Abstract
During development, neural circuits are initially generated by exuberant innervation and are rapidly refined by selective preservation and elimination of axons. The establishment and maintenance of functional circuits therefore requires coordination of axon survival and degeneration pathways. Both developing and mature circuits rely on interdependent mitochondrial and cytoskeletal components to maintain axonal health and homeostasis; injury or diseases that impinge on these components frequently cause pathologic axon loss. Here, we review recent findings that identify mechanisms of axonal preservation in the contexts of development, injury, and disease.
Keywords: axon injury, axon maintenance, axon survival, neurodegeneration, neurotrophins, Wallerian degeneration
Establishing and maintaining circuits
Functional neural circuits depend on proper interconnections formed by long range axonal projections. As the fundamental connective unit of neural circuits, axons must be protected and maintained in the face of multiple potential threats. Axonal maintenance is particularly important because most neurons cannot be replaced and must therefore be preserved throughout the life of the organism. Axon degeneration is a broad term applied to various modes of axon death, with distinct instigators but similar final morphology of axon fragmentation. Though much progress has been made toward elucidating the mechanisms underlying axonal degeneration, a complete understanding requires study of the mechanisms opposing degeneration. Axon regeneration, the recovery and regrowth of axons following acute or chronic trauma, is not the opposite of axon degeneration, but rather a response to it. In reality the opposite of axon degeneration is the process of axonal survival.
It was classically thought that axons degenerate as a result of cell body death, due to a lack of support from the cell body. This theory was first challenged by the discovery of the Wallerian degeneration slow (WldS) mutant mouse (see Glossary), where neuronal expression of the WldS fusion gene delays degeneration of severed axons for weeks. More recent studies have provided direct evidence for active axonal death mechanisms, such as the pro-degeneration molecule dSARM/SARM1 [1,2], as well as for pro-survival mechanisms, such as the Bcl-2 family member Bcl-w (Bcl2l2) [3–5]. Thus, it is now apparent that the axonal compartment relies on distinctive pathways for survival and degeneration, and these exhibit similarities to and differences from cell body survival and death mechanisms [5–13]. In this review, we first examine mechanisms of developmental axon survival and pruning. We then discuss pathways promoting lifelong axonal maintenance and health, and the opposing degenerative processes triggered by injury and disease. Recent reviews have addressed axon regeneration [14,15] and dendritic degeneration [11].
Developmental axon preservation
A common theme in neural development is overproduction followed by elimination and refinement. This mechanism allows for great flexibility in potential circuit configuration [7]. In both the central and peripheral nervous systems, neurons initially extend excess axonal connections, and refinement into a mature circuit requires coordinated pruning of inappropriate connections and preservation of appropriate connections. Pruning must therefore be induced in a selective subset of axons while the remaining axons are protected and maintained. Further, the scale of axonal elimination must be closely regulated. Pruning can remove segments as small as axon terminals or as large as whole axons, and can even include subsequent apoptosis of the cell body.
Extracellular cues
Extracellular cues from other neurons within a circuit or from nearby glial or target cells often determine which axons will initiate intracellular axon pro-survival pathways and which will be removed. Critical cues that have been identified include network activation and secretion of growth factors. During early postnatal development of the neuromuscular junction (NMJ), muscle cells are initially innervated by multiple motor neuron terminal arbors. These overlapping inputs compete for survival in an activity-dependent manner. Inputs delivering stronger and more correlated activity are strengthened, and the remaining inputs are eliminated, such that each muscle cell is ultimately innervated by a single motor neuron [7]. A similar activity-dependent mechanism is used in the developing cerebellum to select for survival of a single climbing fiber input onto a single Purkinje cell [16]. Activity regulated mechanisms including changes in transcription as well as cytoskeletal and morphological adaptation, enable maintenance of axons connected within a functional circuit.
Neurotrophins, nerve growth factor (NGF), brain derived growth factor (BDNF), and neurotrophin 3 and 4 (NT3 and NT4), constitute the most well recognized growth factor family that promotes axonal and neuronal survival. In the peripheral nervous system, survival of sympathetic and sensory neurons depends on successful competition for a limited supply of target-derived neurotrophins. Furthermore, local stimulation with neurotrophins regulates axonal growth, branching, and terminal arborization [8,17–20]. Neurotrophins secreted by target cells bind to tropomyosin-receptor-kinase (Trk) receptors located on innervating axon terminals and initiate both local and retrograde signaling events in the axon. This paradigm has been studied in vitro through the use of various compartmented culture platforms that spatially and fluidically isolate cell bodies and distal axons, and so replicate the separation between axon terminals and cell bodies that occurs within normal neuronal circuits. In these compartmented culture platforms, cell bodies and axons can be independently deprived of or stimulated with neurotrophins, and changes within cell bodies and axons can be assayed separately. In pioneering studies using sympathetic neurons grown in compartmented cultures, Campenot first demonstrated that local axonal neurotrophin stimulation, a correlate of in vivo target-derived neurotrophin stimulation, is required to promote axonal survival, whereas cell body survival is supported by either somatic or axonal neurotrophin stimulation [21].
Inhibitors of axonal apoptosis
Until recently, the involvement of the apoptotic cascade in developmental axon degeneration was largely discounted [22]. Seminal work from several groups has since described an apoptotic caspase cascade within axons that is induced by neurotrophin withdrawal, and identified anti-apoptotic proteins that promote developmental axon survival by inhibiting this specialized cascade (Figure 1).
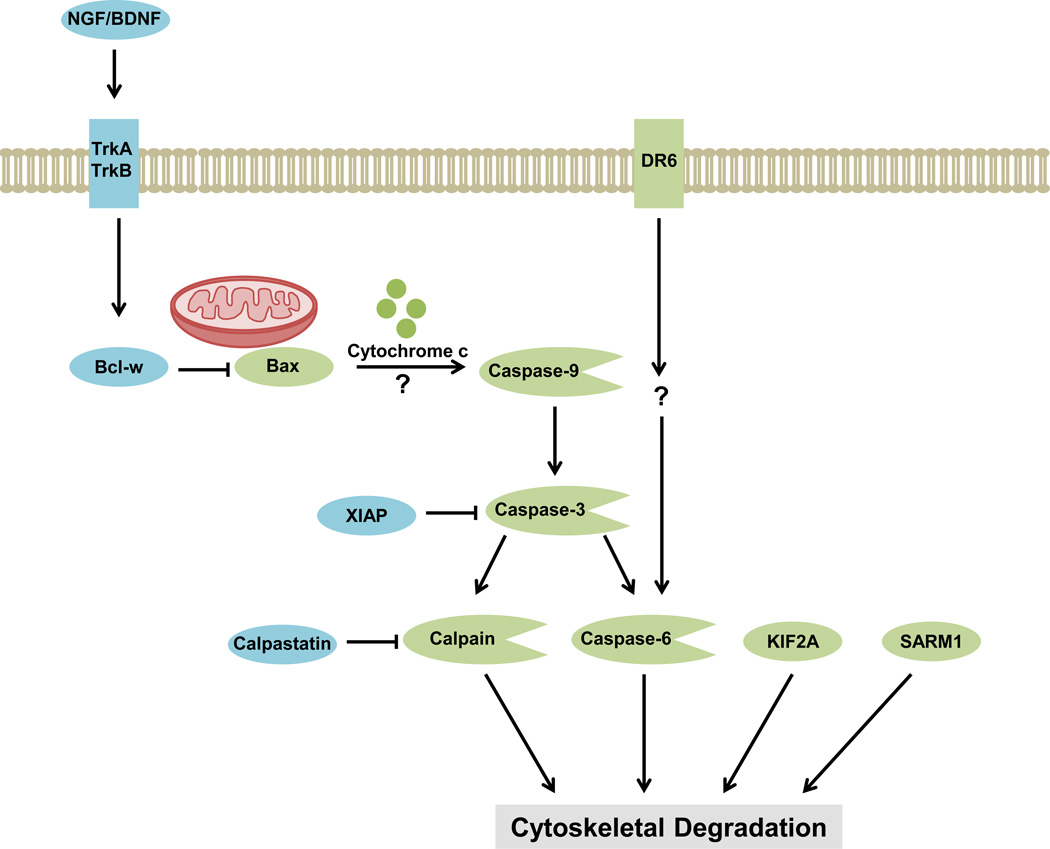
Figure 1.
