Abstract
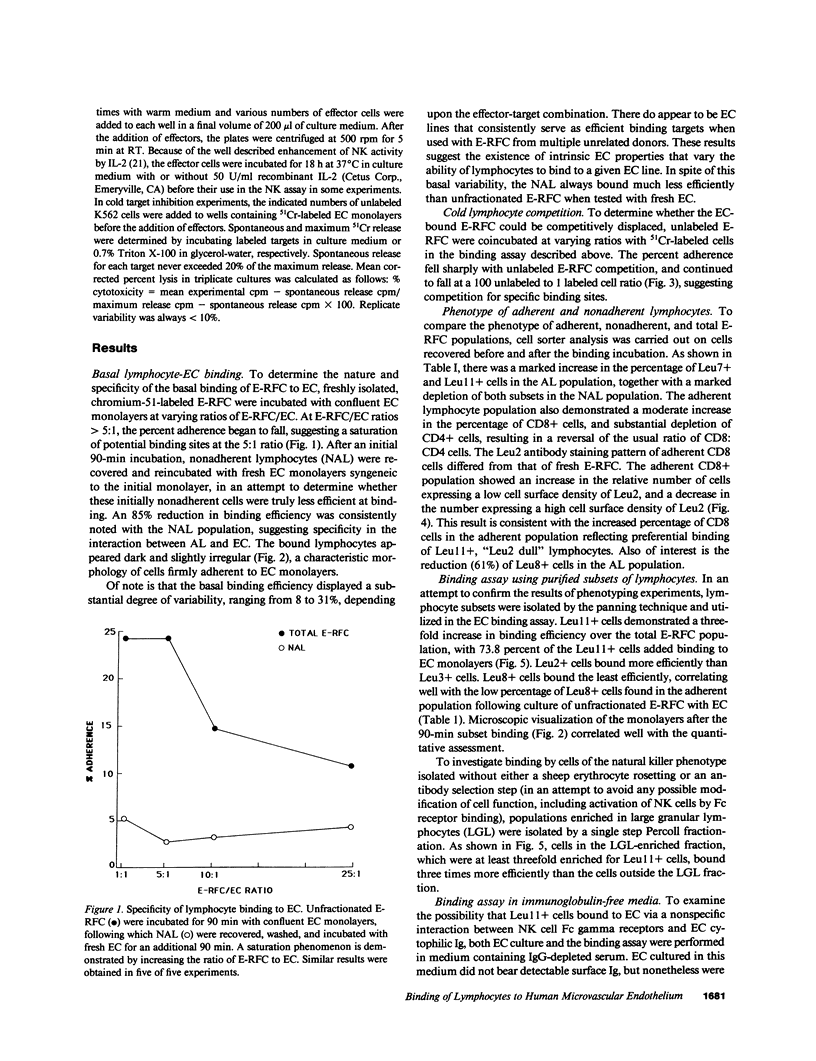
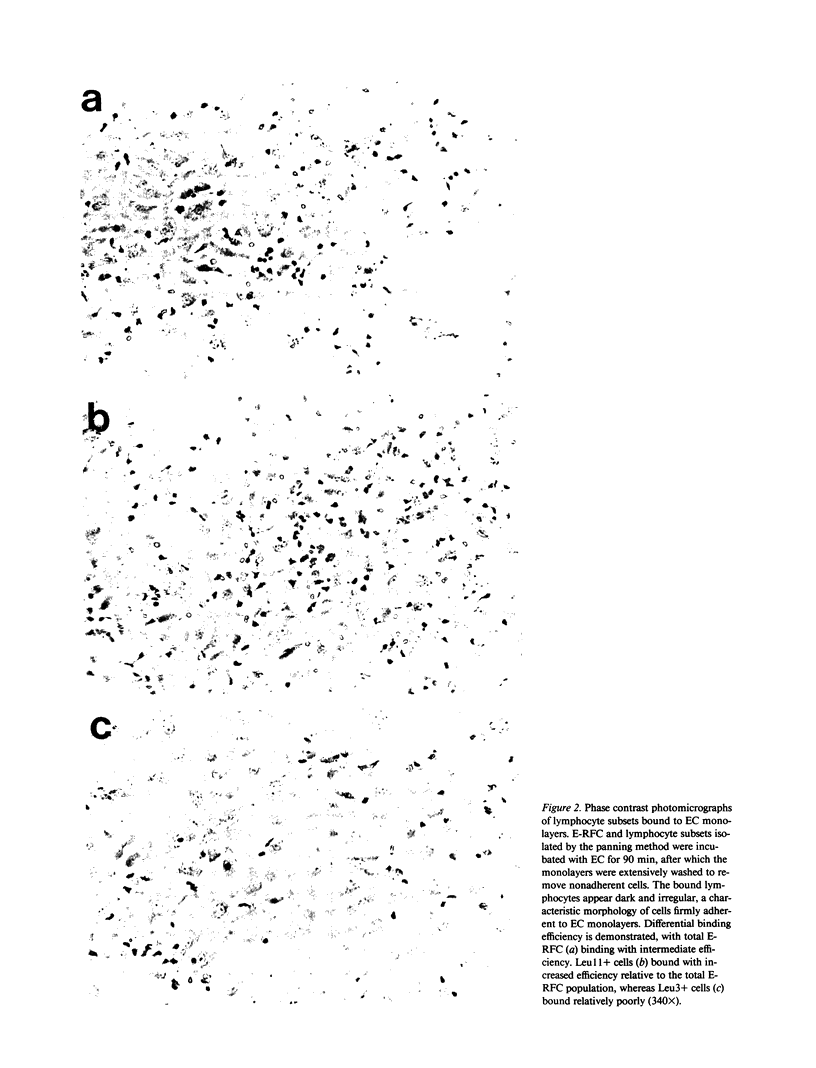
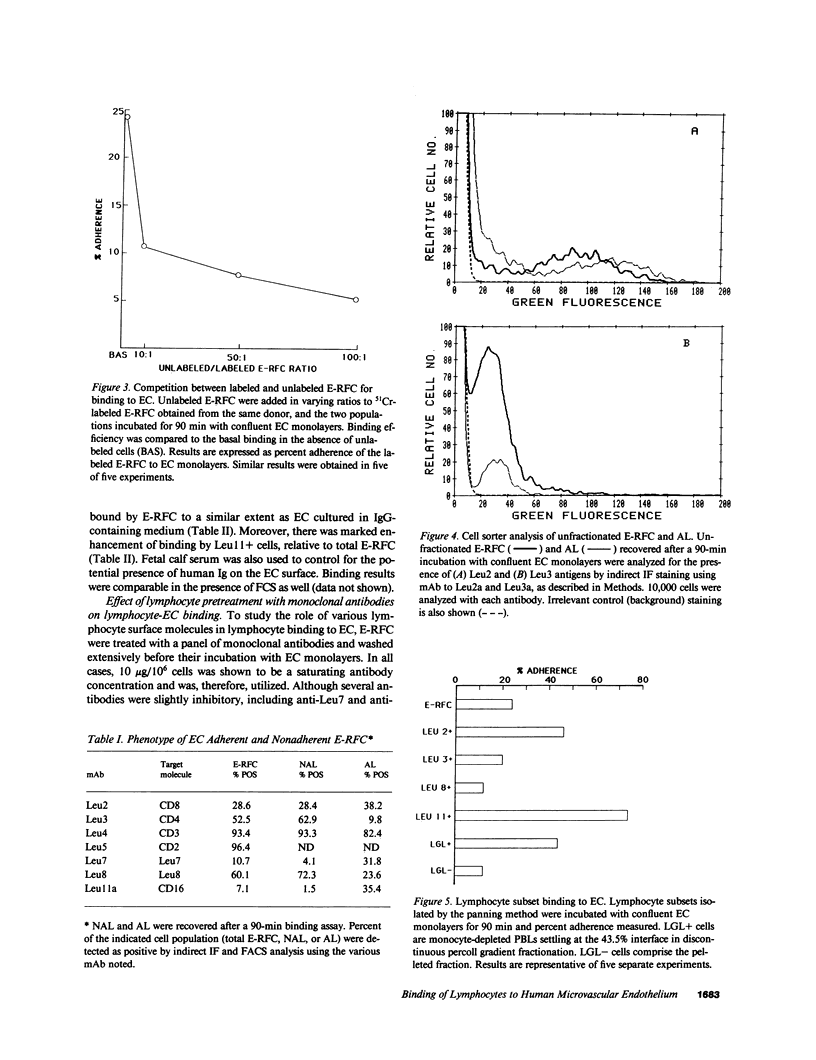
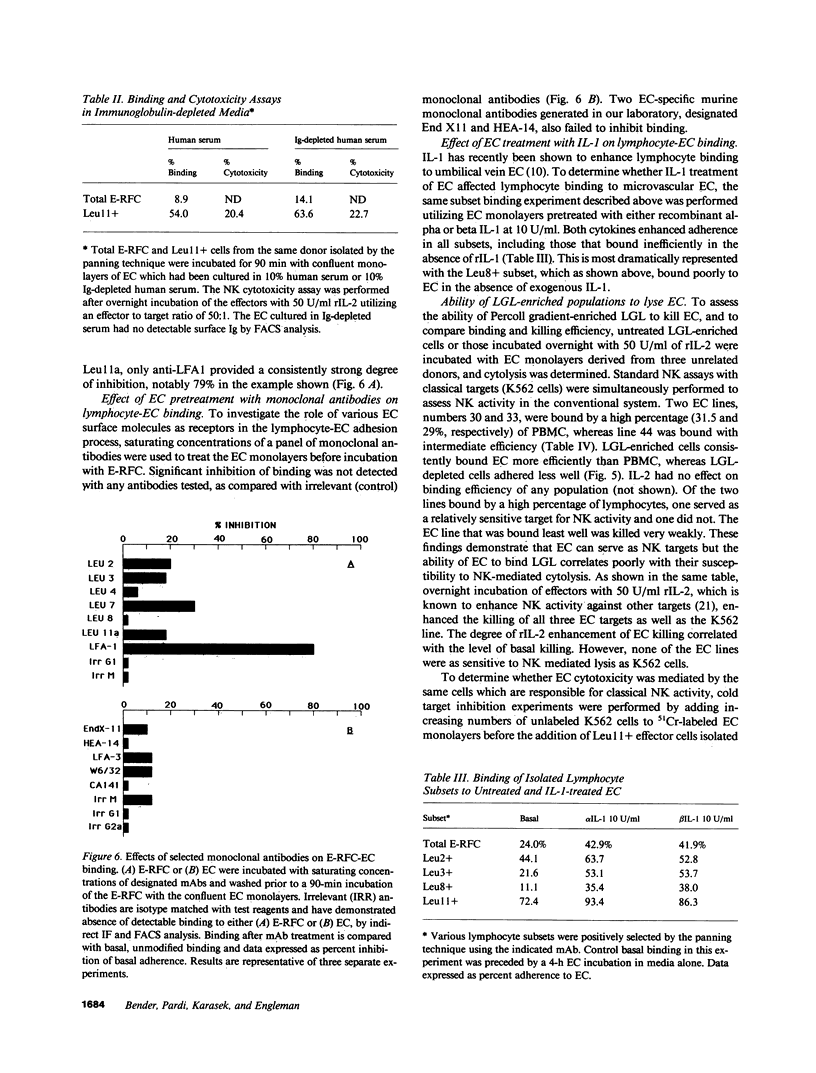
The microvascular endothelium has been postulated to be a critical target in the rejection of vascularized allografts. This study was undertaken to examine the ability of human sheep erythrocyte rosette forming lymphocytes (E-RFC) to form stable conjugates with microvascular endothelial cells (EC), and to assess whether a receptor-ligand interaction mediates this event. Human foreskin microvascular EC monolayers were used as targets of chromium-51-labeled E-RFC in a quantitative adherence assay. Binding was saturable, displaceable by unlabeled E-RFC, augmented by recombinant interleukin 1 (rIL-1) and inhibited by anti-LFA1 antibody. The Leu-11+ lymphocyte subset, known to be enriched for natural killer (NK) cells, bound preferentially. Only the EC-adherent lymphocyte fraction contained NK effectors, which lysed EC and classical NK targets. Thus, NK cells adhere to microvascular EC via a specific receptor-ligand interaction. The possibility exists that such binding occurs in recipients of vascularized allografts, representing the initial stage of graft rejection.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cavender D. E., Haskard D. O., Joseph B., Ziff M. Interleukin 1 increases the binding of human B and T lymphocytes to endothelial cell monolayers. J Immunol. 1986 Jan;136(1):203–207. [PubMed] [Google Scholar]
- Chin Y. H., Carey G. D., Woodruff J. J. Lymphocyte recognition of lymph node high endothelium. IV. Cell surface structures mediating entry into lymph nodes. J Immunol. 1982 Nov;129(5):1911–1915. [PubMed] [Google Scholar]
- Collins T., Krensky A. M., Clayberger C., Fiers W., Gimbrone M. A., Jr, Burakoff S. J., Pober J. S. Human cytolytic T lymphocyte interactions with vascular endothelium and fibroblasts: role of effector and target cell molecules. J Immunol. 1984 Oct;133(4):1878–1884. [PubMed] [Google Scholar]
