Abstract
Diffusion tensor imaging (DTI) is a magnetic resonance imaging technique that allows for the visualization and characterization of the white matter tracts of the brain in vivo. DTI does not assess white matter directly. Rather, it capitalizes on the fact that diffusion is isotropic (equal in all directions) in cerebral spinal fluid and cell bodies but anisotropic (greater in one direction than the other directions) in axons that comprise white matter. It provides quantitative information about the degree and direction of water diffusion within individual units of volume within the magnetic resonance image, and by inference, about the integrity of white matter. Measures from DTI can be applied throughout the brain or to regions of interest. Fiber tract reconstruction, or tractography, creates continuous 3-dimensional tracts by sequentially piecing together estimates of fiber orientation from the direction of diffusion within individual volume units. DTI has increased our understanding of white matter structure and function. DTI shows nonlinear growth of white matter tracts from childhood to adulthood. Delayed maturation of the white matter in the frontal lobes may explain the continued growth of cognitive control into adulthood. Relative to good readers, adults and children who are poor readers have evidence of white matter differences in a specific region of the temporo-parietal lobe, implicating differences in connections among brain regions as a factor in reading disorder. Measures from DTI changed in poor readers who improved their reading skills after intense remediation. DTI documents injury to white matter tracts after prematurity. Measures indicative of white matter injury are associated with motor and cognitive impairment in children born prematurely. Further research on DTI is necessary before it can become a routine clinical procedure.
Index Terms: diffusion tensor imaging, magnetic resonance imaging, tractography, children, white matter development, white matter associations with reading disorder, white matter injury in prematurity
Contemporary neuroscience conceptualizes complex thoughts and emotions as arising from widely distributed and highly interactive neural networks.1–4 Localized cortical regions within the gray matter contribute specialized or semispecialized computations to mental activity, functioning like microprocessors. The structure of these regions can be assessed with conventional magnetic resonance imaging. Activity within these regions during cognitive or emotional processing can be assessed using functional magnetic resonance imaging. Axons arising from cell bodies connect neurons to other neurons. Axons are organized into white matter fiber bundles that link cortical regions, acting like cables connecting the microprocessors. Diffusion tensor imaging is an magnetic resonance imaging technique that allows for the visualization and characterization of white matter tracts in living animals and humans. Understanding structural and functional features of white matter has become increasingly important in neuroscience and in clinical medicine.
The goal of this review is to provide pediatric clinicians and researchers with basic understanding of how diffusion tensor imaging works, its contributions to understanding the neural basis of child development and developmental disorders, limitations of the methodology, and future directions. Until the advent of diffusion tensor imaging, our understanding of white matter was based primarily on postmortem histology and on visual inspection of conventional magnetic resonance images. Diffusion tensor imaging, by contrast, generates quantitative measurements of white matter microstructure. In this review, we provide background about how the method facilitates understanding about white matter. After this foundation, we focus on 3 applications: (1) developmental changes in white matter, documenting locations and patterns of change as a function of age; (2) differences in white matter structure in good and poor readers, suggestive of the important role of white matter in human cognitive variation; and (3) associations of white matter injuries and neurodevelopmental outcomes in children born prematurely, demonstrating that white matter injury may be an important reason for neurodevelopmental disability after preterm delivery.
WHAT IS WHITE MATTER?
White matter is the compartment of the nervous system comprised of myelinated axons. Axons are the long, slender projections that arise from the cell bodies of neurons and that transmit electrical signals from neuron to neuron. Axons are sheathed in myelin, a compound composed primarily of lipids. Serving as insulation, myelin increases electrical resistance and prevents dispersion of electrical signals as they course down axons. Small gaps in the membrane sheath, known as the nodes of Ranvier, allow electrical impulses to skip down the axon, resulting in increased speed of transmission. Within the central nervous system, myelin is produced by glial cells called oligoden-drocytes. Axons are organized into fiber bundles. Grouped together, their myelin sheaths appear white, and hence the term white matter. Long white matter fiber bundles are called fasciculi or tracts. White matter comprises more than half of the volume of the cerebral hemispheres.5
Clinical magnetic resonance imaging scanning permits a qualitative assessment of the volume and appearance of white matter. Clinical reports may describe white matter as diminished in volume, immature, or under myelinated. Diffusion tensor imaging is a specific magnetic resonance imaging technique that generates quantitative measures of white matter at the level of each voxel, the small, arbitrary unit of volume in magnetic resonance imaging scanning (see Glossary). Using specific analytic strategies, it can generate qualitative and quantitative data about the architecture and organization of fiber tracts.
HOW IS THE SIGNAL FOR DIFFUSION TENSOR IMAGING GENERATED?
Diffusion Weighted Magnetic Resonance Imaging
Conventional magnetic resonance images are generated by applying a radio frequency pulse to the brain in a homogeneous magnetic field and measuring the resulting signal emitted by hydrogen atoms in the water molecules. Diffusion weighted magnetic resonance imaging measures the diffusion of water in tissues by applying a gradient that increases the strength of the magnetic field evenly in one direction (see Glossary).6 Based on the physics of magnetic resonance imaging, the emitted signal decreases as a function of the movement of water molecules in the direction of this gradient; however, it is unaffected by the movement perpendicular to the direction of the gradient.7–10 The rate of water diffusion in a given direction is calculated by comparing the signal when diffusion weighting is applied to the signal at the same location when no diffusion weighting is applied (called the B = 0 measurement, see Glossary). The rate of water diffusion in other directions can be measured by changing the direction of the gradient.
Diffusion Tensor Imaging
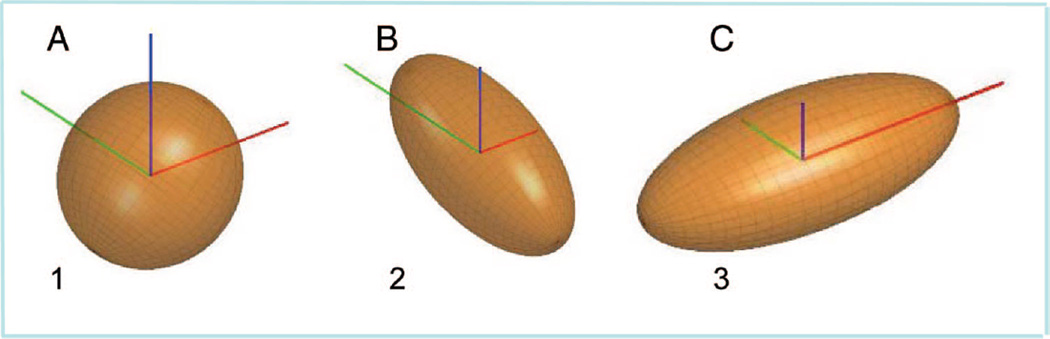
Diffusion tensor imaging provides information about the degree and direction of water diffusion in multiple directions within individual voxels of the magnetic resonance image9,10 providing clues to the structure of the tissues.11,12 Diffusion weighted measurements are collected in at least different directions using gradient pulses. The rate and direction of diffusion within each voxel is the integration of these six measurements, summarized into a “tensor” model (see Glossary).10 The diffusion tensor is best visualized as an ellipsoid (Fig. 1) oriented so its long axis is parallel with the direction of greatest diffusion, known as the principal diffusion direction. Two additional vectors represent the rate of diffusion perpendicular to the principal diffusion direction (Fig. 1). The tensor model generates measures of the structure of cerebral tissue at a microscopic level that are based on characteristics of diffusion in those tissues.
Figure 1.
Three diffusion ellipsoids represent the diffusion profile of 3 different structures, marked 1, 2, and 3 on Figures 2C and 2D. The axes represent the x- (left-right, red), y- (posterior-anterior, green), and z-(inferior-superior, blue) directions. (A) Isotropic diffusion ellipsoid, representing a region of cerebral spinal fluid. (B) Anisotropic diffusion ellipsoid, representing a white matter tract parallel to the y-axis (superior longitudinal fasciculus). (C) Anisotropic diffusion ellipsoid, representing a white matter tract parallel to the x-axis (corpus callosum).
