Abstract
Background
rs895819 and rs11671784 are 2 SNPs in miR-27a that can influence the expression of mature miRNA. However, their role in gastric cancer development is still not well understood. This study aimed to determine whether these 2 polymorphisms are associated with gastric cancer risk in a Chinese population and how they influence the expression of miR-27a.
Material/Methods
This was a case-control study and recruited 278 gastric cases and 278 healthy matched controls. Genotyping of these 2 SNPs among the participants were performed to assess their association with gastric cancer risk. Tumor samples from 59 patients who had physical resection were used for qRT-PCR analysis of miR-27a expression. To further valid the effects of these 2 SNPs, findings of previous studies were pooled to generate integrated evidence.
Results
Individuals with rs895819 G variants exhibited significantly increased risk of gastric cancer, while subjects with rs11671784 A variants had significantly reduced gastric cancer risk. Among the patients, rs895819 G variants were moderately associated with lymphatic invasion and lymph node metastasis, while rs11671784 A variants were associated with significantly reduced risk of lymphatic invasion. qRT-PCR results demonstrated rs895819 polymorphism contributed to an aberrant process from pri-miR-27a to pre-miR-27a, but rs11671784 did not affect the transcription and post-transcription processes of the miR-27a gene. The subsequent meta-analysis largely confirmed the effects of these 2 SNPs on gastric cancer risk.
Conclusions
rs895819 and rs11671784 inversely affect gastric cancer risk and the influence was closely related to their effects on miR-27a expression.
MeSH Keywords: MicroRNAs, Polymorphism, Single Nucleotide, Stomach Neoplasms
Background
Gastric cancer, which has a 5-year survival rate below 25%, is the second leading cause of cancer death worldwide [1]. It has a particularly high occurrence rate in Eastern Europe, East Asia, and some parts of Central and South America [1,2]. In China, gastric cancer is a serious public health problem. Approximately 40% of gastric cancer cases in developing countries occur in China [3].
Gastric carcinogenesis is influenced by multiple genetic and environmental factors [4]. Accumulating evidence shows that individual genetic susceptibility can significantly affect occurrence rate of cancer [5]. The regulative roles of microRNAs (miRNAs) in multiple biological processes, including cell proliferation, cell differentiation, cell death, and stress resistance, were widely reported in previous studies [6,7]. In human carcinogenesis, miRNAs can be either tumor suppressors or oncogenes [8,9]. Previous studies reported that in gastric cancer, miR-27a was upregulated and functioned as an oncogene [10,11]. An in vitro study showed that down-regulation of miR-27a reduced the growth rate of MGC-803, a gastric adenocarcinoma cell line [10]. Katada et al. also observed that in undifferentiated gastric cancer, miR-27a expression was positively correlated with lymph node metastasis [12].
Recent studies have reported that single-nucleotide polymorphisms (SNPs) in miRNA were related to susceptibility to several cancers [13,14]. SNPs in miRNA could alter the expression and/or maturation of miRNA and ultimately affect its functioning [14–16]. In pri-miR-27a, rs895819 and rs11671784 are 2 SNPs that can influence the expression of mature miRNA. However, few studies have explored the association between these 2 SNPs and gastric cancer susceptibility [17–19]. Two studies of rs895819 and gastric cancer risk even reported conflicting results [17,18]. In addition, these 2 SNPs were only 4 nucleotides away from each other, and their combined effects on gastric cancer are still unclear. Considering the important role of miR-27a in gastric cancer, this study aimed to explore whether these 2 SNPs were related to gastric cancer and how they influence the expression of mature miR-27a.
Material and Methods
Study participants
This was a hospital-based case-control study with 278 participants who were diagnosed with gastric cancer, of which 59 patients received surgical resection of gastric tumor tissues at the Central Hospital of Wuhan of Tongji Medical College and the Huashan Hospital of Fudan University from 2010 to 2013. The protocol of this study was approved by the ethics committees of Huazhong University of Science and Technology and Fudan University and all study procedures were performed according to the Declaration of Helsinki principles. Informed consent was obtained from all participants at recruitment. All participants were ethnic Han Chinese without direct blood relationship. All of the gastric cancer patients were newly diagnosed, without historical cancer experience and had not received preoperative radiotherapy or chemotherapy before the surgical gastrectomy. The case group included 196 male and 82 female patients, with an average age of 58.5±9.6 years (range 28–79 years). To match the case group, 278 healthy individuals matched for ethnicity, sex, and age were randomly selected from the blood donation center of the hospital. All control individuals were without malignancy or autoimmune disease. Demographic characteristics of all participants, including age, sex, alcohol and smoking habits, and family history of cancer were obtained and recorded through a structured questionnaire. Individuals who consumed 40 g or more of pure alcohol per day were defined as drinkers [20]. Individuals who smoked at least 1 cigarette per day during the past 1 year were defined as smokers. Fifty-nine gastric tumor tissues were obtained from gastric cancer patients during surgery and were frozen in liquid nitrogen until further use. All clinic-pathological data of these 59 patients were obtained from clinical and pathology records.
