Abstract
We tested 114 faecal samples from wild simian immunodeficiency virus (SIV)-positive (n=43) and SIV-negative (n=71) chimpanzees (Pan troglodytes troglodytes) in southeast Cameroon for the presence of gastrointestinal parasites by direct smear. We observed cysts from different protozoa (Entamoeba coli and Entamoeba histolytica/Entamoeba dispar, Endolimax nana, Iodamoeba butschlii, Chilomastix mesnili, Balantidium coli and Blastocystis cells) and trophozoites from Troglodytella abrassarti and Balantidium coli. Eggs from different helminths (strongylids, Ascaris lumbricoides, Abbreviata caucasica, Trichuris sp., Capillaria sp., Enterobius anthropopeci, Bertiella sp., Hymenolepis diminuta and an undetermined fluke) were also observed. Finally, we observed eggs that could not be properly identified and classified. We did not observe any differences between the SIV+ and SIV− samples except for the unidentified eggs. The studied chimpanzees were highly parasitised by strongylid (85.1 % of prevalence), Troglodytella (43.8 %) and Blastocystis (2.9 %), and the frequency of the other parasites ranged from 0.9 to 8.8 %. These high levels of parasite infections could represent an additional burden in a population where there is a high rate of the SIV virus in circulation.
Keywords: Chimpanzees, Parasites, Coprology, SIV, Helminths, Protozoa
Introduction
Chimpanzees (Pan troglodytes) are classified as a highly endangered species (http://www.iucnredlist.org/details/15936/0) due to expanding human activities that have direct (through poaching and bush meat hunting) or indirect (through logging, mining and human-induced habitat fragmentation) detrimental effects on their survival. In addition, infectious diseases, such as Ebola haemorrhagic fever, paramyxovirosis, anthrax and pneumonic streptococcosis, have additional impacts on the already fragile populations (Formenty et al. 1999; Leendertz et al. 2006; Chi et al. 2007; Kaur et al. 2008). Moreover, chimpanzees are also widely infected with the simian immunodeficiency virus (SIVcpz), the ancestor of HIV-1, the prevalence of which can reach up to 40 % in certain populations. Until recently, it was commonly accepted that SIVs were not pathogenic for their primate hosts (Hirsch 2004; Pandrea et al. 2008; Silvestri et al. 2007; Pandrea and Apetrei 2010). However, several studies have indicated that SIVcpz infection can lead to a severe immunodepression and ultimately to an AIDS-like syndrome, challenging this theory of non-pathogenicity. Indeed, CD4 T-cell depletion was retrospectively observed in necroscopy samples from infected chimpanzees; additionally, one of the tested chimpanzees showed skeletal muscle and hepatocellular atrophy and abdominal lesions due to a massive nematode infestation (Keele et al. 2009; Terio et al. 2011). A decrease in CD4+ T cells, which is associated with various protozoan, helminthic and fungal infections, was also reported for a naturally infected chimpanzee in a sanctuary in Cameroon (Etienne et al. 2011). These studies show that chimpanzees may be much more affected by SIV infections than previously thought. Thus, immunodepressed chimpanzees may be more susceptible to other pathogens, including gastrointestinal parasites. Indeed, several parasites that have been described in chimpanzees, including Balantidium coli (Kim et al. 1978; Nakauchi 1999), Isospora belli (Rijpstra 1967), Cryptosporidium sp. (Mbaya and Udendeye 2011; Locatelli et al. 2012; Gonzalez-Moreno et al. 2013), Microsporidia (Sak et al. 2011; Locatelli et al. 2012) and Blastocystis hominis (Petrášová et al. 2010; Mbaya and Udendeye 2011), are known to be pathogenic in immunosuppressed HIV-infected humans (Karp and Auwaerter 2007). Here, we performed a pilot study on a highly SIV-infected chimpanzee population in southeast Cameroon to study the extent to which they are infected with gastrointestinal parasites.
Material and methods
Study site and samples collection
A total of 220 faecal samples were collected between 2003 and 2011, as previously described, around the village of Mambele in southeast Cameroon, an area where chimpanzees are known to be highly infected with SIVcpz (Keele et al. 2006; Van Heuverswyn et al. 2007) (Fig. 1). Briefly, stool samples were collected on tracks or in the vicinity of night nests. The GPS position was recorded, and the species was inferred in the field according to shape, size and texture of the faecal samples as well as presence of footprints or nearby nests. For each sample, approximately 20 g of stool was collected in a 50-ml tube containing 20 ml of RNAlater® (Ambion, Austin, TX) The samples were kept at ambient temperature at the base camp for a maximum of 3 weeks. These samples were then shipped to a laboratory in Montpellier and stored at −80 °C.
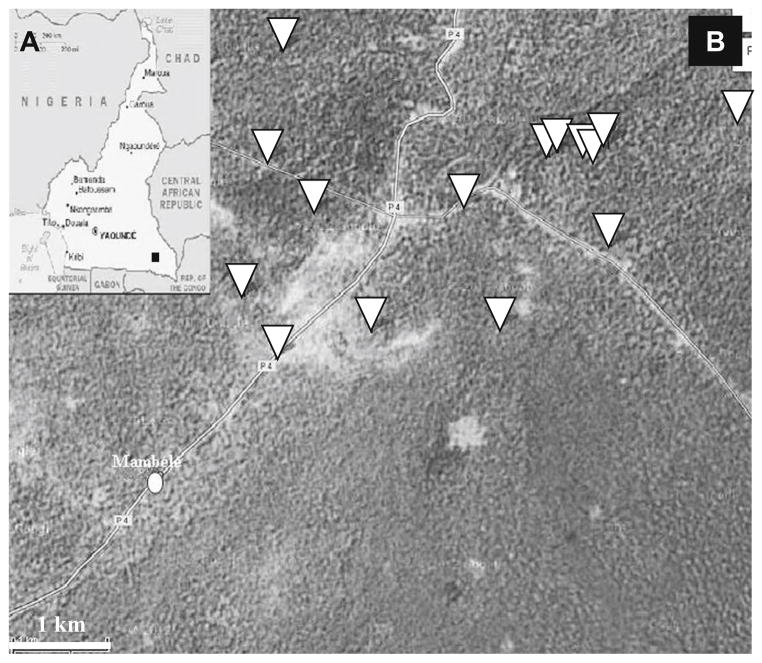
Fig. 1.
Sample collection points. a Cameroon map. Sample collection area is indicated by square. b Mambele area view. GPS collection points are indicated by inverted triangle. Mambele village centre is indicated by circle. The distance bar indicates 1 km
DNA extraction from faecal samples and species identification
The host species was confirmed by mtDNA analyses, as described previously (van der Kuyl et al. 1995; Keele et al. 2006; Van Heuverswyn et al. 2006). Briefly, a QIAamp stool DNA MiniPrep Kit (Qiagen, Valencia, CA) was used to extract the faecal DNA. Two millilitres of the faecal sample was used to obtain a final elution volume of 100 μl of faecal DNA. A ~450- to 500-bp fragment spanning the hyper variable D-loop region was amplified using the primers L15997 and H16498, and/or a 386-bp fragment spanning the 12S gene was amplified using the primers 12S-L1091 and 12S-H1478. The sequences obtained using a 3130xl Genetic Analyzer (Applied Biosystems, France) were aligned using the SeqMan DNAStar (Lasergene, Madison, USA) software. The species from which the samples were obtained was confirmed by performing neighbour-joining analysis using the CLUSTAL X 2.0 program (Thompson et al. 1997).