Developmental axon survival and degeneration pathways. Following trophic withdrawal, parallel pro-degenerative cascades converge on a common pathway of cytoskeletal degradation to induce axon degeneration. Pro-survival molecules (blue) actively inhibit pro-degenerative molecules (green). The neurotrophins NGF and BDNF stimulate TrkA and TrkB receptors on the growing axon and induce axonal expression of the anti-apoptotic Bcl-2 family member Bcl-w. Bcl-w inhibits the pro-apoptotic Bcl-2 family member Bax, preventing activation of the axonal apoptotic cascade [3,5]. The endogenous inhibitors XIAP and calpastatin also inhibit the degenerative proteases caspase-3 and calpain respectively, preventing downstream cytoskeletal degradation [25,26,30]. In the absence of neurotrophins, Bax elicits mitochondrial release of cytochrome c and activation of the protease caspase-9 by an unknown mechanism [26,27]. Caspase-9 cleaves and activates caspase-3, which itself activates caspase-6 and the calcium-sensitive protease calpain [13,25,27–30]. In addition, the receptor DR6 can initiate activation of caspase-6 [27,45]. The proteins KIF2A [46] and SARM1 [2] also induce cytoskeletal degradation in the absence of trophic support, though it is unknown how they are regulated. Abbreviations: Bcl-w, Bcl-2-like protein 2; BDNF, brain derived growth factor; DR6, death receptor 6; KIF2A, Kinesin superfamily protein 2A; NGF, nerve growth factor; TrkA, tropomyosin-receptor-kinase A receptor; TrkB, tropomyosin-receptor-kinase B receptor; SARM1, sterile α-motif-containing and armadillo-motif containing protein; XIAP, X-linked inhibitor of apoptosis protein.
Anti-apoptotic Bcl-2 family members Bcl-2, Bcl-xL (also known as Bcl2l1), and Bcl-w (Bcl2l2) avert somatic apoptosis by binding and sequestering pro-apoptotic Bcl-2 family members, thus preventing mitochondrial release of cytochrome c and subsequent activation of caspases. Of these closely related family members, only Bcl-w mRNA and protein are enriched in axons [4,5]. Bcl-w expression can be detected in late embryonic and early postnatal development as axon terminals reach their peripheral targets, and expression continues throughout adulthood [4]. Moreover, target-derived neurotrophins selectively stimulate a pathway that relies on the MAP kinase ERK5 to induce transcription of Bcl-w and of a set of retrograde response genes [3,23]. Bcl-w binds and inhibits the anti-apoptotic Bcl-2 family member Bax, thus preventing changes in the mitochondrial membrane potential, cytochrome c release, and subsequent initiation of the axonal caspase cascade [5]. Genetic studies indicate Bcl-w functions within the axon to promote axon survival and prevent degeneration [3,5].
In contrast to Bcl-2 proteins, the inhibitor of apoptosis (IAP) proteins prevent initiation of the apoptotic cascade by direct inhibition of caspases, as they bind to activated, cleaved caspase-3 [24,25]. Thus, X-linked inhibitor of apoptosis protein (XIAP) provides a second mode of control that restrains axonal caspase activity and subsequent axonal pruning. Cultured sensory neurons lacking XIAP degenerate more rapidly when deprived of neurotrophin, and embryonic XIAP−/− mice show decreased epidermal sensory innervation without a concomitant loss of cell bodies [25,26]. Furthermore, XIAP−/− neurons grown in compartmented cultures exhibit increased somatic levels of active caspase-3 when neurotrophins are withdrawn only from axons, suggesting a role for XIAP in spatially restricting apoptotic cascade activation to the axon [26].
The caspase cascade activated in axonal degeneration involves an initial and essential catalytic function for caspase-3, followed by activation of caspase-6 [13,25,27–30]. The roles of these two caspases in axonal degeneration have been demonstrated by analysis of genetic models. The calcium-activated calpain family constitutes a second set of proteases implicated in both developmental and pathological axon degeneration. In neurotrophin-supported axons, calpastatin inhibits calpain activation. Upon neurotrophin deprivation, calpastatin is degraded by activated caspase-3, allowing downstream activation of calpain and subsequent cleavage of calpain targets such as neurofilaments [30].
Although primarily regarded as mediating pathological axon degeneration, there is also some evidence that an NAD+-sensitive pathway operates in parallel with the caspase cascade during axon pruning [13,31]. Combined treatment with NAD+ and caspase inhibitors completely protects wildtype axons from neurotrophin withdrawal, while the individual inhibitors alone only exert incomplete protective effects [13]. These results suggest that NAD+ can promote survival of neurotrophin-deprived axons, but it is not yet known whether an NAD+-sensitive pathway is endogenously activated during axon pruning, and how it might interact with the axonal apoptotic cascade.
Regulating protein levels
Regulation of axonal degenerative cascades requires precisely controlled localization and quantities of protective factors. New axonal proteins are supplied by anterograde microtubule-dependent transport as well as by local translation. Inhibition of axonal protein synthesis with local cycloheximide treatment abolishes the axon survival effect of neurotrophins, suggesting that neurotrophins rely in part on locally synthesized factors to mediate axon survival [5]. Two such factors have been recently identified: Bcl-w and IMPA1 (myo-inositol monophosphatase-1). As indicated previously, Bcl-w plays a role in local inhibition of the caspase cascade, and also regulates mitochondrial morphology and function [5]. IMPA1 is a key enzyme of the inositol cycle and therefore necessary for proper induction of phosphoinositide pathways triggered by neurotrophin signaling [32]. Given the thousands of mRNAs identified in embryonic axons, it is likely additional protective factors are locally translated to stabilize axons connected within a circuit [33].
Protein turnover is frequently regulated by the Ubiquitin Proteasome System (UPS). Global inhibition of the proteasome with pharmacological agents or genetic mutations have yielded conflicting results, with some studies finding it prevents axon pruning [30,34] and others finding it accelerates pruning [26]. One mechanism by which proteasome inhibition may be protective is by preventing degradation of pro-survival molecules. Axonal XIAP and calpastatin are both degraded upon neurotrophin withdrawal, resulting in loss of axon viability [25,30]. XIAP is degraded by the UPS, thus releasing caspase-3 and allowing induction of the axonal apoptotic cascade [13,25] and activation of calpain [30]. Conversely, the UPS may also promote axonal survival by degrading pro-apoptotic proteins.
Developmental axon pruning
Axons or axonal segments that are not selected to survive can be eliminated by three distinct mechanisms (Figure 2A–B). The most well studied is axon degeneration, which culminates in cytoskeletal degradation, axon fragmentation, and removal of debris by glia and possibly epidermal cells (Figure 2A) [6–8,11,12,35]. In contrast, both axon retraction (Figure 2B) and axosome shedding (Figure 2C) have only been observed during small-scale pruning of synapses or terminal branches. Axon retraction is the pulling back and absorption of small segments of axon [7,8], and axosome shedding involves axon tip swelling and shedding of membrane-bound axonal remnants called axosomes [6,7,36]. While axon remnants are absorbed by the neuron itself during retraction, axosomes are engulfed by adjacent cells, such as the surrounding Schwann cells [36].
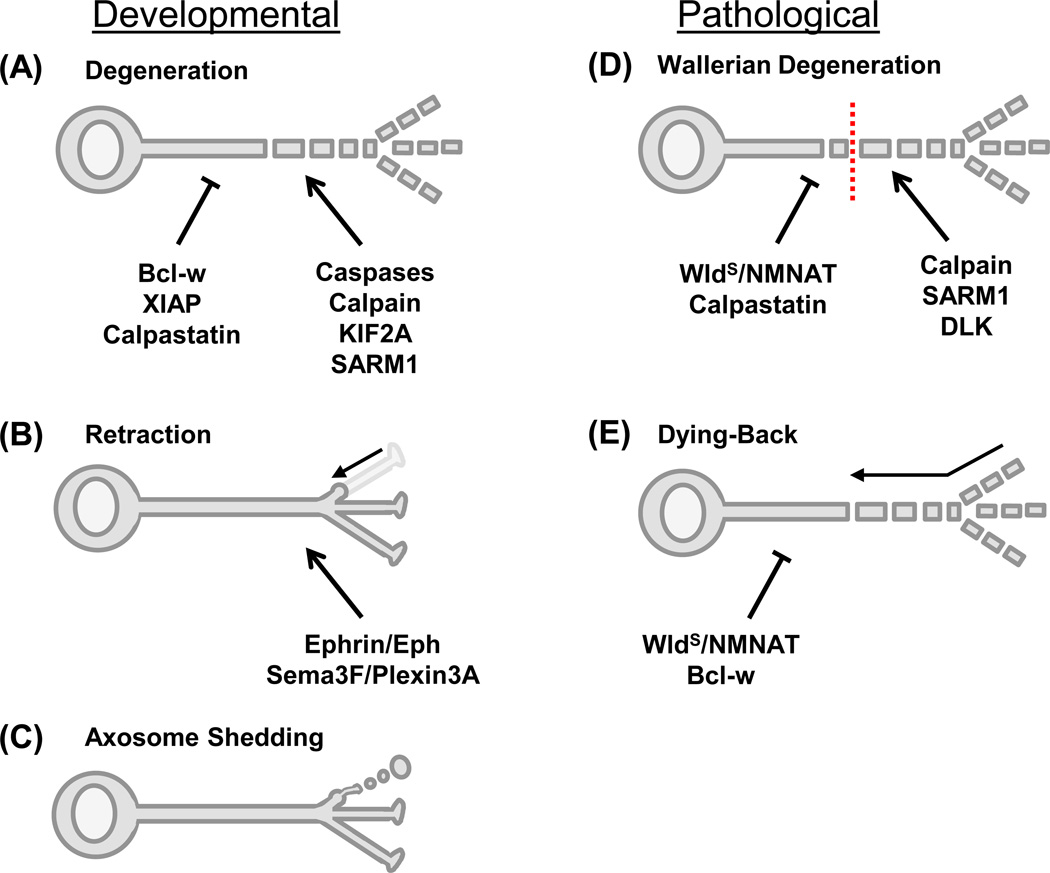
Figure 2.