Diffusion in Cerebral Tissues
At body temperature, water molecules are in a constant state of random Brownian motion. In cerebral spinal fluid, diffusion is isotropic; the molecules diffuse in all directions equally at a rate of ~3 µm/ms. In cerebral gray matter, internal cellular structures and cell membranes slow the rate of diffusion in comparison with the rate in cerebral spinal fluid. However, diffusion remains isotropic. In white matter, cell structure impedes the rate of diffusion perpendicular to the directional orientation of the axon fibers, while leaving the rate of diffusion parallel to the axon relatively unhindered. Diffusion in white matter is thus anisotropic, greater in one direction than in other directions (see Glossary).13 Diffusion tensor imaging measures are more indicative of intercellular rather than intracellular diffusion.14 Three factors that are thought to increase the efficiency of neural conduction—dense axonal packing, large axonal diameters, and high levels of myelination— contribute to a reduction in the amount of intercellular space and restrict water diffusion perpendicular to the orientation of the axons. Therefore, a high index of anisotropy and restricted diffusion perpendicular to the principal diffusion direction are considered indications of healthy or mature white matter microstructure.11–13,15
HOW IS DIFFUSION TENSOR IMAGING ANALYZED?
Measures
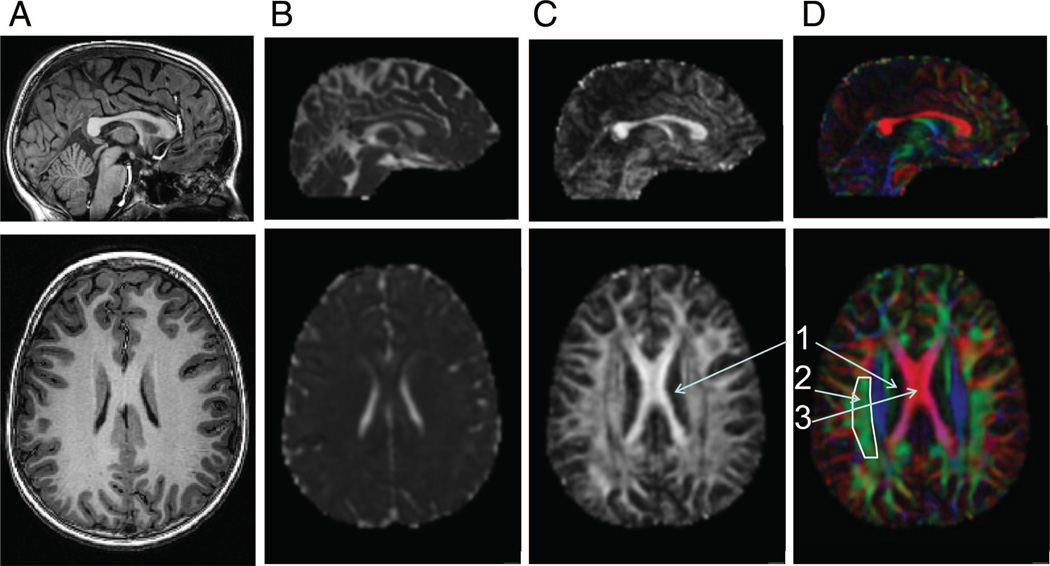
In research and clinical practice, it may be important to evaluate the integrity of white matter at a specific location. The data from the tensor model at the level of each individual voxel and subsequently in a region can be summarized and visualized in three different ways: mean diffusivity, degree of anisotropy, and the main direction of diffusivity. Mean diffusivity characterizes the net degree of displacement of the water molecules, measured in micrometers per millisecond. The mean diffusivity of individual voxels can be overlaid on an anatomical image (Fig. 2A); high mean diffusivity is represented as white appearance and low mean diffusivity as dark appearance (Fig. 2B). Mean diffusivity in both gray and white matter decreases with age because increasing structure within cells impedes water diffusion. Thus, mean diffusivity can be a useful measure for assessing maturation of white matter.16
Figure 2.
Sagittal (top) and axial (bottom) slices from a healthy 12-year-old girl. (A) Conventional T1 weighted anatomical image. (B) Mean diffusivity map calculated from diffusion tensor imaging data. High signal (white areas) represents high diffusion (cerebral spinal fluid); low signal (gradations of dark areas) represents reduced diffusion (gray and white matter). (C) Fractional anisotropy map calculated from diffusion tensor imaging data. High signal (white areas) represents high fractional anisotropy (white matter); low signal (dark areas) represents reduced anisotropy (gray matter and cerebral spinal fluid). (D) Red-Green-Blue map calculated from diffusion tensor imaging data. Voxels displayed as red represent tracts with primarily left-right orientation (x-axis); voxels displayed as green represent tracts with primarily anterior-posterior orientation (y-axis); voxels displayed as blue represent tracts with primarily superior-inferior orientation (z-axis). The superior longitudinal fasciculus, a tract containing fibers projecting along the y-axis (outlined in white, see Fig. 1B) is represented in green. The corpus callosum, a tract containing fibers projecting along the x-axis, is represented in red (see Fig. 1C).
The degree to which diffusion is anisotropic is represented by a fractional anisotropy (FA), which is a nondirectional proportion from 0.0 to 1.0 (see Glossary).12 Light axonal packing would leave more intercellular water than dense packing, resulting in less restriction of diffusion and therefore lowering FA. By contrast, a high degree of myelination would cause axons to be more tightly packed together, increasing FA. The FA of all the voxels of the brain can be overlaid on an anatomical map; high values are represented as white, whereas low values are represented as dark (Fig. 2C). Many studies use FA as the primary measure of white matter integrity.
The final measure that can be extracted from the tensor is based on the orientation in space of the tissue structure. The principal diffusion direction is used to infer the underlying orientation of axonal projections. The rate of diffusion in the principal diffusion direction is called axial diffusivity and the rate of diffusion perpendicular to the principal diffusion direction is called radial diffusivity (See Glossary). Color-coding each voxel based on the principal diffusion direction shows the principal diffusion direction of each voxel: red indicates diffusion along the x-axis, left-right orientation; green indicates diffusion along the y-axis, posterior-anterior orientation; and blue indicates diffusion along the z-axis, inferior-superior orientation (Fig. 2D). The resulting maps provide an indication of whether the white matter tracts are in proper orientation.
Diffusion tensor imaging (DTI) is better suited for analyzing large fiber bundles than small tracts. Difficulties in interpretation arise when two tracts with different principal diffusion directions cross, merge, branch, or touch within a voxel. The FA of that voxel is lower than it would be if it contained only one of those tracts and therefore does not reflect either of the contributing fibers. Future research must continue investigating the neurobiological basis of magnetic resonance diffusion signals and find measurements that can overcome these limitations. Table 1 summarizes the patterns of measures that might be obtained under different underlying neurological conditions.
Table 1.
The Relations Among Fractional Anisotropy, Axial Diffusivity, and Radial Diffusivity Under Different Conditions
| White Matter Characteristics/ Conditions |
Fractional Anisotropy |
Axial Diffusivity |
Radial Diffusivity |
|---|---|---|---|
| Dense axonal packing | High | Unaffected | Low |
| Large axonal diameter/ high myelination |
High | High | Low |
| Axonal degeneration | Low | Low | High |
| Demyelination | Low | Unaffected | High |
Analytic Strategies
Diffusion metrics can be summarized globally for the whole brain. However, most researchers are interested in identifying the anatomical locations at which differences are found. Multiple analytic strategies provide spatial localization for the measures from DTI. We will summarize 4 strategies, discussing the decision about which of these techniques to use based on hypothetical research questions.