Detection of HIV cross-reactive antibodies in faecal samples
IgGs were recovered after dialyses of the faecal samples using methods previously adopted for antibody detection in faecal samples of gorillas and chimpanzees (Keele et al. 2006; Van Heuverswyn et al. 2006). Briefly, RNAlater®-precipitated immunoglobulins were resolubilised by diluting the faecal/RNAlater® mixtures (1.5 ml) in PBS–Tween 20 (7.5 ml) followed by inactivation of this solution for 1 h at 60 °C and centrifugation (3,500g for 10 min) to eliminate the presence of salt contained in the RNAlater® medium. The samples were then dialysed against PBS overnight at 4 °C. The reconstituted extracts were tested for HIV cross-reactive antibodies using the INNO-LIA HIV confirmation test (Innogenetics, Ghent, Belgium) according to the manufacturer’s instructions. Samples were scored as INNO-LIA positive when they showed cross-reactivity with at least one HIV antigen.
Coprology
Once the species identification of the host was confirmed, we assessed the parasite content of the samples by using the method described in Drakulovski et al. 2013. For each sample, 1 ml of 50:50 stool/RNAlater® mix was thawed at room temperature. Each sample was then vortexed briefly. Then, 100 μl was mixed with 100 μl of physiological serum. Next, 50 μl of the sample–physiological serum mix were smeared on a slide and observed directly under a microscope (Olympus BX41). A second slide was prepared using Para-selles KOP Color II (Fumouze diagnostics, France) dye (10 μl of dye for 50 μl of stool/physiological serum mix). When the slides results were both negative or when a significant discrepancy was found, either in type or in number of parasitic elements, between coloured and not coloured slides, two additional smears were performed. The species, number and the shape of the parasitic elements, as well as their ability to be dyed, were recorded. The parasitic elements were photographed using a Nikon Coolpix 5400 digital camera.
Results
Species confirmation and SIV infection rates
Among the 220 faecal samples collected between June 2003 and February 2011 and identified in the field as potentially originating from chimpanzees, mitochondrial DNA and phylogenetic analyses confirmed that 114/220 samples were indeed from the Pan troglodytes troglodytes subspecies. The remaining samples were either from other non-human primate species (11 Cercocebus agilis, 9 Cercopithecus cephus, 4 Cercopithecus nictitans, 4 Lophocebus albigena) of human origin (3 samples) or could not be identified, most likely because of DNA degradation.
A total of 43 (37.7 %) of the 114 chimpanzee samples cross-reacted with one or more HIV-1 antigens in the INNO-LIA HIV confirmation test.
Gastrointestinal parasitic content
Precise species identification of some parasite elements, especially strongylid eggs and amoeba cysts, was difficult because of the hyperosmotic nature of the RNAlater® medium and the low temperature (−80 °C) the samples were preserved in (Drakulovski et al. 2013). Therefore, all the strongylid eggs were regrouped under that same label. For Entamoeba sp. amoeba, some cysts were identifiable as either Entamoeba coli or Entamoeba histolytica/Entamoeba dispar (Fig. 2), but in most cases, the internal features that are used for identification were too degraded to determine the species. Therefore, we decided to regroup all such cysts under the label Entamoeba sp. The same limiting factor made the detection and identification of smaller amoebas (Endolimax, Iodamoeba, Chilomastix) difficult. As a consequence, their prevalence was most likely underestimated.
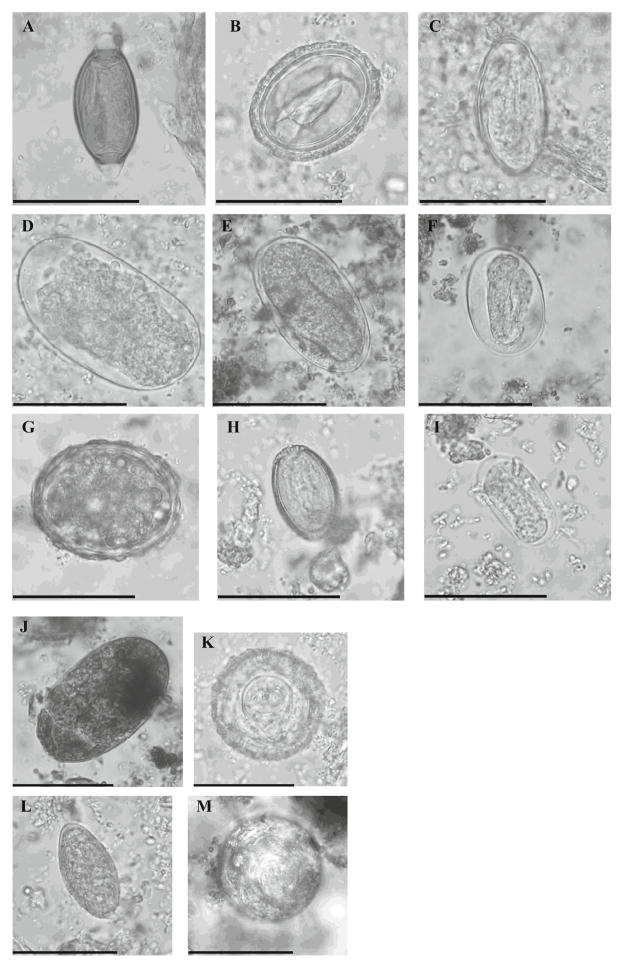
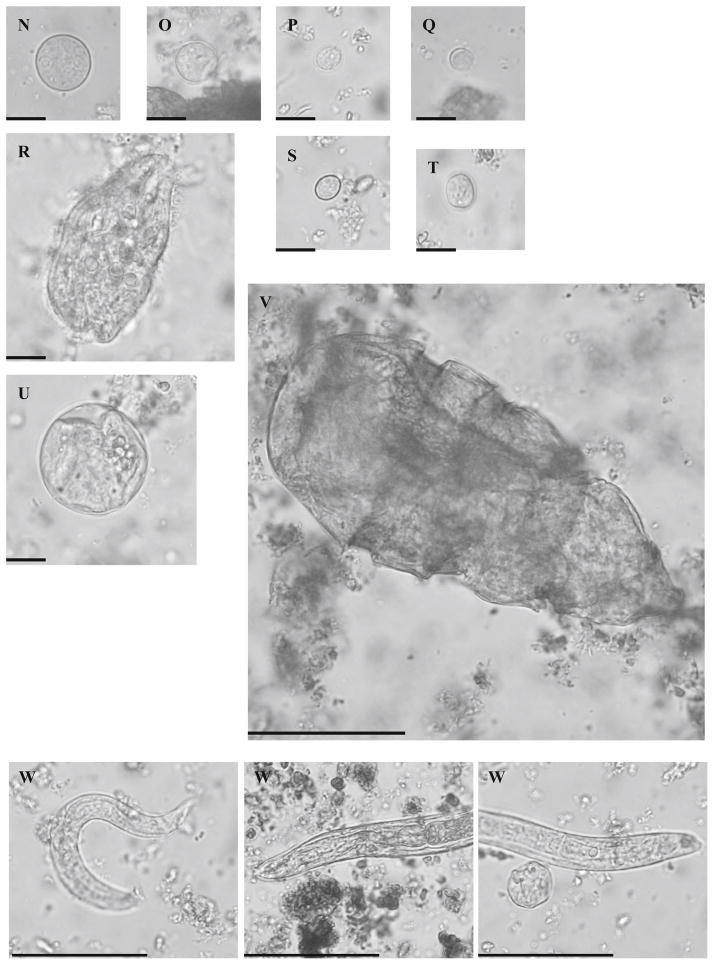
Fig. 2.
