Abstract
Background
Shear wave elastography (SWE) is widely used in breast, liver, prostate and thyroid evaluations. Elastography provides additional information if used to assess parotid gland pathology. We assessed parotid glands by means of SWE to compare the parenchyma properties in different types of inflammation.
Material/Methods
Prospective analysis included 78 consecutive patients with parotid gland pathology: sialolithiasis (33), Stensen’s duct stenosis (15), chronic inflammation (10), and primary Sjögren syndrome (pSS) (20) treated at the Department of Otolaryngology, Head and Neck Surgery of PUMS. The primary predictor variable was type of parotid pathology, and secondary predictor variables were patient age and the duration and intensity of complaints. Ultrasound pictures were compared with elastography values of parotid parenchyma.
Results
Mean elasticity values for pSS (111 Kilopascals (kPa), Stensen’s duct stenosis (63 kPa), sialolithiasis (82 kPa), and chronic inflammation (77 kPa) were significantly higher than the mean value for healthy patients (24 kPa). Elasticity increased proportionally to the intensity of complaints: mild (51 kPa), moderate (78 kPa), and strong (90 kPa). Increased elasticity did not correspond with ultrasonographic pictures. In pSS the parenchyma was almost twice as stiff as in chronic inflammation (p=0.02), although subjective complaints were mostly mild or moderate, and the ultrasonographic picture did not present features of fibrosis.
Conclusions
Sonoelastography, by improving routine ultrasonographic assessment, might be a useful tool for parotid evaluations during the course of chronic inflammation. An extraordinarily high degree of stiffness was revealed in pSS despite lack of fibrosis by ultrasonography and moderate subjective complaints, suggesting that sonoelastography could be a valuable diagnostic tool.
MeSH Keywords: Elasticity Imaging Techniques, Sialadenitis, Ultrasonography
Background
Salivary glands are small organs in the head and neck region; their impact on quality of life is high. Proper saliva production and moisturization of the whole oral cavity have a basic role in preparing the bolus, deglutition, dental status, and subjective well-being. Aesthetic issues are also of importance. The volume of the parotid glands, situated in the preauricular region, influence the shape of the face. Salivary dysfunction is one of the major manifestations in the wide clinical spectrum of Sjögren’s syndrome (SS). The diagnosis of SS is complex and assessment of the involvement of salivary glands in this slowly progressing condition is difficult. The latest American-European criteria (AEC) for SS include: 1. A questionnaire to assess oral and ocular symptoms, 2. Evidence of dry eye (Schimer test), 3. Presence of anti-SS-A/B antibodies, 4. Sialoscintigraphy (sSC), and 5. Biopsy of the minor salivary gland [1–3]. A high diagnostic accuracy of ultrasonography (US) was demonstrated and a novel scoring system for parenchyma inhomogeneity presented [4]. US pictures of the major salivary glands have high diagnostic accuracy for primary Sjögren syndrome (pSS), notably improving diagnostic performance [5]. US pictures, as a highly developed imaging technique [6], could potentially replace sSC [7] or sialography [8], and could be a new item in the AEC for diagnosis of SS [9]. Another issue is treatment efficiency assessment; recent advances via repeated biopsies, sialometry, sialochemistry, biomarkers, secretion, EULAR (European League Against Rheumatism) SS disease activity index, and patient’s related index, reliably judge and compare the value of the therapy [10].