Whole Brain Analysis or Voxel-Based Morphometry
In our first hypothetical experiment, we want to compare measures of white matter in school-aged children (7–12 years old) and adolescents (13–18 years old), and we have no underlying theory about where we might find group differences. A reasonable approach in this case is to analyze all of the voxels in the brain in terms of one of the diffusion measures, such as FA.
The challenge of whole brain analysis is that individuals vary substantially in terms of the size and shape of their brains. It is necessary to align individual brains to assure that the same structures are being compared in all subjects. Voxel-based morphometry implies that the images have been aligned using a protocol called spatial normalization (see Glossary). Spatial normalization uses computer-based algorithms to turn, stretch, shrink, and warp each brain image to make the best fit possible to a template. Unfortunately, normalization may be imperfect, compromising the accuracy of experimental comparisons. Normalization is particularly likely to distort the brains of individuals from clinical populations with underlying structural abnormalities. Smoothing is a process that corrects for some of the errors in normalization by averaging the results from a single voxel with the corresponding results of its neighbors (see Glossary).17 Smoothing not only reduces the effect of noise in the data but also decreases anatomical specificity.
Typically, the next step in voxel-based morphometry is the analysis of voxels that meet a threshold value of FA, such as 0.20, to avoid including areas of gray matter or cerebral spinal fluid. These voxels are processed individually and the differences between groups, (that is, in our hypothetical study, school-aged children compared with adolescents) are analyzed using simple univariate statistics. Alternatively, the measure of interest can be correlated with a relevant factor, in this case age or in other cases test scores. In a DTI study with voxels that are 2 mm3, there are more than 100,000 voxels in an individual brain. Statistical tests generally apply corrections because with this many comparisons some difference are likely to be significant on the basis of chance.18 The results of the analyses are represented in images in which the greatest differences are represented by the most intense colors and typically overlaid on an anatomical image.
Tract-Based Spatial Statistics
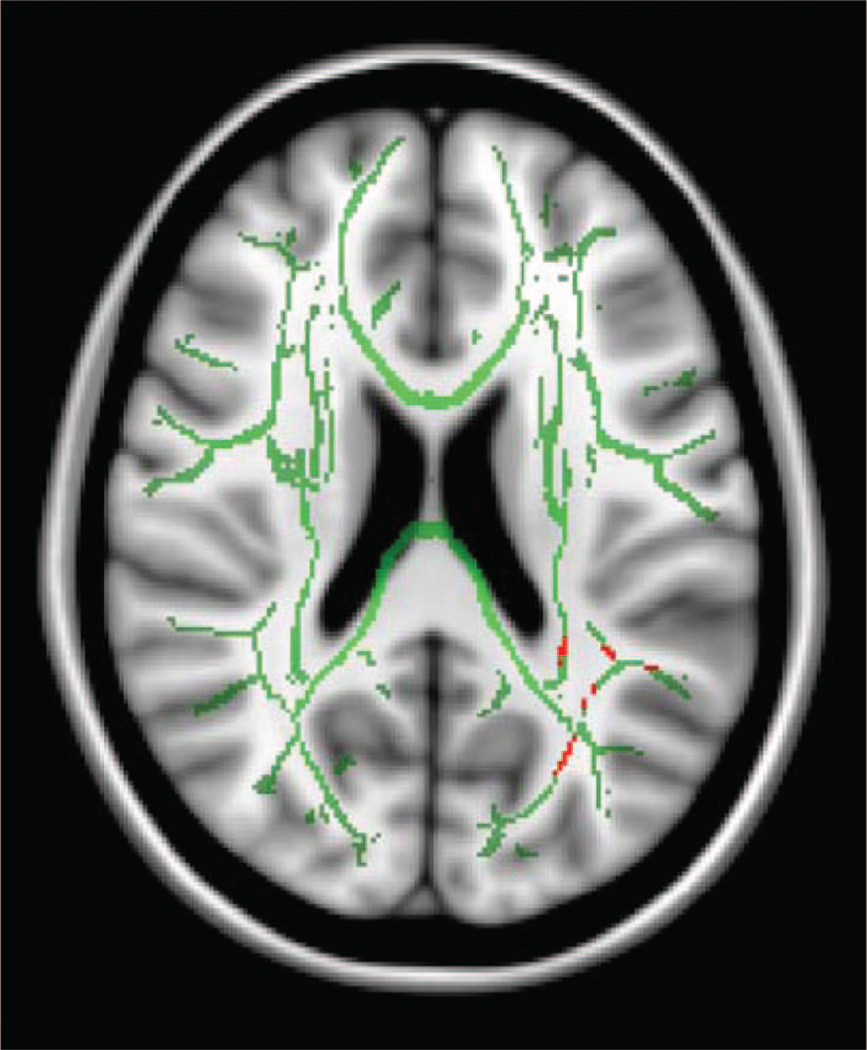
A variation of the whole brain approach is called tract-based spatial statistics. Tract-based spatial statistics roughly aligns the FA images from multiple subjects to a common space using an algorithm. It then creates a tract representation, called the “mean FA skeleton”19 including only those voxels assumed to be at the center of tracts common to all the members of the group and excluding regions of poor alignment.20 The resulting FA skeleton data for all subjects are analyzed the same way as in voxel-based morphometry, by computing statistics at each voxel and identifying regions with significant differences between groups (Fig. 3). A limitation of tract-based spatial statistics is that it may miss variations in the periphery of white matter tracts, which are not included in the analyses. Whole brain techniques such as voxel-based morphometry and tract-based spatial statistics may be a good first attempt at identifying areas of major difference across subjects that can then be followed up on with other methods.
Figure 3.
Mean fractional anisotropy skeleton, in green, generated from Tract Based Spatial Statistics for a sample of 13 children born prematurely and 12 age-matched full-term controls, overlaid on a Montreal Neurologic Institute template. Regions of significant difference in fractional anisotropy (p < .05, uncorrected for multiple comparisons) between children born prematurely and controls are shown in red within the skeleton.
Region of Interest Analysis
In our next hypothetical experiment, we hypothesize that white matter differences will be found in the frontal lobes in a comparison of school-aged children with adolescents. In this case, a region of interest (ROI) approach is appropriate. This method relies on a priori spatial definitions of brain structures of interest21 and analyzes only those voxels within the region. In the ROI approach, the number of statistical comparisons is considerably lower than in a whole brain approach. The advantages are therefore that statistical power increases and small differences not apparent on whole brain analyses may be found. A major disadvantage of this method is that it may miss differences in regions not included in the analysis.