Genotyping
We used the NucleoSpin Blood kit (Macherey-Nagel, GmbH & Co. KG, Germany) for genomic DNA extraction according to the manufacturer’s recommended protocol. The DNA concentration was determined with a Nanodrop Spectrophotometer (ND-1000, USA) by using the full wave-length. rs895819 and rs11671784 are only 4 nucleotides away from each Other, thus the TaqMan probe overlapping these 2 SNPs would give false calls in SNP constellations [21]. To avoid possible false calls in SNP constellations, direct sequencing (SNPseq™, GeneSky) was used for genotyping of rs895819 and rs11671784 in this study.
Quantitative RT-PCR
Total RNA from tumor tissues were extracted with TRIzol isolation reagent (Invitrogen) according to the instructions. TaqMan microRNA assays (Applied Biosystems) were used to quantify the expression of mature miR-27a. U6B was used as normalization control. Briefly, pri- and pre-miRNA levels were determined by qRT-PCR with gene-specific primers and Power SYBR Green PCR Master Mix (Applied Biosystems). The primers for qRT–PCR of pri-miR-27a and both pre- and pri-miR-27a followed the design of Yang et al. [19]. For pri-miR-27a, the primers were 5′-ATATGAGAAAAGAGCTTCCCTGTG-3′ (F) and 5′-CAAGGCCAGAGGAGGTGAG-3′ (R). For both pre- and pri-miR-27a, the primers were: 5′-AGGGCTTAGCTGCTTGTGAG-3′ (F) and 5′-CAAGGCCAGAGGAGGTGAG-3′ (R). All qRT–PCRs were performed in triplicate with the ABI Prism 7300 sequence detection system (Applied Biosystems).
Meta-analysis
Relevant literature about the association between rs895819 and rs11671784 polymorphism and gastric cancer risk were searched for in PubMed, Web of Science, and Medline from January 2002 to March 2014. The key words used were “miR-27a”, “microRNA-27a”, “rs11671784”, “rs895819“, “gastric cancer/carcinoma/tumor/neoplasm”, and “polymorphism’”. Two authors independently performed the search process. For inclusion, studies had to simultaneously meet the following criteria: (1) case-control study; (2) the study explored the association between miR-27a polymorphism and gastric cancer risks; and (3) genotype frequency information was available. Original information, including method of genotyping, total number of cases and controls, source of control groups, and genotype distributions in cases and controls, were extracted for further meta-analysis. Research data obtained from this study were also integrated into the meta-analysis.
Statistical analysis
Quantitative variables with normal distribution are reported with median ±SD. Genotype frequencies and categorical variables between gastric cancer patients and control individuals were analyzed with Pearson χ2 test. Hardy-Weinberg equilibrium of the control group was analyzed by goodness-of-fit χ2 test. The association between SNP rs895819, rs11671784, and risk of gastric carcinoma was estimated by odds ratios (OR) and 95% confidence intervals (CI). For rs895819, the association between genetic variants and gastric cancer risk was analyzed between allelic contrast (A vs. G), homozygote comparisons, (AA vs. GG), heterozygote comparisons (AG vs. GG), dominant model (AA+AG vs. GG), and recessive models (AA vs. AG+GG). The same analysis was applied to rs11671784. The crude OR was assessed by the Woolf approximation method and adjusted OR was calculated by unconditional logistic regression with adjustment of sex, age, residence, smoking status, and alcohol consumption status. Group difference was compared using the t test. In meta-analysis, statistical heterogeneity among studies were assessed by the chi-square-based Q test and I2 [22]. χ2 tests p<0.1 and I2 >50% indicate significant heterogeneity [22]. When significant heterogeneity existed, the source of heterogeneity was further analyzed. If no significant clinical or method heterogeneity identified, a random-effects model was be applied to make estimates. Where necessary, sensitivity analyses were performed to test the stability of outcomes. All statistical analyses were carried out with the STATA software package version 12.0 (Stata Corporation, College Station, TX). P value <0.05 was considered as statistically significant.
