Abstract
Two categories of evolutionary challenges result from escalating human impacts on the planet. The first arises from cancers, pathogens and pests that evolve too quickly, and the second from the inability of many valued species to adapt quickly enough. Applied evolutionary biology provides a suite of strategies to address these global challenges that threaten human health, food security, and biodiversity. This review highlights both progress and gaps in genetic, developmental and environmental manipulations across the life sciences that either target the rate and direction of evolution, or reduce the mismatch between organisms and human-altered environments. Increased development and application of these underused tools will be vital in meeting current and future targets for sustainable development.
Human influence on the biosphere (1,2) has profound consequences for both the rate and direction of evolution (3). Among the consequences are the challenges billions of people face from the effects of cancers, pests and pathogens that adapt quickly to our interventions against them. At the same time, humans and other organisms that we value for economic, ecological or aesthetic reasons are often not able to adapt quickly enough to keep pace with human alterations of the environment. These contemporary dilemmas increasingly threaten human health, food security and biological diversity (4,5,6,7,8,9,10,11,12). For example, the World Health Organization (WHO) warns that microbial resistance to antimicrobial drugs threatens the achievements of modern medicine (13). Likewise, more than 11,000 documented cases of pesticide resistance in nearly 1,000 species of insects, weeds, and plant pathogens jeopardize agricultural economies and food supplies worldwide (14). Failure to adapt may be equally dire and costly, as in the prevalent mismatch between modern human nutritional and lifestyle behaviors and those of our evolutionary past, which generally considered a major contributing factor to the high incidence of obesity and associated illnesses such as type 2 diabetes mellitus and cardiovascular disease (15). Meanwhile, the prospect of earth's sixth mass extinction of species becomes imminent as species are unable to adapt quickly enough to environmental change (16). A growing application of principles from evolutionary biology to challenges such as these may improve our ability to meet many of the most pressing problems of the 21st century (12,17,18,19).
Here we review current and prospective applications of evolutionary biology that may provide solutions for major societal challenges. We examine management approaches that attempt to either improve or undermine adaptation to modern environments by manipulating the relationships between the traits of organisms and the patterns of selection imposed by their environments. These manipulations include tools that may be widely considered evolutionary, such as selective breeding and emerging technologies in genetics, but also manipulations that are often overlooked as evolutionary, specifically manipulations of development that modify traits independent of genetic change, and altering environments in ways that can modulate selection itself. A conceptual framework linking all of these genetic, developmental and environmental manipulations is likely to lead to greater implementation and cross-disciplinary integration of applied evolutionary methods. We highlight how evolutionary strategies may be used to achieve policy targets of sustainable development for improved human health, food production, natural resource use and biodiversity conservation, including how stakeholder conflicts may be reduced to achieve desired outcomes. Throughout, we underscore the merits of building a more unified and integrated field of applied evolutionary biology to address global challenges.
Core evolutionary concepts and their relevance to global challenges
Evolution, defined as the change in genetic makeup of a population over successive generations, requires genetic variation, which arises from mutation and recombination (20). Most important for adaptation is genetic variation that affects variation in functional traits (21), such that alternate genotypes produce alternate phenotypes. Selection increases the frequency of genes that improve fitness – the ability to survive and reproduce. The specific genetic basis for most traits is not known, but trait differences among individuals typically have a significant heritable (genotypic) basis. This basis includes heritable aspects of development, which also may evolve and give rise to adaptive phenotypic plasticity (22). A population with low fitness may experience strong natural selection that favors better- adapted genotypes. However, strong selection will not necessarily ‘rescue’ a population if there are too few adapted individuals or suitable genes for the population to persist (23). Movement of genes between populations (gene flow) and random changes in gene frequency in small populations (genetic drift) can also cause evolution and influence the outcome of natural selection (20). These concepts apply not only to organisms from bacteria to humans, but also to viruses and cancer cells (24).
The core concepts of evolutionary biology are best known for explaining the unity, diversity, and adaptive characteristics of organisms (17). Phylogenetic methods that establish the relatedness of organisms are central to understanding the patterns and processes of evolution underlying the function and diversity of living systems (25). The practical applications of phylogenetic methods have been thoroughly reviewed by others, and include such diverse objectives as reconstructing invasion routes of harmful organisms, conservation planning and combating crime (17,26). Here we focus on the manipulation of processes that determine the adaptedness of individuals, populations and other biological systems in order to meet management objectives (Fig. 1).
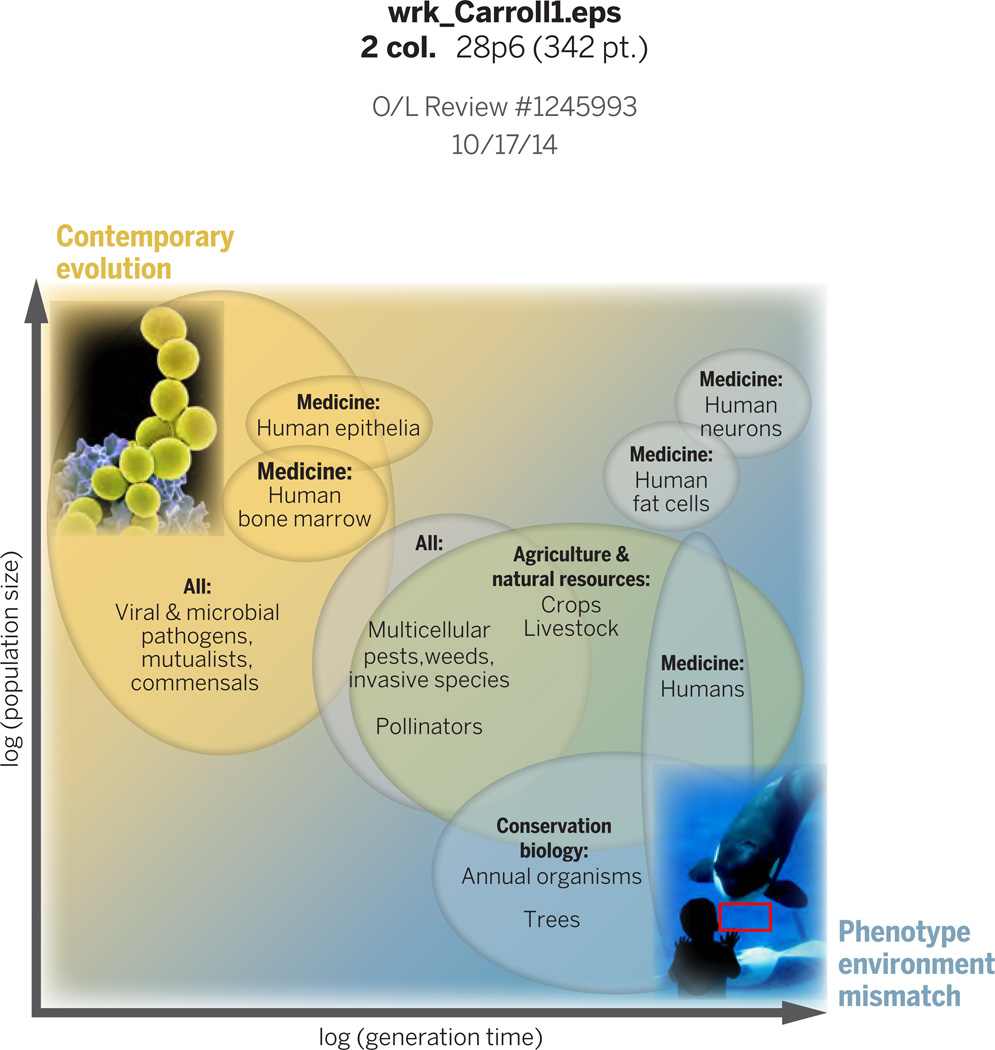
Fig. 1.
The two central paradigms of applied evolution are managing contemporary evolution and phenotype-environment mismatch. Managing contemporary evolution is critical for rapidly reproducing organisms with large population sizes, such as the methicillin-resistant Staphylococcus aureus (MRSA) pictured in the upper left. Altering phenotype-environment mismatch is most relevant for organisms with relatively long generation times and low population sizes, such the large mammals shown the lower right. Labels in ovals refer to example organisms, viruses or cell types in specified management sectors. ‘All’ indicates relevance to all management sectors (food, health and environment).
Agriculture, medicine, and conservation address different challenges, but nonetheless share common strategies to manage evolutionary mismatch and the associated risks to populations experiencing strong selection. Those strategies can be classified as genotypic, developmental, or those related to environmental manipulations (Fig. 2). The potential sustainability of such practices may be assessed by comparing the intensity of selection with the adaptive capacity of a target population (27). For example, the widespread use of antibiotics that exert strong selection on bacteria is typically not sustainable for controlling highly adaptable microbe populations because they rapidly evolve resistance (28). Accordingly, the sustainability of antibiotics use can be increased by either reducing s election, e.g. through regulated use of particularly strong antibiotics, or by attempts to surpass the adaptive capacity of microbes through drug combinations (29). Below, we review successes and emerging methods in applied evolutionary biology, highlighting commonalities across the sectors of health, food and environmental management (Fig. 3).
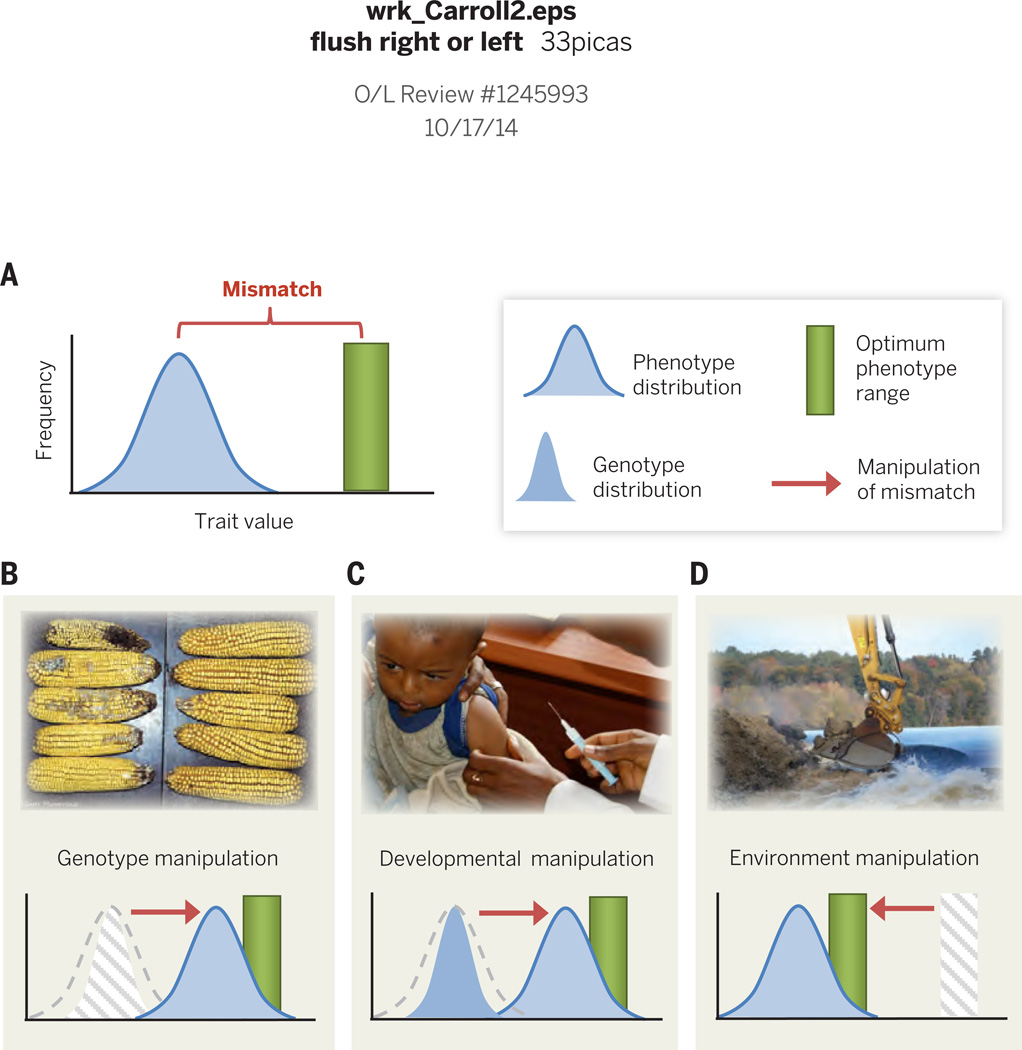
Fig. 2.