The ROIs are sometimes defined based on anatomical landmarks within individual brains, respecting individual variation in brain morphology.22 However, manually drawing the ROIs can be labor intensive and time-consuming and becomes less feasible as the sample size increases. Automated approaches to drawing regions are more standardized and less subjective but are vulnerable to the inaccuracies that arise from normalization. The ROI analysis can be combined with other methods. For example, whole-brain statistical analyses may identify particular structures of interest, which can then be investigated further with the ROI analysis.20 The ROI approach has also been used for assessing the microstructure of white matter tracts within deep gray matter and subcortical gyri, such as within the basal ganglia.21
Fiber Tract Reconstruction or Tractography
In our final hypothetical experiment, we want to evaluate changes in the superior longitudinal fasciculus from school age to adolescence, because we think that this structure may be linked to age-related improvements in cognitive control. Fiber tract reconstruction, or diffusion tensor tractography, is a method used to identify and visualize a continuous 3-dimensional trajectory by sequentially piecing together the estimates of fiber orientation from the directionality of individual voxels.23–25 Tractography identifies the tracts and fibers that connect specific cortical or subcortical structures within and across individuals (Figs. 4A and 4B). Tractography allows the researcher to compare equivalent fiber connections across individuals, even if the precise location of the tract varies. Moreover, using tractography, apparently equivalent anatomical regions in different individuals may be found to contain axons projecting to different brain areas.26
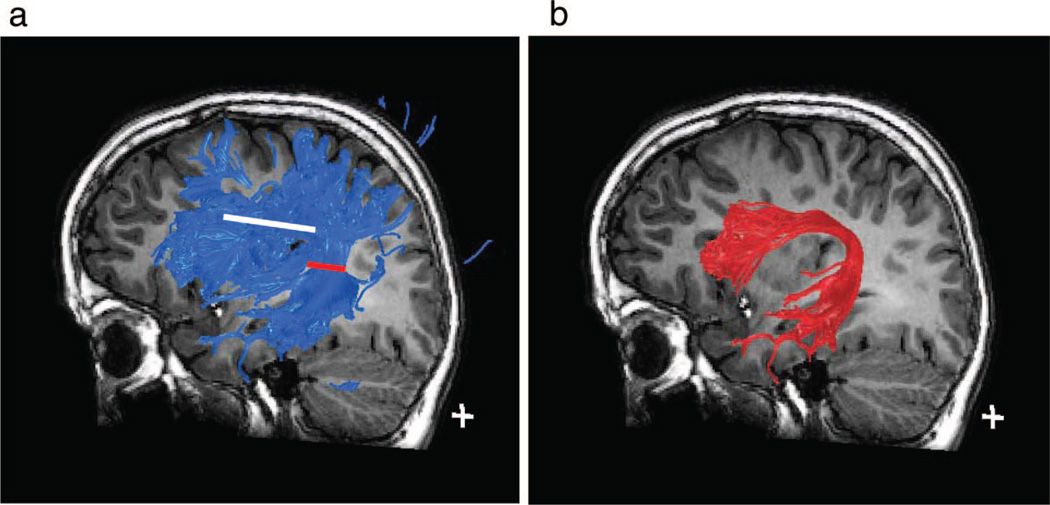
Figure 4.
Fiber tracking. (A) Three-dimensional visualization of superior longitudinal fasciculus fibers, tracked from the region of interest outlined in white in Figure 2D. The location of the "seed" region of interest is depicted in this sagittal view as a white line. The blue fiber tract contains fibers connecting the temporal, parietal, and frontal lobes. (B) The arcuate fasciculus, the portion of the superior longitudinal fasciculus that connects the temporal lobe to the frontal lobe. Tract was defined by limiting the blue fiber group in Figure 4A to only those fibers passing through a second region of interest, depicted by the red line, thereby using a 2 region of interest approach.
The two broad categories of tractography are deterministic and probabilistic.27,28 Deterministic algorithms move from voxel to voxel based on principal diffusion direction until they reach voxels unlikely to be in the tract. This method is problematic in voxels where fiber tracts cross and the primary diffusion direction of the voxel no longer represents the direction of the fiber of interest. Then, the algorithm may proceed along an incorrect trajectory.29 Probabilistic algorithms take into account uncertainty in the orientation of axonal bundles by calculating a distribution of probable fiber orientations in each voxel based on the variability in the diffusion measurements.30,31 Probabilistic tractography has the advantage that it can identify multiple pathways in regions of crossing fibers, allowing an experimenter to study connections that are known to exist but are not found with deterministic algorithms.
Tractography often uses experimenter-defined ROIs to identify the tracts of interest. Seeding is the term used for choosing the initial voxels from which the tract will originate; target regions represent areas through which the fibers must pass to be included as part of the pathway (Figs. 2D, 4A, and 4B). Fibers that pass through specified exclusion regions are selectively removed from analysis (Figs. 4A and 4B). These regions may be manually selected, usually according to a priori information on tract location or may be chosen based on published atlases of DTI-based white matter anatomy.32,33 The multiple ROI approach uses spatially separated target regions that can, for example, allow dissection of different sets of fibers within a bundle.34 Automated methods for defining fiber tracts can also be used and are advantageous for large samples. However, these methods rely on spatial normalization, with its inherent limitations.35
The measures of diffusion we described previously, such as FA or mean diffusivity, can be averaged over the entirety of each tract21 or calculated for different segments of a tract to reveal variations within the tract.36 Diffusion metrics can also be weighted averages, with the number of fiber trajectories passing through a voxel comprising a weight for the value of that voxel.36 In these methods, voxels with many fiber trajectories, assumed to be in the core of the tract, contribute more to the measurements than voxels with few fiber trajectories, likely to represent the edges of tracts.
Tractography is most accurate in identifying large tracts. However, it too has limitations. It should be considered a proxy technique to infer the direction of white matter pathways because it is not a direct visualization.34 It cannot identify the location or direction at the level of the individual axons. Each estimate of fiber orientation is subject to errors that can accumulate.37 Errors in tract reconstruction are particularly likely where fibers cross, merge, or branch.38 Despite these limitations, many DTI-based tract definitions are consistent with postmortem dissections.
APPLICATIONS OF DIFFUSION TENSOR IMAGING
The addition of diffusion tensor imaging (DTI) to the armamentarium of neural imaging techniques has revolutionized our understanding of white matter anatomy, structure-function associations in the nervous system, and disease states. We will focus on three broad areas of interest to the readers of this journal: normal development, reading disorders, and developmental problems after preterm delivery.
Applications of DTI to Brain Development
Anatomical Studies
Between birth and 5 years of age, whole brain volume nearly quadruples,39 mostly due to reductions in gray matter volume and extensive increases in white matter fibers. Postmortem histology found that most white matter tracts appear to be in place by birth. Magnetic resonance imaging (MRI) studies detected the beginning of myelination as early as 29 weeks gestational age and described the progression of maturation in established tracts throughout the first year of life in an inferior-to-superior and posterior-to-anterior fashion.40 A large longitudinal MRI study of children and adolescents described white matter growth as linear,41 although others described variations in the nature of white matter volume increases by region.42
DTI studies have changed, expanded, and refined understanding of white matter development. Subtle structures of the fetal brain have now been visualized in utero beginning at 18 weeks gestational age,43 and normal white matter maturation has been observed to evolve from inner to outer layers and from anterior to posterior. In the third trimester and extending through the neonatal period, the limbic system develops first. This development is followed by connections between the two hemispheres (commissural fibers) and connections between spinal cord and lower brain regions to cortex (projection fibers). Finally, short and long range connections within the hemisphere (association fibers) develop last.39 Commissural tracts and frontotemporal fibers develop anterior-to-posterior. Beyond the regional differences in timing of microstructural change in white matter, DTI methods have also documented maturation to be nonlinear, with the greatest rates of change observed in the majority of tracts by 5 years of age.21,44 In a large, meticulous study of 202 typically developing children, adolescents, and young adults, Lebel et al.21 used DTI-based tractography and region of interest (ROI) analyses to describe the exponential changes in microstructural development of white matter from childhood to adulthood. They determined that 90% of maximal (adult) levels of fractional anisotropy (FA) are achieved by 11 years of age for the splenium anterior and genu posterior of the corpus callosum, and the inferior longitudinal fasciculus (connecting occipital and temporal lobes); by 13 to 20 years for the superior longitudinal fasciculi (connecting frontal, parietal, and temporal lobes), the superior and inferior fronto-occipital fasciculi (connecting occipital and frontal lobes), and the anterior limb of the internal capsule (connecting cortex to medulla); by 21 to 24 years for projection fibers in the external capsule (coursing between basal ganglia and cortex), posterior limb of the internal capsule (containing corticospinal and sensory fibers), and corticospinal tracts; and after 25 years of age for the uncinate (connecting the limbic system and frontal lobes) and cingulum (connecting components of the limbic system). It is possible that white matter changes continue throughout life because white matter growth is activity dependent. However, our current methods of DTI may be insensitive to small changes or changes in small regions.
