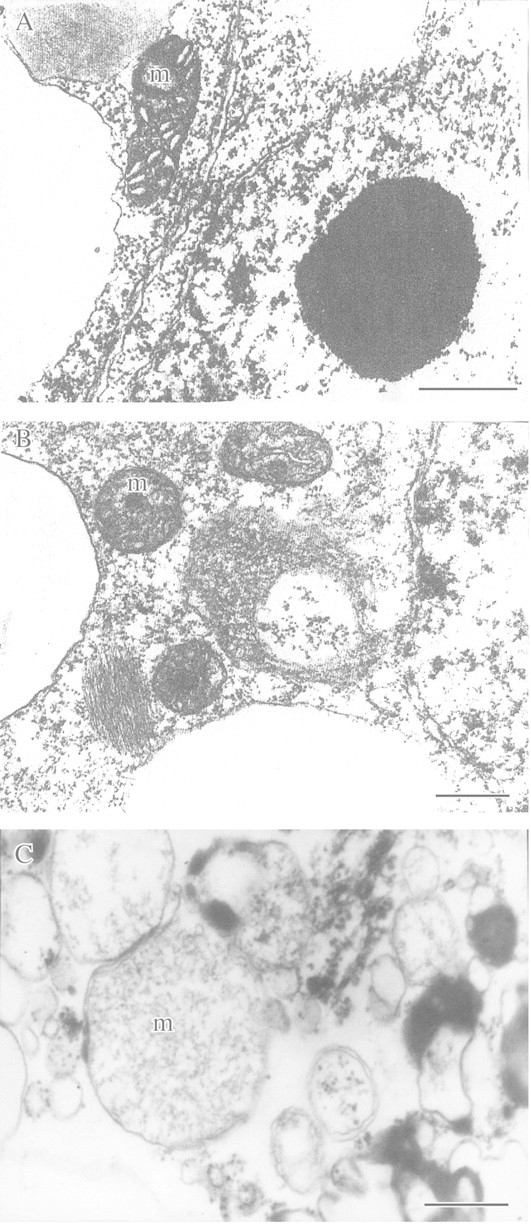
Fig. 10. Ultrastructure of callus cells of Saccharum officinarum with and without glucose feeding under anoxia. A, Before anaerobic incubation (control). B, After 96 h anaerobic incubation in the presence of exogenous glucose (3 %). C, After 48 h anaerobic incubation in glucose‐free medium. m, Mitochondrion. Bars = 0.5 µm.
Fig. 11. Growth index after 1 month of post‐anaerobic growth of cell lines of Saccharum officinarum before and after selection of tolerant cells. Data are means ± standard errors.
CONCLUSIONS
The principal goal of this review has been to show the potential of functional electron microscopy in studies of plant environment stresses, particularly anaerobiosis. The concept of two main strategies of higher plant adaptation to anaerobic stress (apparent and true tolerance), first advanced more than 20 years ago (Vartapetian, 1978; Vartapetian et al., 1978a), was based on functional electron microscopy. It revealed the paradoxical phenomenon of hypersensitivity, rather then hyper‐resistance, of root cell ultrastructure in flooding‐tolerant species, and the protective rather than damaging role of enhanced energy metabolism (substrate provision and glycolysis). This view is now widely accepted and contrasts with the earlier metabolic theory of plant adaptation to anaerobiosis (McManmon and Crawford, 1971; Crawford, 1977, 1978). Mechanisms for the realization of apparent and true tolerance have been studied intensively over the last two decades by numerous research groups using physiological and biochemical experiments, and have been discussed in several helpful reviews (Kennedy et al., 1992; Perata et al., 1993; Ricard et al., 1994; Crawford and Brändle, 1996; Drew, 1997; Vartapetian and Jackson, 1997; Jackson and Armstrong, 1999). There is a fundamental difference between the two mechanisms of adaptation. Apparent tolerance is an avoidance strategy. It is realized on the level of the whole plant and is based on oxygen translocation from aerated parts of plants to organs localized in anaerobic medium. This provides long‐term tolerance and supports high growth rates in wetland species. True tolerance is based on metabolic adaptation. This is realized at the cellular and molecular level. It takes place even in the complete absence of oxygen and can provide short‐term tolerance (several days or even weeks) and, in some species (e.g. rhizomes and leaves of amphibious Acorus calamus), can provide for several months’ survival in the complete absence of oxygen (Henzi and Brändle, 1993; Crawford and Brändle, 1996) preserving the growth capacity. Energy metabolism plays a key role in true tolerance. This view is supported by numerous experiments: (a) stimulation of glycolysis with exogenous sugar stabilizes markedly cell fine structure (Vartapetian et al., 1976, 1977) and prolongs plant survival under anoxia (Webb and Armstrong, 1983; Waters et al., 1991); (b) plant transfer from aerobic to anaerobic medium results in synthesis of anaerobic proteins (Maslova et al., 1975; Sachs et al., 1980) that are mostly enzymes involved in energy metabolism, glycolysis and fermentation (Sachs, 1993); (c) blocking the synthesis of anaerobic proteins destabilizes cell ultrastructure (Vartapetian and Polyakova, 1994) and results in a substantial decrease of anoxia tolerance (Subbaiah et al., 1994); (d) increasing plant tolerance to anoxia by hypoxic pre‐treatment is associated with stimulation of glycolysis and fermentation as well as with improved cell energy status (Saglio et al., 1988; Andrews, 1997); and (e) there is a threshold for ATP production below which hypoxic enhancement of anoxia tolerance may not be possible (Xia et al., 1995; Rawyler et al., 1999).