- Daar A. S., Fuggle S. V., Fabre J. W., Ting A., Morris P. J. The detailed distribution of HLA-A, B, C antigens in normal human organs. Transplantation. 1984 Sep;38(3):287–292. doi: 10.1097/00007890-198409000-00018. [DOI] [PubMed] [Google Scholar]
- Daar A. S., Fuggle S. V., Fabre J. W., Ting A., Morris P. J. The detailed distribution of MHC Class II antigens in normal human organs. Transplantation. 1984 Sep;38(3):293–298. doi: 10.1097/00007890-198409000-00019. [DOI] [PubMed] [Google Scholar]
- Davignon D., Martz E., Reynolds T., Kürzinger K., Springer T. A. Monoclonal antibody to a novel lymphocyte function-associated antigen (LFA-1): mechanism of blockade of T lymphocyte-mediated killing and effects on other T and B lymphocyte functions. J Immunol. 1981 Aug;127(2):590–595. [PubMed] [Google Scholar]
- Davison P. M., Bensch K., Karasek M. A. Isolation and growth of endothelial cells from the microvessels of the newborn human foreskin in cell culture. J Invest Dermatol. 1980 Oct;75(4):316–321. doi: 10.1111/1523-1747.ep12530941. [DOI] [PubMed] [Google Scholar]
- Djeu J. Y., Stocks N., Zoon K., Stanton G. J., Timonen T., Herberman R. B. Positive self regulation of cytotoxicity in human natural killer cells by production of interferon upon exposure to influenza and herpes viruses. J Exp Med. 1982 Oct 1;156(4):1222–1234. doi: 10.1084/jem.156.4.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak H. F., Mihm M. C., Jr, Dvorak A. M., Barnes B. A., Manseau E. J., Galli S. J. Rejection of first-set skin allografts in man. the microvasculature is the critical target of the immune response. J Exp Med. 1979 Aug 1;150(2):322–337. doi: 10.1084/jem.150.2.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes R. D., Guttmann R. D., Gomersall M., Hibberd J. A controlled serial ultrastructural tracer study of first-set cardiac allograft rejection in the rat. Evidence that the microvascular endothelium is the primary target of graft destruction. Am J Pathol. 1983 May;111(2):184–196. [PMC free article] [PubMed] [Google Scholar]