Modes and mechanisms of axon elimination. Schematics of neurons during developmental pruning (A–C) and pathological axon loss (D–E). (A) Developmental axon degeneration, as occurs with trophic deprivation, results in cytoskeletal degradation and axon fragmentation. Degeneration is prevented by Bcl-w, XIAP, and/or calpastatin and driven by caspases, calpain, KIF2A, and SARM1 [2–5,13,25–30]. (B) Retraction involves pulling back and absorption of small axon segments, such as axon terminal branches. It can be driven by Ephrin/Eph [40,41] or Sema3F/Plexin3A [39] signaling pathways. (C) Axosome shedding also removes small axon segments, with swelling of the axon tip and shedding of membrane-bound axon remnants called axosomes. The underlying molecular mechanisms are still unknown. [36]. (D) Wallerian degeneration is a type of pathological axon elimination induced by axon severing (indicated by a dotted red line). Following a latency phase, the axon distal to the injury site undergoes swelling, cytoskeletal breakdown, and fragmentation. Various NMNAT isoforms, the WldS fusion protein, and calpastatin prevent degeneration, while SARM1, DLK, and calpain drive degeneration [1,2,30,95]. (E) In many neurodegenerative diseases axons are eliminated by a dying-back process that involves axon swelling and fragmentation beginning distally and propagating in a proximal direction. Various NMNAT isoforms, the WldS fusion protein, and Bcl-w can oppose this process [4]. Abbreviations: Bcl-w, Bcl-2-like protein 2; DLK, duel leucine zipper kinase; KIF2A, Kinesin superfamily protein 2A; NMNAT, nicotinamide mononucleotide adenylyltransferase; Sema3F, semaphorin 3F; SARM1, sterile α-motif-containing and armadillo-motif containing protein; WldS, Wallerian degeneration slow protein; XIAP, X-linked inhibitor of apoptosis protein.
Extracellular signaling
Some cells in a circuit promote axon survival, while others actively induce axon pruning. In sympathetic neurons, competition results in the more active axons eliminating their competitors. NGF-TrkA and activity dependent signaling cascades maintain the winning axons. Meanwhile, the winning axons release proBDNF, which binds to p75 neurotrophin receptor (p75NTR) in losing axons and induces axon degeneration [37]. A similar mechanism of p75NTR- mediated axon degeneration and Trk-mediated axon survival is critical for correct circuit connectivity in adult septal cholinergic neurons [38]. Several guidance molecules secreted in target regions are also known to induce axon pruning. These include Semaphorin 3F and its receptor Plexin3A, which is involved in pruning of hippocampal mossy fiber collaterals [39], and Ephrins and their receptor Ephs, which mediate RGC topographical mapping [7] and axon retraction [40,41].
Luo and colleagues demonstrated that ecdysone hormone signaling instigates axon pruning of mushroom body (MB) γ neuron during Drosophila metamorphosis. Ecdysone hormone stimulates ecdysone receptor B1 (EcR-B1) expressed selectively on γ neurons and induces axon pruning [42]. Glial cells control neuronal expression of EcR-B1 by secreting the TGF-β ligand myoglianin, activating TGF-β signaling pathways in γ neurons [43,44].
Neurotrophin deprivation engages additional pathways to mediate axonal destruction. In particular, TNF family receptors such as DR6 contribute to axonal degeneration via downstream activation of caspase-6 [27,45]. This cascade may be particularly relevant in Alzheimer’s disease.
Intracellular signaling
Several intracellular signaling mechanisms play a role in axon pruning. As discussed, the apoptotic cascade mediates developmental axon degeneration and is controlled by both negative (XIAP, Bcl-w) and positive (TNF-receptor DR6) regulators. Neurotrophin withdrawal pathways converge on the pro-apoptotic Bcl-2 family member Bax, which causes cytochrome c release from mitochondria [26,27]. Interestingly, while cytochrome c binds Apaf-1 to activate caspase-9 in cell soma apoptosis, apparently Apaf-1 is not required for axon degeneration, and so the mechanism for activation of caspase-9 in axons is not yet clear [26]. Following activation of caspase-9, caspase-3 is cleaved and activated, and caspase-3 directly activates caspase-6 and indirectly activates calpain via calpastatin cleavage [29,30]. Together these results suggest that there are both similarities and distinctions between the axonal and somatic apoptotic cascades that will be important to decipher. Furthermore, while the apoptotic machinery is involved in some types of developmental axon degeneration (neurotrophin deprivation-induced degeneration, retinocollicular axon pruning) [13,25,27–30], it does not appear to be involved in others (MB γ neuron pruning) [34]. These findings suggest there may be multiple mechanisms of developmental axon degeneration that converge on a common pathway of cytoskeletal breakdown.
Developmental axon degeneration can be initiated by the mammalian Toll receptor adaptor SARM1 [2]. SARM1 is activated in parallel with the caspase cascade during neurotrophin deprivation and appears to function primarily at an early stage of degeneration [2]. The mechanism by which SARM1 mediates axon degeneration is unknown, but its Toll interleukin-1 receptor and sterile α motif domains are necessary for its destructive function [2]. Like the caspase cascade, the Drosophila ortholog dSARM does not appear to play a role in MB γ neuron axon pruning [1], but both SARM1 and dSARM promote injury-induced axon degeneration [1,2].
Cytoskeletal breakdown is a common, late feature of axon degeneration and does not seem to be involved in the more restricted processes of axosome shedding or axon retraction. Recent advances have provided some insight into the mechanisms of cytoskeletal breakdown during degeneration. The Kinesin superfamily protein 2A (KIF2A), a microtubule depolymerizing protein, is a key executor of microtubule breakdown and axonal degeneration during neurotrophin withdrawal-induced axon pruning, and so mice lacking KIF2A exhibit delayed degeneration of sensory neurons innervating the skin [46]. In the future it will be important to ascertain how KIF2A is regulated to selectively depolymerize the microtubule cytoskeleton in degenerating axons.
Lifelong axon maintenance
Numerous mechanisms control the health and homeostasis of axons throughout life, and oppose stressors such as excitation and aging. Injury and disease induce axon degeneration both by compromising maintenance mechanisms and promoting active self-destruction pathways. Expression of the Wallerian degeneration slow (WldS) mutant protein, a chimeric fusion of the NAD+ biosynthetic enzyme NMNAT1 and a fragment of the ubiquitination factor E4B (UBE4B), delays axonal degeneration induced by numerous pathological insults (see Conforti et. al. 2014 for an extensive summary of the effects of WldS/NMNAT on various axon pathologies [47]) [48–50]. Study of the WldS protein and its constituents has provided great insight into mechanisms of axonal viability that rely on the interrelated processes of metabolic homeostasis, calcium buffering, axonal transport, and protein synthesis.
Metabolic maintenance
Proper metabolism is integral to axon viability and functionality. Axons require large amounts of energy and metabolites to support membrane depolarization and synaptic transmission [51], and thus the NAD+ biosynthetic NMNAT enzymes are essential in axon maintenance [48–50]. Mammalian NMNAT isoforms include the nuclear NMNAT1, the golgi-associated NMNAT2, and the mitochondrial NMNAT3 [50]. WldS is a chimeric version of NMNAT1, and its ability to delay axon degeneration requires NAD+ synthetic activity in the cytoplasm, and possibly in the axon [52–54]. While overexpression of either NMNAT2 [55–57] or NMNAT3 [54] delays degeneration of injured axons, only endogenous NMNAT2 is required for maintenance of healthy axons [55,58–60]. WldS may confer its protection by directly substituting for the more labile NMNAT2, as NMNAT2 is rapidly degraded following injury while WldS is degraded more slowly [55,59]. Furthermore, mutant forms of NMNAT2 with prolonged half-life exhibit a level of axon protection comparable to WldS [59].
While NMNAT enzymatic activity is necessary for axon viability, it is less clear whether this effect requires the enzymatic product NAD+ [52–54]. Injured axons exhibit a dramatic decrease in NAD+ prior to morphological degradation, and degeneration is mitigated in vitro by exogenous NAD+ [61,62]. However, protection by exogenous NAD+ requires supra-physiological levels [52,61,63,64], increasing cellular NAD+ levels by inhibiting NAD+-consuming enzymes does not protect injured axons [65], nor does the inhibition of NAD+ biosynthesis abolish WldS-mediated protection [52]. Furthermore, WldS does not detectably increase overall cellular levels of NAD+ [65,66]. A possible explanation that reconciles these disparate results is that WldS and NMNATs induce a local increase in NAD+, perhaps at mitochondria, rather than increasing total cytoplasmic content of NAD+ [12,54]. This hypothesis is supported by evidence that cytoplasmic WldS copurifies with mitochondria [53,54,67].