Functional electron microscopy has also demonstrated the existence of a general adaptation syndrome in plants and suggested a molecular mechanism for its realization under anaerobic stress. The key role of energy metabolism both in reversible damage and subsequent restoration of mitochondrial ultrastructure is emphasized here. Results of EM studies, confirmed by biochemical analysis, have revealed unexpected differences in anaerobic metabolism of lipids in such seemingly similar tolerant species as germinating rice and Echinochloa phyllopogon. In addition, a physiological role for nitrate, utilized by both dissimilative and assimilative pathways under anoxia, has been demonstrated. Finally, functional electron microscopy has also been useful in in vitro selection of anoxia‐tolerant cell lines and regenerating plants from callus. This work gives reason to believe that this approach, in parallel with genetic transformation, will help create plants that are better able to tolerate flooding in field conditions.
ACKNOWLEDGEMENTS
We thank Professors W. Armstrong and M. C. Drew for reading the manuscript and for invaluable comments. This work was supported by the Russian Foundation for Basic Research (grant N 00‐04‐48234).
Fig. 12. Post‐anaerobic growth of callus of Saccharum officinarum; only selected cells lines survived anoxic treatment for 96 h.
Supplementary Material
Received: 28 August 2001; Returned for revision: 29 November 2001; Accepted 13 June 2002. This article is dedicated to Professor A. L. Kursanov on the occasion of his 100th birthday.
References
- AldrichHC, Ferl RJ, Hils MH, Akin DE.1985. Ultrastructural correlates of anaerobic stress in corn roots. Tissue and Cell 17: 341–348. [DOI] [PubMed] [Google Scholar]
- AndreevVYu, Generozova IP, Vartapetian BB.1991. Energy status and mitochondrial ultrastructure of excised pea root at anoxia and postanoxia. Plant Physiology and Biochemistry 29: 171–176. [Google Scholar]
- AndreevaIN, Grineva GM.1970. Ultrastructure of endoplasmic reticulum in the cells of maize root under conditions of hypoxia. Plant Physiology 17: 956–961 (in Russian). [Google Scholar]
- AndrewsCJ.1997. A comparison of glycolytic activity in wheat and two forage grasses in relation to their tolerance to ice encasement. Annals of Botany 79 (Suppl. A): 87–92. [Google Scholar]
- AndrewsCJ, Pomeroy MK.1981. The effect of flooding pretreatment on cold hardiness and survival of winter cereals in ice encasement. Canadian Journal of Plant Science 61: 507–513. [Google Scholar]
- AndrewsCJ, Pomeroy MK.1983. The influence of flooding pretreatment on metabolic changes in winter cereal seedlings during ice encasement. Canadian Journal of Botany 61: 142–147. [Google Scholar]
- Ap Rees T, Wilson P.1984. Effect of reduced supply of oxygen on the metabolism of roots of Glyceria maxima and Pisum sativum Zeitschrift für Pflanzenphysiologie 114: 493–503. [Google Scholar]
- Ap ReesT, Jenkin LET, Smith AM, Wilson PM.1987. The metabolism of flood‐tolerant plants. In: Crawford RMM, ed. Plant life in aquatic and amphibious habitats Oxford: Blackwell Scientific Publications, 227–238.