- Gallatin W. M., Weissman I. L., Butcher E. C. A cell-surface molecule involved in organ-specific homing of lymphocytes. Nature. 1983 Jul 7;304(5921):30–34. doi: 10.1038/304030a0. [DOI] [PubMed] [Google Scholar]
- Goding J. W. Use of staphylococcal protein A as an immunological reagent. J Immunol Methods. 1978;20:241–253. doi: 10.1016/0022-1759(78)90259-4. [DOI] [PubMed] [Google Scholar]
- Haskard D., Cavender D., Beatty P., Springer T., Ziff M. T lymphocyte adhesion to endothelial cells: mechanisms demonstrated by anti-LFA-1 monoclonal antibodies. J Immunol. 1986 Nov 1;137(9):2901–2906. [PubMed] [Google Scholar]
- Kohl S., Springer T. A., Schmalstieg F. C., Loo L. S., Anderson D. C. Defective natural killer cytotoxicity and polymorphonuclear leukocyte antibody-dependent cellular cytotoxicity in patients with LFA-1/OKM-1 deficiency. J Immunol. 1984 Dec;133(6):2972–2978. [PubMed] [Google Scholar]
- Krensky A. M., Robbins E., Springer T. A., Burakoff S. J. LFA-1, LFA-2, and LFA-3 antigens are involved in CTL-target conjugation. J Immunol. 1984 May;132(5):2180–2182. [PubMed] [Google Scholar]
- Lanier L. L., Benike C. J., Phillips J. H., Engleman E. G. Recombinant interleukin 2 enhanced natural killer cell-mediated cytotoxicity in human lymphocyte subpopulations expressing the Leu 7 and Leu 11 antigens. J Immunol. 1985 Feb;134(2):794–801. [PubMed] [Google Scholar]
- Lanier L. L., Engleman E. G., Gatenby P., Babcock G. F., Warner N. L., Herzenberg L. A. Correlation of functional properties of human lymphoid cell subsets and surface marker phenotypes using multiparameter analysis and flow cytometry. Immunol Rev. 1983;74:143–160. doi: 10.1111/j.1600-065x.1983.tb01088.x. [DOI] [PubMed] [Google Scholar]
- Lanier L. L., Le A. M., Phillips J. H., Warner N. L., Babcock G. F. Subpopulations of human natural killer cells defined by expression of the Leu-7 (HNK-1) and Leu-11 (NK-15) antigens. J Immunol. 1983 Oct;131(4):1789–1796. [PubMed] [Google Scholar]