Parasite elements observed by direct microscopy in stool samples collected from wild chimpanzees between 2003 and 2011 in the Mambele area of Cameroon. a Trichuris sp. egg. b Abbreviata caucasica egg. c Enterobius anthropopeci egg. d Large strongylid egg (>50 μm in length). e Large strongylid egg with formed lava (>50 μm in length). f Small strongylid egg with formed larva (<50 μm in length ). g Ascaris lumbricoides egg. h Capillaria sp. egg. i Small strongylid egg (<50 μm in length) with formed larva and thin wall, probably Ankylostomid egg. j Undetermined fluke egg. k Bertiella sp. egg. l Unidentified egg. m Hymenolepis diminuta egg. n Entamoeba coli cyst. o Entamoeba histolytica/Entamoeba dispar cyst. p Endolimax nana cyst. q Blastocystis cell. r Balantidium coli trophozoite. s Chilomastix mesnili cyst. t Iodamoeba butschilii cyst. u Balantidium coli cyst. v Troglodytella abrassarti trophozoite. w Various nematode larvae. Size bars indicate respectively 50 μm for elements a to m and v to w and 10 μm for elements n to u
We found eggs from different helminth species at the following rates of infection: strongylid eggs of different sizes, possibly belonging to different species, in 87/114 samples (85.1 %); eggs of Ascaris lumbricoides (1/114, 0.9 %), Abbreviata caucasica (1/114, 0.9 %), Trichuris sp. (4/114, 4.5 %), Capillaria sp. (2/114, 1.8 %), Enterobius anthropopeci (3/114, 2.7 %), Bertiella sp. (3/114, 2.7 %), Hymenolepis diminuta (1/114, 0.9 %), and an unidentified fluke (2/114, 1,8 %); and different larvae. Regarding the presence of protozoa, we found cysts of Entamoeba sp. (10/114, 8.8 %), Endolimax nana (5/114, 4.4 %), Iodamoeba, butschlii (2/114, 1.8 %), Chilomastix mesnili (6/114, 5.2 %) and Balantidium coli (1/114, 0.9 %). We also observed Troglodytella abrassarti (50/114, 43.8 %), Balantidium coli trophozoites and Blastocystis hominis cells (25/114, 21.9 %) (Table 1 and Fig. 2).
Table 1.
Prevalence of various parasite elements found in chimpanzee stool sample set collected from 2003 to 2011 in the Mambele area of Cameroon
| Parasite elements (eggs or cysts) | Number of SIV+ samples positive for parasite elements (/43) | Prevalence among SIV+ samples | Number of SIV− positive for parasite elements (/71) | Prevalence among SIV− samples | Overall prevalence (SIV++ SIV− samples) |
|---|---|---|---|---|---|
| Strongylidae | 35 | 81.4 % | 62 | 87.3 % | 85.1 % |
| Ascaris | 1 | 2.3 % | 0 | 0 % | 0.9 % |
| Abbreviata | 0 | 0 % | 1 | 1.4 % | 0.9 % |
| Trichuris | 1 | 2.3 % | 3 | 4.2 % | 4.5 % |
| Capillaria | 2 | 4.6 % | 0 | 0 % | 1.8 % |
| Enterobius | 0 | 0 % | 3 | 4.2 % | 2.7 % |
| Bertiella | 2 | 4.6 % | 1 | 1.4 % | 2.7 % |
| Hymenolepis | 0 | 0 % | 1 | 1.4 % | 0.9 % |
| Fluke | 1 | 2.3 % | 1 | 1.4 % | 1.8 % |
| Unindentified egg | 8 | 18.6 %* | 4 | 5.6 %* | 10.5 % |
| Entamoeba | 3 | 6.9 % | 7 | 9.9 % | 8.8 % |
| Endolimax | 1 | 2.3 % | 4 | 5.6 % | 4.4 % |
| Iodamoeba | 1 | 2.3 % | 1 | 1.4 % | 1.8 % |
| Chilomastix | 2 | 4.6 % | 4 | 5.6 % | 5.2 % |
| Giardia | 0 | 0 % | 0 | 0 % | 0 % |
| Balantidium | 0 | 0 % | 1 | 1.4 % | 0.9 % |
| Troglodytella | 19 | 44.2 % | 31 | 43.6 % | 43.8 % |
| Blastocystis | 8 | 18.6 % | 17 | 23.4 % | 21.9 % |
Prevalence of the various helminths (A) or protozoa (B) elements was calculated for SIV+, SIV− and total chimpanzee samples. High prevalence (>20 %) is indicated in bold. Significant difference ((|ε|>1.96)) between SIV+ and SIV− groups is indicated by asterisk
In addition, unknown elements were observed in ten samples. They were characterised by an oval shape, 48–50 μm in length by 24–25 μm in width, with a thick wall (Fig. 2) and were stained with KOP Color II dye, as were other parasitological elements. Pollen database (PalDat, www.paldat.org) browsing did not link them to any identified pollen. The modified Ziehl stain method did not stain these samples as it would for coccidian oocysts. Thus, by default, these elements were classified as unknown helminth eggs. Their overall prevalence was 10.5 %, with several samples presenting a high number of “eggs” (2–3/microscope field at ×40).
The rates of parasite infection in SIV-positive versus SIV-negative chimpanzees were presented in Table 1. None of the rates were significantly different between SIV+ and SIV− groups except for the small unidentified eggs (|ε|=2.19>1.96).
Discussion
Helminth infections
In this study, we found that the wild chimpanzees in the southeast Cameroon population are infected with 17 different parasite elements: 10 different types of helminths and 7 different types of protozoa. Among the helminth eggs, we found strongylid eggs of different sizes: large eggs (60–80 μm in length), possibly belonging to Hyostrongylus, Libyostrongylus, Trichostrongylus, Nematodirus, Ternidens, Oesophagostomum and/or Gongylonema genera; and small eggs (40 to 60 μm of length), possibly belonging to Strongyloides and/or Ankylostoma genera (Rothman et al. 2006) (see Fig. 2). The rate of global strongylid infection was 85.1 %. This rate was significantly higher than the 10.9 to 41.3 % strongylid prevalence in wild Pan troglodytes schweinfurthii populations in Rubondo Island National Park, Tanzania (Petrášová et al. 2010; Petrzelková et al. 2010), 19.53 % prevalence in wild Pan troglodytes verus population in Fongoli, Senegal, (Howells et al. 2011) and 13.04 % prevalence found in captive Pan troglodytes elliotii from Calabar, Nigeria (Mbaya and Udendeye 2011). However, this rate of infection was similar to what was found in Pan troglodytes schweinfurthii populations of Gombe National Park, Tanzania (Gillespie et al. 2010), and Kibale National Park, Uganda (Krief et al. 2005; Muehlenbein 2005), with a strongylid prevalence ranging from 74.32 up to 100 %. The rate of infection found was also relatively higher than in free-ranging baboons and vervet monkeys in Ethiopia (Legesse and Erko 2004); forest baboons in Senegal (Howells et al. 2011); red-tailed, blue and L’Hoest’s monkeys in Uganda (Gillespie et al. 2004); blue monkeys in South Africa (Gillespie et al. 2004) and mountain gorillas in Uganda (Rothman et al. 2008). This high level of strongylid infection could represent a threat to the health of the chimpanzee population, as strongylids and Ankylostomatidae are known to be mildly to highly pathogenic in humans and are responsible for a wide range of symptoms, from mucosal ulcerations to abscesses, anaemia, weight loss and ultimately death in non-human primates (NHPs) (Guttierez 2000; Rothman et al. 2006; Krief et al. 2008; Terio et al. 2011).