One of the key medical methods for detection and characterization of pathologies is the assessment of tissue stiffness by palpation. A new technique reflecting the elasticity of tissue is sonoelastography [11–14]. Shear-wave elastography (SWE) overlaps morphological ultrasound criteria and potentially gives more information about the status of gland parenchyma in a “one touch” examination. This quantitative technique, developed by Supersonic Imaging (SSI), is available on superficial high-frequency probes. Ultrasound scanners are used to generate short-duration acoustic radiation forces that impart small (1–10 μ) localized displacements in the tissue, correlating with the local stiffness [15,16]. This phenomenon provides information about the local viscoelastic properties of the tissue. Images are shown with a color scale linked to the value in kPa [12,17] (Figure 1). Although the method has been widely used in breast [18,19], liver [20–22], pancreas [23], prostate [24], and thyroid [25,26] parenchyma evaluation, there are no established cut-off values to differentiate parotid gland lesions. Thus, sonoelastography could deliver more information in different parotid inflammatory processes and might help to improve qualification for management strategies, treatment intensification, and efficient monitoring. To date, an investigation of parotid elasticity in the course of pSS has not been conducted. The aim of this study was to assess the parotid by means of SWE and to compare the parenchyma properties in pSS, Stensen’s duct stenosis, sialolithiasis, and chronic non-specific inflammation (Figure 2).
Figure 1.
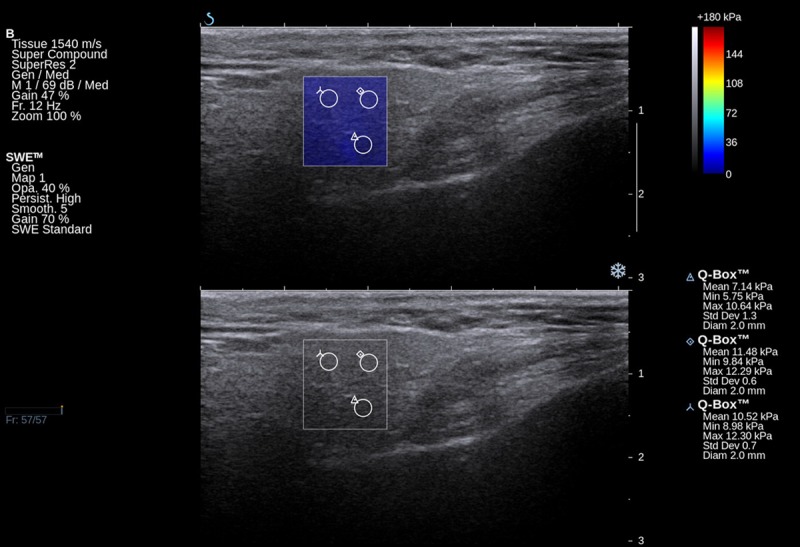
Elastographic and B-mode image of a normal parotid gland of a healthy volunteer.
Figure 2.
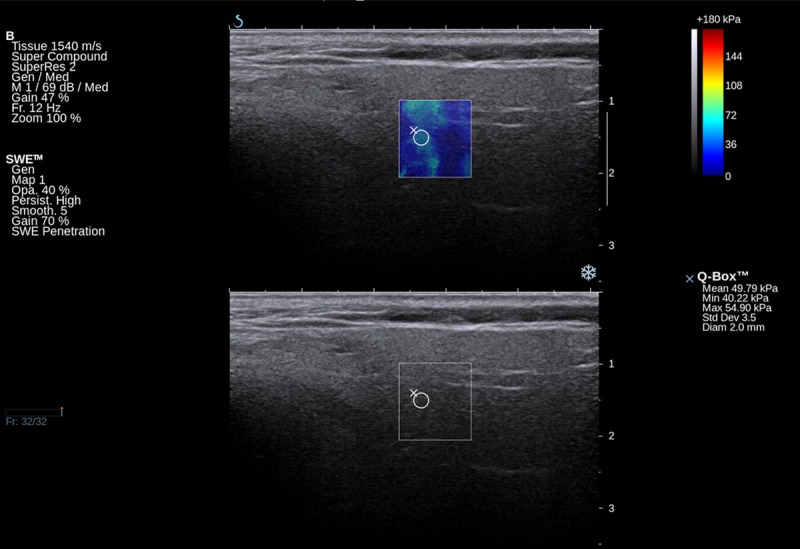
Elastographic and B-mode image of inflammatory changed parotid gland in a chronic sialolithiasis patient.