Phenotype-environment mismatch. (A) Mismatch between phenotypes and an environment occurs when a population's phenotypic trait distribution differs from the optimum; greater mismatch increases selection for adaptation, but also implies greater costs through reduced survival and reproduction. (B) Genotypic manipulations reduce mismatch by managing existing genetic variation or introducing new genes. For example, conventional corn is damaged by insect pests (left) that are killed by bacterial proteins produced by genetically engineered Bt corn (right). Alternatively, evolutionary mismatch can also be managed by (C) Developmental manipulations of phenotypes, such as vaccination to enhance immunity against pathogens, or (D) Environmental manipulations, such as habitat restoration. These examples demonstrate methods to reduce mismatch, but these same tactics can be reversed to impose greater mismatch where beneficial to human interests (e.g., pest eradication).
Fig. 3.
Two management intervention categories of applied evolutionary biology: 1. Controlling adversaries and 2. Protecting valued populations. Together they are enabled by four strategies (boldface). A core set of eight evolutionary principles guides the execution of these strategies and underlies tactics (left hand columns) used to meet management objectives in the food and fiber production, health and environmental sectors (right hand columns). Colored squares show different treatments; curves show frequency distributions of phenotypes; double helices are genomes; green arrows show change through space or time; green wedges show point interventions using selection or GE. Semicolons separate multiple management examples. Hypothetical applications are given in two cases that lack empirical examples. Expanded treatments for each cell and references are provided in Supplementary Table S2.
Successes and prospects in applied evolutionary biology
Applied evolutionary biology encompasses widely different manipulations that may together achieve a broad range of goals. From protecting biodiversity with conventional environmental management that increases fitness in wild environments, to medical recommendations for traditional diets, some methods of applied evolutionary biology have a long history of use even if they are not often seen as evolutionary in nature. In contrast, the synthesis of wholly novel genomes with emerging technologies represents obvious evolutionary manipulation that deliberately adds new organisms to the tree of life, but with little history of application, it involves unknown risks and public controversy. Here we review some of the most recent successes and leading prospects for the application of evolutionary biology, in a progression from relatively well-established methods to underexplored strategies. We first consider manipulations of selection to improve population productivity and individual health, and to delay the emergence of resistance (Fig. 2). We then examine less developed methods for the cultivation of populations inherently pre-adapted to impending environmental changes, and for innovative applications of group selection in crops and wildlife. We end this section with urgent considerations for managing evolutionary factors that span disciplinary boundaries, as in cases of emerging zoonotic disease.
Environmental alignment to secure biodiversity and human health
A common application of evolutionary principles is to manage current environments to be more like the historical habitats in which selection shaped the genetic makeup of humans and other species. Conventional habitat protection and restoration recognize that threatened species often adapt poorly to changing environments in the wild (26,30). Conversely, rapid adaptation to captive rearing programs used to rebuild populations of rare species, contributes to a 50 to 90 percent failure rate of reintroductions (31). Reintroduction success has been improved with enclosures and rearing methods that mimic wild conditions and by limiting the number of captive generations to minimize adaptation to artificial conditions (32).
Some of the most serious non-communicable diseases in humans may be prevented by better aligning current environments with those in which our hunter-gatherer ancestors evolved (33). Sedentary modern lifestyles and diets with high glycemic processed foods are increasingly implicated in the rapidly rising rates of obesity, diabetes and cardiovascular disorders (34). These mismatch disorders are estimated to contribute to about two-thirds of all deaths in Western societies (35) and to a growing proportion of deaths in developing countries (36,37). In 2012, the economic burden of type 2 diabetes alone was estimated at $500 billion globally, nearly 1% of world GDP (38). To restore conditions to which people are better adapted physiologically, while retaining the desired elements of a modern lifestyle (35), public health scientists recommend greater phys ical activity (39) with reduced consumption of refined carbohydrates (36), that is, diets and activity levels closer to those of the past, to which we are better adapted. More generally, a number of evolutionarily-based tools are available to prevent chronic non-communicable diseases, including the 19% of global cancer incidents that the WHO attributes to environmental exposure (40). These tools include life course approaches, which manage the timing and duration of environmental exposures to minimize risks of subsequent chronic disease (41). From a public health standpoint, environmental approaches to disease prevention may often be most cost-effective when applied outside of health care settings and when simultaneously targeting groups of people rather one individual at a time, such as through price regulation on goods, or public information campaigns (42). Further, systematic population scans that associate disease phenotypes with human genotypes (43, 44) are an important tool for determining the genetic basis of lifestyle diseases, and therefore in assessing heritable risk and treatment options. Such assessments however run the risk of identifying false positives and underestimating the complexity of genetic and epigenetic regulation (45, 46). For example, it is estimated that 90% of chronic disease risk cannot currently be directly linked to genetic factors, but is more likely to be understood in the context of human environmental exposures such as diet and toxicants (47). Thus, future prevention and treatment of chronic diseases will combine enhanced genotype-phenotype association scans with improved monitoring of toxic compounds in the surrounding environment and in human tissues (47). Such genotype-phenotype association studies search simultaneously for associations across the hundreds of disease phenotypes included in electronic medical registers (45). This expanded approach reduces the rate of false positives and helps to identify genetic factors that contribute to multiple diseases as well as diseases controlled by multiple genes.
Altering genomes for improved food security and human health
Climate change and environmental degradation compromise the productivity of agricultural systems that must feed a rapidly growing human population (48). Genetic modification of crops, through enhanced artificial selection methods and perhaps genetic engineering, will likely be important in meeting these challenges. Genetically engineered (GE) crops were first grown on a large scale in 1996, and during 2013, 18 million farmers in 27 countries planted GE crops on approximately 10% of the world's cultivated land (175 million hectares) (49). More than 99% of this area was planted with soybean, corn, cotton or canola into which genes were inserted to confer tolerance to herbicides, protection against insects, or both (50). These engineered varieties are extreme examples of apparently effective genotypic manipulations to reduce mismatch to specific environments. However, societal acceptance is an important factor, and GE crops remain controversial (51,52). They have not been adopted widely in some regions including Europe, where alternative manipulations of evolutionary mismatch, such as use of non-GE lines with some degree of tolerance, pesticide applications and integrated pest management serve as alternative genotypic and environmental manipulations (53).
An alternative to GE is enhanced artificial selection and hybridization of superior cultivated varieties with molecular genetic tools that identify individuals and gene regions conveying preferred traits (54). A priority application, where GE has until now been less successful Carroll et al. 10 (55), is to improve abiotic tolerance due to more frequent weather extremes under climate change. For example, flood tolerant rice, which is grown by two million farmers in Bangladesh and India (49), was developed with marker-assisted breeding using molecular markers of quantitative traits to identify targets for hybridization and selection (56). At the same time, candidate drought tolerance genes for GE crops have also recently been identified in rice as well as corn (57,58), with corn hybrids putatively tolerant to both drought and herbicides brought to market in 2013 (55,59). Regardless, whether produced via artificial selection or genetic engineering, the potential for genetic manipulations of mismatch to improve food security may be greatest when technology allows growers to select or customize crop varieties for adaptation in their local agroecosystems (60).
In contrast to the advances in agriculture, genetic modification to treat human disease is currently in a trial phase. Gene therapy is under development mainly for diseases with high heritability and simple genetic control, in which replacing or complementing parts of a patient’s genome can improve their health (61,62,63). Therapies in advanced trial stages include the targeting of retinal cells to prevent expression of heritable blindness (64,65), and oral administration of p53 gene for tumor suppression (66). However, even as targeted DNA analysis and whole-genome sequencing of patients becomes increasingly routine (67), few efforts have met the promise of their pre-clinical and clinical trials to reach final approval phase of ‘post-marketing’ surveillance trials (68,69).
Using environmental heterogeneity to delay resistance evolution
One of the most costly and widespread outcomes of efforts to limit populations is the rapid evolution of resistance to control measures in insect pests (14), weeds (70), pathogens, and cancers (71). For example, intensive use of the herbicide glyphosate by farmers, particularly those who grow glyphosate-tolerant GE crops, has selected for resistance in 24 weed species in 18 countries since 1996 (72,73). In contrast, strategies that vary selection in space or time have delayed the evolution of resistance in some pests (Fig. 3). For example, scientists and farmers have proactively developed and implemented strategies to slow pest adaptation to GE crops that produce insecticidal proteins from Bacillus thuringiensis (Bt) (74,75). The primary strategy employs ‘refuges’ of host plants that do not produce Bt toxins to promote survival of susceptible pests (74). In principle, the rare resistant pests that survive on Bt crops are more likely to mate with the comparatively abundant, susceptible pests from the nearby refuges. If resistance is inherited as a recessive trait, the heterozygous offspring from such matings will be susceptible and will die on the transgenic plants. The U.S. EPA and regulatory agencies in many other countries have mandated refuges since Bt crops were first commercialized (76,77). Retrospective analysis after more than a decade of monitoring indicates that refuges do indeed delay resistance, particularly when resistance is a recessive trait (77,78).
The success of refuge tactics in agriculture is now drawing attention in other management sectors, including fisheries, where refuges may impede costly life history and body size evolution resulting from harvest selection (79). Likewise, in cancer management, portions of tumors with low vascularization and consequently low delivery of chemotoxins may serve as refuges that sustain chemosensitive tumor genotypes (80,81) and slow the evolution of resistance to chemotherapy in metastatic cancer (82,83). Such resistance accounts for a large proportion of current treatment failures (84). Compared w ith typical failures when oncologists try to eradicate a patient's cancer with high drug doses, lower doses could be more successful if they favor survival of chemosensitive cell lines that can outcompete chemoresistant lines (85). Increasingly sophisticated models of tumor evolution may eventually support implementation of such non-eradication therapies (86).
While refuges delay resistance with genetic swamping of resistant lineages by susceptible lineages, another strategy attempts to curb resistance through selection that combines multiple modes of action (also known as “stacking” or “pyramiding”). In many human diseases, including HIV, tuberculosis, malaria and cancer, resistance frequently evolves under selection from individual drugs (87). Combination therapies are based on the evolutionary principle that if genes conferring resistance to each selection pressure are rare and inherited independently, individuals with all of the genes required for full resistance will be rare or even absent in target populations (4,14,88,89). For example, resistance evolved rapidly to potent antiretroviral drugs administered singly in patients with HIV, but combinations of three such drugs have provided long-term efficacy and have become the standard of care (90,91). The potential tradeoffs associated with combining two or more drugs or pesticides to delay resistance include short-term increases in costs (92) and negative side effects (93), as well as the concern that such combinations will also ultimately favor the evolution of multiple resistance (87,94,95,96). For example, incorporating two or more toxins together in GE varieties slows resistance evolution (97,98), but this advantage may diminish when less resistant single-toxin varieties are planted in the same area as multi-toxin varieties, and provide stepping stones for multiple-resistance evolution (99). Combined selection pressures are most likely to be durable when implemented as a facet of more broadly integrated systems, such as integrated pest management (IPM). IPM combines selection pressures from a diverse suite of tactics for pest suppression including various forms of biological control and optimized spatiotemporal cropping schemes (100). By increasing treatment durability, combinatorial strategies are among the most important instruments for the control of highly adaptable pests, pathogens and cancers (Fig. 3).