- ArmstrongW.1978. Root aeration in the wetland condition. In: Hook DD, Crawford RMM, eds. Plant life in anaerobic environments. Ann Arbor: Ann Arbor Science, 269–297.
- ArmstrongW.1979. Aeration in higher plants. Advances in Botanical Research 7: 225–332. [Google Scholar]
- ArmstrongW,Brändle R, Jackson MB.1994. Mechanisms of flood tolerance in plants. Acta Botanica Neerlandica 43: 307–358. [Google Scholar]
- BlomCWPM, Voesenek LACJ, Banga M, Engelaar WMHG, Rijnders JHGM, Van de Steeg HM, Visser EJW.1994. Physiological ecology of riverside species: adaptive responses of plants to submergence. Annals of Botany 74: 253–263. [Google Scholar]
- BotrelA, Kaiser WM.1997. Nitrate reductase activation state in barley roots in relation to the energy and carbohydrate status. Planta 201: 496–501. [DOI] [PubMed] [Google Scholar]
- BrändleR.1991. Flooding resistance of rhizomatous amphibious plants. In: Jackson MB, Davies DD, Lambers H, eds. Plant life under oxygen deprivation Ecology, physiology and biochemistry The Hague: SPB Academic, 35–46.
- BucherM, Brändle R, Kuhlemeier C.1996. Glycolytic gene expression in amphibious Acorus calamus L. under natural conditions. Plant and Soil 178: 75–82. [Google Scholar]
- CampbellR, Drew MC.1983. Electron microscopy of gas space (aerenchyma) formation in adventious roots of Zea mays L. subjected to oxygen shortage. Planta 157: 350–357. [DOI] [PubMed] [Google Scholar]
- ChirkovaTV.1978. Some regulatory mechanisms of plant adaptation to temporal anaerobiosis. In: Hook DD, Crawford RMM, eds. Plant life in anaerobic environments. Ann Arbor: Ann Arbor Science, 137–154.
- ChirkovaTV.1988. The modes of plant adaptation to hypoxia and anoxia. Leningrad: Publishing House of Leningrad University (in Russian).
- CostesC, Vartapetian BB.1978. Plant grown in a vacuum: the ultrastructure and functions of mitochondria. Plant Science Letters 11: 115–119. [Google Scholar]
- CouéeI, Defontaine S, Carde J‐P, Pradet A.1992. Effects of anoxia on mitochondrial biogenesis in rice shoots. Plant Physiology 98: 411–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CrawfordRMM.1977. Tolerance of anoxia and ethanol metabolism in germinating seeds. New Phytologist 79: 511–517. [Google Scholar]
- CrawfordRMM.1978. Metabolic adaptation to anoxia. In: Hook DD, Crawford RMM, eds. Plant life in anaerobic environments. Ann Arbor: Ann Arbor Science, 119–136.
- CrawfordRMM, Brändle R.1996. Oxygen deprivation stress in a changing environment. Journal of Experimental Botany 47: 145–159. [Google Scholar]
- DaviesDD.1980. Anaerobic metabolism and the production of organic acids. In: Davies DD, ed. The biochemistry of plants, vol. 2. New York, London: Academic Press, 581–611.
- DaviesDD, Kenworthy P, Mocquot B, Roberts K.1985. The metabolism of pea roots under hypoxia. Current Topics of Plant Biochemistry and Physiology 4: 141–155. [Google Scholar]
- DrewMC.1997. O2 deficiency and root metabolism: injury and acclimation under hypoxia and anoxia. Annual Review of Plant Physiology and Plant Molecular Biology 48: 223–250. [DOI] [PubMed] [Google Scholar]
- FanTWM, Higashi RM, Lane AN.1988. The investigation of the effects of nitrate on hypoxic metabolism in maize roots. Archives of Biochemistry and Biophysics 266: 592–606. [DOI] [PubMed] [Google Scholar]
- FanTWM, Higashi RM, Frenkiel T, Lane AN.1997. Anaerobic nitrate and ammonium metabolism in flood tolerant rice coleoptiles. Journal of Experimental Botany 48: 1655–1666. [Google Scholar]
- FoxTC, Kennedy RA, Rumpho ME.1994. Energetics of plant growth under anoxia: metabolic adaptation of Oryza sativa and Echinochloa phyllopogon. Annals of Botany 74: 445–455. [Google Scholar]
- FrancisCM, Devitt AC, Steele P.1974. Influence of flooding on the alcohol dehydrogenase activity of roots of Trifolium subterraneum.Australian Journal of Plant Physiology 93: 1094–1101. [Google Scholar]
- GambrellRP, Delaune RD, Patrick WH Jr.1991. Redox processes in soils following oxygen depletion. In: Jackson MB, Davies DD, Lambers H, eds. Plant life under oxygen deprivation Ecology, physiology and biochemistry. The Hague: SPB Academic, 101–117.