Material and Methods
We performed a prospective analysis of 78 patients with parotid gland inflammatory conditions. The study population was composed of a cohort of patients with sialolithiasis (33), Stensen’s duct stenosis (15), chronic inflammation (10), and pSS (20) treated at the tertiary referral center of the Department of Otolaryngology, Head and Neck Surgery, Poznan University of Medical Science, between January 2011 and December 2011. To be enrolled to the study, the patient had to have a history of chronic parotid gland disease, and preauricular swelling and pain and be eligible for ultrasound and elastography examination. Patients under suspicion of parotid tumor or an acute inflammatory episode were excluded.
Ultrasound elastography was performed with a SuperSonic Imagine Aixplorer using a SuperLinear™ SL15-4 transducer operating with frequency of 4–15 MHz. In measurements, 11 MHz frequency was used. The detailed elasticity values (in kPa) were collected on elastograms. The minimal, maximal, and mean values from 3 measurement points in different parts of the parotid were taken into consideration. The places of measurements were chosen by an experienced sonographer as being most representative of the whole glandular parenchyma. Next, the mean value was obtained. The depth from the skin surface to the external border of the sample box was 5–8 mm as a result of subcutaneous tissue thickness. The transducer location needed to be chosen properly. Because of the small space between the mandible and temporal bone, the examination was vulnerable to the artifacts as a result of ultrasound wave reflection. Choosing the appropriate location and size of the elastographic frame, an experienced sonographer could avoid disturbances arising from both surrounding bone structures and widened salivary ducts, and executed all sonoelastographic surveys with repetitive outcomes. The stiffness score range was 9–269 kPa, depending on glandular pathology. The gland in each case was also examined by high resolution B-mode ultrasonography. The basic parameter was the parenchyma echogenicity. The normoechogenic, isoechoic images of normal parotid tissue were in contrast with the heteroechogenic, hyperechoic, high-density areas of pathological parenchyma. Additional US findings included hyperechoic stone deposits, widened Stensen’s ducts, and multiple hypoechoic areas of enlarged secondary ducts.
For all patients, their history was taken with special regard to the duration of complaints: <12, 12–24, and >24 months. The intensity of swelling, pain, and xerostomia were categorized into a 3-step subjective scale as mild, moderate, and strong. Patients with pSS completed the AEC questionnaire to assess the oral and ocular symptoms, had the Schimer test for evidence of dry eye, were tested for the presence of anti-SS-A/B antibodies, and had sSC. Eight of the 20 pSS patients had sialoendoscopy with steroid infusion. In all other patients, the final diagnosis was obtained by performing both diagnostic and therapeutic sialoendoscopy using a Karl Storz sialoendoscope of 1.6 mm and 1.3 mm diameter.
Fifty-four healthy patients aged 43–78 years, mean 60 years, constituted the reference group for the elastography values. The mean elasticity of salivary glands from the reference group was compared with the mean values obtained for the each of the 4 pathological entities.
The main predictor variable was the type of parotid pathology: sialolithiasis, duct stenosis, or chronic inflammation. Secondary predictor variables were ultrasound features of parenchyma, patient age, and the duration and intensity of complaints. Primary outcome variables were the mean elastography values of parotid gland parenchyma. The interdependence between predictor and outcome variables was analyzed. Statistical analysis performed by means of Spearman’s rank correlation coefficient was used to describe interdependence between elastography results and: patient age, patient complaints, and duration of complaints. The Kruskal-Wallis test was used to compare patient complaints and pathology of salivary glands; elastography results: and duration of complaints, severity of complaints, pathology of salivary glands, and USG picture. This test was also used for correlation of treatment success (defined as endoscopic stone evacuation or Stensen’s duct dilatation) with duration of complaints and pathology of salivary glands. The Mann-Whitney test was used for statistical analysis of elastography results of the whole examined group, patients with Sjögren syndrome, and sialolithiasis cases, in comparison to the control group, treatment success, and elastography results.
Results
Parotid pathology (primary predictor variables)
Table 1 shows data from the 33 patients with sialolithiasis, 15 with Stensen’s duct stenosis, 10 with chronic inflammation, and 20 with diagnosed pSS, regarding their age, the duration and intensity of complaints, sonographic pictures, and elastography values of parotid parenchyma.