Choosing population sources to anticipate climate change
While some strategies of applied evolutionary biology are established or rapidly increasing, other rarely used strategies are of interest because of their underexplored potential to replace or complement longstanding management practices. These include using non-local seeding sources for re-planting in environmental restoration and forestry, and the exploitation of group selection-based designs in crop and livestock breeding.
The mismatch of valued plants to new climates is an overarching challenge in forestry, agriculture and conservation biology. A widespread debate concerns whether to use local versus external sources of genetic material for replanting to best anticipate climate change in forestry, agriculture, wildlife and environmental restoration. The massive scale of many replanting efforts – 400,000 ha of production forest is planted each year in Canada alone (101) – plus the long intervals between plantings for many perennial species and restoration projects, means that these choices may have broad economic and ecological consequences. Traditionally, resident stocks have been favored to capture locally valuable adaptations. In forestry this approach is exemplified by established bioclimatic ‘seed-transfer zones’ that steer local seed sourcing for planting of some of the world’s largest production systems (102,103). Evidence from wild plant restoration programs indicates however that local sources are not always best, particularly in altered environments (104,105,106,107,108, 109). This may arise when nearby sources share some of the vulnerabilities responsible for the declines of the original populations (103). In these situations, climate mismatches may be better relieved by translocating genotypes that are pre-adapted to expected conditions (110,111), for example more tolerant to heat, drought or pest stresses (112). When single sources do not show the range of adaptations required at a given site, reintroduction may be improved with propagules pooled from a diversity of sources to increase overall genetic variation, and thus the odds that some individuals will be suited for changing conditions (104,105,113). A recent meta-analysis in restoration ecology underscores shortcomings of the 'local-is-best' dictum (114) and comparable analyses of sourcing successes and failures in forestry and perennial agriculture are needed to find ways to sustain productivity under climate change.
Exploiting group versus individual performance in crops and livestock
In most agriculture and aquaculture, productivity is measured at the level of groups (e.g., field or herd) rather than in individual performance. More attention to traits that improve group performance may thus offer a broader suite of tactics to increase production while demanding fewer resources, including pesticides, to meet basic human needs (115) (Fig. 3). In the majority of natural systems, group selection is considered weak relative to selection among individuals (116). Consequently, past natural selection in the ancestors of domesticated species may have favored traits that promote individual performance but are costly to group productivity. One important consequence may be greater current opportunities for artificial selection of individual traits that improve group performance while avoiding inadvertent evolution of ‘uncooperative’ individuals (8), such as those with competitive root structures in dryland field crops (117). Artificial selection for group yield in maize has produced lines with reduced male function and that bear more-vertical leaves, which reduce the shading of neighbors. Both of these traits decrease individual plant performance while enhancing group productivity (118,119), but in the absence of strategic breeding to favor these changes directly, they have evolved only slowly, requiring 60 years to appear as unplanned responses to selection on group yield alone (120). Weiner and colleagues (121) have proposed a proactive evolutionary design for wheat production that selects for traits that increase collective shading of weeds within specific planting configurations, in order to increase overall crop yield while reducing herbicide use. Similar group-based perspectives apply in animal husbandry, where traits like reduced aggressiveness favor group productivity under domestication, but might have been selected against in the wild (122). By combining agronomy and environmental physiology with evolutionary modeling, group-based agricultural systems may offer new and more sustainable paths to meet global production goals.
Addressing evolution across management sectors
One of the most significant outcomes of the scale of human activity is that evolutionary concerns in one management sector often spill over into, or depend on, others (Fig. 4). These connections result from novel biotic interactions due to natural, intentional or inadvertent transport of organisms and their genes by trade, infrastructure, and waste streams (123,124). Further coordination of prevention, control and monitoring will be required to address growing interdependencies among management sectors. Increased exchange of emerging pathogens between health, agricultural and natural systems is a key case in point (125,126,127,128). For example, while domestic pigs are the principal reservoir of ‘swine influenza’ (H1N1), they simultaneously host other influenza strains, including those associated with human hosts and domestic and migratory avian hosts (129). The intensive communal raising of pigs and poultry for food therefore encourages virus strains to exchange genes and adapt to more host species (130). One overarching concern is that pigs hosting highly pathogenic wild avian strains (H5N1) could contribute to selection for the direct mammal-to-mammal transmission that underlies human epidemics. The consequences of such evolution (131) are foreshadowed by the recent global outbreaks of H5N1 in 2004 and H1N1 in 2009 (132). These events underscore the need for initiatives in prevention and control that cross traditional disciplinary boundaries, including coordinated surveillance of viral evolution and the monitoring of pathogen reservoir species across the food, health and environment spectrum (127,133).
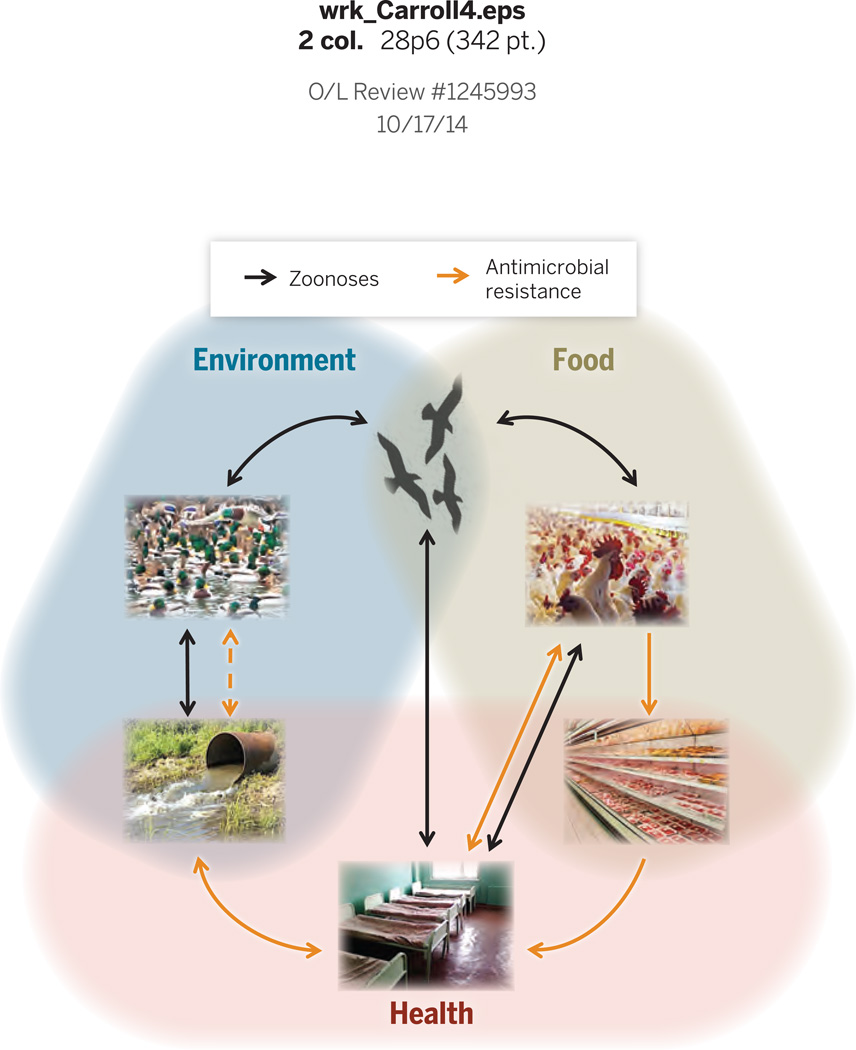
Fig. 4.
Emerging pathogens such as zoonoses (black arrows) and resistant bacteria (gray arrows) illustrate interdependencies generated by gene flow among the economic sectors of food, health and the environment. In zoonoses, vertebrates such as birds act as reservoirs for pathogens that can infect humans. Through direct transmission or via domesticated animals, zoonoses are passed to humans and cause regular local and rare global epidemics (such as the flu outbreaks of H5N1-2004 and H1N1-2009). ‘Reverse zoonoses’ are transmitted from infected humans to wildlife (179). Antimicrobial resistance in bacterial stains associated with livestock evolves in response to widespread use of antibiotics in agriculture and to a lesser degree due to treatment in humans. Via food items, industry workers and waste disposal, resistant strains enter other human contexts. In a public health context resistant strains constitute a growing extra risk during treatment of illnesses, e.g., in hospitals. Antibiotics in human effluent cause widespread resistance selection in natural and semi-natural environments, which together with resistance reservoirs in natural environments further increase the risks of resistant pathogens in humans. In the figure, the dashed line indicates a variety of poorly known interactions among wild species.
The unresolved problem of rapidly evolving antimicrobial resistance is another pressing example of interdependence among management sectors, particularly between systems managed for food production and human health. Annual estimated costs of combatting multidrug resistant microbes in the US alone total $35 billion (134,135), and the failure to produce new antimicrobials as quickly as their predecessors lose efficacy (136,137) places a premium on stewardship of the few drugs that remain broadly effective (138,139). Although overprescribing of antibiotics for human treatment is a very real concern, the major use of antimicrobial drugs in many parts of the world is to promote the health and growth of livestock (140,141). This use selects for antimicrobial resistant microbes that may infect humans (Fig. 4) (141,142). Antibiotic-treated animals that are raised to feed people are now implicated in the origins of the most extensively resistant Escherichia coli encountered in human sepsis (143). Particularly worrisome is that once free in the environment, resistance genes do not dissipate with distance like many abiotic environmental pollutants. Resistance genes can replicate, and thus they can transfer horizontally among bacterial taxa, travel intact over great distances via hosts, and rise to new abundances in the presence of antimicrobials with similar modes of action. As pools of resistance genes become more prevalent and disseminated through human activities, they are likely to become increasingly important in new regions and management sectors (144). Because coupled evolutionary dynamics operate over such large spatial scales and multiple management sectors, their management requires political coordination, as exemplified by the Transatlantic Taskforce on Antimicrobial Resistance (145). Regulatory bodies have also taken the first steps to restrict use of some antibiotics to single management sectors (146,147). Broader and more rigorous implementation of such restrictions will be needed to sustain the most critical public benefits of our modern antibiotic era.
Next Steps
Applied evolutionary biology in international policy
Applied evolutionary biology addresses both the rapidly evolving and the mismatched biological systems that underlie many global challenges (148). Meeting international objectives for sustainable development (Millennium Development Goals and the anticipated Sustainable Development Goals (149)) and biodiversity conservation (the Convention for Biological Diversity’s 2020 ‘Aichi’ targets (150)) will require much greater integration of evolutionary principles into policy than has been widely acknowledged. Box 1 summarizes potential policy contributions of cases reviewed here. For example, we must implement resistance management strategies for pesticides and antibiotics to meet newly proposed Sustainable Development Goals for human health, food and water security (149). Likewise, choices of adaptable source populations will improve the resilience of restored habitats (Aichi target 15: ‘restore 15 percent of degraded habitats before 2020’) and increase the reliability of crop supplies. Further, sustainable harvest strategies (151,152) and early warning signs of unsustainable harvest (153) will help to achieve lasting stocks of fish and aquatic invertebrates (Aichi target 6: all stocks should be harvested sustainably). The identification and protection of diverse genotypes is also critical to the future of crop improvement and for the discovery of chemical compounds such as new therapeutics. In this realm, the international Nagoya protocol on Access and Benefit-Sharing of genetic resources (154) may assist in securing public access to resources for adaptation to local conditions while coordinating with global research and development efforts (155,156,157).
Box 1: Recommended contributions of applied evolutionary biology to proposed themes of new international sustainable development goals (149), based on examples presented in this review.