Concerning the other helminth infections (Ascaris, Trichuris, Abbreviata, Capillaria, Enterobius, Bertiella, Fluke and Hymenolepis), the prevalence was low and ranged from 0.9 to 4.5 %, depending on the species, and was similar to or below the average rate of infection found in other chimpanzee communities in Tanzania, Nigeria, Uganda, and Central African Republic (Lilly et al. 2002; Krief et al. 2003; Gillespie et al. 2010; Howells et al. 2011; Petrášová et al. 2010; Petrzelková et al. 2010; Mbaya and Udendeye 2011).
Protozoan infections
In our samples set, we found up to seven different protozoan elements belonging to the Entamoeba, Endolimax, Iodamoeba, Chilomastix, Balantidium, Troglodytella and Blastocystis genera. The amoeba cyst prevalence was low (8.8 % for the Entamoeba sp. and ranging from 1.75 to 5.2 % for the smaller amoebas) and similar to what was detected in the chimpanzee communities in Fongoli, Senegal (Howells et al. 2011), Rubondo Island, Tanzania (Petrzelková et al. 2010), and Kibale National Park, Uganda (Muehlenbein 2005), and slightly lower than what was found in Gombe National Park, Tanzania (Gillespie et al. 2010), and Dzanga-Ndoki National Park, Central African Republic (Masi et al. 2012). The smaller amoebas (Endolimax, Iodamoeba, Chilomastix) do not represent a health hazard: They are commensal non-pathogenic parasites. However, even if the prevalence of the Entamoeba group (including the highly pathogenic Entamoeba histolytica) was low, its presence could have consequences, as amoebic dysentery outbreaks are potentially lethal in chimpanzees (Miller and Bray 1966).
In respect to ciliates detection, we found no Giardia intestinalis shedding in our sample set. Instead, we found high prevalence of Troglodytella abrassarti (prevalence 43.8 %), consistent with other studies of chimpanzees (Pomajbíková et al. 2010a). We also found one positive sample for Balantidium coli cysts and trophozoites. Balantidium coli is considered to be mildly pathogenic and is rarely detected in wild chimpanzees (Pomajbíková et al. 2010b). However, clinical balantidiasis has been reported in captive African great apes (Teare and Loomis 1982; Lee et al. 1990; Lankester et al. 2008). The possible clinical significance in a high rate SIV-infected population will be discussed below.
Finally, we also detected Blastocystis elements at relatively high prevalence (21.9 %). This prevalence was likely underestimated because Blastocystis are small elements easier to observe after concentration methods not applicable to our sample set. Indeed, a pilot study using molecular detection methods on the chimpanzee stool samples collected from the same area showed presence of Blastocystis in 87–88 % of the samples (Locatelli et al. 2012). Infection with Blastocystis has already been reported in the captive and free-roaming chimpanzee population of the Afi Mountain Conservation area, Calabar, Nigeria (Mbaya and Udendeye 2011), and in Rubondo Island, Tanzania (Petrášová et al. 2010; Petrášová et al. 2011), with prevalence ranging from 13.04 to 60.9 %. They were also detected in other NHP species including vervets and baboons in Ethiopia (Legesse and Erko 2004) as well as orangutans in Borneo, Indonesia (Labes et al. 2010). The infectious role of Blastocystis remains unclear so far, as asymptomatic shedding is highly common. However, Blatocystis presence is also associated with gastrointestinal symptoms in humans including diarrhoea and abdominal pain (Qadri et al. 1989). Blastocystis could also be responsible for chronic gastrointestinal illnesses, including irritable bowel syndrome (Boorom et al. 2008; Poirier et al. 2012). However, the clinical significance of Blastocystis infection for NHPs populations is as unclear as it is for the human population and, in certain cases, may be dependent on the subtype involved (Hussein et al. 2008; Parija and Jeremiah 2013).
Gastrointestinal parasites in SIV-positive versus SIV-negative chimpanzees
This is the first study that compares the gastrointestinal parasites of wild-living chimpanzees in respect to their SIV serological status. Concerning the helminths, there was no significant difference in the prevalence of strongylid, Ascaris, Abbreviata, Trichuris, Capillaria, Enterobius, Bertiella, fluke or Hymenolepis infections between the groups. However, there was a slightly significant difference (|ε|=2.19>1.96) in the prevalence of individuals shedding the unidentified helminth eggs. Helminths are poor lentivirus infection sentinels, as their rate of infection is not particularly influenced by HIV-induced immunodepression in humans (Wiwanitkit 2006; Karp and Auwaerter 2007). Thus, it is not clear why such a difference exists in this specific case, especially for a still unidentified helminth. We can only speculate that it is most likely more related to the feeding habits of the chimpanzee population and ecological/environmental factors that are more likely to influence a helminth infection (Gillespie et al. 2010) rather than their immunological status.
Regarding protozoan infection, there were no significant differences between SIV+ and SIV− samples sets for any of the detected amoeba (Entamoeba sp., Iodamoeba, Chilomastix or Endolimax), ciliates (Troglodytella or Balantidium) or heterokonts (Blastocystis).
Results for gastrointestinal amoebas were not unexpected, as these protozoa are not considered to be opportunistic, and their prevalence is not influenced by HIV serological status in humans (Wiwanitkit 2006; Karp and Auwaerter 2007). The ciliate Balantidium coli is not considered to be an opportunistic parasite in humans with AIDS either. However, this protozoon can be responsible for chronic, life-threatening diarrhoea in HIV+ patients (Clyti et al. 1998; Cermeño et al. 2003). As such, its presence in a SIV hotspot could be of concern for the health and survival of the apes’ population. The other ciliate detected in our sample set, Troglodytella abrassarti, is commonly found in great ape (gorillas, chimpanzees and bonobos) faeces (Pomajbíková et al. 2010a). This ciliate was previously considered to be pathogenic and associated with diarrhoeas (Mortelmans et al. 1971). However, more recent studies have suggested a symbiotic function and participation in nourishment degradation ((Pomajbíková et al. 2010a; Profousová et al. 2011). Indeed, occurrence and shedding intensity of Troglodytella trophozoites seem linked to the level of starch in the food intake in chimpanzees (Petrželková et al. 2012). Thus, this entodiniomorphid does not seem to be harmful in healthy apes. However, its health impact on SIV-infected chimpanzees is still to be determined, especially given that ape-to-ape transmission may occur (Modrý et al. 2009). Finally, concerning Blastocystis sp., there are contradictory observations concerning the Blastocystis sp. prevalence in HIV+ versus HIV− patients. In some studies, increased prevalence of these protozoa has been described in an HIV+ population (Storgaard et al. 1996), but other studies did not find this correlation (Morgan et al. 1996). Whether these observations could be extrapolated to chimpanzees, where the prevalence of Blastocystis is high (18.6 %) in a heavily SIV-infected population, should be a concern, especially in young animals because Blastocystis has been associated with chronic diarrhoeas in HIV+ children (Cegielski et al. 1993; Idris et al. 2010).