If NAD+ is a pro-survival factor, how does it act to protect axons? Axonal energy is primarily supplied by mitochondria, which use NAD+ to produce ATP. Axonal injury decreases both NAD+ and ATP levels even before morphological degeneration [61]. Exogenous application of NAD+ or expression of WldS sustains both NAD+ and ATP levels and delays subsequent degeneration [61]. In addition, WldS mitochondria exhibit enhanced ability to generate ATP, suggesting that NMNAT/WldS may therefore protect axons by maintaining mitochondrial bioenergetics (Figure 3) [54].
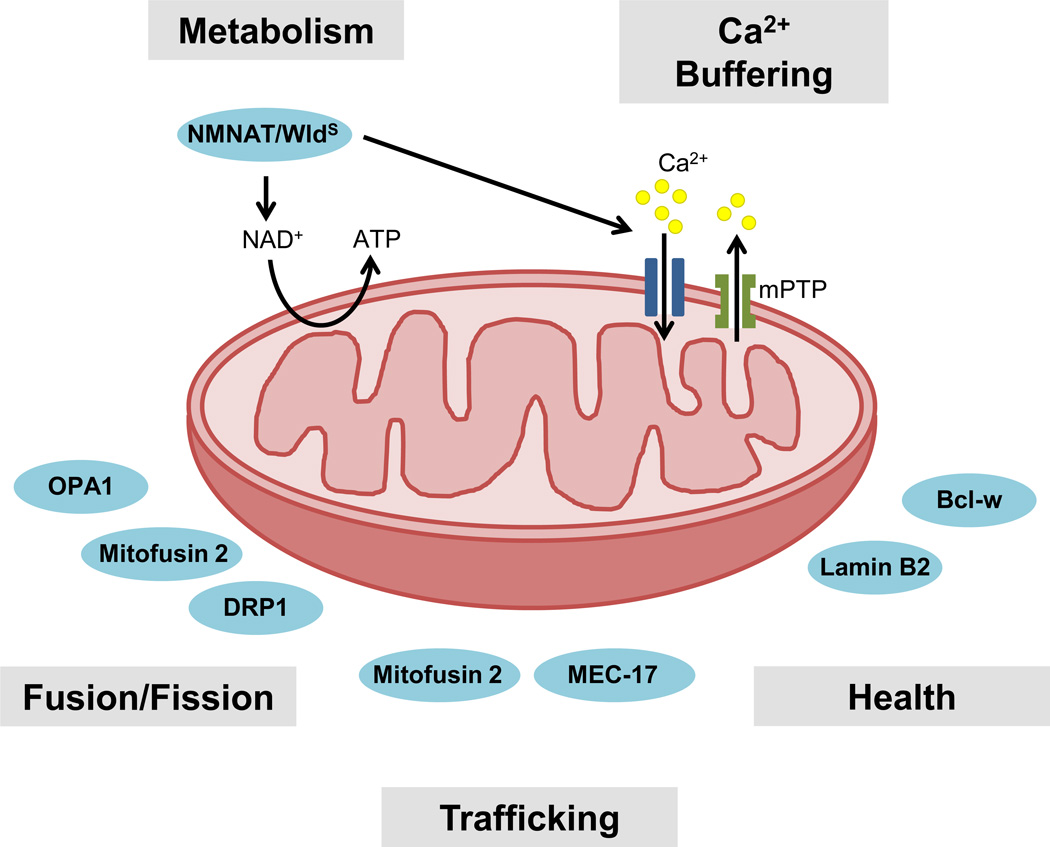
Figure 3.
Mitochondria are central to axon health and homeostasis. The interdependent mitochondrial processes of metabolism and calcium buffering maintain axonal health and are impaired by injury and disease. WldS, a chimeric fusion of the enzyme NMNAT1 and the ubiquitination factor E4B, delays axonal degeneration induced by injury and disease. NMNAT/WldS produces NAD+, which is used by mitochondria to produce ATP. NMNAT/WldS may promote axonal survival by preventing injury-induced loss of NAD+ and ATP [61]. Innate mitochondrial calcium buffering maintains calcium homeostasis and is overwhelmed by axon injury, resulting in formation of the mPTP and increased axoplasmic calcium levels [12,70,71,73]. NMNAT/WldS increases the calcium buffering capacity of axonal mitochondria [67], possibly by increasing ATP synthesis [61]. Several processes are necessary for mitochondrial function and localization, including fusion and fission, mitochondrial trafficking, and maintenance of mitochondrial health by the proteins Lamin B2 [76] and Bcl-w [4]. Mutations in the α tubulin acetyltransferase MEC-17 and the fusion and fission proteins OPA1, Mitofusin2, and DRP1 are implicated in axon degenerative diseases [79–81]. Abbreviations: ATP, adenosine triphosphate; Ca2+, calcium ion; DRP1, GTPase dynamin-related protein 1; mPTP, mitochondrial permeability transition pore; NAD+, nicotinamide adenine dinucleotide; NMNAT, nicotinamide mononucleotide adenylyltransferase; OPA1, optic atrophy 1; WldS, Wallerian degeneration slow protein.
Calcium homeostasis
Calcium, an important regulator of synaptic transmission, mitochondrial transport and function, and of diverse signaling cascades [51,68], also activates pro-degenerative axonal components [30,69]. Traumatic injury elevates intra-axonal calcium by impairing both extracellular calcium efflux and mitochondrial calcium sequestration, and thereby promotes axon degeneration [12,70,71]. Mitochondria normally control calcium levels by cytosolic calcium uptake (Figure 3) [72]. However, excess intracellular calcium levels can cause mitochondrial overloading, formation of the mitochondrial permeability transition pore (mPTP), and subsequent release of calcium from intra-mitochondrial stores [68]. Therefore, inhibiting mPTP activation or decreasing intracellular calcium prevents degeneration of injured axons [73].
NMNAT/WldS functions upstream of increased intra-axonal calcium, since inhibition of mPTP does not further delay degeneration of WldS axons [73] and exogenous calcium treatment abolishes WldS-mediated axon protection [74]. A recent study demonstrated that WldS suppresses injury-induced axonal calcium elevation, and that WldS mitochondria have increased calcium buffering capacity [67]. Given that mitochondrial ATP production, membrane potential, and calcium buffering are highly interrelated [68], it is possible that NMNAT/WldS increases mitochondrial calcium buffering as a result of increasing ATP synthesis [67]. However, detailed temporal and spatial examinations of mitochondrial ATP and calcium levels are needed to test this theory.
Mitochondrial quality control and localization
Given the importance of mitochondria in metabolism and calcium buffering, maintaining a population of healthy mitochondria is essential for axon viability. Numerous mechanisms promote mitochondrial health and remove dysfunctional mitochondria; impairment of these processes is central to many neurodegenerative diseases (Figure 3) [75].
Several proteins contribute to the health of axonal mitochondria. Bcl-w is necessary for axonal maintenance in mature animals, in addition to its function in development [4]. Mice lacking Bcl-w exhibit elongated axonal mitochondria together with adult-onset degeneration of peripheral sensory axons [4]. Axonal Lamin B2, a nuclear skeleton protein, is essential for axon viability in Xenopus retinal ganglion cells [76]. In the absence of Lamin B2, axonal mitochondria are elongated and exhibit impaired membrane potential [76]. As previously discussed, NMNAT/WldS promotes mitochondrial function, enhancing mitochondrial ATP synthesis [54], calcium buffering capacity [67], and motility [67,77]. Mitochondrial fusion and fission are opposing processes whose balance maintains mitochondrial morphology and function [78]. Mutations in components controlling fusion and fission are responsible for several axon degenerative diseases. Notably, mutations affecting the mitochondrial fusion protein optic atrophy 1 (OPA1) or the mitochondrial fission protein GTPase dynamin-related protein 1 (DRP1) result in atrophy of optic nerve axons [79,80]. In addition, mutation of the mitochondrial fusion protein Mitofusin 2 suppresses both mitochondrial fusion and transport and causes Charcot-Marie Tooth Disease [81].
Axonal transport both delivers new mitochondria and removes dysfunctional mitochondria, and so disruptions in mitochondrial transport contribute to multiple neurodegenerative diseases [75,82]. Mitochondria are actively transported to regions with high energetic demands, such as synapses, in order to maintain proper circuit connectivity [75,82]. Mitophagy, or mitochondrial autophagy, which removes damaged mitochondria from axons, also relies on axonal transport [75,83]. While axon injury causes cessation of mitochondrial movement, WldS prevents mitochondrial stalling after injury and increases basal mitochondrial motility in uninjured axons [67]. The ability of WldS to enhance mitochondrial motility correlates with improved calcium buffering capacity by mitochondria [67]. Similarly, cytoplasmically-targeted NMNAT1 partially protects against chemotoxic injury-induced deficits in mitochondrial transport [84]. Mitochondrial motility is essential for WldS or NMNAT1 to protect axons after injury, highlighting the essential role of mitochondrial transport in axon viability [67,84]. Mitochondrial transport is closely tied to cytoskeletal balance, as demonstrated by a recent study in C. elegans showing that loss of the α-tubulin acetyltransferase MEC-17 causes microtubule destabilization, reduced axonal mitochondria numbers, and spontaneous axon degeneration [85].