Goal 1: Thriving lives and livelihoods
-
-
Reduce chronic lifestyle disease through environmental alignment of human lifestyle.
-
-
Reduce environmental levels of human toxicants through application of reduced selection response techniques* to pesticides/biocides.
-
-
Apply reduced selection response techniques to maintain long-term efficacy of antimicrobials and avert the anbiotics crisis.
-
-
Reconcile individual and group incentives in health systems to reduce virulence and resistance of emerging and re-emerging pathogens.
Goal 2: Sustainable food security
-
-
Increase crop yield through continued selection of varieties and improved access to these.
-
-
Prolong efficacy of pesticides and artificially selected or GE crops through reduced selection response techniques.
-
-
Improve yields through integration of group selection in production of novel crop varieties.
-
-
Reduce climate change impact by choosing crop varieties resilient to drought, flooding and other extremes.
Goal 3: Secure sustainable water
-
-
Increase water security through use of reduced selection response techniques to water polluting pesticides/biocides
-
-
Use genetic manipulation to produce crop varieties with improved water economy.
Goal 4: Universal clean energy
-
-
Improve biofuels through genetic manipulation with the aim to reduce CO2 emissions and land area for energy production.
-
-
Assess risks and benefits of synthetic organisms for biofuel production taking taking gene flow, land use and property rights issues into account.
Goal 5: Healthy and productive ecosystems
-
-
Reduce biodiversity extinction rates through environmental alignment and genetic manipulation of fitness.
-
-
Retain naturalness of captive biodiversity through environmental alignment.
-
-
Choose pre-adapted or high diversity sources for increased habitat restoration success.
Goal 6: Governance for sustainable societies
-
-
Incorporate externalities from rapid evolution as well as the loss of evolutionary history and potential into green accounting for sustainable governance of the earth system.
-
-
Coordinate strategies of SDG’s in a coupled systems framework to reduce conflicts from inadvertent contemporary evolution and phenotype-environment mismatch.
* Reduced selection response techniques refer to the four tactics in Figure 3 that slows evolution by varying selection in space and time, diversifying selection, and targeting of specific traits, and additionally adoption of alternatives to strong selection agents such as toxins.
The extensive and targeted genetic manipulations permitted through recent advances in biotechnology are setting the stage for novel biological functions for which we either lack an understanding of potential risks, or knowledge of how best to assess them (158). Thus, perhaps the area of applied evolutionary biology where development of international policy is most urgent is the area of synthetic biology. Synthesizing wholly or partially novel organisms offers tremendous opportunities in many areas such as biofuels, medicine, environmental restoration and conservation (159,160,161), but national and international guidelines are needed to avert potentially harmful outcomes (158,162). Segments of medicine and agriculture include social scientists and economists in systematic risk assessment (76,163). Similar practices would benefit conservation biology and natural resource management, as increasingly proactive and intensive manipulations appear on the horizon. These prospects include resurrected species and wild populations genetically engineered for resistance to lethal diseases such as chytrid fungus in frogs and white-nose syndrome in bats (161,162).
Implementing applied evolutionary biology locally and globally
Reconciliation of individual and group stakeholder interests plays a central role in the effort to achieve sustainability through applied evolutionary biology (164,165,166,167). Anthropogenic evolutionary change often has consequences that extend beyond the immediate vicinity of the causal agents and pose dilemmas in achieving cooperation from local to global scales (168). Thus, in some applied evolutionary strategies, individuals must exchange their private short-term gains for the long-term public good. In managing pest resistance to transgenic Bt crops, farmers who plant refuges of conventional crops contribute to the long-term public good of sustained pest susceptibility to Bt toxin, but may incur the short-term private cost of pest damage to their refuges. For example, farmers that planted only non-Bt corn in five midwestern states of the U.S. accrued nearly two-thirds of the estimated $6.8 billion in Bt corn benefits between 1996 and 2009 (169). This benefit arose from a combination of less expensive non-Bt corn and because widespread adoption of Bt corn caused regional suppression of the major target pest (169). Perhaps in part due to the latter, farmer compliance with the refuge strategy for Bt corn in the U.S. has steadily declined and threatens the sustainability of resistance management (170). Such conflicts between individual and public good may be the rule rather than the exception in the implementation of applied evolutionary biology.
The economic theories of public choice provide tools for reconciling individual and group conflicts (171) (Box 1). Governments can tax undesirable actions, subsidize desirable ones, regulate activities (146,147), and create tradable property rights. For example, subsidies and regulated access to public schools can increase participation in vaccination programs that benefit public health but may increase risks to unvaccinated individuals (172). Theoretical modeling suggests that an unregulated vaccination market will yield too little advance vaccination and too much vaccination at the time of infection, which could select for increased virulence (163). With pathogen resistance, both the relative fitness of resistant genotypes in untreated environments (173,174) and the prevalence of resistance in natural environments (175) may increase the cost of lost susceptibility to a drug. Improved policies that reduce public costs may emerge from better accounting of the causes and consequences of such evolutionary externalities (163,176).
Toward a unified discipline
As demonstrated by many of the examples above, applied evolutionary biology uses principles common to all areas of biology, and because of this, progress in one area may often enable solutions in others. New approaches in this developing field may best be generated and assessed through collaborations that span disciplinary boundaries (177) (Fig. 3). Promoting greater adoption and consistency in the use of evolutionary terminology, which is inconsistent across disciplines (178), will therefore be an important first step toward a more unified field of applied evolutionary biology.
The global scale of human impacts is now more widely appreciated than ever before. Successful governance of living systems requires understanding evolutionary history as well as contemporary and future evolutionary dynamics. Our current scientific capacity for evolutionarily-informed management does not match the need, but it can be increased through new and more widespread training and collaboration, monitored experimentation, and context-sensitive implementation. Like engineering, which is a multifaceted applied science with common core principles, shared vocabulary and coordinated methods, applied evolutionary biology has the potential to serve society as a predictive and integrative framework for addressing practical concerns in applied biology which share at their core the basic evolutionary principles governing life.
Supplementary Material
Acknowledgements
M.T.K., R.F.D., S.P.C., S.Y.S., and T.B.S. were supported by grants from NSF. C.T.B. is supported by NIGMS grant number U54GM088558. M.T.K., is supported by Maine Agricultural and Forest Experiment Station; P.D.G. by the National Research Centre for Growth and Development; S.P.C. by CSIRO and the Australian-American Fulbright Commission; S.Y.S. by grants from the College of Biological Sciences, UC Davis; T.B.S. by grants from NIH, and B.E.T. by USDA Biotechnology Risk Assessment Grant 2011-33522-30729. P.S.J. acknowledges the Danish National Research Foundation for support to the Center for Macroecology, Evolution and Climate, and the American-Scandinavian Foundation. Sonal Singhal, Jacobus Boomsma, Carsten Rahbek, Niels Strange and Trevon Fuller provided useful comments on previous versions of the manuscript.
Footnotes
References and notes
- 1.Ecosystems and Human Well-being: Synthesis. Washington, DC: Island Press; 2005. Millennium Ecosystem Assessment. [Google Scholar]
- 2.Ellis EC. Used planet: A global history. Proc. Natl. Acad. Sci. U.S.A. 2013;110:7978–7985. doi: 10.1073/pnas.1217241110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Latta RG. Conservation genetics as applied evolution: from genetic pattern to evolutionary process. Evol. Appl. 2008;1:84–94. doi: 10.1111/j.1752-4571.2007.00008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palumbi SR. Evolution - Humans as the world's greatest evolutionary force. Science. 2001;293:1786–1790. doi: 10.1126/science.293.5536.1786. [DOI] [PubMed] [Google Scholar]
- 5.Gluckman PD, Bergstrom CT. Christmas 2011: Food for thought, evolutionary biology within medicine: a perspective of growing value. Brit. Med. J. 2011;343:d7671. doi: 10.1136/bmj.d7671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gould F. Broadening the application of evolutionarily based genetic pest management. Evolution. 2008;62:500–510. doi: 10.1111/j.1558-5646.2007.00298.x. [DOI] [PubMed] [Google Scholar]
- 7.Thrall PH, et al. Evolution in agriculture: the application of evolutionary approaches to the management of biotic interactions in agro-ecosystems. Evol. Appl. 2011;4:200–215. doi: 10.1111/j.1752-4571.2010.00179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denison RF. Darwinian Agriculture: How Understanding Evolution Can Improve Agriculture. Princeton Univ. Press; 2012. [Google Scholar]
- 9.Hollomon DW. Do we have the tools to manage resistance in the future? Pest Manag Sci. 2012;68:149–154. doi: 10.1002/ps.2291. [DOI] [PubMed] [Google Scholar]
- 10.Stockwell CA, Hendry AP, Kinnison MK. Contemporary evolution meets conservation biology. Trends Ecol. Evol. 2004;18:94–101. [Google Scholar]
- 11.Hendry AP, et al. Evolutionary biology in biodiversity science, conservation, and policy: A call to action. Evolution. 2010;64:1517–1528. doi: 10.1111/j.1558-5646.2010.00947.x. [DOI] [PubMed] [Google Scholar]
- 12.Hendry AP, et al. Evolutionary principles and their practical application. Evol. Appl. 2011;4:159–183. doi: 10.1111/j.1752-4571.2010.00165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO. Antimicrobial resistance: global report on surveillance. [Retrieved on May 5, 2014];2014 http://www.who.int/drugresistance/documents/surveillancereport/en/
- 14.Tabashnik BE, et al. Defining terms for proactive management of resistance to Bt crops and pesticides. J. Econ. Entomol. 2014;107:496–507. doi: 10.1603/ec13458. [DOI] [PubMed] [Google Scholar]