- GuglielminettiL, Perata P, Alpi A.1995. Effect of anoxia on carbohydrate metabolism in rice seedlings. Plant Physiology 108: 735–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HanhijarviAM, Fagerstedt KV.1995. Comparison of carbohydrate utilization and energy charge in the yellow flag iris (Iris pseudacorus) and garden iris (Iris germanica) under anoxia. Physiologia Plantarum 93: 493–497. [Google Scholar]
- HenziT, Brändle R.1993. Long‐term survival of rhizomatous species under oxygen deprivation. In: Jackson MB, Black CR, eds. Interacting stresses on plants in a changing climate. NATO ASI Series, Berlin and Heidelberg: Springer‐Verlag, 305–314.
- HoleDJ, Cobb BG, Hole P, Drew MC.1992. Enhancement of anaerobic respiration in root tips of Zea mays following low oxygen (hypoxic) acclimation. Plant Physiology 99: 213–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IvanovBF, Andreev VYu.1992. On the role of ‘nitrate respiration’ in higher plants resistance to anoxia. In: Lambers H, van der Plas LHW, eds. Molecular, biochemical and physiological aspects of plant respiration The Hague: SPB Academic, 559–566.
- JacksonMB.1991. Ethylene in root growth and development. In: Mattoo AK, Suttle JC, eds. The plant hormone ethylene. Boca Raton: CRC Press, 160–181.
- JacksonMB, Armstrong W.1999. Formation of aerenchyma and the processes of plant ventilation in relation to soil flooding and submergence. Plant Biology 1: 274–287. [Google Scholar]
- JohnsonJR, Cobb BG, Drew MC.1989. Hypoxic induction of anoxia tolerance in root tips of Zea mays Plant Physiology 91: 837–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KennedyRA, Rumpho ME, Fox ThC.1992. Anaerobic metabolism in plants. Plant Physiology 100: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KennedyRA, Barret SC, Van der Zee D, Rumpho ME.1980. Germination and seedling growth under anaerobic conditions in Echinochloa crus‐galli (barnyard grass). Plant, Cell and Environ ment 3: 243–248. [Google Scholar]
- KennedyRA, Fox ThC, Everard JD, Rumpho ME.1991. Biochemical adaptations to anoxia: potential role of mitochondrial metabolism to flood tolerance in Echinochloa phyllopogon (barnyard grass). In: Jackson MB, Davies DD, Lambers H, eds. Plant life under oxygen deprivation. Ecology, physiology and biochemistry. The Hague: SPB Academic, 217–227.
- LarcherW.1980. Physiological plant ecology. Berlin: Springer‐Verlag.
- LeeRB.1979. The release of nitrite from barley roots in response to metabolic inhibitors, uncoupling agents, and anoxia. Journal of Experimental Botany 30: 119–133. [Google Scholar]
- LuzikovVN.1985. The mitochondrial biogenesis and breakdown. New York: Plenum Press.
- LuzikovVN, Zubatov AS, Rainina EI.1973. Formation and degradation of mitochondria during aerobic growth of Saccharomyces cerevisiae Journal of Bioenergetics 5: 129–149. [Google Scholar]
- McManmonM, Crawford RMM.1971. A metabolic theory of flooding tolerance: the significance of enzyme distribution and behaviour. New Phytologist 70: 299–306. [Google Scholar]
- MarshallDR, Broué P, Pryor AJ.1973. Adaptive significance of alcohol dehydrogenase isozymes in maize. Nature New Biology 244: 16–18. [DOI] [PubMed] [Google Scholar]
- MaslovaIP, Tchernyadeva IF, Vartapetian BB.1975. Soluble proteins and alcohol dehydrogenase of rice seedlings in anoxia. Twelfth International Botanical Congress, Abstracts, vol. 2. Leningrad: Nauka, 365.