Cytoskeletal stability
The axonal cytoskeleton consists of microtubules, actin, and neurofilaments. Microtubules, the main highway for long-range retrograde and anterograde transport, are in a constant state of dynamic instability, with continuous depolymerization and repolymerization [86]. Pharmacological hyperstabilization [87] or destabilization [88] of axonal microtubules causes degeneration. Furthermore, injured [89], MEC-17 mutant [85], or neurotrophin-deprived [46] axons, which typically undergo microtubule destabilization, can be protected by the microtubule stabilizing agent paclitaxel. Thus, paclitaxel, which is toxic to healthy axons [87], may act to restore cytoskeletal balance in degenerating axons. These results suggest that carefully balanced microtubule stability is critical for axon health (Figure 4).
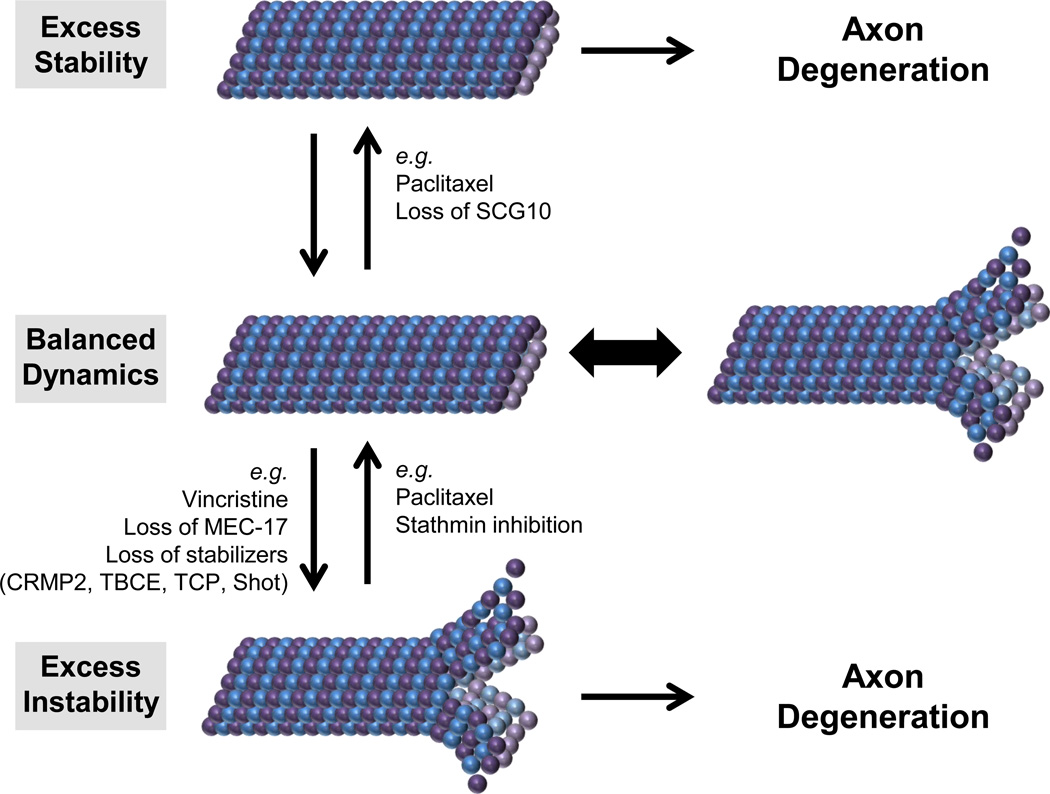
Figure 4.
Balanced cytoskeletal dynamics are essential for axon maintenance. Microtubules are in a constant state of depolymerization and polymerization. Genetic and pharmacological insults that either stabilize microtubules (such as the chemotherapeutic paclitaxel or loss of the microtubule destabilizing protein SCG10) or destabilize microtubules (such as the chemotherapeutic vincristine, loss of MEC-17, or loss of microtubule stabilizing proteins CRMP2, TBCE, TCP, or Shot) cause axon degeneration [87,88,90,91,93,94]. Axons with pathologically destabilized microtubules can be protected by treatment with the microtubule stabilizing agent paclitaxel [46,85,89] or inhibition of the microtubule destabilizing protein stathmin [92]. Abbreviations: CRMP2, collapsin response mediator protein 2; SCG10, superior cervical ganglion 10; TBCE, tubulin-specific chaperone E; TCP, T-complex protein; Shot, short stop.
Cytoskeletal stability is regulated by several protective or degenerative molecules. For example, superior cervical ganglion 10 (SCG10) is a microtubule destabilizing protein that promotes axonal health. SCG10 is rapidly degraded following axon injury, and maintaining SCG10 levels preserves mitochondrial motility and delays axon degeneration [90]. While SCG10 knockdown does not cause spontaneous axon degeneration, it does accelerate injury-induced degeneration [90]. Conversely, inhibition of microtubule destabilizing proteins can protect axons with pathological microtubule instability. Progressive motor neuronopathy (pmn) mice have a mutation in the tubulin chaperone Tbce gene that impairs microtubule polymerization and causes motor axon degeneration [91]. The transcription factor STAT3 locally inhibits axonal stathmin, a microtubule destabilizing protein in the same family as SCG10, and rescues axonal pathology in cultured pmn motor neurons [92]. Microtubule stabilizing proteins have also been shown to be necessary for axon health. The collapsin response mediator protein 2 (CRMP2) promotes microtubule stability. Axon injury causes CRMP2 degradation, and maintaining CRMP2 levels delays degeneration [93].
Accumulating evidence suggests that balanced cytoskeletal stability regulates the pro-degeneration molecule duel leucine zipper kinase (DLK) and its Drosophila ortholog Wallenda. Genetic loss of the cytoskeletal stabilizing protein spectraplakin short stop (Shot) or the tubulin chaperone TCP1 leads to cytoskeletal instability and DLK activation [94]. DLK/Wallenda mediates a degenerative response by activating the c-Jun N-terminal kinase (JNK) [95]. Notably, DLK function is context dependent and can promote both axon regeneration [96–99] and presynaptic bouton development [100].
Maintaining protein levels
As in developmental axon preservation, axonal maintenance requires supply, localization, and turnover of protein. Axonal transport and local translation provide maintenance factors to the axon. Conversely, UPS-mediated protein degradation promotes axon degeneration by reducing levels of several axonal maintenance factors [101–104]. While local translation is required for mitochondrial function and axon viability [105], only the protective factor Lamin B2 is known to be translated locally in mature axons [76]. In contrast, the maintenance factors NMNAT2 and SCG10 are rapidly degraded in axons by the UPS and must be constantly renewed by anterograde transport from the cell body [55,59].
Pathological axon degeneration
Axons damaged by injury or disease must be actively eliminated. In some cases, removal of damaged axons enables axon regrowth and maintains neural circuitry, such as following lesions of peripheral axons. However, when axon trauma is more widespread or axons are not capable of regenerating, pathological axon degeneration compromises neural circuit functionality.
Following nerve transection, axons undergo Wallerian degeneration (Figure 2D). During an initial latency stage, the injured axon remains intact and electrically functional; this is followed by a rapid degenerative phase involving swelling, cytoskeletal degradation, and axon fragmentation. Because the precise timing and location of injury can be controlled, nerve transection is commonly used as a simple model of axon degenerative diseases. In Wallerian degeneration, transport is interrupted by the physical severing of the axon, resulting in a loss of labile pro-survival factors such as NMNAT2 [59,60,103,104] and SCG10 [90]. Axonal ATP levels rapidly decline [61], axonal mitochondria stall [67], and the innate calcium buffering capabilities of the axon fail [12,70,71]. Future studies will be needed to define the exact temporal and causal relationships among these events. Many neurodegenerative diseases also exhibit axon loss, with a distal to proximal gradient of swelling and fragmenting that resembles Wallerian degeneration, described as a dying-back axonopathy (Figure 2E). Impaired axonal mitochondrial integrity, function, and transport are common pathologies of neurodegenerative diseases, including Alzheimer’s Disease, Huntington’s Disease, Parkinson’s Disease, Amyotrophic Lateral Sclerosis, and others [75].
Concluding remarks and future directions
The establishment and functionality of neural networks requires precise control of axon survival and elimination in development and throughout life. Recent studies describe core mechanisms that preserve connections between cells in a circuit and eliminate surplus or damaged connections. The mechanisms that govern axon survival and elimination have similarities to and differences from cell soma viability and death. In particular, interdependent mitochondrial and cytoskeletal processes are central to axon survival, and impairment of these processes by injury or disease leads to pathological axon degeneration.