- 15.Gluckman PD, Hanson MA. Mismatch: Why Our World No Longer Fits Our Bodies. Oxford Univ. Press; 2006. [Google Scholar]
- 16.Barnosky AD, et al. Has the Earth's sixth mass extinction already arrived? Nature. 2011;471:51–57. doi: 10.1038/nature09678. [DOI] [PubMed] [Google Scholar]
- 17.Bull JJ, Wichman HA. Applied evolution. Annu. Rev. Ecol. Syst. 2001;32:183–217. [Google Scholar]
- 18.Carroll SP, Fox CW. Conservation biology: evolution in action. Oxford Univ. Press; 2008. [Google Scholar]
- 19.Gluckman PD, Hanson MA, Buklijas T, Low FM, Beedle AS. Epigenetic mechanisms that underpin metabolic and cardiovascular diseases. Nat. Rev. Endocrinol. 2009;5:401–408. doi: 10.1038/nrendo.2009.102. [DOI] [PubMed] [Google Scholar]
- 20.Hartl D. A Primer of Population Genetics. Sunderland, MA: Sinauer Associates, Inc.; 1988. [Google Scholar]
- 21.Abrams PA. Modelling the adaptive dynamics of traits involved in inter- and intraspecific interactions: an assessment of three methods. Ecol. Lett. 2001;4:166–175. [Google Scholar]
- 22.Ghalambor CK, McKay JK, Carroll SP, Reznick DN. Adaptive versus non**-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct. Ecol. 2007;21:394–407. [Google Scholar]
- 23.Kinnison MT, Hairston NG. Eco-evolutionary conservation biology: contemporary evolution and the dynamics of persistence. Funct. Ecol. 2007;21:444–454. [Google Scholar]
- 24.Aktipis C, et al. Overlooking evolution: a systematic analysis of cancer relapse and therapeutic resistance research. PLoS One. 2011;6:e26100. doi: 10.1371/journal.pone.0026100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mouquet N, et al. Ecophylogenetics: advances and perspectives. Biol. Rev. 2012;87:769–785. doi: 10.1111/j.1469-185X.2012.00224.x. [DOI] [PubMed] [Google Scholar]
- 26.Mace GM, Purvis A. Evolutionary biology and practical conservation: bridging a widening gap. Molec. Ecol. 2008;17:9–19. doi: 10.1111/j.1365-294X.2007.03455.x. [DOI] [PubMed] [Google Scholar]
- 27.Chevin L-M, Gallet R, Gomulkiewicz R, Holt RD, Fellous S. Phenotypic plasticity in evolutionary rescue experiments. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2013;368 doi: 10.1098/rstb.2012.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Read AF, Huijben S. Evolutionary biology and the avoidance of antimicrobial resistance. Evol. Appl. 2009;2:40–51. doi: 10.1111/j.1752-4571.2008.00066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Read AF, Day T, Huijben S. The evolution of drug resistance and the curious orthodoxy of aggressive chemotherapy. Proc. Natl. Acad. Sci. U.S.A. 2011;108(Suppl):10871–10877. doi: 10.1073/pnas.1100299108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ashley MV, et al. Evolutionarily enlightened management. Biol. Cons. 2003;111:115–123. [Google Scholar]
- 31.Williams SE, Hoffman EA. Minimizing genetic adaptation in captive breeding programs: A review. Biol. Cons. 2009;142:2388–2400. [Google Scholar]
- 32.Leus K, Traylor-Holzer K, Lacy RC. Genetic and demographic population management in zoos and aquariums: recent developments, future challenges and opportunities for scientific research. Intl. Zoo Yearbook. 2011;45:213–225. [Google Scholar]
- 33.Gluckman PD, Low FM, Buklijas T, Hanson Ma, Beedle AS. How evolutionary principles improve the understanding of human health and disease. Evol. Appl. 2011;4:249–263. doi: 10.1111/j.1752-4571.2010.00164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cordain L, et al. Origins and evolution of the Western diet: health implications for the 21st century. Amer. J. Clin. Nutrit. 2005;81:341–354. doi: 10.1093/ajcn.81.2.341. [DOI] [PubMed] [Google Scholar]
- 35.Brownson RC, Haire-Joshu D, Luke DA. Shaping the context of health: a review of environmental and policy approaches in the prevention of chronic diseases. Annu. Rev. Publ. Health. 2006;27:341–370. doi: 10.1146/annurev.publhealth.27.021405.102137. [DOI] [PubMed] [Google Scholar]
- 36.Gaziano T, Pagidipati N. Scaling Up Chronic Disease Prevention Interventions in Lower- and Middle-Income Countries. Annu. Rev. Publ. Health. 2013;34:317–335. doi: 10.1146/annurev-publhealth-031912-114402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bloom DE, et al. The global economic burden of noncommunicable diseases. Program on the Global Demography of Aging. 2012;8712 [Google Scholar]
- 39.Woodcock J, Franco OH, Orsini N, Roberts I. Non-vigorous physical activity and all-cause mortality: systematic review and meta-analysis of cohort studies. Int. J. Epidemiol. 2011;40:121–138. doi: 10.1093/ije/dyq104. [DOI] [PubMed] [Google Scholar]
- 40.WHO. Environmental and occupational cancers. [accessed Feb 6, 2014]; http://www.who.int/mediacentre/factsheets/fs350/en/
- 41.Lynch J, Smith GD. A life course approach to chronic disease epidemiology. Annu. Rev. Public Health. 2005;26:1–35. doi: 10.1146/annurev.publhealth.26.021304.144505. [DOI] [PubMed] [Google Scholar]
- 42.Chokshi DA, Farley TA. The cost-effectiveness of environmental approaches to disease prevention. New Engl. J. Med. 2012;367:295–297. doi: 10.1056/NEJMp1206268. [DOI] [PubMed] [Google Scholar]
- 43.Rolph T. Loss-of-function mutations in SLC30A8 protect against type 2 diabetes. Nature Genetics. 2014 Mar 2; doi: 10.1038/ng.2915. published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Omenn GS. Evolution and public health. Proc. Natl. Acad. Sci. U.S.A. 2010;107(Suppl):1702–1709. doi: 10.1073/pnas.0906198106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Denny JC, et al. Systematic comparison of phenome-wide association study of electronic medical record data and genome-wide association study data. Nat. Biotechnol. 2013;31:1102–1111. doi: 10.1038/nbt.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Travisano M, Shaw RG. Lost in the map. Evolution. 2013;67:305–314. doi: 10.1111/j.1558-5646.2012.01802.x. [DOI] [PubMed] [Google Scholar]
- 47.Rappaport SM. Implications of the exposome for exposure science. J. Expo. Sci. Env. Epid. 2011;21:5–9. doi: 10.1038/jes.2010.50. [DOI] [PubMed] [Google Scholar]
- 48.Godfray HCJ, et al. Food security: the challenge of feeding 9 billion people. Science. 2010;327:812–818. doi: 10.1126/science.1185383. [DOI] [PubMed] [Google Scholar]
- 49.James C. Executive summary. Global status of commercialized biotech/GM crops. 2013:46. ISAAA Brief. [Google Scholar]
- 50.Ashraf M. Inducing drought tolerance in plants: recent advances. Biotechnol. Adv. 2010;28:169–183. doi: 10.1016/j.biotechadv.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 51.Pauwels E. Public Understanding of Synthetic Biology. BioSci. 2013;63:79–89. [Google Scholar]
- 52.Aerni P. Resistance to agricultural biotechnology: The importance of distinguishing between weak and strong public attitudes. Biotech. J. 2013;8:1129–1132. doi: 10.1002/biot.201300188. [DOI] [PubMed] [Google Scholar]
- 53.Jacobsen S-E, et al. Feeding the world: genetically modified crops versus agricultural biodiversity. Agron. Sustain. Devel. 2013;33:651–662. [Google Scholar]
- 54.Varshney RK, Bansal KC, Aggarwal PK, Datta SK, Craufurd PQ. Agricultural biotechnology for crop improvement in a variable climate: hope or hype? Trends. Plant Sci. 2011;16:363–371. doi: 10.1016/j.tplants.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 55.Marshall A. Drought-tolerant varieties begin global march. Nat. Biotechnol. 2014;32:308–308. [Google Scholar]
- 56.Varshney R, Ribaut J, Buckler E. Can genomics boost productivity of orphan crops? Nat. Biotechnol. 2012;30:1172–1176. doi: 10.1038/nbt.2440. [DOI] [PubMed] [Google Scholar]
- 57.Yang S, Vanderbeld B, Wan J, Huang Y. Narrowing down the targets: towards successful genetic engineering of drought-tolerant crops. Molec. Plant. 2010;3:469–490. doi: 10.1093/mp/ssq016. [DOI] [PubMed] [Google Scholar]
- 58.Gaudin ACM, et al. Taking transgenic rice drought screening to the field. J. Exptl. Bot. 2012;64:109–118. doi: 10.1093/jxb/ers313. [DOI] [PubMed] [Google Scholar]
- 59.International Service for the Acquisition of Agri-Biotech Applications. Approval Database, 2013. 2013 http://www.isaaa.org/gmapprovaldatabase/default.asp. [Google Scholar]
- 60.Mercer KL, Perales HR, Wainwright JD. Climate change and the transgenic adaptation strategy: Smallholder livelihoods, climate justice, and maize landraces in Mexico. Global Environ. Change. 2012;22:495–504. [Google Scholar]
- 61.Tachibana M. Towards germline gene therapy of inherited mitochondrial diseases. Nature. 2013;493:627–631. doi: 10.1038/nature11647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kordower JH, Kirik D. Introduction: gene therapy has gone from a pipe dream to clinical reality. Neurobiol. Disease. 2012;48:151–152. doi: 10.1016/j.nbd.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 63.Choi I-K, Yun C-O. Recent developments in oncolytic adenovirus-based immunotherapeutic agents for use against metastatic cancers. Cancer Gene Therapy. 2013;20:70–76. doi: 10.1038/cgt.2012.95. [DOI] [PubMed] [Google Scholar]
- 64.Lipinski DM, Thake M, MacLaren RE. Clinical applications of retinal gene therapy. Progress Retin. Eye Res. 2013;32:22–47. doi: 10.1016/j.preteyeres.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 65.Wert KJ, Davis RJ, Sancho-Pelluz J, Nishina PM, Tsang SH. Gene therapy provides long-term visual function in a pre-clinical model of retinitis pigmentosa. Human Molec. Gen. 2013;22:558–567. doi: 10.1093/hmg/dds466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Suzuki K, Matsubara H. Recent advances in p53 research and cancer treatment. J. Biomed. Biotechnol. 2011;2011:978312. doi: 10.1155/2011/978312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kilpivaara O, Aaltonen LA. Diagnostic Cancer Genome Sequencing and the Contribution of Germline Variants. Science. 2013;339:1559–1562. doi: 10.1126/science.1233899. [DOI] [PubMed] [Google Scholar]
- 68.Lowenstein P, Castro M. Uncertainty in the translation of preclinical experiments to clinical trials. Why do most phase III clinical trials fail? Curr. Gene Ther. 2009;9:368–374. doi: 10.2174/156652309789753392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Edelstein M. M. Gene Therapy Clinical Trials Worldwide. [accessed April 13 2014];J. Gene Medicine. 2014 database at http://www.abedia.com/wiley/index.html. [Google Scholar]
- 70.Vigueira CC, Losen KM, Caicedo AL. The red queen in the corn: agricultural weeds as models of rapid adaptation. Heredity. 2013;110:303–311. doi: 10.1038/hdy.2012.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Greene SE, Reid A. Moving Targets: Fighting the Evolution of Resistance in Infections, Pests, and Cancer. American Society of Microbiology. 2013 http://bit.ly/YeNhoS. [PubMed] [Google Scholar]
- 72.Powles SB, Yu Q. Evolution in action: plants resistant to herbicides. Annu. Rev. Plant Biol. 2010;61:317–347. doi: 10.1146/annurev-arplant-042809-112119. [DOI] [PubMed] [Google Scholar]
- 73.Heap I. The International Survey of Herbicide Resistant Weeds. 2013 www.weedscience.com. [Google Scholar]
- 74.Tabashnik BE. Evolution of resistance to Bacillus thuringiensis. Annu. Rev. Entomol. 1994;39:47–79. [Google Scholar]