- MooreP.1982. Survival mechanism in wetland plants. Nature 299: 581–582. [Google Scholar]
- MorissetC.1978. Structural and cytoenzymological aspects of the mitochondria in excised roots of oxygen‐deprived Lycopersicon cultivated in vitro. In: Hook DD, Crawford RMM, eds. Plant life in anaerobic environments. Ann Arbor: Ann Arbor Science, 497–538.
- MorissetC.1983. Effect of energetic shortage upon the ultrastructure of some organelles in excised roots of Lycopersicon esculentum cultivated in vitro Part I: reversible structural modification of the endoplasmic reticulum. Cytologia 48: 349–362. [Google Scholar]
- MüllerE, Albers B, Janiesch P.1994. Influence of NO3 and NH4 nutrition on fermentation, nitrate reductase activity and adenylate energy charge of roots of Carex pseudocyperus L. and Carex sylvatica Huds. exposed to anaerobic nutrient solutions. Plant and Soil 166: 221–230. [Google Scholar]
- NeueHU, Becker‐Heidmann P, Scharpenseel HW.1990. Organic matter dynamics, soil properties, and cultural practices in rice lands and their relationship to methane production. In: Bouwman AF, ed. Soils and greenhouse effect. Chichester: John Wiley and Sons, 457–466.
- ObersonJ, Pavelic D, Brändle R, Rawyler A.1999. Nitrate increases membrane stability of potato cells under anoxia. Journal of Plant Physiology 155: 792–794. [Google Scholar]
- OliveiraL.1977. Changes in the ultrastructure of mitochondria of roots of Triticale subjected to anaerobiosis. Protoplasma 91: 267–280. [Google Scholar]
- ÖpikH.1973. Effect of anaerobiosis on respiration rate, cytochrome oxidase activity and mitochondrial structures in coleoptiles of rice (Oryza sativa L.). Journal of Cell Science 12: 725–739. [DOI] [PubMed] [Google Scholar]
- PerataP, Guglielminetti L, Alpi A.1997. Mobilization of endosperm reserves in cereal seeds under anoxia. Annals of Botany 79 (Suppl. A): 49–56. [Google Scholar]
- PerataP, Geshi N, Akazawa T, Yamaguchi J.1993. Effect of anoxia on the induction of α‐amylase in cereal seeds. Planta 191: 402–408. [Google Scholar]
- PerataP, Pozueta‐Romero J, Akazawa T, Yamaguchi J.1992. Effect of anoxia on starch breakdown in rice and wheat seeds. Planta 188: 611–618. [DOI] [PubMed] [Google Scholar]
- PonnamperumaFN.1984. Effect of flooding on soils. In: Kozlowski TT, ed. Flooding and plant growth. Physiological ecology. Orlando, San Diego, San Francisco: Academic Press, 9–45.
- RawylerA, Pavelic D, Gianinazzi Ch, Oberson J, Brändle R.1999. Membrane lipid integrity relies on a threshold of ATP production rate in potato cell cultures submitted to anoxia. Plant Physiology 120: 293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ReggianiR, Brambilla I, Bertani A.1985. Effect of exogenous nitrate on anaerobic metabolism in excised rice roots. II Fermentative activity and adenylate energy charge. Journal of Experimental Botany 36: 1698–1704. [Google Scholar]
- ReggianiR, Mattana M, Aurisano N, Bertani A.1993. Utilization of stored nitrate during the anaerobic germination of rice seeds. Plant Cell Physiology 34: 379–383. [Google Scholar]
- RicardB, Van Toai T, Chourey P, Saglio PH.1998. Evidence for the critical role of sucrose synthase for anoxic tolerance of maize roots using a double mutant. Plant Physiology 116: 1323–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RicardB, Couee I, Raymond P, Saglio PH, Saint‐Ges V, Pradet A.1994. Plant metabolism under hypoxia and anoxia. Plant Physiology and Biochemistry 32: 1–10. [Google Scholar]
- RobertsJKM, Andrade FH, Anderson IC.1985. Further evidence that cytoplasmic acidosis is a determinant of flooding intolerance in plants. Plant Physiology 77: 492–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SachsMM.1993. Molecular genetic basis of metabolic adaptation to anoxia in maize and its possible utility for improving tolerance of crops to waterlogging. In: Jackson MB, Black CR, eds. Interacting stresses on plants in a changing climate. NATO ASI Series, Berlin and Heidelberg: Springer‐Verlag, 375–395.