Table 1.
Type of parotid pathology with regard to: patient’s age, the duration and intensity of complaints, ultrasound images and the primary outcome variable: elastography values (p value presented only for characteristics for which statistical tests were performed).
| Diagnosis/number of patients | pSS/20 | Stensen’s duct stenosis/15 | Sialolithiasis/33 | Chronic inflammation/10 | All/78 | p |
|---|---|---|---|---|---|---|
| Age (years) | ||||||
| Mean | 46 | 49 | 47 | 42 | 46 | |
| Median | 48 | 55 | 49 | 48 | 49 | |
| Std. dev. | 17 | 13 | 16 | 15 | ||
|
| ||||||
| Duration of complaints (months) | ||||||
| Mean | 18 | 14 | 15 | 13 | 15 | |
| Median | 24 | 12 | 12 | 9 | 12 | |
| Std. dev. | 8 | 9 | 9 | 10 | ||
|
| ||||||
| Intensity of complaints (No. of patients) | ||||||
| Mild | 8 | 5 | 4 | 7 | 24 | |
| Moderate | 6 | 7 | 12 | 3 | 28 | |
| Strong | 6 | 3 | 17 | 0 | 26 | |
|
| ||||||
| Ultrasound picture (No. of patients) | ||||||
| Hypoechoic areas | 14 (70%) | 0 (0%) | 0 (0%) | 0 (0%) | 14 (18%) | |
| Normoechogenic | 6 (30%) | 13 (86.7%) | 21 (63.6%) | 10 (100%) | 50 (64%) | |
| Hyperechogenic | 0 (0%) | 2 (13.3%) | 12 (36.4%) | 0 (0%) | 14 (18%) | |
|
| ||||||
| Elasticity values (kPa) | ||||||
| Mean | 111 | 63 | 82 | 48 | 77 | |
| Median | 106 | 60 | 66 | 21 | 62 | 0.05 |
| Std. dev. | 60 | 40 | 64 | 50 | 59 | |
Patient characteristics (secondary predictor variables)
There may be interdependence between the parenchyma stiffness and patient age, as well as the duration and intensity of complaints. Thus, these 3 variables were analyzed first. The patient age range was 16–72 years, with a mean of 46 years and a median of 49 years; particular etiological subgroups did not differ statistically. The duration of the complaints for the whole group ranged from 2 to 24 months. Complaints from the pSS, Stensen’s duct stenosis, sialolithiasis, and chronic inflammation lasted 24, 12, 12, and 9 months, respectively. The intensity of complaints categorized as strong occurred in 6/20 (30%) pSS patients, in 3/15 (20%) Stensen’s duct stenosis patients, in 17/33 (52%) sialolithiasis patients, and in 0/10 (0%) chronic inflammation patients.
Hyperechogenicity of salivary gland parenchyma and widening of excretory ducts were the next variables taken into consideration. Ultrasonographic findings revealed normoechoic parenchyma in 50 patients, hypoechoic foci in 14 patients, and hyperechoic tissue in 14 patients. Hyperechogenicity was found in 2/15 (13.3%) Stensen’s duct stenosis patients, and in 12/33 (36.4%) sialolithiasis patients. All 14 multiple hypoechoic lesions were found in the pSS patients group (Figure 3). There was no interdependence between patient age, the intensity and duration of complaints, and the ultrasound picture of the gland parenchyma.
Figure 3.