Inter-species (apes to humans and humans to apes) transmission risk
We performed our study on faecal samples collected from wild chimpanzees. Because these primates were not habituated to humans, contact with humans and inter-species transmission of pathogens should be minimal. The absence of Giardia intestinalis in our samples supports this theory. Indeed, Giardia intestinalis is the model example of a gastrointestinal parasite transmitted by faecal contamination of the environment (water and farming). The baseline prevalence of Giardia intestinalis contamination of free-living chimpanzees in undisturbed areas was set at 3 % (Gillespie et al. 2009). As we have prevalence of 0 % in our sample set (Table 1), it would be easy to conclude that interactions between humans and great apes in the area are below minimal. However, several facts are challenging this observation. First, three faecal samples collected on chimpanzee trails or near a nest were of human origin. Secondly, one sample was detected positive for Balantidium coli. While this protozoa is almost never detected in wild apes (Pomajbíková et al. 2010b), Balantidium coli is known to infect a wide range of hosts from pigs (wild or domesticated) to rats but also humans. Terrestrial or semi-arboreal NHPs are likely to enter in contact with contaminated soil or faeces where habitats overlap (Levecke et al. 2007; Schuster and Ramirez-Avila 2008). Gastrointestinal pathogen exchanges between humans and apes have been already documented in African regions (Goldberg et al. 2008; Rwego et al. 2008). Thus, this infected individual could be the sentinel case showing that interaction between humans or human livestock and wild chimpanzee occurs around Mambele, leading to a risk of contamination for the apes. Finally, farmers frequently report chimpanzees raiding crops around the village of Mambele. Indeed, GPS data showed that some stool samples were collected less than 1,500 to 1,000 m from the centre of the village (see Fig. 1). The proximity to human settlements can increase the chances of parasite contamination from chimpanzees to humans, especially for strongylid/Ankylostomid. Cross-species transmission of Strongyloides fülleborni has been described in Africa (Hira and Patel 1977; Hira and Patel 1980; Evans et al. 1991) and could be a concern with the vicinity of such a highly strongylid-infected chimpanzee population.
Conclusion
This is the first study assessing parasitic coprology in wild chimpanzees from southeast Cameroon and the first study that compares the frequency of parasites between SIV+ and SIV− chimpanzees. Because SIVcpz has a negative impact on the health and survival of chimpanzees, it is important to document the gastrointestinal pathogens present in this chimpanzee population. Studying this chimpanzee population showed that it is highly infected with gastrointestinal nematodes of the strongylid type (85.1 % of prevalence), Troglodytella (43.8 % of prevalence) and Blastocystis (21.9 % of prevalence) as well as several others helminths and protozoa. We did not find any significant difference in the prevalence between SIV+ and SIV− samples; however, this chimpanzee population is clearly infected with mildly to potentially highly pathogenic helminths and protozoa that could be lethal in HIV-infected humans and with pathogens responsible for deleterious symptoms in HIV+ children and possibly also for SIVcpz-infected chimpanzees.
Acknowledgments
This work was supported in part by grants from the National Institute of Health (RO1 AI 50529) and the Agence Nationale de Recherches sur le SIDA (ANRS 12255). We thank the staff and the SIV team from PRESICA for logistical support in Cameroon and the Cameroonian Ministries of Health, Environment and Forestry, and Research for permission to collect samples in Cameroon. We also thank Dr. L. Mondolot, Pr. Y. Pelissier and Dr. S. Bertout for their useful help with the identification of false positives. We thank Pr. D. Basset for his useful help with identification of several parasite elements.
Footnotes
Ethical standards The research conducted here complies with host country and institutional policies of ethical research on non-human primates.
Conflict of interest The authors declare no conflict of interest.
Contributor Information
Pascal Drakulovski, Email: pascal.drakulovski@univ-montp1.fr, UMI 233 “TransVIHMI” Institut de Recherche pour le Developpement (IRD) and University of Montpellier 1 (UM1), Montpellier, France. Laboratoire de Parasitologie et Mycologie Médicale, UFR de Pharmacie, 15 Avenue Charles Falhault, 34093 Montpellier, Cedex 05, France.
Sébastien Bertout, Virology Laboratory, CREMER/IMPM/IRD, Yaoundé, Cameroon.
Sabrina Locatelli, UMI 233 “TransVIHMI” Institut de Recherche pour le Developpement (IRD) and University of Montpellier 1 (UM1), Montpellier, France.
Christelle Butel, UMI 233 “TransVIHMI” Institut de Recherche pour le Developpement (IRD) and University of Montpellier 1 (UM1), Montpellier, France.
Sébastien Pion, UMI 233 “TransVIHMI” Institut de Recherche pour le Developpement (IRD) and University of Montpellier 1 (UM1), Montpellier, France.
Eitel Mpoudi-Ngole, Virology Laboratory, CREMER/IMPM/IRD, Yaoundé, Cameroon.
Eric Delaporte, UMI 233 “TransVIHMI” Institut de Recherche pour le Developpement (IRD) and University of Montpellier 1 (UM1), Montpellier, France.
Martine Peeters, UMI 233 “TransVIHMI” Institut de Recherche pour le Developpement (IRD) and University of Montpellier 1 (UM1), Montpellier, France.
Michèle Mallié, UMI 233 “TransVIHMI” Institut de Recherche pour le Developpement (IRD) and University of Montpellier 1 (UM1), Montpellier, France.