- 75.Gould F. Sustainability of transgenic insecticidal cultivars: integrating pest genetics and ecology. Annu. Rev. Entomol. 1998;43:701–726. doi: 10.1146/annurev.ento.43.1.701. [DOI] [PubMed] [Google Scholar]
- 76.US Environmental Protection Agency. The Environmental Protection Agency’s White Paper on Bt Plant-pesticide Resistance Management. 1998 [Google Scholar]
- 77.Tabashnik BE, Brévault T, Carrière Y. Insect resistance to Bt crops: lessons from the first billion acres. Nat. Biotechnol. 2013;31:510–521. doi: 10.1038/nbt.2597. [DOI] [PubMed] [Google Scholar]
- 78.Tabashnik BE, Gassmann AJ, Crowder DW, Carriere Y. Insect resistance to Bt crops: evidence versus theory. Nat. Biotechnol. 2008;26:199–202. doi: 10.1038/nbt1382. [DOI] [PubMed] [Google Scholar]
- 79.Dunlop ES, Baskett ML, Heino M, Dieckmann U. The propensity of marine reserves to reduce the evolutionary effects of fishing in a migratory species. Evol. Appl. 2009;2:371–393. doi: 10.1111/j.1752-4571.2009.00089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gatenby RA, Brown J, Vincent T. Lessons from applied ecology: cancer control using an evolutionary double bind. Cancer Res. 2009;69:7499–7502. doi: 10.1158/0008-5472.CAN-09-1354. [DOI] [PubMed] [Google Scholar]
- 81.Silva AS, Gatenby RA. A theoretical quantitative model for evolution of cancer chemotherapy resistance. Biol. Direct. 2010;5:25. doi: 10.1186/1745-6150-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gillies RJ, Verduzco D, Gatenby RA. Evolutionary dynamics of carcinogenesis and why targeted therapy does not work. Nat. Rev. Cancer. 2012;12:487–493. doi: 10.1038/nrc3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Greaves M, Maley CC. Clonal evolution in cancer. Nature. 2012;481:306–313. doi: 10.1038/nature10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Longley DB, Johnston PG. Molecular mechanisms of drug resistance. J. Pathol. 2005;205:275–292. doi: 10.1002/path.1706. [DOI] [PubMed] [Google Scholar]
- 85.Gatenby RA. A change of strategy in the war on cancer. Nature. 2009;459:508–509. doi: 10.1038/459508a. [DOI] [PubMed] [Google Scholar]
- 86.Almendro V. Inference of Tumor Evolution during Chemotherapy by Computational Modeling and In Situ Analysis of Genetic and Phenotypic Cellular Diversity. Cell Rep. 2014;6:514–527. doi: 10.1016/j.celrep.2013.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Michel J-B, Yeh PJ, Chait R, Moellering RC, Kishony R. Drug interactions modulate the potential for evolution of resistance. Proc. Natl. Acad. Sci. U.S.A. 2008;105:14918–14923. doi: 10.1073/pnas.0800944105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bourguet D. Heterogeneity of selection and the evolution of resistance. Trend Ecol. Evol. 2013;28:110–118. doi: 10.1016/j.tree.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 89.Cunningham JJ, Gatenby RA, Brown JS. Evolutionary dynamics in cancer therapy. Molec. Pharmaceutics. 2011;8:2094–2100. doi: 10.1021/mp2002279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gulick R. Antiretroviral treatment 2010: progress and controversies. J. Acquir. Immune Defic. Syndr. 2010;55:S43–S48. doi: 10.1097/QAI.0b013e3181f9c09e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gupta R, Van de Vijver D, Manicklal S, Wainberg MA. Evolving uses of oral reverse transcriptase inhibitors in the HIV-1 epidemic: from treatment to prevention. Retrovirology. 2013;10:82. doi: 10.1186/1742-4690-10-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brun H, et al. Quantitative resistance increases the durability of qualitative resistance to Leptosphaeria maculans in Brassica napus. New Phytol. 2010;185:285–299. doi: 10.1111/j.1469-8137.2009.03049.x. [DOI] [PubMed] [Google Scholar]
- 93.Tabashnik BE. Managing resistance with multiple pesticide tactics: Theory, evidence and recommendations. J. Econ. Entomol. 1989;82:1263–1269. doi: 10.1093/jee/82.5.1263. [DOI] [PubMed] [Google Scholar]
- 94.Bergstrom CT, Lo M, Lipsitch M. Ecological theory suggests that antimicrobial cycling will not reduce antimicrobial resistance in hospitals. Proc. Natl. Acad. Sci. U.S.A. 2004;101:13285–13290. doi: 10.1073/pnas.0402298101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Torella JP, Chait R, Kishony R. Optimal drug synergy in antimicrobial treatments. PLoS Computat. Biol. 2010;6:e1000796. doi: 10.1371/journal.pcbi.1000796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Michel J-B, Yeh PJ, Chait R, Moellering RC, Kishony R. Drug interactions modulate the potential for evolution of resistance. Proc. Natl. Acad. Sci. U.S.A. 2008;105:14918–14923. doi: 10.1073/pnas.0800944105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jacquemin B, Gasquez J, Reboud X. Modelling binary mixtures of herbicides in populations resistant to one of the components: evaluation for resistance management. Pest Mgmt. Sci. 2009;65:113–121. doi: 10.1002/ps.1647. [DOI] [PubMed] [Google Scholar]
- 98.Roush RT. Two–toxin strategies for management of insecticidal transgenic crops: can pyramiding succeed where pesticide mixtures have not? Phil. Trans. R. Soc. Lond. B. 1998;353:1777–1786. [Google Scholar]
- 99.Zhao J-Z. Concurrent use of transgenic plants expressing a single and two Bacillus thuringiensis genes speeds insect adaptation to pyramided plants. Proc. Natl. Acad. Sci. U.S.A. 2005;102:8426–8430. doi: 10.1073/pnas.0409324102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shelton AM. Field manual of techniques in invertebrate pathology. Netherlands: Springer; 2007. Resistance to insect pathogens and strategies to manage resistance: an update. [Google Scholar]
- 101.Pedlar JH, et al. Placing forestry in the assisted migration debate. BioSci. 2012;62:835–842. [Google Scholar]
- 102.Gray LK, Gylander T, Mbogga MS, Chen P-Y, Hamann A. Assisted migration to address climate change: recommendations for aspen reforestation in western Canada. Ecol. Appl. 2011;21:1591–1603. doi: 10.1890/10-1054.1. [DOI] [PubMed] [Google Scholar]
- 103.Potter KM, Hargrove WW. Determining suitable locations for seed transfer under climate change: a global quantitative method. New Forests. 2012;43:581–599. [Google Scholar]
- 104.Godefroid S, et al. How successful are plant species reintroductions? Biol. Cons. 2011;144:672–682. [Google Scholar]
- 105.Broadhurst LM, et al. Seed supply for broadscale restoration: maximizing evolutionary potential. Evol. Appl. 2008;1:587–597. doi: 10.1111/j.1752-4571.2008.00045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Weeks AR, et al. Assessing the benefits and risks of translocations in changing environments: a genetic perspective. Evol. Appl. 2011;4:709–725. doi: 10.1111/j.1752-4571.2011.00192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Byrne M, Stone L, Millar MA. Assessing genetic risk in revegetation. J. Appl. Ecol. 2011;48:1365–1373. [Google Scholar]
- 108.Vander Mijnsbrugge K, Bischoff A, Smith B. A question of origin: Where and how to collect seed for ecological restoration. Basic Appl. Ecol. 2010;11:300–311. [Google Scholar]
- 109.Godefroid S, Vanderborght T. Plant reintroductions: the need for a global database. Biodiv. Cons. 2011;20:3683–3688. [Google Scholar]
- 110.Merritt DJ, Dixon KW. Restoration seed banks– a matter of scale. Science. 2011;332:424–425. doi: 10.1126/science.1203083. [DOI] [PubMed] [Google Scholar]
- 111.AdapTRee website. ( http://adaptree.sites.olt.ubc.ca/),
- 112.Hicke JA, Jenkins JC. Mapping lodgepole pine stand structure susceptibility to mountain pine beetle attack across the western United States. For. Ecol. Mgmt. 2008;255:1536–1547. [Google Scholar]
- 113.Moritz C, et al. Conservation units and translocations: strategies for conserving evolutionary processes. Hereditas. 1999;130:217–228. [Google Scholar]
- 114.Godefroid S, Vanderborght T. Plant reintroductions: the need for a global database. Biodiv. Cons. 2011;20:3683–3688. [Google Scholar]
- 115.Donald CM. The breeding of crop ideotypes. Euphytica. 1968;17:385–403. [Google Scholar]
- 116.Gardner A, Grafen A. Capturing the superorganism: a formal theory of group adaptation. J. Evol. Biol. 2009;22:659–671. doi: 10.1111/j.1420-9101.2008.01681.x. [DOI] [PubMed] [Google Scholar]
- 117.Kumar A, Turner NC, Singh DP, Singh P, Barr M. Diurnal and seasonal patterns of water potential, photosynthesis, evapotranspiration and water use efficiency of clusterbean. Photosynthet. 1999;37:601–607. [Google Scholar]
- 118.Jennings PR. Plant type as a rice breeding objective. Crop Sci. 1964;4:13–15. [Google Scholar]
- 119.Duncan WG, Williams WA, Loomis RS. Tassels and productivity of maize. Crop Sci. 1967;7:37–39. [Google Scholar]
- 120.Duvick DN, Cassman KG. Post-green-revolution trends in yield potential of temperate maize in the north-central United States. Crop Sci. 1999;39:1622–1630. [Google Scholar]
- 121.Weiner J, Andersen SB, Wille WKM, Griepentrog HW, Olsen JM. Evolutionary Agroecology: the potential for cooperative, high density, weed-suppressing cereals. Evol. Appl. 2010;3:473–479. doi: 10.1111/j.1752-4571.2010.00144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wade MJ, Bijma P, Ellen ED, Muir W. Group selection and social evolution in domesticated animals. Evol Applic. 2010;3:453–465. doi: 10.1111/j.1752-4571.2010.00147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liu J, et al. Coupled Human and Natural Systems. AMBIO. 2007;36:639–649. doi: 10.1579/0044-7447(2007)36[639:chans]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 124.Rabinowitz P, Conti L. Links Among Human Health, Animal Health, and Ecosystem Health. Annu. Rev. Publ. Health. 2013;34:189–204. doi: 10.1146/annurev-publhealth-031912-114426. [DOI] [PubMed] [Google Scholar]
- 125.Woolhouse MEJ, Gowtage-Sequeria S. Host range and emerging and reemerging pathogens. Emerg. Infect. Dis. 2005;11:1842. doi: 10.3201/eid1112.050997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Olsen B, et al. Global patterns of influenza A virus in wild birds. Science. 2006;312:384–388. doi: 10.1126/science.1122438. [DOI] [PubMed] [Google Scholar]
- 127.Smith GJD, et al. Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza A epidemic. Nature. 2009;459:1122. doi: 10.1038/nature08182. [DOI] [PubMed] [Google Scholar]
- 128.Jones KE, et al. Global trends in emerging infectious diseases. Nature. 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ma W, Kahn RE, Richt JA. The pig as a mixing vessel for influenza viruses: human and veterinary implications. J. Molec. Genet. Med. 2009;3:158–166. [PMC free article] [PubMed] [Google Scholar]
- 130.Yassine HM, Lee CW, Saif YM. Interspecies Transmission of Influenza A Viruses Between Swine and Poultry. Curr. Topics Microbiol. Immunol. 2013;370:227–240. doi: 10.1007/82_2011_180. [DOI] [PubMed] [Google Scholar]
- 131.Altizer S, Bartel R, Han BA. Animal migration and infectious disease risk. Science. 2011;331:296–302. doi: 10.1126/science.1194694. [DOI] [PubMed] [Google Scholar]
- 132.Rios-Soto KR, Song BJ, Castillo-Chavez C. Epidemic spread of influenza viruses: the impact of transient populations on disease dynamics. Math. Biosci. Eng. 2011;8:199–222. doi: 10.3934/mbe.2011.8.199. [DOI] [PubMed] [Google Scholar]