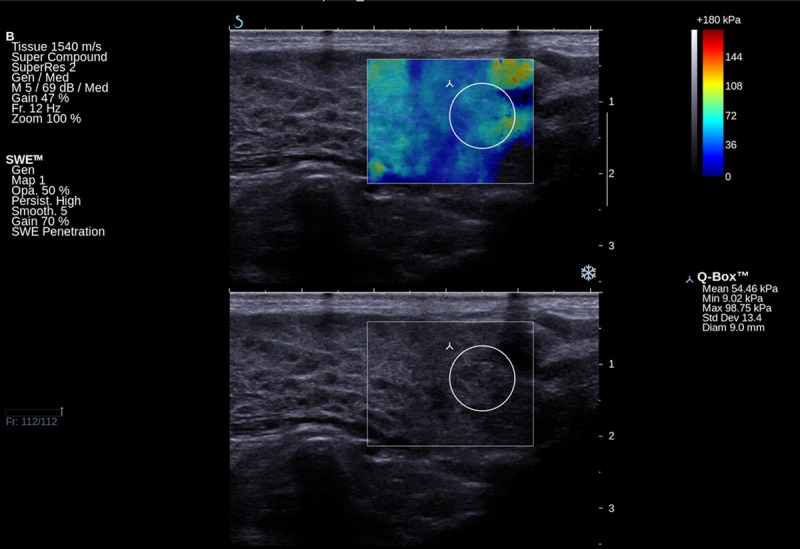
Elastographic and B-mode image of hypoechoic areas in a pSS patient.
Elastography values (primary outcome variable)
The sonoelastography values with regard to patient age, intensity and duration of complaints, sonographic picture, and type of pathology were analyzed. Forty-two patients were above and 36 were below the mean age of 46 years. Mean elastography values did not differ significantly in either age category (70 kPa and 83 kPa, respectively) (p=0.27). The mean duration of symptoms was 15 months, mean elastography values for the longer and shorter observation time were 85 kPa and 68 kPa, respectively, but the difference was not significant (p=0.13). Twenty-six patients with complaints subjectively described as strong had mean elastography values of 90 kPa, the 28 with moderate complaints had mean elastography values of 78 kPa and the 24 with mild complaints had mean elastography values of 51 kPa; the differences in all 3 categories obtained with Kruskal-Wallis test were statistically significant (p=0.03).
Parenchyma inhomogeneity was present in 28 US examinations. In 14 patients with images assessed as hyperechoic, the mean parenchyma stiffness was 104 kPa; in 14 patients with hypoechoic foci, mean parenchyma stiffness was 127 kPa; and in 50 patients with normoechogenic parenchyma, mean parenchyma stiffness was 62 kPa. The differences in all 3 were statistically significant (p=0.02).
Elasticity values in the 4 etiologic groups were analyzed. For the pSS, Stensen’s duct stenosis, sialolithiasis, and chronic inflammation groups, mean values were 111 kPa, 63 kPa, 82 kPa, and 48 kPa, respectively, and median values were 106 kPa, 60 kPa, 66 kPa, and 21 kPa, respectively. The difference between the pSS group and the other groups was significant (p=0.039); interestingly, the glandular parenchyma of pSS patients was almost twice as stiff as in the chronic inflammation group, although the subjective complaints were mostly mild or moderate and the sonographic picture did not present hyperechoic changes. In the sialolithiasis patients, the measurements of parenchyma stiffness were not influenced by the presence of the stone. The elasticity measurements were taken away from the stone, which in all cases was impacted in the Stensen’s duct. The elasticity did not differ statistically between men (n=32, mean 81 kPa, median 61 kPa, Q1=42 kPa, Q3=89 kPa (first and third quartile) and women (n=46, mean 74 kPa, median 64 kPa, Q1=25 kPa, Q3=112 kPa). The mean value of elasticity for the healthy patients was 24 kPa. It was comparable in the right and left gland for all individuals and was significantly lower than in all considered disease conditions (pSS p=0.00001, Stensen’s duct stenosis p=0.003, sialolithiasis p=0.002, and chronic inflammation p=0.005).
Discussion
Diagnostic tools for the salivary glands are well established, but efforts to introduce new methods are still ongoing. US, CT, and MR are the methods of choice in imaging the status of parenchyma. Sialography, sialoendoscopy, and MR sialography are useful in Stensen’s duct visualization. US seems the most attractive, available, inexpensive, and non-invasive technique. It can be performed repeatedly, and could replace X-ray sialography and sSC [7]. SWE as the adjunct tool in parotid US is easy to perform, adds objective information about the tissue properties, and indicates the extent of fibrotic areas. Stiffness measurements are reproducible over time.