The field of axonal survival and death has been very active recently, including studies across multiple organisms and multiple types of neurons. These studies have benefited from improved in vivo methodologies as well as improved spatially compartmented culture systems that will now enable future studies to address the critical questions that remain (Box 1). A major question is the degree of overlap between developmental and mature axon survival pathways as well as their opposing developmental and pathological axon destruction mechanisms. In identifying key players in these processes, recent studies have already defined some commonalities and differences. Another major question is whether pro-degeneration factors or other pro-survival factors are translated locally in the axon. Despite the abundance of mRNAs in both developing and mature axons [34], few pro-survival factors are known to be locally synthesized. Investigations into these questions and others will elucidate mechanisms that maintain healthy axons within neural networks.
Box 1. Outstanding Questions.
What are the similarities and differences between developmental and pathological axon survival and death pathways?
Are multiple axon survival or death factors locally synthesized in the axon?
How is axonal caspase-9 activated in the absence of Apaf-1?
Is the axonal apoptotic cascade involved in pathological axon degeneration?
What is the significance of the similarities and differences between cell soma survival/death and axon survival/death? Do these differences restrict degeneration to the axon?
What is the role of electrical activity in maintaining mature axons in a functional circuit?
Highlights.
Pro-survival molecules protect axons from developmental pruning.
Apoptotic machinery mediates axonal pruning during development.
Lifelong axon maintenance requires proper mitochondrial and cytoskeletal function.
Impaired axon maintenance results in pathological axon degeneration.
Acknowledgments
Our research is supported by the National Institutes of Health grant R01NS050674, the Barr Weaver Award, and the Harvard/MIT Joint Research Grant in Basic Neuroscience to R.A.S., and by the Edward R. and Anne G. Lefler Center Predoctoral Fellowship to S.E.P. We thank Dr. Katharina Cosker, Sara Fenstermacher, and Maria Pazyra-Murphy for critical reading of the manuscript.
Glossary
- Apaf-1 (Apoptotic protease activating factor 1)
A key constituent of the apoptotic machinery that binds cytochrome c and subsequently activates caspase-9.
- Bcl-w (Bcl-2-like protein 2)
A pro-survival Bcl-2 family member that binds pro-apoptotic Bcl-2 family members to prevent initiation of the axonal apoptotic caspase cascade [4].
- Calpains
A family of calcium-activated cysteine proteases that degrade cytoskeletal components and are activated in both developmental and pathological axon degeneration [30].
- Calpastatin
An endogenous calpain inhibitor, which is degraded during developmental and pathological axon degeneration [30].
- Caspases (cysteine-aspartic proteases)
A family of cysteine proteases essential for cell body apoptosis and developmental axon degeneration. Caspases are first synthesized as inactivate pro-caspases, which are activated upon cleavage.
- DLK (dual leucine zipper kinase)
A mitogen-activated protein kinase kinase kinase involved in axon degeneration and regeneration [94–99]. Its Drosophila ortholog is Wallenda.
- DR6 (death receptor 6)
A tumor necrosis factor (TNF) receptor whose activation induces apoptosis and axon degeneration [27,45]
- IMPA1 (myo-inositol monophosphatase-1)
An enzyme involved in synthesis of myo-inositol and therefore essential for phosphophatidylinositol signaling pathways such as neurotrophin signaling.
- Lamin B2
A component of the nuclear skeleton that also localizes to axons and is essential for axon maintenance [76].
- MEC-17
An enzyme that catalyzes tubulin acetylation, a posttranslational modification, and which also stabilizes microtubules and preserves axons independent of its acetyltransferase activity [85].
- Neurotrophins
A family of secreted growth factors that promote axonal and neuronal survival by binding to transmembrane tropomyosin-receptor-kinase (Trk) receptors.
- NMNAT (nicotinamide mononucleotide adenylyltransferase)
A family of NAD+ biosynthetic enzymes involved in axon maintenance, and a component of the WldS fusion gene.
- NAD+ (nicotinamide adenine dinucleotide)
An essential coenzyme for cellular metabolism and signaling, which functions in ATP metabolism and as a substrate for several protein-modifying enzymes [48]
- mPTP (mitochondrial permeability transition pore)
A protein pore formed in the inner membrane of the mitochondria under pathological conditions, allowing mitochondrial release of calcium stores and reactive oxygen species.
- SARM1 (sterile α-motif-containing and armadillo-motif containing protein)
A Toll-like receptor adaptor essential for axon degeneration by an unknown mechanism [1,2]. Its Drosophila ortholog is dSARM.
- SCG10 (superior cervical ganglion 10)
A microtubule destabilizing protein that promotes axonal health. It is degraded in a JNK-dependent manner following axon injury [90].
- UPS (Ubiquitin Proteasome System)
The major non-lysosomal mechanism by which the cell degrades proteins. Proteins are targeted for degradation by the small protein tag ubiqituin via ubiquitin ligase enzymes, and tagged proteins are subsequently degraded by the proteasome complex.
- Wallerian degeneration
A form of axon degeneration resulting from focal nerve transection wherein the axon distal to the injury site swells and fragments.
- WldS (Wallerian degeneration slow)
A chimeric fusion gene of the NAD+ biosynthetic enzyme NMNAT1 and the ubiquitination factor E4B, whose expression delays various forms of pathological axon degeneration.
- XIAP (X-linked inhibitor of apoptosis protein)
An inhibitor of apoptosis protein that binds to and inhibits caspases to prevent initiation of cell body apoptosis and the axonal apoptotic cascade [25,26].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Osterloh J, et al. dSarm/Sarm1 is required for activation of an injury-induced axon death pathway. Science (New York, NY.) 2012;337:481–484. doi: 10.1126/science.1223899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gerdts J, et al. Sarm1-mediated axon degeneration requires both SAM and TIR interactions. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:13569–13580. doi: 10.1523/JNEUROSCI.1197-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pazyra-Murphy M, et al. A retrograde neuronal survival response: target-derived neurotrophins regulate MEF2D and bcl-w. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:6700–6709. doi: 10.1523/JNEUROSCI.0233-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Courchesne S, et al. Sensory neuropathy attributable to loss of Bcl-w. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:1624–1634. doi: 10.1523/JNEUROSCI.3347-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cosker K, et al. Target-derived neurotrophins coordinate transcription and transport of bclw to prevent axonal degeneration. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:5195–5207. doi: 10.1523/JNEUROSCI.3862-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Low L, Cheng H. A little nip and tuck: axon refinement during development and axonal injury. Current opinion in neurobiology. 2005;15:549–556. doi: 10.1016/j.conb.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 7.Luo L, O'Leary D. Axon retraction and degeneration in development and disease. Annual review of neuroscience. 2005;28:127–156. doi: 10.1146/annurev.neuro.28.061604.135632. [DOI] [PubMed] [Google Scholar]
- 8.Saxena S, Caroni P. Mechanisms of axon degeneration: from development to disease. Progress in neurobiology. 2007;83:174–191. doi: 10.1016/j.pneurobio.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Coleman M. Axon degeneration mechanisms: commonality amid diversity. Nature reviews. Neuroscience. 2005;6:889–898. doi: 10.1038/nrn1788. [DOI] [PubMed] [Google Scholar]
- 10.Yan T, et al. Axon degeneration: Mechanisms and implications of a distinct program from cell death. Neurochem Int. 2010;56:529–534. doi: 10.1016/j.neuint.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 11.Maor-Nof M, Yaron A. Neurite pruning and neuronal cell death: spatial regulation of shared destruction programs. Current opinion in neurobiology. 2013;23:990–996. doi: 10.1016/j.conb.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 12.Wang J, et al. Axon degeneration: molecular mechanisms of a self-destruction pathway. The Journal of cell biology. 2012;196:7–18. doi: 10.1083/jcb.201108111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schoenmann Z, et al. Axonal degeneration is regulated by the apoptotic machinery or a NAD+-sensitive pathway in insects and mammals. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:6375–6386. doi: 10.1523/JNEUROSCI.0922-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Z, et al. Peripheral regeneration. Annual review of neuroscience. 2007;30:209–233. doi: 10.1146/annurev.neuro.30.051606.094337. [DOI] [PubMed] [Google Scholar]
- 15.Massimo AH. Axonal degeneration and regeneration: a mechanistic tug-of-war. Journal of neurochemistry. 2009 doi: 10.1111/j.1471-4159.2008.05754.x. [DOI] [PubMed] [Google Scholar]
- 16.Hashimoto K, Kano M. Functional differentiation of multiple climbing fiber inputs during synapse elimination in the developing cerebellum. Neuron. 2003;38:785–796. doi: 10.1016/s0896-6273(03)00298-8. [DOI] [PubMed] [Google Scholar]
- 17.LeMaster A, et al. Overexpression of brain-derived neurotrophic factor enhances sensory innervation and selectively increases neuron number. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19:5919–5931. doi: 10.1523/JNEUROSCI.19-14-05919.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Causing C, et al. Synaptic innervation density is regulated by neuron-derived BDNF. Neuron. 1997;18:257–267. doi: 10.1016/s0896-6273(00)80266-4. [DOI] [PubMed] [Google Scholar]