- SachsMM, Freeling M, Okimoto R.1980. The anaerobic proteins of maize. Cell 20: 761–767. [DOI] [PubMed] [Google Scholar]
- SachsMM, Subbaiah CC, Saab IN.1996. Anaerobic gene expression and flooding tolerance in maize. Journal of Experimental Botany 47: 1–15. [Google Scholar]
- SaglioPH, Drew MC, Pradet A.1988. Metabolic acclimation to anoxia induced by low (2–4 kPa) partial pressure oxygen pretreatment (hypoxia) in root tips of Zea mays.Plant Physiology. 86: 61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SaglioPH, Germain V, Ricard B.1999. The response of plants to oxygen deprivation: role of enzyme induction in the improvement of tolerance to anoxia. In: Lerner HR, ed. Plant responses to environ mental stresses: from phytohormones to genome reorganization. Books in soils, plants and the environment. Vol. 72. New York, Basel: Marcel Dekker, 373–393.
- SaglioPH, Raymond P, Pradet A.1980. Metabolic activity and energy charge of excised maize root tips under anoxia. Plant Physiology 66: 1053–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SelyeH.1950. The physiology and pathology of exposure to stress. Montreal: Medical Publishers.
- ShibasakaM, Tsuji H.1991. Changes in respiratory system of submerged rice seedlings after exposure to air. In: Jackson MB, Davies DD, Lambers H, eds. Plant life under oxygen deprivation Ecology, physiology and biochemistry. The Hague: SPB Academic, 169–186.
- SmithAM, Ap Rees T.1979. Pathways of carbohydrate fermentation in the roots of marsh plants. Planta 146: 327–334. [DOI] [PubMed] [Google Scholar]
- SoldatenkovSV, Chirkova TV.1963. The role of leaves in root respiration under anaerobic conditions. Plant Physiology 10: 452–458 (in Russian). [Google Scholar]
- SubbaiahCC, Bush DC, Sachs MM.1994. Elevation of cytosolic calcium precedes anoxic gene expression in maize suspension‐cultured cells. Plant Cell 6: 1747–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SubbaiahCC, Bush DC, Sachs MM.1998. Mitochondrial contribution to the anoxic Ca2+ signal in maize suspension‐cultered cells. Plant Physiology 118: 759–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SummersJE, Ratcliffe RG, Jackson MB.2000. Anoxia tolerance in the aquatic monocot Potamogeton pectinatus: absence of oxygen stimulates elongation in association with an unusually large Pasteur effect. Journal of Experimental Botany 51: 1413–1422. [PubMed] [Google Scholar]
- TadegeM, Brandle R, Kuhlemeier C.1998. Anoxia tolerance in tobacco roots: effect of overexpression of pyruvate decarboxylase. The Plant Journal 14: 327–335 [Google Scholar]
- UedaK, Tsuji H.1971. Ultrastructural changes of organelles in coleoptile cells during anaerobic germination of rice seeds. Protoplasma 73: 203–215. [Google Scholar]
- VartapetianBB.1973. Aeration of roots in relation to molecular oxygen transport in plants. In: Slater RO, ed. Plant response to climatic factors Proceedings of Uppsala Symposium1970. Paris: UNESCO, 259–265.
- VartapetianBB.1978. Introduction – life without oxygen. In: Hook DD, Craford RMM, eds. Plant life in anaerobic environments. Ann Arbor: Ann Arbor Science, 1–12.
- VartapetianBB.1991. Flood‐sensitive plants under primary and secondary anoxia: ultrastructural and metabolic responses. In: Jackson MB, Davies DD, Lambers H, eds. Plant life under oxygen deprivation Ecology, physiology and biochemistry. The Hague: SPB Academic, 201–216.
- VartapetianBB.1993. Flood tolerant and flood sensitive plants under primary and secondary anoxia. In: Jackson MB, Black CR, eds. Interacting stresses on plants in a changing climate. NATO ASI Series, Berlin and Heidelberg: Springer‐Verlag, 231–241.