References
- Boorom KF, Smith H, Nimri L, Viscogliosi E, Spanakos G, Parkar U, Li LH, Zhou XN, Ok UZ, Leelayoova S, Jones MS. Oh my aching gut: irritable bowel syndrome, Blastocystis, and asymptomatic infection. Parasit Vectors. 2008;1:40. doi: 10.1186/1756-3305-1-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cegielski JP, Msengi AE, Dukes CS, Mbise R, Redding-Lallinger R, Minjas JN, Wilson ML, Shao J, Durack DT. Intestinal parasites and HIV infection in Tanzanian children with chronic diarrhea. AIDS. 1993;7:213–221. doi: 10.1097/00002030-199302000-00009. [DOI] [PubMed] [Google Scholar]
- Cermeño JR, Hernández De Cuesta I, Uzcátegui O, Páez J, Rivera M, Baliachi N. Balantidium coli in an HIV-infected patient with chronic diarrhoea. AIDS. 2003;17:941–942. doi: 10.1097/00002030-200304110-00030. [DOI] [PubMed] [Google Scholar]
- Chi F, Leider M, Leendertz F, Bergmann C, Boesch C, Schenk S, Pauli G, Ellerbrok H, Hakenbeck R. New Streptococcus pneumoniae clones in deceased wild chimpanzees. J Bacteriol. 2007;189:6085–6088. doi: 10.1128/JB.00468-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clyti E, Aznar C, Couppie P, el Guedj M, Carme B, Pradinaud R. A case of coinfection by Balantidium coli and HIV in French Guiana]. [Article in French. Bull Soc Pathol Exot. 1998;91:309–311. [PubMed] [Google Scholar]
- Drakulovski P, Locatelli S, Butel C, Pion S, Krasteva D, Mougdi-Pole E, Delaporte E, Peeters M, Mallié M. Use of RNAlater as a preservation method for parasitic coprology studies in wild-living chimpanzees. Exp Parasitol. 2013;135:257–261. doi: 10.1016/j.exppara.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne L, Nerrienet E, LeBreton M, Bibila GT, Foupouapouognigni Y, Rousset D, Nana A, Djoko CF, Tamoufe U, Aghokeng AF, Mpoudi-Ngole E, Delaporte E, Peeters M, Wolfe ND, Ayouba A. Characterization of a new simian immunodeficiency virus strain in a naturally infected Pan troglodytes troglodytes chimpanzee with AIDS related symptoms. Retrovirology. 2011;8:4. doi: 10.1186/1742-4690-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans AC, Markus MB, Joubert JJ, Gunders AE. Bushman children infected with the nematode Strongyloides fülleborni. S Afr Med J. 1991;80:410–411. [PubMed] [Google Scholar]
- Formenty P, Boesch C, Wyers M, Steiner C, Donati F, Dind F, Walker F, Le Guenno B. Ebola virus outbreak among wild chimpanzees living in a rain forest of Côte d’Ivoire. J Infect Dis. 1999;179:120–126. doi: 10.1086/514296. [DOI] [PubMed] [Google Scholar]
- Gillespie TR, Greiner EC, Chapman CA. Gastrointestinal parasites of the guenons of western Uganda. J Parasitol. 2004;90:1356–1360. doi: 10.1645/GE-311R. [DOI] [PubMed] [Google Scholar]
- Gillespie TR, Morgan D, Deutsch JC, Kuhlenschmidt MS, Salzer JS, Cameron K, Reed T, Sanz C. A legacy of low-impact logging does not elevate prevalence of potentially pathogenic protozoa in free-ranging gorillas and chimpanzees in the Republic of Congo: logging and parasitism in African apes. Ecohealth. 2009;6:557–564. doi: 10.1007/s10393-010-0283-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie TR, Lonsdorf EV, Canfield EP, Meyer DJ, Nadler Y, Raphael J, Pusey AE, Pond J, Pauley J, Mlengeya T, Travis DA. Demographic and ecological effects on patterns of parasitism in eastern chimpanzees (Pan troglodytes schweinfurthii) in Gombe National Park, Tanzania. Am J Phys Anthropol. 2010;143:534–544. doi: 10.1002/ajpa.21348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg TL, Gillespie TR, Rwego IB, Estoff EL, Chapman CA. Forest fragmentation as cause of bacterial transmission among non-human primates, humans, and livestock, Uganda. Emerg Infect Dis. 2008;14:1375–1382. doi: 10.3201/eid1409.071196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Moreno O, Hernandez-Aguilar RA, Piel AK, Stewart FA, Gracenea M, Moore J. Prevalence and climatic associated factors of Cryptosporidium sp. infections in savanna chimpanzees from Ugalla, Western Tanzania. Parasitol Res. 2013;1:393–399. doi: 10.1007/s00436-012-3147-8. [DOI] [PubMed] [Google Scholar]
- Guttierez Y. In Diagnostic pathology of parasitic infections with clinical correlations. Oxford University Press; 2000. pp. 287–342. [Google Scholar]
- Hira PR, Patel BG. Strongyloides fülleborni infections in man in Zambia. Am J Trop Med Hyg. 1977;4:640–643. doi: 10.4269/ajtmh.1977.26.640. [DOI] [PubMed] [Google Scholar]
- Hira PR, Patel BG. Human strongyloidiasis due to the primate species Strongyloides fülleborni. Trop Geogr Med. 1980;1:23–29. [PubMed] [Google Scholar]
- Hirsch VM. What can natural infection of African monkeys with simian immunodeficiency virus tell us about the pathogenesis of AIDS? AIDS Rev. 2004;1:40–53. [PubMed] [Google Scholar]
- Howells ME, Pruetz J, Gillespie TR. Patterns of gastro-intestinal parasites and commensals as an index of population and ecosystem health: the case of sympatric western chimpanzees (Pan troglodytes verus) and guinea baboons (Papio hamadryas papio) at Fongoli, Senegal. Am J Primatol. 2011;73:173–179. doi: 10.1002/ajp.20884. [DOI] [PubMed] [Google Scholar]
- Hussein EM, Hussein AM, Eida MM, Atwa MM. Pathophysiological variability of different genotypes of human Blastocystis hominis Egyptian isolates in experimentally infected rats. Parasitol Res. 2008;102:853–860. doi: 10.1007/s00436-007-0833-z. [DOI] [PubMed] [Google Scholar]
- Idris NS, Dwipoerwantoro PG, Kurniawan A, Said M. Intestinal parasitic infections of immunocompromised children with diarrhoea: clinical profile and therapeutic response. J Infect Dev Ctries. 2010;5:309–317. doi: 10.3855/jidc.275. [DOI] [PubMed] [Google Scholar]
- Karp CL, Auwaerter PG. Coinfection with HIV and tropical infectious diseases. I. Protozoal pathogens. Clin Infect Dis. 2007;45:1208–1213. doi: 10.1086/522181. [DOI] [PubMed] [Google Scholar]