- 133.Pepin KM, et al. Identifying genetic markers of adaptation for surveillance of viral host jumps. Nature Rev. Microbiol. 2010;8:802–813. doi: 10.1038/nrmicro2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.ECDC and EMEA. The bacterial challenge: time to react. 2009 ECDC/EMEA Joint Technical Report. EMEA/576176/2009 (ISBN 978-92-9193-193-4). [Google Scholar]
- 135.APUA. The cost of antibiotic resistance to US families and the healthcare system. Alliance for the Prudent Use of Antibiotics (APUA); 2010. [Google Scholar]
- 136.Cooper MA, Shlaes D. Fix the antibiotics pipeline. Nature. 2011;472:32–32. doi: 10.1038/472032a. [DOI] [PubMed] [Google Scholar]
- 137.Butler MS, Blaskovich MA, Cooper MA. Antibiotics in the clinical pipeline in 2013. J. Antibiot. 2013;66:571–591. doi: 10.1038/ja.2013.86. [DOI] [PubMed] [Google Scholar]
- 138.Bal AM, Gould IM. Antibiotic stewardship: overcoming implementation barriers. Curr. Opin. Infect. Dis. 2011;24:357–362. doi: 10.1097/QCO.0b013e3283483262. [DOI] [PubMed] [Google Scholar]
- 139.Macdougall C, Polk RE. Antimicrobial stewardship programs in health care systems. Clin. Microbiol. Rev. 2005;18:638–656. doi: 10.1128/CMR.18.4.638-656.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.US FDA. 2009 Summary Report on Antimicrobials Sold or Distributed for Use in Food-Producing Animals. US Food and Drug Administration; 2010. Dec 9, [Google Scholar]
- 141.Silbergeld EK, Graham J, Price LB. Industrial food animal production, antimicrobial resistance, and human health. Annu. Rev. Public Health. 2008;29:151–169. doi: 10.1146/annurev.publhealth.29.020907.090904. [DOI] [PubMed] [Google Scholar]
- 142.Woolhouse MEJ, Ward MJW. Sources of antimicrobial resistance. Science. 2013 doi: 10.1126/science.1243444. [DOI] [PubMed] [Google Scholar]
- 143.Manges AR, Johnson JR. Food-borne origins of Escherichia coli causing extraintestinal infections. Clin. Infect. Dis. 2012;55:712–719. doi: 10.1093/cid/cis502. [DOI] [PubMed] [Google Scholar]
- 144.Martinez JL, Olivares J. In: Antimicrobial Resistance in the Environment. Keen PL, Montforts MHMM, editors. chap. 9. Wiley-Blackwell; 2011. pp. 157–172. [Google Scholar]
- 145.Transatlantic Taskforce on Antimicrobial Resistance. [Retrieved on December 29, 2012];Recommendations for future collaboration between the US. and EU. (TATFAR Report, 2011), ( http://www.ecdc.europa.eu/en/activities/diseaseprogrammes/TATFAR/Documents/210911_TATFAR_Report.pdf) [Google Scholar]
- 146.European Commission. [Retrieved on December 29, 2012];Communication from the Commission to the European Parliament and the Council: Action plan against the rising threats from Antibmicrobial Resistance. 2011 COM 748 ( http://ec.europa.eu/dgs/health_consumer/docs/communication_amr_2011_748_en.pdf) [Google Scholar]
- 147.USFDA. [Retrieved on December 29, 2012];New Animal Drugs; Cephalosporin Drugs; Extralabel Animal Drug Use; Order of Prohibition. 2012 77 FR 735 ( https://federalregister.gov/a/2012-35)
- 148.Carroll SP, Kinnison MT, Bernatchez L. In light of evolution: interdisciplinary challenges in food, health, and the environment. Evol. Appl. 2011;4:155–158. doi: 10.1111/j.1752-4571.2011.00182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Griggs D, et al. Policy: Sustainable development goals for people and planet. Nature. 2013;495:305–307. doi: 10.1038/495305a. [DOI] [PubMed] [Google Scholar]
- 150.Convention of Biological Diversity. Strategic Plan for Biodiversity 2011–2020. Aichi Biodiversity Targets; 2010. http://www.cbd.int/sp/targets/ [Google Scholar]
- 151.Jørgensen C, et al. Ecology: managing evolving fish stocks. Science. 2007;318:1247–1248. doi: 10.1126/science.1148089. [DOI] [PubMed] [Google Scholar]
- 152.Laugen AT, et al. Evolutionary impact assessment: accounting for evolutionary consequences of fishing in an ecosystem approach to fisheries management. Fish Fish. 2012;15:1–32. doi: 10.1111/faf.12007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Baskett ML, Waples RS. Evaluating alternative strategies for minimizing unintended fitness consequences of cultured individuals on wild populations. Conserv. Biol. 2013;27:83–94. doi: 10.1111/j.1523-1739.2012.01949.x. [DOI] [PubMed] [Google Scholar]
- 154.Convention on Biological Diversity. The Nagoya Protocol on Access and Benefit-sharing. 2013 http://www.cbd.int/abs/ [Google Scholar]
- 155.Santamaría L, Méndez PF. Evolution in biodiversity policy - current gaps and future needs. Evol. Appl. 2012;5:202–218. doi: 10.1111/j.1752-4571.2011.00229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Welch EW. Potential implications of the Nagoya Protocol for the livestock sector. J. Anim. Breed. Genet. 2012;129:423–424. doi: 10.1111/jbg.12013. [DOI] [PubMed] [Google Scholar]
- 157.Jackson JA, Laikre L, Baker CS, Kendall KC. Guidelines for collecting and maintaining archives for genetic monitoring. Conserv. Genet. Resour. 2011;4:527–536. [Google Scholar]
- 158.Dana GV, Kuiken T, Rejeski D, Snow AA. Synthetic biology: Four steps to avoid a synthetic-biology disaster. Nature. 2012;483:29–29. doi: 10.1038/483029a. [DOI] [PubMed] [Google Scholar]
- 159.Snow AA, Smith VH. Genetically engineered algae for biofuels: a key role for ecologists. Bioscience. 2012;62:765–768. [Google Scholar]
- 160.Mali P, et al. RNA-guided human genome engineering via Cas9. Science. 2013;339:823–826. doi: 10.1126/science.1232033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Thomas MA. Gene tweaking for conservation. Nature. 2013;501:485–486. doi: 10.1038/501485a. [DOI] [PubMed] [Google Scholar]
- 162.Redford KH, Adams W, Mace GM. Synthetic Biology and Conservation of Nature: Wicked Problems and Wicked Solutions. PLoS Biol. 2013;11:2–5. doi: 10.1371/journal.pbio.1001530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Althouse BM, Bergstrom TC, Bergstrom CT. A public choice framework for controlling transmissible and evolving diseases. Proc. Natl. Acad. Sci. U.S.A. 2010;107:1696–1701. doi: 10.1073/pnas.0906078107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Liu J, et al. Complexity of coupled human and natural systems. Science. 2007;317:1513–1516. doi: 10.1126/science.1144004. [DOI] [PubMed] [Google Scholar]
- 165.Ostrom E. A general framework for analyzing sustainability of social-ecological systems. Science. 2009;325:419–422. doi: 10.1126/science.1172133. [DOI] [PubMed] [Google Scholar]
- 166.Ostrom E. A diagnostic approach for going beyond panaceas. Proc. Natl. Acad. Sci. U.S.A. 2007;104:15181–15187. doi: 10.1073/pnas.0702288104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hardin G. The tragedy of the commons. Science. 1968;162:1243–1248. [PubMed] [Google Scholar]
- 168.Baquero F, Campos J. Editorial The tragedy of the commons in antimicrobial chemotherapy. 2003;16:11–13. [PubMed] [Google Scholar]
- 169.Hutchison WD, et al. Area wide suppression of European corn borer with Bt maize reaps savings to non-Bt maize growers. Science. 2010;330:222–225. doi: 10.1126/science.1190242. [DOI] [PubMed] [Google Scholar]
- 170.Jaffe G. Complacency on the farm: significant noncompliance with EPA's refuge requirements threatens the future effectiveness of genetically engineered pest-protected corn. Washington, DC: Center for Science in the Public Interest; 2009. [Google Scholar]
- 171.Samuelson PA. The pure theory of public expenditure. Rev. Econ. Stat. 1954;36:387–389. [Google Scholar]
- 172.Nokes DJ, Anderson RM. Vaccine safety versus vaccine efficacy in mass immunisation programmes. The Lancet. 1991;338:1309–1312. doi: 10.1016/0140-6736(91)92601-w. [DOI] [PubMed] [Google Scholar]
- 173.Kaier K, Frank U. Measuring the externality of antibacterial use from promoting antimicrobial resistance. Pharmaco. Econom. 2010;28:1123–1128. doi: 10.2165/11535640-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 174.Kaier K. Economic modeling of the persistence of antimicrobial resistance. Nat. Resour. Model. 2011;25:388–402. [Google Scholar]
- 175.Wright GD. Antibiotic resistance in the environment: a link to the clinic? Curr. Opin. Microbiol. 2010;13:589–594. doi: 10.1016/j.mib.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 176.Herrmann M, Laxminarayan R. Antibiotic Effectiveness: New Challenges in Natural Resource Management. Annu. Rev. Resource Econom. 2010;2:125–138. [Google Scholar]
- 177.Cook CN, Mascia MB, Schwartz MW, Possingham HP, Fuller RA. Achieving conservation science that bridges the knowledge-action boundary. Conserv. Biol. 2013;27:669–678. doi: 10.1111/cobi.12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Antonovics J, et al. Evolution by any other name: Antibiotic resistance and avoidance of the E-word. PLoS Biol. 2007;5:e30. doi: 10.1371/journal.pbio.0050030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Messenger AM, Barnes AN, Gray GC. Reverse zoonotic disease transmission (Zooanthroponosis): a systematic review of seldom-documented human biological threats to animals. PloS one. 9:e89055. doi: 10.1371/journal.pone.0089055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Salguero-Gomez R. ComPADRe III demographic database. [Retrieved on May 15th, 2014];Unpublished. ( http://www.demogr.mpg.de/en/laboratories/evolutionary_biodemography_1171/projects/compadre_plant_matrix_database_comadre_animal_matrix_database_1867.htm) [Google Scholar]
- 181.Todar K. The Growth of Bacterial Populations. [Retrieved on December 29, 2012];Online textbook of Bacteriology. ( http://textbookofbacteriology.net/growth_3.html)
- 182.Rodrigo AG, et al. Coalescent estimates of HIV-1 generation time in vivo. Proc. Natl. Acad. Sci. U.S.A. 1999;96:2187–2191. doi: 10.1073/pnas.96.5.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Boyle NR, Morgan JA. Flux balance analysis of primary metabolism in Chlamydomonas reinhardtii. BMC Syst. Biol. 2009;3:4. doi: 10.1186/1752-0509-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Goulding K, Merrett M. The photometabolism of acetate by Chlorella pyrenoidosa. J. Exp. Bot. 1966;17:678–689. [Google Scholar]
- 185.Truman RW, Krahenbuhl JL, Viable M. leprae as a research reagent. Int. J. Leprosy. 2001;69:1–12. [PubMed] [Google Scholar]
- 186.Zwart MP, Daròs JA, Elena SF. One is enough: In vivo effective population size is dose-dependent for a plant RNA virus. PLoS Pathog. 2011;7:e1002122. doi: 10.1371/journal.ppat.1002122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Lee SY, Bollinger J, Bezdicek D, Ogram A. Estimation of the abundance of an uncultured soil bacterial strain by a competitive quantitative PCR method. Appl. Environ. Microbiol. 1996;62:3787–3793. doi: 10.1128/aem.62.10.3787-3793.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Danks H. Short life cycles in insects and mites. Can Entomol. 2006;138:407–463. [Google Scholar]
- 189.Cartwright G, Athens J, Wintrobe M. Analytical review: The kinetics of granulopoiesis in normal man. Blood. 1964;24:780–803. [PubMed] [Google Scholar]
- 190.Marshman E, Booth C, Potten CS. The intestinal epithelial stem cell. Bioessays. 2002;24:91–98. doi: 10.1002/bies.10028. [DOI] [PubMed] [Google Scholar]