- 19.Albers K, et al. Overexpression of nerve growth factor in epidermis of transgenic mice causes hypertrophy of the peripheral nervous system. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1994;14:1422–1432. doi: 10.1523/JNEUROSCI.14-03-01422.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou F, et al. NGF-induced axon growth is mediated by localized inactivation of GSK-3beta and functions of the microtubule plus end binding protein APC. Neuron. 2004;42:897–912. doi: 10.1016/j.neuron.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 21.Campenot R. Development of sympathetic neurons in compartmentalized cultures. II. Local control of neurite survival by nerve growth factor. Developmental biology. 1982;93:13–21. doi: 10.1016/0012-1606(82)90233-0. [DOI] [PubMed] [Google Scholar]
- 22.Finn J, et al. Evidence that Wallerian degeneration and localized axon degeneration induced by local neurotrophin deprivation do not involve caspases. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:1333–1341. doi: 10.1523/JNEUROSCI.20-04-01333.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watson F, et al. Neurotrophins use the Erk5 pathway to mediate a retrograde survival response. Nature neuroscience. 2001;4:981–988. doi: 10.1038/nn720. [DOI] [PubMed] [Google Scholar]
- 24.Salvesen G, Duckett C. IAP proteins: blocking the road to death's door. Nature reviews. Molecular cell biology. 2002;3:401–410. doi: 10.1038/nrm830. [DOI] [PubMed] [Google Scholar]
- 25.Unsain N, et al. XIAP regulates caspase activity in degenerating axons. Cell Reports. 2013;4:751–763. doi: 10.1016/j.celrep.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 26.Cusack C, et al. Distinct pathways mediate axon degeneration during apoptosis and axon-specific pruning. Nature communications. 2013;4:1876. doi: 10.1038/ncomms2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nikolaev A, et al. APP binds DR6 to trigger axon pruning and neuron death via distinct caspases. Nature. 2009;457:981–989. doi: 10.1038/nature07767. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Vohra B, et al. Amyloid precursor protein cleavage-dependent and - independent axonal degeneration programs share a common nicotinamide mononucleotide adenylyltransferase 1-sensitive pathway. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:13729–13738. doi: 10.1523/JNEUROSCI.2939-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simon D, et al. A caspase cascade regulating developmental axon degeneration. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:17540–17553. doi: 10.1523/JNEUROSCI.3012-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang J, et al. Regulation of axon degeneration after injury and in development by the endogenous calpain inhibitor calpastatin. Neuron. 2013;80:1175–1189. doi: 10.1016/j.neuron.2013.08.034. [DOI] [PubMed] [Google Scholar]
- 31.Deckwerth T, Johnson E. Neurites can remain viable after destruction of the neuronal soma by programmed cell death (apoptosis) Developmental biology. 1994;165:63–72. doi: 10.1006/dbio.1994.1234. [DOI] [PubMed] [Google Scholar]
- 32.Andreassi C, et al. An NGF-responsive element targets myo-inositol monophosphatase-1 mRNA to sympathetic neuron axons. Nature neuroscience. 2010;13:291–301. doi: 10.1038/nn.2486. [DOI] [PubMed] [Google Scholar]
- 33.Gumy LF, et al. Transcriptome analysis of embryonic and adult sensory axons reveals changes in mRNA repertoire localization. Rna. 2011;17:85–98. doi: 10.1261/rna.2386111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watts R, et al. Axon pruning during Drosophila metamorphosis: evidence for local degeneration and requirement of the ubiquitin-proteasome system. Neuron. 2003;38:871–885. doi: 10.1016/s0896-6273(03)00295-2. [DOI] [PubMed] [Google Scholar]
- 35.Han C, et al. Epidermal cells are the primary phagocytes in the fragmentation and clearance of degenerating dendrites in Drosophila. Neuron. 2014;81:544–560. doi: 10.1016/j.neuron.2013.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bishop D, et al. Axon branch removal at developing synapses by axosome shedding. Neuron. 2004;44:651–661. doi: 10.1016/j.neuron.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 37.Singh K, et al. Developmental axon pruning mediated by BDNF-p75NTR-dependent axon degeneration. Nature neuroscience. 2008;11:649–658. doi: 10.1038/nn.2114. [DOI] [PubMed] [Google Scholar]
- 38.Park K, et al. p75NTR-dependent, myelin-mediated axonal degeneration regulates neural connectivity in the adult brain. Nature neuroscience. 2010;13:559–566. doi: 10.1038/nn.2513. [DOI] [PubMed] [Google Scholar]
- 39.Bagri A, et al. Stereotyped pruning of long hippocampal axon branches triggered by retraction inducers of the semaphorin family. Cell. 2003;113:285–299. doi: 10.1016/s0092-8674(03)00267-8. [DOI] [PubMed] [Google Scholar]
- 40.Xu N-J, Henkemeyer M. Ephrin-B3 reverse signaling through Grb4 and cytoskeletal regulators mediates axon pruning. Nature neuroscience. 2009;12:268–276. doi: 10.1038/nn.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Petros TJ, et al. Ephrin-B2 elicits differential growth cone collapse and axon retraction in retinal ganglion cells from distinct retinal regions. Developmental neurobiology. 2010 doi: 10.1002/dneu.20821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee T, et al. Cell-autonomous requirement of the USP/EcR-B ecdysone receptor for mushroom body neuronal remodeling in Drosophila. Neuron. 2000 doi: 10.1016/s0896-6273(00)00155-0. [DOI] [PubMed] [Google Scholar]
- 43.Takeshi A, et al. Glia instruct developmental neuronal remodeling through TGF-β signaling. Nature neuroscience. 2011 doi: 10.1038/nn.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu X, et al. Plum, an immunoglobulin superfamily protein, regulates axon pruning by facilitating TGF-β signaling. Neuron. 2013;78:456–468. doi: 10.1016/j.neuron.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Olsen O, et al. Genetic Analysis Reveals that Amyloid Precursor Protein and Death Receptor 6 Function in the Same Pathway to Control Axonal Pruning Independent of beta-Secretase. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:6438–6447. doi: 10.1523/JNEUROSCI.3522-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maor-Nof M, et al. Axonal pruning is actively regulated by the microtubule-destabilizing protein kinesin superfamily protein 2A. Cell Reports. 2013;3:971–977. doi: 10.1016/j.celrep.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 47.Conforti L, et al. Wallerian degeneration: an emerging axon death pathway linking injury and disease. Nature reviews. Neuroscience. 2014;15:394–409. doi: 10.1038/nrn3680. [DOI] [PubMed] [Google Scholar]
- 48.Wang J, He Z. NAD and axon degeneration: from the Wlds gene to neurochemistry. Cell adhesion & migration. 2009;3:77–87. doi: 10.4161/cam.3.1.7483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coleman M, Freeman M. Wallerian degeneration, wld(s), and nmnat. Annual review of neuroscience. 2010;33:245–267. doi: 10.1146/annurev-neuro-060909-153248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ali Y, et al. NMNATs, evolutionarily conserved neuronal maintenance factors. Trends in neurosciences. 2013;36:632–640. doi: 10.1016/j.tins.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sheng Z-H. Mitochondrial trafficking and anchoring in neurons: New insight and implications. The Journal of cell biology. 2014;204:1087–1098. doi: 10.1083/jcb.201312123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Conforti L, et al. Wld S protein requires Nmnat activity and a short N-terminal sequence to protect axons in mice. The Journal of cell biology. 2009;184:491–500. doi: 10.1083/jcb.200807175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beirowski B, et al. Non-nuclear Wld(S) determines its neuroprotective efficacy for axons and synapses in vivo. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:653–668. doi: 10.1523/JNEUROSCI.3814-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yahata N, et al. Nicotinamide mononucleotide adenylyltransferase expression in mitochondrial matrix delays Wallerian degeneration. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:6276–6284. doi: 10.1523/JNEUROSCI.4304-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gilley J, Coleman M. Endogenous Nmnat2 is an essential survival factor for maintenance of healthy axons. PLoS biology. 2010;8 doi: 10.1371/journal.pbio.1000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Feng Y, et al. Overexpression of Wld(S) or Nmnat2 in mauthner cells by single-cell electroporation delays axon degeneration in live zebrafish. Journal of neuroscience research. 2010;88:3319–3327. doi: 10.1002/jnr.22498. [DOI] [PubMed] [Google Scholar]
- 57.Yan T, et al. Nmnat2 delays axon degeneration in superior cervical ganglia dependent on its NAD synthesis activity. Neurochem Int. 2010;56:101–106. doi: 10.1016/j.neuint.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 58.Milde S, et al. Deletions within its subcellular targeting domain enhance the axon protective capacity of Nmnat2 in vivo. Scientific reports. 2013;3:2567. doi: 10.1038/srep02567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Milde S, et al. Subcellular localization determines the stability and axon protective capacity of axon survival factor Nmnat2. PLoS biology. 2013;11 doi: 10.1371/journal.pbio.1001539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gilley J, Coleman MP. Endogenous Nmnat2 is an essential survival factor for maintenance of healthy axons. PLoS biology. 2009;8 doi: 10.1371/journal.pbio.1000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang J, et al. A local mechanism mediates NAD-dependent protection of axon degeneration. The Journal of cell biology. 2005;170:349–355. doi: 10.1083/jcb.200504028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sasaki Y, et al. Stimulation of nicotinamide adenine dinucleotide biosynthetic pathways delays axonal degeneration after axotomy. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:8484–8491. doi: 10.1523/JNEUROSCI.2320-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Araki T, et al. Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science (New York, NY) 2004;305:1010–1013. doi: 10.1126/science.1098014. [DOI] [PubMed] [Google Scholar]