Most studies of brain anatomy combine data from male and female subjects. However, whole-brain analysis of DTI data on a sample of typically developing children between the ages of 5 and 18 years found that white matter volume increases faster in male than in female children.45 Males were also found to have proportionally greater white matter than gray matter, even after controlling for total cerebral volume. Differences in FA and volume underscore the need to consider gender differences in neural development and have implications for the study of brain-behavior relationships in disordered development.
Structure-Function Associations
DTI methods have been used to link features of white matter maturation to cognitive function. In a whole-brain analysis of 47 participants, Schmithorst et al.46 found positive correlations between intelligence quotient (IQ) and FA in several structures—left arcuate fasciculus, the genu of the corpus callosum, right parietal regions, and the frontal lobes. This finding suggests that increases in fiber organization and axonal density support or reflect increased global cognitive ability. Of particular interest is the association between cognitive measures and increases in white matter maturation within the frontal lobes, because these are regions that are among the last to myelinate and are known to support planning, organizing, inhibiting automatic responses, and other executive functions. It is generally thought that myelination limits developmental change and plasticity in axons.5 Therefore, delayed myelination, particularly in areas of the brain associated with higher order cognitive functions, may explain the continued growth of these abilities into adulthood. DTI tractography is consistent with this concept; increased restriction in diffusion measured as radial diffusivity in frontostriatal regions predicted faster reaction times on a go-no-go task, a paradigm that requires response inhibition, in children and adults aged 7 to 31 years, even after controlling for age and accuracy.47 Tractography and ROI analytic methods have also shown increases in FA of right hemisphere frontoparietal pathways to be associated with spatial working memory ability.48
Applications of DTI to Reading
Reading is a highly complex cognitive skill, requiring the coordination of multiple, distributed brain regions. Approximately 5% to 10% of otherwise typically developing children have a reading disability or dyslexia. Functional MRI studies have found that good readers show a high degree of functional activation in left temporoparietal cortex, whereas poor readers show minimal left temporoparietal activation and a high degree of frontal activation with reading, primarily on tasks involving phonological decoding.49
DTI has expanded our understanding of the neural basis of reading and reading disorder. Klingberg et al.50 used DTI to compare the whole-brain FA values in adults with good versus poor reading ability. They found that relative to good readers, poor readers had lower FA bilaterally in a region of the temporoparietal lobes. The values of FA in this critical region positively correlated with reading skill.50 These findings suggest that dyslexia represents a disruption in the communication between brain regions critical for reading.51,52 Deutsch et al.53 replicated these results in children aged 7 to 13 years with a range of reading abilities. They found that FA in left temporoparietal white matter only correlated with Word Identification scores. DTI studies have further identified 3 major white matter pathways that seem to be critical to the development of reading skill: the superior corona radiata, the corpus callosum, and the superior longitudinal fasciculus (in particular, the arcuate).22
Other pathways may be important to reading, but the results are contradictory. Andrews et al.,54 in a study of adolescents born prematurely and controls, found that FA in the corpus callosum was positively associated with reading skills. However, Doughtery et al.29 found that FA in the corpus callosum was negatively associated with phonological scores. The latter group interpreted their findings to suggest that better readers may have reduced interhemispheric activity. More research on the meaning of FA may be required to reconcile differences. More recently, studies have used a combination of ROI approaches and tractography to localize the area of greatest correlation between FA and reading skill; these studies have linked Word Identification scores to the superior corona radiata, located in the posterior limb of the internal capsule.55 However, an intriguing case study found that selective damage to the arcuate fasciculus, a part of the superior longitudinal fasciculus (Fig. 4B), in an adolescent with radiation-induced tissue necrosis was associated with profound dyslexia,57 although another adolescent with a missing arcuate fasciculus after premature delivery had normal reading abilities (Yeatman et al., in preparation). Again, more research on both methods and structure-function correlations is needed.
A recent and exciting study has documented microstructural measures of white matter on DTI in children who were poor readers and then showed functional improvements in reading after intensive remediation.57 White matter development and myelination are thought to be activity-dependent, increasing as pathways are used more frequently or more intensively.5 Changes in white matter after remediation may be either a cause or a result of improvements in reading skill. In either case, in the coming years, DTI may contribute to the evaluation of education and therapy in a variety of clinical conditions.
Applications of DTI to Neurodevelopmental Outcomes of Prematurity
Structural Abnormalities
White matter tracts in the parietal and periventricular regions are highly susceptible to injury from premature birth.58 This predilection is due to multiple factors: the immature vascular supply to cerebral white matter; impairment in the regulation of cerebral blood flow; and vulnerability of the precursors of oligodendrocytes, the myelin-producing cells, to hypoxia, ischemia, reperfusion, and inflammation between 24 and 32 weeks gestation.58–60 Recent reviews emphasize that white matter injury from prematurity falls on a spectrum from large or multiple destructive cystic lesions to diffuse, noncystic injury.59,60 Improvements in neonatal intensive care in the past decade have resulted in a reduction in the prevalence of cystic white matter injuries.59 However, there has not been corresponding decreases in the prevalence of noncystic white matter injury or improvements in neurodevelopmental outcomes.61 DTI holds considerable promise for detecting and monitoring the degree and location of diffuse injuries.
DTI obtained when the premature infant approaches term age seems to be sensitive to diffuse white matter injury prior to detection of such injury on cerebral ultrasound or conventional MRI.62 A study using tract-based spatial statistics to analyze DTI in preterm infants without evidence of lesions on conventional MRI at term-equivalent age found several areas of lower FA in pre-terms compared to term controls, including the centrum semiovale, frontal white matter, and the genu of the corpus callosum. In infants born at less than 28 weeks gestation, additional areas had reductions in FA, including the external capsule, the posterior aspect of the posterior limb of the internal capsule, and the isthmus and middle portions of the body of the corpus callosum.20 Diffuse white matter signal abnormalities on clinical imaging at term correlate with increased radial diffusivity and decreased FA, particularly in the internal capsule and sensorimotor regions of the infant brain.63 Animal studies find that injury to myelin results in increased radial diffusivity. At term age, when differentiating oligodendrocytes are just beginning to wrap around axons in preparation for myelination, increased radial diffusivity may represent a failure of the cells to develop fully and create the sheath around the axon that ultimately would allow myelination.63
Dudink et al.64 sought to determine reference values for the FA in the white matter tracts of very low birth weight infants. They retrospectively analyzed DTIs that were obtained in 28 very low birth weight infants between 26 and 32 weeks gestational age who did not have evidence of white matter abnormalities on conventional MRI and who had normal developmental outcome when assessed at 1 to 3 years of age. They found positive correlations between the gestational age and FA of the posterior limb of the internal capsule and the pyramidal tracts in these infants. Although FA measurements should be similar across scanners, it is important to document the reliability of FA values across different centers, scanners, and scanning protocols before applying DTI clinically to predict outcomes of prematurity.
Associations of Structural Findings with DTI and Motor Function
Abnormal FA in the posterior limb of the internal capsule on DTI when preterm children are near term age has been associated with later neurologic and gait abnor-malities,65 even in children who had no significant findings on conventional MRI at the same gestational age.66 DTI has also documented that thalamocortical connections may be disrupted in addition to descending corticospinal tracts in cerebral palsy.67,68 Injury to these tracts has been found to correlate with sensory and motor findings.67
DTI Findings in Older Children, Adolescents, and Adults Born Prematurely
DTI studies demonstrate that white matter microstructure disturbances in children born prematurely persist into school age and beyond.69–71 One study found that school-aged children born prematurely have lower whole-brain mean FA than their full-term peers; in addition, preterm males have lower FA than females.71 Apart from prematurity, the children in this study had no history of poor health and no major behavioral or cognitive deficits. The mean FA of their total white matter was a significant predictor of IQ score after adjusting for birth weight, gestational age, and gender. One possible confounding factor was the lower SES and parental education level in the preterm group versus controls.