There has been no earlier work on parotid parenchyma SWE, and this paper is to our knowledge the first one describing objective stiffness measurements in the chronic inflammatory process of salivary glands. Elasticity is a new parameter in parotid assessment; a few papers have discussed the specificity and sensitivity in preoperative parotid tumors differentiation [27–29], but the range of elasticity values measured (in kPa) in different conditions has not yet been established. The mean elasticity for the healthy subjects was 24 kPa and was 77 kPa for the examined group.
Fibrosis is synonymous with stiffness for all organ parenchyma, although there 2 terms are semantically different. The imaging modalities (MR, CT, and US) have the potential to assess the fibrosis as high-density or echoes of some parts of the organ or the whole organ. In our study, in 14 cases the parotids had the sonographic features of fibrosis with mean elasticity values 104 kPa, while the normoechogenic parenchyma had mean elasticity of 62 kPa. The interdependence between the palpation properties of tissue, the sonographic picture, and objectively measured elasticity is clear; the first 2 methods are subjective, unlike elastography in which reproducible values were obtained.
Based upon the presented examinations, the elasticity of the parotid parenchyma does not depend on patient age or complaint duration. It was interesting that in chronic inflammatory conditions, the elasticity values increased proportionally with the intensity of complaints; for the range of subjective complaints (mild, moderate, and strong), the mean elasticity values increased proportionally (51 kPa, 78 kPa, and 90 kPa, respectively). But most striking was the noticeable difference in the mean parenchyma stiffness of the particular disease entities: 111 kPa for pSS, 63 kPa for Stensen’s duct stenosis, 82 kPa for sialolithiasis, and 48 kPa for chronic inflammation. The enormously high values in the pSS group cannot be explained by the greater intensity of ailments – complaints categorized as strong occurred in 6 of the 20 pSS group and in half of the group with sialolithiasis. In the pSS group, there was no hyperechogenicity in US images, but there were normoechogenic parenchyma (6 patients), or hypoechoic areas (14 patients). It appears that elasticity did correspond closely with hypoechoic areas, but the findings were not proven statistically.
Conclusions
Our study showed that sonoelastography improved the standard sonographic assessment of salivary glands during the course of chronic inflammation. Although the method did not provide detailed information about the internal architecture and morphological changes of the gland, it might be a useful adjunct to ultrasonography, primarily to confirm certain tissue characteristics suggested by ultrasound. Increased elasticity of the gland did not correspond to the ultrasonographic picture of parenchyma, but agreed with the severity of complaints. In all pathological conditions taken into consideration, the ES values were significantly higher then in healthy controls. Extraordinary high stiffness was discovered in pSS, although no fibrosis in US was detected and subjective complaints were moderate. Thus, for patients with pSS, this method seems to be especially valuable. Further investigations will be needed to determine if this high or increasing stiffness may predict the progressive course of the sialadenitis and if elasticity might change independently from ultrasound appearance over a period of extended longitudinal observation.
There is no potential conflict of interest regarding this manuscript. There are no commercial benefits from the work reported on in the manuscript.