- Kaur T, Singh J, Tong S, Humphrey C, Clevenger D, Tan W, Szekely B, Wang Y, Li Y, Alex Muse E, Kiyono M, Hanamura S, Inoue E, Nakamura M, Huffman MA, Jiang B, Nishida T. Descriptive epidemiology of fatal respiratory outbreaks and detection of a human-related metapneumovirus in wild chimpanzees (Pan troglodytes) at Mahale Mountains National Park, Western Tanzania. Am J Primatol. 2008;70:755–765. doi: 10.1002/ajp.20565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keele BF, Van Heuverswyn F, Li Y, Bailes E, Takehisa J, Santiago ML, Bibollet-Ruche F, Chen Y, Wain LV, Liegeois F, Loul S, Ngole EM, Bienvenue Y, Delaporte E, Brookfield JF, Sharp PM, Shaw GM, Peeters M, Hahn BH. Chimpanzee reservoirs of pandemic and nonpandemic HIV-1. Science. 2006;313:523–526. doi: 10.1126/science.1126531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keele BF, Jones JH, Terio KA, Estes JD, Rudicell RS, Wilson ML, Li Y, Learn GH, Beasley TM, Schumacher-Stankey J, Wroblewski E, Mosser A, Raphael J, Kamenya S, Lonsdorf EV, Travis DA, Mlengeya T, Kinsel MJ, Else JG, Silvestri G, Goodall J, Sharp PM, Shaw GM, Pusey AE, Hahn BH. Increased mortality and AIDS-like immunopathology in wild chimpanzees infected with SIVcpz. Nature. 2009;460:515–519. doi: 10.1038/nature08200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JC, Abee CR, Wolf RH. Balantidiosis in a chimpanzee (Pan troglodytes) Lab Anim. 1978;4:231–233. doi: 10.1258/002367778781088620. [DOI] [PubMed] [Google Scholar]
- Krief S, Bories C, Hladik CM. Results of fecal parasitologic examination performed on a population of wild chimpanzees (Pan troglodytes schweinfurthii) in Uganda. Bull Soc Pathol Exot. 2003;96:80–82. [Article in French] [PubMed] [Google Scholar]
- Krief S, Huffman MA, Sevenet T, Guillot J, Bories C, Hladik CM, Wrangham RW. Noninvasive monitoring of the health of Pan troglodytes schweinfurthii in the Kibale National Park, Uganda. Int J Primatol. 2005;26:467–490. [Google Scholar]
- Krief S, Jamart A, Mahé S, Leendertz FH, Mätz-Rensing K, Crespeau F, Bain O, Guillot J. Clinical and pathologic manifestation of oesophagostomosis in African great apes: does self-medication in wild apes influence disease progression? J Med Primatol. 2008;4:188–195. doi: 10.1111/j.1600-0684.2008.00285.x. [DOI] [PubMed] [Google Scholar]
- Labes EM, Hegglin D, Grimm F, Nurcahyo W, Harrison ME, Bastian ML, Deplazes P. Intestinal parasites of endangered orangutans (Pongo pygmaeus) in Central and East Kalimantan, Borneo, Indonesia. Parasitology. 2010;137:123–135. doi: 10.1017/S0031182009991120. [DOI] [PubMed] [Google Scholar]
- Lankester F, Mätz-Rensing K, Kiyang J, Jensen SA, Weiss S, Leendertz FH. Fatal ulcerative colitis in a western lowland gorilla (Gorilla gorilla gorilla) J Med Primatol. 2008;6:297–302. doi: 10.1111/j.1600-0684.2008.00287.x. [DOI] [PubMed] [Google Scholar]
- Lee RV, Prowten AW, Anthone S, Satchidanand SK, Fisher JE, Anthone R. Typhlitis due to Balantidium coli in captive lowland gorillas. Rev Infect Dis. 1990;12:1052–1059. doi: 10.1093/clinids/12.6.1052. [DOI] [PubMed] [Google Scholar]
- Leendertz FH, Lankester F, Guislain P, Néel C, Drori O, Dupain J, Speede S, Reed P, Wolfe N, Loul S, Mpoudi-Ngole E, Peeters M, Boesch C, Pauli G, Ellerbrok H, Leroy EM. Anthrax in Western and Central African great apes. Am J Primatol. 2006;68:928–933. doi: 10.1002/ajp.20298. [DOI] [PubMed] [Google Scholar]
- Legesse M, Erko B. Zoonotic intestinal parasites in Papio anubis (baboon) and Cercopithecus aethiops (vervet) from four localities in Ethiopia. Acta Trop. 2004;90:231–236. doi: 10.1016/j.actatropica.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Levecke B, Dorny P, Geurden T, Vercammen F, Vercruysse J. Gastrointestinal protozoa in non-human primates of four zoological gardens in Belgium. Vet Parasitol. 2007;148:236–246. doi: 10.1016/j.vetpar.2007.06.020. [DOI] [PubMed] [Google Scholar]
- Lilly AA, Mehlman PT, Doran D. Intestinal Parasites in Gorillas, Chimpanzees, and Humans at Mondika Research Site, Dzanga-Ndoki National Park, Central African Republic. Int J Primatol. 2002;23:555–573. [Google Scholar]
- Locatelli S, Butel C, Esteban A, Drakulovski P, Krasteva D, Mpoudi Ngole E, Mallié M, Delaporte E, Peeters M. Long-term monitoring of opportunistic diseases in SIV infected non-habituated apes from Cameroon: a pilot study. Communication. XXIV Congress of the international Primatological Society; August 12–17; Mexico. 2012. [Google Scholar]
- Masi S, Chauffour S, Bain O, Todd A, Guillot J, Krief S. Seasonal effects on great ape health: a case study of wild chimpanzees and western gorillas. PLoS ONE. 2012;7(12):e49805. doi: 10.1371/journal.pone.0049805. Epub 2012 Dec 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbaya AW, Udendeye UJ. Gastrointestinal parasites of captive and free-roaming primates at the Afi Mountain Primate Conservation Area in Calabar, Nigeria and their zoonotic implications. Pak J Biol Sci. 2011;14:709–714. doi: 10.3923/pjbs.2011.709.714. [DOI] [PubMed] [Google Scholar]
- Miller MJ, Bray RS. Entamoeba histolytica infections in the Chimpanzee (Pan satyricus) J Parasitol. 1966;52:386–388. [Google Scholar]
- Modrý D, Petrzelková KJ, Pomajbíková K, Tokiwa T, Krízek J, Imai S, Vallo P, Profousová I, Slapeta J. The occurrence and ape-to-ape transmission of the entodiniomorphid ciliate Troglodytella abrassarti in captive gorillas. J Eukaryot Microbiol. 2009;1:83–87. doi: 10.1111/j.1550-7408.2008.00369.x. [DOI] [PubMed] [Google Scholar]
- Morgan D, Whitworth J, Eotu H, Omoding N, Moore M. Gastrointestinal parasite infections. Lancet. 1996;348:965–966. doi: 10.1016/S0140-6736(05)65384-6. [DOI] [PubMed] [Google Scholar]
- Mortelmans J, Vercruysse J, Kageruka P. Three pathogenic intestinal protozoa of anthropoid apes:Entamoeba histolytica, Balantidium coli and Troglodytella abrassarti. Proceeding of the Third International Congress of Primatology; Zürich. 1971. pp. 187–191. [Google Scholar]
- Muehlenbein MP. Parasitological analyses of the male chimpanzees (Pan troglodytes schweinfurthii) at Ngogo, Kibale National Park, Uganda. Am J Primatol. 2005;65:167–179. doi: 10.1002/ajp.20106. [DOI] [PubMed] [Google Scholar]
- Nakauchi K. The prevalence of Balantidium coli infection in fifty-six mammalian species. J Vet Med Sci. 1999;61:63–65. doi: 10.1292/jvms.61.63. [DOI] [PubMed] [Google Scholar]