DTI has also detected differences in FA in specific white matter areas, both regions associated with periventricular leukomalacia in preterm infants and regions indicating a distal extension of white matter injury. One whole-brain analysis identified 13 white matter areas (clusters of at least 100 voxels) with reduced FA in preterm adolescents.70 A ROI analysis was then carried out, in which the mean FA values from each significant cluster were extracted and compared with results from clinical neuropsychological tests.72 Visual perceptual deficits were associated with low FA in the external capsule, posterior part of the internal capsule, and in the inferior fasciculus; these findings suggest damage of association tracts containing secondary visual fibers that carry information for processing in temporal and frontal areas. Low IQ was associated with low FA in the external capsule and inferior and middle superior fasciculus, all areas containing long association fibers. Performance IQ was correlated with FA in the right posterior internal capsule and right superior longitudinal fasciculus. Fine motor impairment was related to low FA in the internal and external capsule and superior fasciculus, which may reflect disturbed connectivity of motor projection fibers and long association tracts. Mild social deficits, as measured by the Autism Spectrum Screening Questionnaire, correlated with reduced FA in the external capsule and superior fasciculus. Inattention symptoms and the diagnosis of Attention-Deficit/Hyperactivity Disorder were related to lower FA in several white matter areas. Impairments in more complex functioning such as attention, arithmetic, and visual motor integration seemed to be related to more extensive injury to white matter microstructure. A limitation of this study is that the regions of interest were not defined in native space but on normalized brains. MRI showed structural abnormalities in a large percentage of the preterms, such as thinning of the posterior corpus callosum and reduced periventricular occipital and parietal white matter tissue, so that inaccuracies in the spatial normalization process may have occurred.
DTI studies of the corpus callosum in older children and adolescents born prematurely show variable results. A ROI analysis of preterm and full-term children from 10 to 16 years of age found associations between regions of the corpus callosum and reading scores. A tractography study of young adults with a mean age of 19.3 years compared measures of the corpus callosum with IQ.73 It found no significant difference between preterms and controls for FA or mean diffusivity in the corpus callosum but found that preterm females had higher mean diffusivity in the genu than control females, which was also associated with lower performance IQ. These findings differ from a study that found reduced FA in both the genu and splenium70; another that found lower FA in the splenium69; and a third that found no associations between IQ and corpus callosum microstructure.72 One possible confounding factor is the ongoing development of the corpus callosum, which may attenuate group differences between adolescence and early adulthood. Differences in age of assessment, the socioeconomic status of the subject groups, and analysis methods also limit the comparability of these studies.
CONCLUSIONS AND FUTURE DIRECTIONS
Diffusion tensor imaging (DTI) is a magnetic resonance imaging technique that generates measures of white matter microstructure. The data from DTI have greatly expanded our understanding of the normal development of the nervous system in childhood and adolescence. Associations between structural measures on DTI and functional measures, such as reading scores, suggest that variations in white matter structure may be associated with important variations in human functioning. In addition, mechanisms of improvements after intense remediation may become clear if replication studies find that changes in DTI measures correlate with functional improvements.57 This recent finding opens up the possibility that DTI can be used to supplement behavioral measures to investigate the impact of education and therapy on clinical or functional status. DTI has also contributed to understanding the nature of neural injury in prematurity and the variation in neurodevelopmental outcome after preterm delivery.
Despite these advances, further research is needed before DTI can be used routinely for clinical purposes. At the present time, it is unclear whether measures from different centers and different scanners are comparable or whether each center, scanner and scanning protocol will require its own norms for DTI. Centers often use their own customized software to analyze DTI data, further complicating the full-scale application of DTI in the clinical arena, although sharing of the algorithms is possible.74 Several studies, as we have reviewed, yield contradictory data in terms of core measures such as fractional anisotropy. In some cases, increasing fractional anisotropy is associated with positive findings but in other cases fractional anisotropy is associated with unfavorable outcomes. The reason for such variation must be understood, particularly before DTI is used for prediction of long-term outcomes. New scanning protocols under development may be able to differentiate the contribution of myelination from neuronal size or packing density, thereby explaining disparate findings. Overall, we anticipate continued improvements in the acquisition, analysis, and interpretation of data with corresponding advances in our understanding of neuroscience and clinical medicine.
ACKNOWLEDGMENTS
We gratefully acknowledge Irene M. Loe, Lynne C. Huffman, Brian G. Tang, and Maria Salinas for helpful comments on earlier versions of the article.
This work was supported by a grant from the National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development, RO1 HD046500 (to H.M.F.).
GLOSSARY
- Anisotropic diffusion
Diffusion that is not equal in all directions (isotropic) but greater in one direction than in other directions
- Axial diffusivity
Rate of diffusion in the direction that is parallel to the white matter tract.
- B = 0 image
An image collected without diffusion weighting. The B0 image is used as a reference for diffusion weighted images when calculating the diffusion tensor.
- Fractional anisotropy (FA)
A ratio ranging from 0 to 1 that represents the degree to which diffusion is anisotropic. High FA values indicate that diffusion is much greater in one direction that others, whereas low FA values indicate that diffusion is nearly equal in every direction.
- Gradient
A gradual increase (or decrease) in the strength of the magnetic field in a given direction, causing different effects on the biological substrate at different locations.
- Normalization (or spatial normalization)
A process that attempts to fit an individual brain to an average brain from a specific sample or from a general population. Normalization may stretch, shrink, or warp the image to achieve the best fit. Normalization aides in the comparison of regions across individuals.
- Principal diffusion direction
The direction in which water diffuses the fastest rate. In white matter, the principal diffusion direction is usually parallel to the orientation of axonal projections.
- Radial diffusivity
Rate of diffusion in the direction that is perpendicular to the white matter tract.
- Smoothing
A method of averaging the data of one voxel with the neighboring voxels. The researcher defines the number of neighboring voxels or the distance over which to average the data.
- Tensor
The tensor model summarizes diffusion weighted imaging data collected in multiple directions. The diffusion tensor is used to calculate summary measures such as fractional anisotropy, principal diffusion direction, and axial and radial diffusivity.
- Voxel
A small arbitrary unit of volume in a magnetic resonance imaging scan. The size of the voxel is a function of the number of slices obtained, the field of view, and the matrix of the magnetic resonance imaging scan. Clinical scans often have voxels that are less than 1 mm3. If a scan has slices that are 5-mm thick, then one dimension of the voxel is 5 mm.