Footnotes
Source of support: The study was approved by the Bioethics Committee of Poznan University of Medical Sciences (no. 494/12)
References
- 1.Vitali C, Carotti M, Salaffi F. Is it the time to adopt salivary gland ultrasonography as an alternative diagnostic tool for the classification of patients with Sjogren’s syndrome? Arthritis Rheum. 2013;65(7):1950. doi: 10.1002/art.37945. [DOI] [PubMed] [Google Scholar]
- 2.Baldini C, Talarico R, Tzioufas AG, Bombardieri S. Classification criteria for Sjogren’s syndrome: a critical review. J Autoimmun. 2012;39(1–2):9–14. doi: 10.1016/j.jaut.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 3.Tzioufas AG, Kapsogeorgou EK, Moutsopoulos HM. Pathogenesis of Sjogren’s syndrome: what we know and what we should learn. J Autoimmun. 2012;39(1–2):4–8. doi: 10.1016/j.jaut.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Milic VD, Petrovic RR, Boricic IV, et al. Major salivary gland sonography in Sjogren’s syndrome: diagnostic value of a novel ultrasonography score (0–12) for parenchymal inhomogeneity. Scand J Rheumatol. 2010;39(2):160–66. doi: 10.3109/03009740903270623. [DOI] [PubMed] [Google Scholar]
- 5.Cornec D, Jousse-Joulin S, Pers JO, et al. Contribution of salivary gland ultrasonography to the diagnosis of Sjogren’s syndrome: toward new diagnostic criteria? Arthritis Rheum. 2013;65(1):216–25. doi: 10.1002/art.37698. [DOI] [PubMed] [Google Scholar]
- 6.Esmadi M, Lone N, Ahmad DS, et al. Multiloculated pleural effusion detected by ultrasound only in a critically-ill patient. Am J Case Rep. 2013;14:63–66. doi: 10.12659/AJCR.883816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Milic V, Petrovic R, Boricic I, et al. Ultrasonography of major salivary glands could be an alternative tool to sialoscintigraphy in the American-European classification criteria for primary Sjogren’s syndrome. Rheumatology (Oxford) 2012;51(6):1081–85. doi: 10.1093/rheumatology/ker431. [DOI] [PubMed] [Google Scholar]
- 8.Takagi Y, Kimura Y, Nakamura H, et al. Salivary gland ultrasonography: can it be an alternative to sialography as an imaging modality for Sjogren’s syndrome? Ann Rheum Dis. 2010;69(7):1321–24. doi: 10.1136/ard.2009.123836. [DOI] [PubMed] [Google Scholar]
- 9.Vitali C, Bootsma H, Bowman SJ, et al. Classification criteria for Sjogren’s syndrome: we actually need to definitively resolve the long debate on the issue. Ann Rheum Dis. 2013;72(4):476–78. doi: 10.1136/annrheumdis-2012-202565. [DOI] [PubMed] [Google Scholar]
- 10.Vissink A, Bootsma H, Spijkervet FK, et al. Current and future challenges in primary Sjogren’s syndrome. Curr Pharm Biotechnol. 2012;13(10):2026–45. doi: 10.2174/138920112802273254. [DOI] [PubMed] [Google Scholar]
- 11.Ferraioli G, Tinelli C, Dal Bello B, et al. Accuracy of real-time shear wave elastography for assessing liver fibrosis in chronic hepatitis C: A pilot study. Hepatology. 2012;56(6):2125–33. doi: 10.1002/hep.25936. [DOI] [PubMed] [Google Scholar]
- 12.Lambrecht M, Nevens D, Nuyts S. Intensity-modulated radiotherapy vs. parotid-sparing 3D conformal radiotherapy. Effect on outcome and toxicity in locally advanced head and neck cancer. Strahlenther Onkol. 2013;189(3):223–29. doi: 10.1007/s00066-012-0289-7. [DOI] [PubMed] [Google Scholar]
- 13.Kumar S, Ram S, Navazesh M. Salivary gland and associated complications in head and neck cancer therapy. J Calif Dent Assoc. 2011;39(9):639–47. [PubMed] [Google Scholar]