- VartapetianBB, Andreeva IN.1986. Mitochondrial ultrastructure of three hydrophyte species at anoxia and in anoxic glucose‐supplemented medium. Journal of Experimental Botany 37: 685–692. [Google Scholar]
- VartapetianBB, Jackson MB.1997. Plant adaptation to anaerobic stress. Annals of Botany 79 (Suppl. A): 3–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VartapetianBB, Polyakova LI.1994. Blocking of anaerobic protein synthesis destabilizes dramatically plant mitochondrial membrane ultrastructure. Biochemistry and Molecular Biology International 33: 405–410. [PubMed] [Google Scholar]
- VartapetianBB, Polyakova LI.1999. Protective effect of exogenous nitrate on the mitochondrial ultrastructure of Oryza sativa coleoptiles under strict anoxia. Protoplasma 206: 163–167. [Google Scholar]
- VartapetianBB, Zakhmilova NA.1990. Ultrastructure of wheat seedling mitochondria under anoxia and postanoxia.Protoplasma 156: 39–44. [Google Scholar]
- VartapetianBB, Andreeva IN, Kozlova GI.1976. The resistance to anoxia and the mitochondrial fine structure of rice seedlings. Protoplasma 88: 215–224. [Google Scholar]
- VartapetianBB, Andreeva IN, Kursanov AL.1974a Appearance of unusual mitochondria in rice coleoptiles at conditions of secondary anoxia. Nature 248: 258–259 (See also Erratum 250: 84). [Google Scholar]
- VartapetianBB, Andreeva IN, Maslova IP.1971. Ultrastructure of rice coleoptile cells under anaerobic and aerobic conditions. Doklady USSR Academy of Sciences 196: 1231–1233 (in Russian). [Google Scholar]
- VartapetianBB, Andreeva IN, Maslova IP.1972. Ultrastructure of root mitochondria under conditions of anoxia and elevated temperatures. Plant Physiology 19: 1105–1111 (in Russian). [Google Scholar]
- VartapetianBB, Andreeva IN, Nuritdinov N.1978a Plant cell under oxygen stress. In: Hook DD, Crawford RMM, eds. Plant life in anaerobic environments. Ann Arbor, Michigan: Ann Arbor Science, 13–88.
- VartapetianBB, Mazliak P, Lance C.1978b Lipid biosynthesis in rice coleoptiles grown in the presence or in the absence of oxygen. Plant Sciences Letters 13: 321–328. [Google Scholar]
- VartapetianBB, Agapova LP, Averianov AA, Veselovsky VA.1974b New approach to study of oxygen transport in plants using chemiluminiscent method. Nature 249: 269. [DOI] [PubMed] [Google Scholar]
- VartapetianBB, Andreeva IN, Kozlova GI, Agapova LP.1977. Mitochondrial ultrastructure in roots of mesophyte and hydrophyte at anoxia and after glucose feeding. Protoplasma 93: 243–256. [Google Scholar]
- VartapetianBB, Andreeva IN, Maslova IP, Davtian NG.1970. The oxygen and ultrastructure of root cells. Agrochimica 15: 1–19. [Google Scholar]
- VoesenekLACJ, Blom CWPM.1996. Plant and hormones: an eco physiological view on timing and plasticity. Journal of Ecology 84: 111–119. [Google Scholar]
- WatersI, Morrell S, Greenway H, Colmer TD.1991. Effects of anoxia on wheat seedlings. II. Influence of O2 supply prior to anoxia on tolerance to anoxia, alcoholic fermentation and sugar levels. Journal of Experimental Botany 42: 1437–1447. [Google Scholar]
- WebbT, Armstrong W.1983. The effects of anoxia and carbohydrates on the growth and viability of rice, pea and pumpkin roots. Journal of Experimental Botany 34: 579–603. [Google Scholar]
- WebbJ, Jackson MB.1986. A transmission and cryo‐scanning electron microscopy study of the formation of aerenchyma (cortical gas‐filled space) in adventitious roots of rice (Oryza sativa).Journal of Experimental Botany 37: 179, 832–841. [Google Scholar]
- XiaJH, Saglio PH, Roberts JKM.1995. Nucleotide levels do not critically determine survival of maize root tips acclimated to a low oxygen environment. Plant Physiology 108: 589–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZalenskiiOV.1977. Ecological and physiological aspects of the study of photosynthesis. Leningrad: Nauka (in Russian).
- ZhangQ, Greenway H.1994. Anoxia tolerance and anaerobic catabolism of aged beetroot storage tissues. Journal of Experimental Botany 45: 567–575. [Google Scholar]
- Zs.‐NagyI, Galli C.1977. On the possible role of unsaturated fatty acids in the anaerobiosis of Anodonta cygnea L. (Mollusca, Pelecypoda). Acta Biologica Academy of Science, Hungary 28: 23–131. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.