REFERENCES
- 1.Just MA, Varma S. The organization of thinking: what functional brain imaging reveals about the neuroarchitecture of complex cognition. Cogn Affect Behav Neurosci. 2007;7:153–191. doi: 10.3758/cabn.7.3.153. [DOI] [PubMed] [Google Scholar]
- 2.McClelland JL, Mirman D, Holt LL. Are there interactive processes in speech perception? Trends Cogn Sci. 2006;10:363–369. doi: 10.1016/j.tics.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomas MSC, McClelland JL. Connectionist Models of Cognition. New York, NY: Cambridge University Press; 2008. [Google Scholar]
- 4.Rogers TT, McClelland JL. A Parallel Distributed Processing Approach to Semantic Cognition: Applications to Conceptual Development. Mahwah, NJ: Lawrence Erlbaum Associates Publishers; 2005. [Google Scholar]
- 5.Fields RD. White matter matters. Sci Am. 2008;298:42–49. [PubMed] [Google Scholar]
- 6.Bammer R. Basic principles of diffusion-weighted imaging. Eur J Radiol. 2003;45:169–184. doi: 10.1016/s0720-048x(02)00303-0. [DOI] [PubMed] [Google Scholar]
- 7.Le Bihan D. Molecular diffusion, tissue microdynamics and microstructure. NMR in Biomed. 1995;8:375–386. doi: 10.1002/nbm.1940080711. [DOI] [PubMed] [Google Scholar]
- 8.Stejskal E, Tanner J. Spin diffusion measurements: spin echoes in the presence of a time dependent field gradient. J Chem Phys. 1965;42:288–292. [Google Scholar]
- 9.Le Bihan D. Looking into the functional architecture of the brain with diffusion MRI. Nat Rev Neurosci. 2003;4:469–480. doi: 10.1038/nrn1119. [DOI] [PubMed] [Google Scholar]
- 10.Le Bihan D, Mangin JF, Poupon C, et al. Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging. 2001;13:534–546. doi: 10.1002/jmri.1076. [DOI] [PubMed] [Google Scholar]
- 11.Basser PJ. Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR Biomed. 1995;8:333–344. doi: 10.1002/nbm.1940080707. [DOI] [PubMed] [Google Scholar]
- 12.Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magn Reson Med. 1996;36:893–906. doi: 10.1002/mrm.1910360612. [DOI] [PubMed] [Google Scholar]
- 13.Beaulieu C. The basis of anisotropic water diffusion in the nervous system—a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- 14.Sen PN, Basser PJ. A model for diffusion in white matter in the brain. Biophys J. 2005;89:2927–2938. doi: 10.1529/biophysj.105.063016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996;111:209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- 16.Snook L, Plewes C, Beaulieu C. Voxel based versus region of interest analysis in diffusion tensor imaging of neurodevelopment. Neuroimage. 2007;34:243–252. doi: 10.1016/j.neuroimage.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 17.Friston KJ, Ashburner J. Generative and recognition models for neuroanatomy. Neuroimage. 2004;23:21–24. doi: 10.1016/j.neuroimage.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 18.Ashburner J, Friston KJ. Voxel-based morphometry—the methods. NeuroImage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 19.Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 20.Anjari M, Srinivasan L, Allsop J, et al. Diffusion tensor imaging with tract-based spatial statistics reveals local white matter abnormalities in preterm infants. Neuroimage. 2007;35:1021–1027. doi: 10.1016/j.neuroimage.2007.01.035. [DOI] [PubMed] [Google Scholar]
- 21.Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage. 2008;40:1044–1055. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- 22.Ben-Shachar M, Dougherty RF, Wandell BA, Ben-Shachar M, Dougherty RF, Wandell BA. White matter pathways in reading. Curr Opin Neurobiol. 2007;17:258–270. doi: 10.1016/j.conb.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 23.Basser PJ, Pajevic S, Pierpaoli C, Duda J, Aldroubi A. In vivo fiber tractography using DT-MRI data. Magn Reson Med. 2000;44:625–632. doi: 10.1002/1522-2594(200010)44:4<625::aid-mrm17>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 24.Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999;45:265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 25.Conturo TE, Lori NF, Cull TS, et al. Tracking neuronal fiber pathways in the living human brain. Proc Natl Acad Sci USA. 1999;96:10422–10427. doi: 10.1073/pnas.96.18.10422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yeatman JD, Ben-Shachar M, Bammer R, Feldman HM. Using diffusion tensor imaging and fiber tracking to characterize diffuse perinatal white matter injury: a case report. J Child Neurol. 2009;24:795–800. doi: 10.1177/0883073808331080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mukherjee P, Berman JI, Chung SW, Hess CP, Henry RG. Diffusion tensor MR imaging and fiber tractography: theoretic underpinnings. AJNR Am J Neuroradiol. 2008;29:632–641. doi: 10.3174/ajnr.A1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mukherjee P, Chung SW, Berman JI, Hess CP, Henry RG. Diffusion tensor MR imaging and fiber tractography: technical considerations. AJNR Am J Neuroradiol. 2008;29:843–852. doi: 10.3174/ajnr.A1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dougherty RF, Ben-Shachar M, Deutsch GK, Hernandez A, Fox GR, Wandell BA. Temporal-callosal pathway diffusivity predicts phonological skills in children. Proc Natl Acad Sci USA. 2007;104:8556–8561. doi: 10.1073/pnas.0608961104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sherbondy AJ, Dougherty RF, Ben-Shachar M, Napel S, Wandell BA. ConTrack: finding the most likely pathways between brain regions using diffusion tractography. J Vis. 2008;8:15.1–16.1. doi: 10.1167/8.9.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ciccarelli O, Parker GJ, Toosy AT, et al. From diffusion tractography to quantitative white matter tract measures: a reproducibility study. Neuroimage. 2003;18:348–359. doi: 10.1016/s1053-8119(02)00042-3. [DOI] [PubMed] [Google Scholar]
- 32.Mori S, Wakana S, van Zijl PC, Nagae-Poetscher LM. MRI Atlas of Human White Matter. Amsterdam, Netherlands: Elsevier; 2005. [Google Scholar]
- 33.Catani M, Mesulam M. The arcuate fasciculus and the disconnection theme in language and aphasia: history and current state. Cortex. 2008;44:953–961. doi: 10.1016/j.cortex.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Catani M, Jones DK, Ffytche DH. Perisylvian language networks of the human brain. Ann Neurol. 2005;57:8–16. doi: 10.1002/ana.20319. [DOI] [PubMed] [Google Scholar]
- 35.Breier JI, Hasan KM, Zhang W, Men D, Papanicolaou AC. Language dysfunction after stroke and damage to white matter tracts evaluated using diffusion tensor imaging. AJNR Am J Neuroradiol. 2008;29:483–487. doi: 10.3174/ajnr.A0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berman JI, Glass HC, Miller SP, et al. Quantitative fiber tracking analysis of the optic radiation correlated with visual performance in premature newborns. AJNR Am J Neuroradiol. 2009;30:120–124. doi: 10.3174/ajnr.A1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jones DK. Determining and visualizing uncertainty in estimates of fiber orientation from diffusion tensor MRI. Magn Reson Med. 2003;49:7–12. doi: 10.1002/mrm.10331. [DOI] [PubMed] [Google Scholar]
- 38.Pierpaoli C, Barnett A, Pajevic S, et al. Water diffusion changes in Wallerian degeneration and their dependence on white matter architecture. Neuroimage. 2001;13:1174–1185. doi: 10.1006/nimg.2001.0765. [DOI] [PubMed] [Google Scholar]
- 39.Huang H, Zhang J, Wakana S, et al. White and gray matter development in human fetal, newborn and pediatric brains. Neuroimage. 2006;33:27–38. doi: 10.1016/j.neuroimage.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 40.Lenroot RK, Giedd JN. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev. 2006;30:718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 41.Giedd JN, Blumenthal J, Jeffries NO, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 42.Paus T, Zijdenbos A, Worsley K, et al. Structural maturation of neural pathways in children and adolescents: in vivo study. Science. 1999;283:1908–1911. doi: 10.1126/science.283.5409.1908. [DOI] [PubMed] [Google Scholar]