- 14.Fleming IN, Kut C, Macura KJ, et al. Ultrasound elastography as a tool for imaging guidance during prostatectomy: initial experience. Med Sci Monit. 2012;18(11):CR635–42. doi: 10.12659/MSM.883540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nieuw Amerongen AV, Veerman EC. Current therapies for xerostomia and salivary gland hypofunction associated with cancer therapies. Support Care Cancer. 2003;11(4):226–31. doi: 10.1007/s00520-002-0409-5. [DOI] [PubMed] [Google Scholar]
- 16.Shahdad SA, Taylor C, Barclay SC, et al. A double-blind, crossover study of Biotene Oralbalance and BioXtra systems as salivary substitutes in patients with post-radiotherapy xerostomia. Eur J Cancer Care (Engl) 2005;14(4):319–26. doi: 10.1111/j.1365-2354.2005.00587.x. [DOI] [PubMed] [Google Scholar]
- 17.Güneri P, Alpöz E, Epstein JB, et al. In vitro antimicrobial effects of commercially available mouth-wetting agents. Spec Care Dentist. 2011;31(4):123–28. doi: 10.1111/j.1754-4505.2011.00194.x. [DOI] [PubMed] [Google Scholar]
- 18.Balleyguier C, Ciolovan L, Ammari S, et al. Breast elastography: The technical process and its applications. Diagn Interv Imaging. 2013;94(5):503–13. doi: 10.1016/j.diii.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 19.Stock M, Dörr W, Stromberger C, et al. Investigations on parotid gland recovery after IMRT in head and neck tumor patients. Strahlenther Onkol. 2010;186(12):665–71. doi: 10.1007/s00066-010-2157-7. [DOI] [PubMed] [Google Scholar]
- 20.Ferraioli G, Lissandrin R, Filice C. Real-time tissue elastography in the assessment of liver stiffness. Hepatology. 2013;58(2):834. doi: 10.1002/hep.26215. [DOI] [PubMed] [Google Scholar]
- 21.Sporea I, Sirli RL. Hepatic elastography for the assessment of liver fibrosis – present and future. Ultraschall Med. 2012;33(6):550–58. doi: 10.1055/s-0032-1313011. [DOI] [PubMed] [Google Scholar]
- 22.Yu H, Wilson SR. New noninvasive ultrasound techniques: can they predict liver cirrhosis? Ultrasound Q. 2012;28(1):5–11. doi: 10.1097/RUQ.0b013e31824a4fc9. [DOI] [PubMed] [Google Scholar]
- 23.Maurer J, Hipp M, Schäfer C, Kölbl O. Dysphagia. Impact on quality of life after radio(chemo)therapy of head and neck cancer. Strahlenther Onkol. 2011;187(11):744–49. doi: 10.1007/s00066-011-2275-x. [DOI] [PubMed] [Google Scholar]
- 24.Zhu Y, Chen Y, Qi T, et al. Prostate cancer detection with real-time elastography using a bi-plane transducer: comparison with step section radical prostatectomy pathology. World J Urol. 2014;32(2):329–33. doi: 10.1007/s00345-012-0922-1. [DOI] [PubMed] [Google Scholar]
- 25.Bhatia KS, Tong CS, Cho CC, et al. Shear wave elastography of thyroid nodules in routine clinical practice: preliminary observations and utility for detecting malignancy. Eur Radiol. 2012;22(11):2397–406. doi: 10.1007/s00330-012-2495-1. [DOI] [PubMed] [Google Scholar]
- 26.Ruchala M, Szczepanek E, Sowinski J. Sonoelastography in de Quervain thyroiditis. J Clin Endocrinol Metab. 2011;96(2):289–90. doi: 10.1210/jc.2010-1595. [DOI] [PubMed] [Google Scholar]
- 27.Wierzbicka M, et al. Is sonoelastography a helpful method for evaluation of parotid tumors? Eur Arch Otorhinolaryngol. 2013;270(7):2101–7. doi: 10.1007/s00405-012-2255-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Celebi I, Mahmutoglu AS. Early results of real-time qualitative sonoelastography in the evaluation of parotid gland masses: a study with histopathological correlation. Acta Radiol. 2013;54(1):35–41. doi: 10.1258/ar.2012.120405. [DOI] [PubMed] [Google Scholar]
- 29.Ying M, Wu VW, Kwong DL. Comparison of sonographic appearance of normal and postradiotherapy parotid glands: a preliminary study. Ultrasound Med Biol. 2007;33(8):1244–50. doi: 10.1016/j.ultrasmedbio.2007.02.016. [DOI] [PubMed] [Google Scholar]