- Libby P., Ordovas J. M., Auger K. R., Robbins A. H., Birinyi L. K., Dinarello C. A. Endotoxin and tumor necrosis factor induce interleukin-1 gene expression in adult human vascular endothelial cells. Am J Pathol. 1986 Aug;124(2):179–185. [PMC free article] [PubMed] [Google Scholar]
- Lifson J., Raubitschek A., Benike C., Koths K., Ammann A., Sondel P., Engleman E. Purified interleukin-2 induces proliferation of fresh human lymphocytes in the absence of exogenous stimuli. J Biol Response Mod. 1986 Feb;5(1):61–72. [PubMed] [Google Scholar]
- Loveland B. E., Hogarth P. M., Ceredig R., McKenzie I. F. Cells mediating graft rejection in the mouse. I. Lyt-1 cells mediate skin graft rejection. J Exp Med. 1981 May 1;153(5):1044–1057. doi: 10.1084/jem.153.5.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loveland B. E., McKenzie I. F. Which T cells cause graft rejection? Transplantation. 1982 Mar;33(3):217–221. doi: 10.1097/00007890-198203000-00001. [DOI] [PubMed] [Google Scholar]
- Masuyama J., Minato N., Kano S. Mechanisms of lymphocyte adhesion to human vascular endothelial cells in culture. T lymphocyte adhesion to endothelial cells through endothelial HLA-DR antigens induced by gamma interferon. J Clin Invest. 1986 May;77(5):1596–1605. doi: 10.1172/JCI112475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miossec P., Cavender D., Ziff M. Production of interleukin 1 by human endothelial cells. J Immunol. 1986 Apr 1;136(7):2486–2491. [PubMed] [Google Scholar]
- Nemlander A., Saksela E., Häyry P. Are "natural killer" cells involved in allograft rejection? Eur J Immunol. 1983 Apr;13(4):348–350. doi: 10.1002/eji.1830130415. [DOI] [PubMed] [Google Scholar]
- Perussia B., Trinchieri G., Jackson A., Warner N. L., Faust J., Rumpold H., Kraft D., Lanier L. L. The Fc receptor for IgG on human natural killer cells: phenotypic, functional, and comparative studies with monoclonal antibodies. J Immunol. 1984 Jul;133(1):180–189. [PubMed] [Google Scholar]