- 191.Flindt R. Biologie in Zahlen. 6th ed. Heidelberg: Elsevier GmbH, Spektrum Akademischer Verlag; 2003. [Google Scholar]
- 192.Spalding KL, et al. Dynamics of fat cell turnover in humans. Nature. 2008;453:783–787. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- 193.Bhardwaj RD, et al. Neocortical neurogenesis in humans is restricted to development. Proc. Natl. Acad. Sci. U.S.A. 2006;103:12564–12568. doi: 10.1073/pnas.0605177103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Azevedo FAC, et al. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J. Comp. Neurol. 2009;513:532–541. doi: 10.1002/cne.21974. [DOI] [PubMed] [Google Scholar]
- 195.Fenner JN. Cross-cultural estimation of the human generation interval for use in genetics-based population divergence studies. Am. J. Phys. Anthropol. 2005;128:415–423. doi: 10.1002/ajpa.20188. [DOI] [PubMed] [Google Scholar]
- 196.World Population Prospects: The 2010 Revision. New York: 2011. [Retrieved on December 29, 2012]. United Nations, Department of Economic and Social Affairs, Population Division. ( http://esa.un.org/unpd/wpp/) [Google Scholar]
- 197.Usher MB. A matrix approach to the management of renewable resources, with special reference to selection forests. J. Appl. Ecol. 1966;3:355–367. [Google Scholar]
- 198.Usher M. A matrix model for forest management. Biometrics. 1969;25:309–315. [Google Scholar]
- 199.Southerton S. personal communication, 29 April. 2014 [Google Scholar]
- 200.Wear RG, Haslett SJ, Alexander NL. Effects of temperature and salinity on the biology of Artemia fransiscana from Lake Grassmere, New Zealand. 2. Maturation, fecundity, and generation times. J. Exp. Mar. Boil. Ecol. 1986;98:167–183. [Google Scholar]
- 201.Winston ML. The Biology of the Honey Bee. Harvard University Press; 1991. [Google Scholar]
- 202.Martin AP, Palumbi SR. Body size, metabolic rate, generation time, and the molecular clock. Proc. Natl. Acad. Sci. U.S.A. 1993;90:4087–4091. doi: 10.1073/pnas.90.9.4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Van Mantgem PJ, Stephenson NL. The accuracy of matrix population model projections for coniferous trees in the Sierra Nevada, California. J. Ecol. 2005;93:737–747. [Google Scholar]
- 204.McPherson K, Williams K. Establishment growth of cabbage palm, Sabal palmetto (Arecaceae) Am. J. Bot. 1996;83:1566–1570. [Google Scholar]
- 205.Ferrero-Serrano Á, Hild AL, Mealor BA. Can Invasive Species Enhance Competitive Ability and Restoration Potential in Native Grass Populations? Restor. Ecol. 2011;19:545–551. [Google Scholar]
- 206.Baskett ML, Levin SA, Gaines SD, Dushoff J. Marine reserve design and the evolution of size at maturation in harvested fish. Ecol. Appl. 2005;15:882–901. [Google Scholar]
- 207.Shaw DR, et al. Benchmark study on glyphosate-resistant cropping systems in the United States. Part 1: Introduction to 2006–2008. Pest Manage. Sci. 2011;67:741–746. doi: 10.1002/ps.2160. [DOI] [PubMed] [Google Scholar]
- 208.Melander AL. Can insects become resistant to sprays? J. Econ. Entomol. 1914;7:167–173. [Google Scholar]
- 209.Niederman MS. Is "crop rotation" of antibiotics the solution to a "resistant" problem in the ICU? Am. J. Respir. Crit. Care Med. 1997;156:1029–1031. doi: 10.1164/ajrccm.156.4.ed-14. [DOI] [PubMed] [Google Scholar]
- 210.Rapp RE, Datta A, Irmak S, Arkebauer TJ, Knezevic SZ. Integrated management of common reed (Phragmites australis) along the Platte River in Nebraska. Weed Technol. 2012;26:326–333. [Google Scholar]
- 211.Bergstrom DM, et al. Indirect effects of invasive species removal devastate World Heritage Island. J. Appl. Ecol. 2009;46:73–81. [Google Scholar]
- 212.Van den Bosch R, Sterrn VM. The integration of chemical and biological control of arthropod pests. Annu. Rev. Entomol. 1962;7:367–386. doi: 10.1146/annurev.en.07.010162.002055. [DOI] [PubMed] [Google Scholar]
- 213.Csermely P, Ágoston V, Pongor S. The efficiency of multi-target drugs: the network approach might help drug design. Trends Pharmacol. Sci. 2005;26:178–182. doi: 10.1016/j.tips.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 214.Mackinnon MJ, Read AF, Gandon S. Virulence evolution in response to vaccination: the case of malaria. Vaccine. 2008;26(Suppl. 3):C42. doi: 10.1016/j.vaccine.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Hanley KA, et al. A trade-off in replication in mosquito versus mammalian systems conferred by a point mutation in the NS4B protein of dengue virus type 4. Virology. 2003;312:222–232. doi: 10.1016/s0042-6822(03)00197-1. [DOI] [PubMed] [Google Scholar]
- 216.Lavergne S, Molofsky J. Control strategies for the invasive reed Canarygrass (Phalaris arundinacea L.) in North American Wetlands: the need for an Integrated Management Plan. Nat. Area. J. 2006;26:208–214. [Google Scholar]
- 217.Schoner CA, Norris RF, Chilcote W. Yellow foxtail (Setaria lutescens) biotype studies: growth and morphological characteristics. Weed. Sci. 1978;26:632–636. [Google Scholar]
- 218.Read AF, Huijben S. Evolutionary biology and the avoidance of antimicrobial resistance. Evol. Appl. 2009;2:40–51. doi: 10.1111/j.1752-4571.2008.00066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Schetelig MR, et al. Site-specific recombination for the modification of transgenic strains of the Mediterranean fruit fly Ceratitis capitata. Proc. Natl. Acad. Sci. U.S.A. 2009;106:18171–18176. doi: 10.1073/pnas.0907264106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Crotty S, et al. The broad-spectrum antiviral ribonucleoside ribavirin is an RNA virus mutagen. Nat. Med. 2000;6:1375–1379. doi: 10.1038/82191. [DOI] [PubMed] [Google Scholar]
- 221.Eigen M. Error catastrophe and antiviral strategy. Proc. Natl. Acad. Sci. U.S.A. 2002;99:13374–13376. doi: 10.1073/pnas.212514799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Wise de Valdez MR, et al. Genetic elimination of dengue vector mosquitoes. Proc. Natl. Acad. Sci. U.S.A. 2011;108:4772–4775. doi: 10.1073/pnas.1019295108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Bardsley D, Hugo G. Migration and climate change: examining thresholds of change to guide effective adaptation decision-making. Popul. Environ. 2010;32:238–262. [Google Scholar]
- 224.Jacobsen T, Adams RM. Salt and silt in ancient Mesopotamian agriculture: progressive changes in soil salinity and sedimentation contributed to the breakup of past civilizations. Science. 1958;128:1251–1258. doi: 10.1126/science.128.3334.1251. [DOI] [PubMed] [Google Scholar]
- 225.Berger J, et al. Chickpea evolution has selected for contrasting phenological mechanisms among different habitats. Euphytica. 2011;180:1–15. [Google Scholar]
- 226.Carrera-Bastos P. The western diet and lifestyle and diseases of civilization. Res. Rep. Clinic. Cardiol. 2011;2:15–35. [Google Scholar]
- 227.Turner LB. A meta-analysis of fat intake, reproduction, and breast cancer risk: An evolutionary perspective. Am. J. Human Biol. 2011;23:601–608. doi: 10.1002/ajhb.21176. [DOI] [PubMed] [Google Scholar]
- 228.Gluckman PD, Hanson MA, Low FM. The role of developmental plasticity and epigenetics in human health. Birth Defects Res. C. Embryo. Today. 2011;93:12–18. doi: 10.1002/bdrc.20198. [DOI] [PubMed] [Google Scholar]
- 229.Rice KR, Emery NC. Managing microevolution: restoration in the face of global change. Front. Ecol. Environ. 2003;1:469–478. [Google Scholar]
- 230.Sgrò CM, Lowe AJ, Hoffmann AA. Building evolutionary resilience for conserving biodiversity under climate change. Evol. Appl. 2011;4:326–337. doi: 10.1111/j.1752-4571.2010.00157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Redpath N, Osgathorpe LM, Park K, Goulson D. Crofting and bumblebee conservation: The impact of land management practices on bumblebee populations in northwest. Scotland. Biol. Conserv. 2010;143:492–500. [Google Scholar]
- 232.Jarvis A, Lane A, Hijmans RJ. The effect of climate change on crop wild relatives. Agric. Ecosys. Environ. 2008;126:13–23. [Google Scholar]
- 233.Trethowan RM, Turner MA, Chattha TM. Breeding strategies to adapt crops to a changing climate. In: Lobell D, Burke M, editors. Climate Change and Food Security. Springer Netherlands: 2010. pp. 155–174. [Google Scholar]
- 234.Dupuy LC, et al. Directed molecular evolution improves the immunogenicity and protective efficacy of a Venezuelan equine encephalitis virus DNA vaccine. Vaccine. 2009;27:4152–4160. doi: 10.1016/j.vaccine.2009.04.049. [DOI] [PubMed] [Google Scholar]
- 235.Yap TA, Carden CP, Kaye SB. Beyond chemotherapy: targeted therapies in ovarian cancer. Nat. Rev. Cancer. 2009;9:167–181. doi: 10.1038/nrc2583. [DOI] [PubMed] [Google Scholar]
- 236.Cho H, et al. Optimized clinical performance of growth hormone with an expanded genetic code. Proc. Natl. Acad. Sci. U.S.A. 2011;108:9060–9065. doi: 10.1073/pnas.1100387108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Bartel MA, Weinstein JR, Schaffer DV. Directed evolution of novel adeno-associated viruses for therapeutic gene delivery. Gene therapy. 2012;19:694–700. doi: 10.1038/gt.2012.20. [DOI] [PubMed] [Google Scholar]
- 238.Venesky MD, Mendelson JR, III, Sears BF, Stiling P, Rohr JR. Selecting for tolerance against pathogens and herbivores to enhance success of reintroduction and translocation. Conserv. Biol. 2012;26:586–592. doi: 10.1111/j.1523-1739.2012.01854.x. [DOI] [PubMed] [Google Scholar]
- 239.Lopez S, Rousset F, Shaw FH, Shaw RG, Ronce O. Joint effects of inbreeding and local adaptation on the evolution of genetic load after fragmentation. Conserv. Biol. 2009;23:1618–1627. doi: 10.1111/j.1523-1739.2009.01326.x. [DOI] [PubMed] [Google Scholar]
- 240.Davis EB, Koo MS, Conroy C, Patton JL, Moritz C. The California Hotspots Project: identifying regions of rapid diversification of mammals. Mol. Ecol. 2008;17:120–138. doi: 10.1111/j.1365-294X.2007.03469.x. [DOI] [PubMed] [Google Scholar]
- 241.Hannah L. A global conservation system for climate-change adaptation. Conserv. Biol. 2010;24:70–77. doi: 10.1111/j.1523-1739.2009.01405.x. [DOI] [PubMed] [Google Scholar]
- 242.Jacobs DF. Toward development of silvical strategies for forest restoration of American chestnut (Castanea dentata) using blight-resistant hybrids. Biol. Conserv. 2007;137:497–506. [Google Scholar]
- 243.Muir WM. Group selection for adaptation to multiple-hen cages: selection program and direct responses. Poult. Sci. 1996;75:447–458. doi: 10.3382/ps.0750447. [DOI] [PubMed] [Google Scholar]
- 244.Denison RF, Kiers ET, West SA. Darwinian agriculture: when can humans find solutions beyond the reach of natural selection? Q. Rev. Biol. 2003;78:145–168. doi: 10.1086/374951. [DOI] [PubMed] [Google Scholar]
- 245.Condon AG, Richards RA, Rebetzke GJ, Farquhar GD. Breeding for high water-use efficiency. J. Exp. Bot. 2004;55:2447–2460. doi: 10.1093/jxb/erh277. [DOI] [PubMed] [Google Scholar]
- 246.Carroll SP, Watters JV. Managing phenotypic variability with genetic and environmental heterogeneity: adaptation as a first principle of conservation practice. In: Carroll SP, Fox CW, editors. Conservation Biology–Evolution in Action. Oxford Univ. Press; 2008. pp. 181–198. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.