- Pandrea I, Apetrei C. Where the wild things are: pathogenesis of SIV infection in African nonhuman primate hosts. Curr HIV AIDS Rep. 2010;7:28–36. doi: 10.1007/s11904-009-0034-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandrea I, Sodora DL, Silvestri G, Apetrei C. Into the wild: simian immunodeficiency virus (SIV) infection in natural hosts. Trends Immunol. 2008;29:419–428. doi: 10.1016/j.it.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parija SC, Jeremiah S. Blastocystis: taxonomy, biology and virulence. Trop Parasitol. 2013;3:17–25. doi: 10.4103/2229-5070.113894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrášová J, Modrý D, Huffman MA, Mapua MI, Bobáková L, Mazoch V, Singh J, Kaur T, Petrželková KJ. Gastrointestinal parasites of indigenous and introduced primate species of Rubondo Island National Park, Tanzania. Int J Primatol. 2010;31:920–936. [Google Scholar]
- Petrášová J, Uzlíková M, Kostka M, Petrželková KJ, Huffman MA, Modrý D. Diversity and host specificity of Blastocystis in syntopic primates on Rubondo Island, Tanzania. Int J Parasitol. 2011;41:1113–1120. doi: 10.1016/j.ijpara.2011.06.010. [DOI] [PubMed] [Google Scholar]
- Petrzelková KJ, Hasegawa H, Appleton CC, Huffman MA, Archer CE, Moscovice LR, Mapua MI, Singh J, Kaur T. Gastrointestinal parasites of the chimpanzee population introduced onto Rubondo Island National Park, Tanzania. Am J Primatol. 2010;72:307–316. doi: 10.1002/ajp.20783. [DOI] [PubMed] [Google Scholar]
- Petrželková KJ, Schovancová K, Profousová I, Kišidayová S, Váradyová Z, Pekár S, Kamler J, Modrý D. The effect of low- and high-fiber diets on the population of entodiniomorphid ciliates Troglodytella abrassarti in captive chimpanzees (Pan troglodytes) Am J Primatol. 2012;74:669–675. doi: 10.1002/ajp.22021. [DOI] [PubMed] [Google Scholar]
- Poirier P, Wawrzyniak I, Vivarès CP, Delbac F, El Alaoui H. New insights into Blastocystis spp.: a potential link with irritable bowel syndrome. PLoS Pathog. 2012;8(3):e1002545. doi: 10.1371/journal.ppat.1002545. Epub 2012 Mar 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomajbíková K, Petrzelková KJ, Profousová I, Petrásová J, Kisidayová S, Varádyová Z, Modrý D. A survey of entodiniomorphid ciliates in chimpanzees and bonobos. Am J Phys Anthropol. 2010a;142:42–48. doi: 10.1002/ajpa.21191. [DOI] [PubMed] [Google Scholar]
- Pomajbíková K, Petrželková KJ, Profousová I, Petrášová J, Modrý D. Discrepancies in the occurrence of Balantidium coli between wild and captive African great apes. J Parasitol. 2010b;96:1139–1344. doi: 10.1645/GE-2433.1. [DOI] [PubMed] [Google Scholar]
- Profousová I, Mihaliková K, Laho T, Váradyová Z, Petrželková KJ, Modrý D, Kišidayová S. The ciliate, Troglodytella abrassarti, contributes to polysaccharide hydrolytic activities in the chimpanzee colon. Folia Microbiol (Praha) 2011;56:339–343. doi: 10.1007/s12223-011-0053-x. [DOI] [PubMed] [Google Scholar]
- Qadri SM, al-Okaili GA, al-Dayel F. Clinical significance of Blastocystis hominis. J Clin Microbiol. 1989;27:2407–2409. doi: 10.1128/jcm.27.11.2407-2409.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijpstra AC. Sporocysts of Isospora sp. in a chimpanzee (Pan troglodytes, L.) Proc K Ned Akad Wet C. 1967;70:395–401. [PubMed] [Google Scholar]
- Rothman JM, Bowman DD, Kalema-Zikusoka G, Nkurunungi JB. The parasites of the gorillas in Bwindi Impenetrable National Park Uganda. In: Newton-Fisher NE, Notman H, Paterson JD, Vernon R, editors. Primates of Western Uganda. Springer; New York: 2006. pp. 171–193. [Google Scholar]
- Rothman JM, Pell AN, Bowman DD. Host-parasite ecology of the helminths in mountain gorillas. J Parasitol. 2008;94:834–840. doi: 10.1645/GE-1454.1. [DOI] [PubMed] [Google Scholar]
- Rwego IB, Isabirye-Basuta G, Gillespie TR, Goldberg TL. Gastrointestinal bacterial transmission among humans, mountain gorillas, and livestock in Bwindi Impenetrable National Park, Uganda. Conserv Biol. 2008;22:1600–1607. doi: 10.1111/j.1523-1739.2008.01018.x. [DOI] [PubMed] [Google Scholar]
- Sak B, Kvác M, Petrzelková K, Kvetonová D, Pomajbíková K, Mulama M, Kiyang J, Modrý D. Diversity of microsporidia (Fungi: Microsporidia) among captive great apes in European zoos and African sanctuaries: evidence for zoonotic transmission? Folia Parasitol (Praha) 2011;58:81–86. doi: 10.14411/fp.2011.008. [DOI] [PubMed] [Google Scholar]
- Schuster FL, Ramirez-Avila L. Current world status of Balantidium coli. Clin Microbiol Rev. 2008;21:626–638. doi: 10.1128/CMR.00021-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestri G, Paiardini M, Pandrea I, Lederman MM, Sodora DL. Understanding the benign nature of SIV infection in natural hosts. J Clin Invest. 2007;117:3148–3154. doi: 10.1172/JCI33034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storgaard M, Lauren AL, Anderson PL. The occurrence of Blastocystis hominis in HIV-infected patients. AIDS. 1996;10:444–445. doi: 10.1097/00002030-199604000-00016. [DOI] [PubMed] [Google Scholar]
- Teare JA, Loomis MR. Epizootic of balantidiasis in lowland gorillas. J Am Vet Med Assoc. 1982;181:1345–1347. [PubMed] [Google Scholar]
- Terio KA, Kinsel MJ, Raphael J, Mlengeya T, Lipende I, Kirchhoff CA, Gilagiza B, Wilson ML, Kamenya S, Estes JD, Keele BF, Rudicell RS, Liu W, Patton S, Collins A, Hahn BH, Travis DA, Lonsdorf EV. Pathologic lesions in chimpanzees (Pan trogylodytes schweinfurthii) from Gombe National Park, Tanzania, 2004–2010. J Zoo Wildl Med. 2011;42:597–607. doi: 10.1638/2010-0237.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Kuyl AC, Kuiken CL, Dekker JT, Goudsmit J. Phylogeny of African monkeys based upon mitochondiral 12S rRNA sequence. J Mol Evol. 1995;40:173–180. doi: 10.1007/BF00167111. [DOI] [PubMed] [Google Scholar]
- Van Heuverswyn F, Li Y, Neel C, Bailes E, Keele BF, Liu W, Loul S, Butel C, Liegeois F, Bienvenue Y, Ngolle EM, Sharp PM, Shaw GM, Delaporte E, Hahn BH, Peeters M. Human immunodeficiency viruses: SIV infection in wild gorillas. Nature. 2006;444:164. doi: 10.1038/444164a. [DOI] [PubMed] [Google Scholar]
- Van Heuverswyn F, Li Y, Bailes E, Neel C, Lafay B, Keele BF, Shaw KS, Takehisa J, Kraus MH, Loul S, Butel C, Liegeois F, Yangda B, Sharp PM, Mpoudi-Ngole E, Delaporte E, Hahn BH, Peeters M. Genetic diversity and phylogeographic clustering of SIVcpzPtt in wild chimpanzees in Cameroon. Virology. 2007;368:155–171. doi: 10.1016/j.virol.2007.06.018. [DOI] [PubMed] [Google Scholar]
- Wiwanitkit V. Intestinal parasite infestation in HIV-infected patients. Curr HIV Res. 2006;4:87–96. doi: 10.2174/157016206775197682. [DOI] [PubMed] [Google Scholar]