- Phillips J. H., Babcock G. F. NKP-15: a monoclonal antibody reactive against purified human natural killer cells and granulocytes. Immunol Lett. 1983 Mar;6(3):143–149. doi: 10.1016/0165-2478(83)90096-2. [DOI] [PubMed] [Google Scholar]
- Pohlman T. H., Stanness K. A., Beatty P. G., Ochs H. D., Harlan J. M. An endothelial cell surface factor(s) induced in vitro by lipopolysaccharide, interleukin 1, and tumor necrosis factor-alpha increases neutrophil adherence by a CDw18-dependent mechanism. J Immunol. 1986 Jun 15;136(12):4548–4553. [PubMed] [Google Scholar]
- Sanchez-Madrid F., Krensky A. M., Ware C. F., Robbins E., Strominger J. L., Burakoff S. J., Springer T. A. Three distinct antigens associated with human T-lymphocyte-mediated cytolysis: LFA-1, LFA-2, and LFA-3. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7489–7493. doi: 10.1073/pnas.79.23.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scala G., Allavena P., Djeu J. Y., Kasahara T., Ortaldo J. R., Herberman R. B., Oppenheim J. J. Human large granular lymphocytes are potent producers of interleukin-1. Nature. 1984 May 3;309(5963):56–59. doi: 10.1038/309056a0. [DOI] [PubMed] [Google Scholar]
- Schmidt R. E., Bartley G., Levine H., Schlossman S. F., Ritz J. Functional characterization of LFA-1 antigens in the interaction of human NK clones and target cells. J Immunol. 1985 Aug;135(2):1020–1025. [PubMed] [Google Scholar]
- Shaw S., Luce G. E., Quinones R., Gress R. E., Springer T. A., Sanders M. E. Two antigen-independent adhesion pathways used by human cytotoxic T-cell clones. Nature. 1986 Sep 18;323(6085):262–264. doi: 10.1038/323262a0. [DOI] [PubMed] [Google Scholar]
- Spits H., van Schooten W., Keizer H., van Seventer G., van de Rijn M., Terhorst C., de Vries J. E. Alloantigen recognition is preceded by nonspecific adhesion of cytotoxic T cells and target cells. Science. 1986 Apr 18;232(4748):403–405. doi: 10.1126/science.3485822. [DOI] [PubMed] [Google Scholar]