- 64.Conforti L, et al. NAD(+) and axon degeneration revisited: Nmnat1 cannot substitute for Wld(S) to delay Wallerian degeneration. Cell death and differentiation. 2007;14:116–127. doi: 10.1038/sj.cdd.4401944. [DOI] [PubMed] [Google Scholar]
- 65.Sasaki Y, et al. Nicotinamide mononucleotide adenylyl transferase-mediated axonal protection requires enzymatic activity but not increased levels of neuronal nicotinamide adenine dinucleotide. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:5525–5535. doi: 10.1523/JNEUROSCI.5469-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mack T, et al. Wallerian degeneration of injured axons and synapses is delayed by a Ube4b/Nmnat chimeric gene. Nature neuroscience. 2001;4:1199–1206. doi: 10.1038/nn770. [DOI] [PubMed] [Google Scholar]
- 67.Avery M, et al. WldS prevents axon degeneration through increased mitochondrial flux and enhanced mitochondrial Ca2+ buffering. Current biology : CB. 2012;22:596–600. doi: 10.1016/j.cub.2012.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brookes P, et al. Calcium, ATP, ROS: a mitochondrial love-hate triangle. American journal of physiology. Cell physiology. 2004;287:33. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- 69.George EB, et al. Axotomy-induced axonal degeneration is mediated by calcium influx through ion-specific channels. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1995;15:6445–6452. doi: 10.1523/JNEUROSCI.15-10-06445.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stirling D, Stys P. Mechanisms of axonal injury: internodal nanocomplexes and calcium deregulation. Trends in molecular medicine. 2010;16:160–170. doi: 10.1016/j.molmed.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Marambaud P, et al. Calcium signaling in neurodegeneration. Molecular neurodegeneration. 2009;4:20. doi: 10.1186/1750-1326-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rizzuto R, et al. Mitochondria as all-round players of the calcium game. J Physiol. 2000;529(1):37–47. doi: 10.1111/j.1469-7793.2000.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Barrientos S, et al. Axonal degeneration is mediated by the mitochondrial permeability transition pore. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:966–978. doi: 10.1523/JNEUROSCI.4065-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Glass J, et al. Calcium-mediated degeneration of the axonal cytoskeleton in the Ola mouse. Journal of neurochemistry. 1994;62:2472–2475. doi: 10.1046/j.1471-4159.1994.62062472.x. [DOI] [PubMed] [Google Scholar]
- 75.Court F, Coleman M. Mitochondria as a central sensor for axonal degenerative stimuli. Trends in neurosciences. 2012;35:364–372. doi: 10.1016/j.tins.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 76.Yoon B, et al. Local translation of extranuclear lamin B promotes axon maintenance. Cell. 2012;148:752–764. doi: 10.1016/j.cell.2011.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fang C, et al. Axonal transport plays a crucial role in mediating the axon-protective effects of NmNAT. Neurobiology of disease. 2014 doi: 10.1016/j.nbd.2014.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chan D. Mitochondrial fusion and fission in mammals. Annual review of cell and developmental biology. 2006;22:79–99. doi: 10.1146/annurev.cellbio.22.010305.104638. [DOI] [PubMed] [Google Scholar]
- 79.Waterham HR, et al. A Lethal Defect of Mitochondrial and Peroxisomal Fission. N Engl J Med. 2007 doi: 10.1056/NEJMoa064436. [DOI] [PubMed] [Google Scholar]
- 80.Delettre C, et al. Nuclear gene OPA1, encoding a mitochondrial dynamin-related protein, is mutated in dominant optic atrophy. Nature. 2000 doi: 10.1038/79936. [DOI] [PubMed] [Google Scholar]
- 81.Misko AL, et al. Mitofusin2 mutations disrupt axonal mitochondrial positioning and promote axon degeneration. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:4145–4155. doi: 10.1523/JNEUROSCI.6338-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schwarz TL. Mitochondrial trafficking in neurons. Cold Spring Harbor perspectives in biology. 2013;5 doi: 10.1101/cshperspect.a011304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Maday S, et al. Autophagosomes initiate distally and mature during transport toward the cell soma in primary neurons. The Journal of cell biology. 2012;196:407–417. doi: 10.1083/jcb.201106120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cheng F, et al. Axonal transport plays a crucial role in mediating the axon-protective effects of NmNAT. Neurobiology of disease. 2014 doi: 10.1016/j.nbd.2014.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Neumann B, Hilliard MA. Loss of MEC-17 leads to microtubule instability and axonal degeneration. Cell Reports. 2014 doi: 10.1016/j.celrep.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mitchison T, Kirschner M. Dynamic instability of microtubule growth. Nature. doi: 10.1038/312237a0. [DOI] [PubMed] [Google Scholar]
- 87.Yang IH, et al. Compartmentalized microfluidic culture platform to study mechanism of paclitaxel-induced axonal degeneration. Experimental neurology. 2009;218:124–128. doi: 10.1016/j.expneurol.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Silva A, et al. Evidence for direct axonal toxicity in vincristine neuropathy. Journal of the peripheral nervous system : JPNS. 2006;11:211–216. doi: 10.1111/j.1529-8027.2006.0090.x. [DOI] [PubMed] [Google Scholar]
- 89.King A, et al. Excitotoxin-induced caspase-3 activation and microtubule disintegration in axons is inhibited by taxol. Acta neuropathologica communications. 2013 doi: 10.1186/2051-5960-1-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shin J, et al. SCG10 is a JNK target in the axonal degeneration pathway. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:705. doi: 10.1073/pnas.1216204109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schaefer MKE, et al. Progressive Motor Neuronopathy: A Critical Role of the Tubulin Chaperone TBCE in Axonal Tubulin Routing from the Golgi Apparatus. Journal of Neuroscience. 2007 doi: 10.1523/JNEUROSCI.1599-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Selvaraj B, et al. Local axonal function of STAT3 rescues axon degeneration in the pmn model of motoneuron disease. The Journal of cell biology. 2012;199:437–451. doi: 10.1083/jcb.201203109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wakatsuki S, et al. ZNRF1 promotes Wallerian degeneration by degrading AKT to induce GSK3B-dependent CRMP2 phosphorylation. Nature cell biology. 2011;13:1415–1423. doi: 10.1038/ncb2373. [DOI] [PubMed] [Google Scholar]
- 94.Valakh V, et al. Loss of the spectraplakin short stop activates the DLK injury response pathway in Drosophila. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:17863–17873. doi: 10.1523/JNEUROSCI.2196-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Miller BR, et al. A dual leucine kinase-dependent axon self-destruction program promotes Wallerian degeneration. Nature neuroscience. 2009;12:387–389. doi: 10.1038/nn.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Xiong X, Collins C. A conditioning lesion protects axons from degeneration via the Wallenda/DLK MAP kinase signaling cascade. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:610–615. doi: 10.1523/JNEUROSCI.3586-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xiong X, et al. Protein turnover of the Wallenda/DLK kinase regulates a retrograde response to axonal injury. The Journal of cell biology. 2010;191:211–223. doi: 10.1083/jcb.201006039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Watkins TA, et al. DLK initiates a transcriptional program that couples apoptotic and regenerative responses to axonal injury. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:4039–4044. doi: 10.1073/pnas.1211074110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shin JE, et al. Dual leucine zipper kinase is required for retrograde injury signaling and axonal regeneration. Neuron. 2012;74:1015–1022. doi: 10.1016/j.neuron.2012.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Klinedinst S, et al. Independent pathways downstream of the Wnd/DLK MAPKKK regulate synaptic structure, axonal transport, and injury signaling. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:12764–12778. doi: 10.1523/JNEUROSCI.5160-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hoopfer E, et al. Wlds protection distinguishes axon degeneration following injury from naturally occurring developmental pruning. Neuron. 2006;50:883–895. doi: 10.1016/j.neuron.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 102.Zhai Q, et al. Involvement of the ubiquitin-proteasome system in the early stages of wallerian degeneration. Neuron. 2003;39:217–225. doi: 10.1016/s0896-6273(03)00429-x. [DOI] [PubMed] [Google Scholar]
- 103.Xiong X, et al. The Highwire ubiquitin ligase promotes axonal degeneration by tuning levels of Nmnat protein. PLoS biology. 2011;10 doi: 10.1371/journal.pbio.1001440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Babetto E, et al. The Phr1 ubiquitin ligase promotes injury-induced axon self-destruction. Cell Reports. 2013;3:1422–1429. doi: 10.1016/j.celrep.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hillefors M, et al. Axon viability and mitochondrial function are dependent on local protein synthesis in sympathetic neurons. Cell Mol Neurobiol. 2007;27:701–716. doi: 10.1007/s10571-007-9148-y. [DOI] [PMC free article] [PubMed] [Google Scholar]