- 43.Kasprian G, Brugger PC, Weber M, et al. In utero tractography of fetal white matter development. Neuroimage. 2008;43:213–224. doi: 10.1016/j.neuroimage.2008.07.026. [DOI] [PubMed] [Google Scholar]
- 44.Barnea-Goraly N, Menon V, Eckert M, et al. White matter development during childhood and adolescence: a cross-sectional diffusion tensor imaging study. Cereb Cortex. 2005;15:1848–1854. doi: 10.1093/cercor/bhi062. [DOI] [PubMed] [Google Scholar]
- 45.Schmithorst VJ, Holland SK, Dardzinski BJ. Developmental differences in white matter architecture between boys and girls. Hum Brain Mapp. 2008;29:696–710. doi: 10.1002/hbm.20431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmithorst VJ, Wilke M, Dardzinski BJ, Holland SK. Cognitive functions correlate with white matter architecture in a normal pediatric population: a diffusion tensor MRI study. Hum Brain Mapp. 2005;26:139–147. doi: 10.1002/hbm.20149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liston C, Watts R, Tottenham N, et al. Frontostriatal microstructure modulates efficient recruitment of cognitive control. Cereb Cortex. 2006;16:553–560. doi: 10.1093/cercor/bhj003. [DOI] [PubMed] [Google Scholar]
- 48.Nagy Z, Westerberg H, Klingberg T. Maturation of white matter is associated with the development of cognitive functions during childhood. J Cogn Neurosci. 2004;16:1227–1233. doi: 10.1162/0898929041920441. [DOI] [PubMed] [Google Scholar]
- 49.Shaywitz SE, Shaywitz BA, Pugh KR, et al. Functional disruption in the organization of the brain for reading in dyslexia. Proc Natl Acad Sci USA. 1998;95:2636–2641. doi: 10.1073/pnas.95.5.2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Klingberg T, Vaidya CJ, Gabrieli JD, Moseley ME, Hedehus M. Myelination and organization of the frontal white matter in children: a diffusion tensor MRI study. Neuroreport. 1999;10:2817–2821. doi: 10.1097/00001756-199909090-00022. [DOI] [PubMed] [Google Scholar]
- 51.Catani M, ffytche DH. The rises and falls of disconnection syndromes. Brain. 2005;128(Part 10):2224–2239. doi: 10.1093/brain/awh622. [DOI] [PubMed] [Google Scholar]
- 52.Geschwind N. Disconnection syndromes in animals and man. Brain. 1965;88:237–294. doi: 10.1093/brain/88.2.237. [DOI] [PubMed] [Google Scholar]
- 53.Deutsch GK, Dougherty RF, Bammer R, Siok WT, Gabrieli JD, Wandell B. Children’s reading performance is correlated with white matter structure measured by diffusion tensor imaging. Cortex. 2005;41:354–363. doi: 10.1016/s0010-9452(08)70272-7. [DOI] [PubMed] [Google Scholar]
- 54.Andrews JS, Ben-Shachar M, Yeatman JD, Flom LL, Luna B, Feldman HM. Reading performance correlates with white matter properties in preterm and full term children. Dev Med Child Neurol. doi: 10.1111/j.1469-8749.2009.03456.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beaulieu C, Plewes C, Paulson LA, et al. Imaging brain connectivity in children with diverse reading ability. Neuroimage. 2005;25:1266–1271. doi: 10.1016/j.neuroimage.2004.12.053. [DOI] [PubMed] [Google Scholar]
- 56.Rauschecker AM, Deutsch GK, Ben-Shachar M, Schwartzman A, Perry LM, Dougherty RF. Reading impairment in a patient with missing arcuate fasciculus. Neuropsychologia. 2009;47:180–194. doi: 10.1016/j.neuropsychologia.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Keller TA, Just MA. Altering cortical connectivity: remediation-induced changes in the white matter of poor readers. Neuron. 2009;64:624–631. doi: 10.1016/j.neuron.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Volpe JJ. Neurobiology of periventricular leukomalacia in the premature infant. Pediatr Res. 2001;50:553–562. doi: 10.1203/00006450-200111000-00003. [DOI] [PubMed] [Google Scholar]
- 59.Back SA. Perinatal white matter injury: the changing spectrum of pathology and emerging insights into pathogenetic mechanisms. Ment Retard Dev Disabil Res Rev. 2006;12:129–140. doi: 10.1002/mrdd.20107. [DOI] [PubMed] [Google Scholar]
- 60.Back SA, Riddle A, McClure MM. Maturation-dependent vulnerability of perinatal white matter in premature birth. Stroke. 2007;38(2 Suppl):724–730. doi: 10.1161/01.STR.0000254729.27386.05. [DOI] [PubMed] [Google Scholar]
- 61.Hamrick SEG, Miller SP, Leonard C, et al. Trends in severe brain injury and neurodevelopmental outcome in premature newborn infants: the role of cystic periventricular leukomalacia.[see comment] J Pediatr. 2004;145:593–599. doi: 10.1016/j.jpeds.2004.05.042. [DOI] [PubMed] [Google Scholar]
- 62.Inder T, Huppi PS, Zientara GP, et al. Early detection of periventricular leukomalacia by diffusion-weighted magnetic resonance imaging techniques.[see comment] J Pediatr. 1999;134:631–634. doi: 10.1016/s0022-3476(99)70251-9. [DOI] [PubMed] [Google Scholar]
- 63.Cheong JL, Thompson DK, Wang HX, et al. Abnormal white matter signal on MR imaging is related to abnormal tissue microstructure. AJNR Am J Neuroradiol. 2009;30:623–628. doi: 10.3174/ajnr.A1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dudink J, Lequin M, van Pul C, et al. Fractional anisotropy in white matter tracts of very-low-birth-weight infants. Pediatric Radiology. 2007;37:1216–1223. doi: 10.1007/s00247-007-0626-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rose J, Mirmiran M, Butler EE, et al. Neonatal microstructural development of the internal capsule on diffusion tensor imaging correlates with severity of gait and motor deficits. Dev Med Child Neurol. 2007;49:745–450. doi: 10.1111/j.1469-8749.2007.00745.x. [DOI] [PubMed] [Google Scholar]
- 66.Arzoumanian Y, Mirmiran M, Barnes PD, et al. Diffusion tensor brain imaging findings at term-equivalent age may predict neurologic abnormalities in low birth weight preterm infants. AJNR Am J Neuroradiol. 2003;24:1646–1653. [PMC free article] [PubMed] [Google Scholar]
- 67.Hoon AH, Jr, Stashinko EE, Nagae LM, et al. Sensory and motor deficits in children with cerebral palsy born preterm correlate with diffusion tensor imaging abnormalities in thalamocortical pathways. Dev Med Child Neurol. 2009;51:697–704. doi: 10.1111/j.1469-8749.2009.03306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nagae LM, Hoon AH, Jr, Stashinko E, et al. Diffusion tensor imaging in children with periventricular leukomalacia: variability of injuries to white matter tracts. AJNR Am J Neuroradiol. 2007;28:1213–1222. doi: 10.3174/ajnr.A0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nagy Z, Westerberg H, Skare S, et al. Preterm children have disturbances of white matter at 11 years of age as shown by diffusion tensor imaging. Pediatr Res. 2003;54:672–679. doi: 10.1203/01.PDR.0000084083.71422.16. [DOI] [PubMed] [Google Scholar]
- 70.Vangberg TR, Skranes J, Dale AM, Martinussen M, Brubakk A-M, Haraldseth O. Changes in white matter diffusion anisotropy in adolescents born prematurely. NeuroImage. 2006;32:1538–1548. doi: 10.1016/j.neuroimage.2006.04.230. [DOI] [PubMed] [Google Scholar]
- 71.Yung A, Poon G, Qiu D-Q, et al. White matter volume and anisotropy in preterm children: a pilot study of neurocognitive correlates. Pediatr Res. 2007;61:732–736. doi: 10.1203/pdr.0b013e31805365db. [DOI] [PubMed] [Google Scholar]
- 72.Skranes J, Vangberg TR, Kulseng S, et al. Clinical findings and white matter abnormalities seen on diffusion tensor imaging in adolescents with very low birth weight. Brain. 2007;130:654–666. doi: 10.1093/brain/awm001. [DOI] [PubMed] [Google Scholar]
- 73.Kontis Db, Catani Ma, Cuddy Mb, et al. Diffusion tensor MRI of the corpus callosum and cognitive function in adults born preterm. Neuroreport. 2009;20:424–428. doi: 10.1097/WNR.0b013e328325a8f9. [DOI] [PubMed] [Google Scholar]
- 74.Nielsen JF. A modular framework for development and interlaboratory sharing and validation of diffusion tensor tractography algorithms. J Digit Imaging. 2006;19:112–117. doi: 10.1007/s10278-006-9948-5. [DOI] [PMC free article] [PubMed] [Google Scholar]