- Stern D. M., Bank I., Nawroth P. P., Cassimeris J., Kisiel W., Fenton J. W., 2nd, Dinarello C., Chess L., Jaffe E. A. Self-regulation of procoagulant events on the endothelial cell surface. J Exp Med. 1985 Oct 1;162(4):1223–1235. doi: 10.1084/jem.162.4.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timonen T., Ortaldo J. R., Herberman R. B. Analysis by a single cell cytotoxicity assay of natural killer (NK) cells frequencies among human large granular lymphocytes and of the effects of interferon on their activity. J Immunol. 1982 Jun;128(6):2514–2521. [PubMed] [Google Scholar]
- Timonen T., Saksela E. Isolation of human NK cells by density gradient centrifugation. J Immunol Methods. 1980;36(3-4):285–291. doi: 10.1016/0022-1759(80)90133-7. [DOI] [PubMed] [Google Scholar]
- Wagner C. R., Vetto R. M., Burger D. R. Expression of I-region-associated antigen (Ia) and interleukin 1 by subcultured human endothelial cells. Cell Immunol. 1985 Jun;93(1):91–104. doi: 10.1016/0008-8749(85)90391-0. [DOI] [PubMed] [Google Scholar]
- Weiner M. S., Bianco C., Nussenzweig V. Enhanced binding of neuraminidase-treated sheep erythrocytes to human T lymphocytes. Blood. 1973 Dec;42(6):939–946. [PubMed] [Google Scholar]
- Wysocki L. J., Sato V. L. "Panning" for lymphocytes: a method for cell selection. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2844–2848. doi: 10.1073/pnas.75.6.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yannelli J. R., Sullivan J. A., Mandell G. L., Engelhard V. H. Reorientation and fusion of cytotoxic T lymphocyte granules after interaction with target cells as determined by high resolution cinemicrography. J Immunol. 1986 Jan;136(2):377–382. [PubMed] [Google Scholar]
- Yu C. L., Haskard D. O., Cavender D., Johnson A. R., Ziff M. Human gamma interferon increases the binding of T lymphocytes to endothelial cells. Clin Exp Immunol. 1985 Dec;62(3):554–560. [PMC free article] [PubMed] [Google Scholar]