Results
Baseline characteristics of participants
Demographic characteristics of the participants in case and control groups are summarized in Table 1. No significant difference was observed in age and sex, suggesting the match between controls and cases was adequate. In addition, no significant differences were found in place of residence and family history of gastric cancer between the case and control groups. However, alcohol consumption in the case group was significantly higher than in the control group (58.3% vs. 52.2%, p=0.04) (Table 1), suggesting it might be a risk factor of gastric cancer.
Table 1.
Demographic information of all participants.
| Variables | Cases (%) n=278 | Controls (%) n=278 | P-value* |
|---|---|---|---|
| Age (mean ±SD) | 58.5±9.6 | 59.3±10.2 | 0.73 |
| Gender | |||
| Male | 196 (70.5) | 196 (70.5) | 0.93 |
| Female | 82 (29.5) | 82 (29.5) | |
| Residence | |||
| Rural | 183 (65.8) | 172 (61.8) | 0.37 |
| Urban | 95 (34.2) | 106 (38.2) | |
| Family history of gastric cancer | |||
| No | 233 (83.8) | 244 (87.8) | 0.11 |
| Yes | 45 (16.2) | 34 (12.2) | |
| Alcohol drinking | |||
| No | 116 (41.7) | 133 (47.8) | 0.09 |
| Yes | 162 (58.3) | 145 (52.2) | |
| Smoking status | |||
| No | 109 (39.2) | 121 (43.5) | 0.21 |
| Yes | 169 (60.8) | 157 (56.5) | |
| H. pylori infection status | |||
| H. pylori (−) | 119 (42.8) | – | – |
| H. pylori (+) | 159 (57.2) | – | |
P-value were calculated by multivariate unconditional logistic regression, adjusted for age, gender, residence, family history of gastric cancer, alcohol drinking and smoking status.
“−” – Not available
rs895819 A/G and rs11671784 G/A polymorphism had an inverse effect on risk of gastric cancer
For rs895819, the frequency of G alleles in the cases was 39.9%, which was significantly higher than 32.9% in controls (χ2=5.90; p=0.02) (Table 2). The distribution of rs895819 genotypes (AA/AG/GG) in cases and controls was slightly different, but was not statistically significant (χ2=5.57; p=0.06) (Table 2). The observed rs895819 genotype frequency in cases and controls was in agreement with Hardy-Weinberg equilibrium (cases: χ2=1.37, p=0.24; controls: χ2=2.55, p=0.11), suggesting no population stratification. Compared with AA homozygotes, the OR after adjustment of potential covariates in GG was 1.67 (95% CI 1.00–2.77, p=0.05) and in GC was 1.36 (95% CI 1.00–1.97, p=0.09) (Table 2). Generally, individuals with variant genotypes (AG + GG) had a 1.43-fold increase in risk of gastric cancer (adjusted OR=1.43, 95% CI 1.01–2.02, p=0.04) (Table 2). These results suggest individuals with rs895819 G allele had increased risk of gastric cancer compared with rs895819 AA carriers.
Table 2.
The association between rs895819/rs11671784 and risk of gastric cancer.
| SNP | Genotype or alleles | Case (%) | Control (%) | Crude OR [95% CI] | P-value | *Adjusted OR [95% CI] | *P-value |
|---|---|---|---|---|---|---|---|
| rs895819 | AA | 105 (37.8) | 131 (47.1) | 1.00 | – | 1.00 | – |
| AG | 124 (44.6) | 111 (39.9) | 1.39 [0.97, 2.00] | 0.07 | 1.36 [1.00, 1.97] | 0.09 | |
| GG | 49 (17.6) | 36 (12.9) | 1.70 [1.03, 2.80] | 0.04 | 1.67 [1.00, 2.77] | 0.05 | |
| AG+GG | 173 (62.2) | 147 (52.8) | 1.47 [1.05, 2.06] | 0.03 | 1.43 [1.01, 2.02] | 0.04 | |
| A | 334 (60.1) | 373 (67.1) | 1.00 | 1.00 | |||
| G | 222 (39.9) | 183 (32.9) | 1.35 [1.06, 1.73] | 0.02 | 1.32 [1.03, 1.70] | 0.03 | |
| rs11671784 | GG | 128 (46.0) | 100 (36.0) | 1.00 | – | 1.00 | – |
| GA | 122 (43.9) | 134 (48.2) | 0.71 [0.50, 1.02] | 0.06 | 0.72[0.51, 1.03] | 0.06 | |
| AA | 28 (10.1) | 44 (15.8) | 0.50 [0.29, 0.85] | 0.01 | 0.52 [0.31, 0.87] | 0.02 | |
| GA+AA | 150 (54.0) | 178 (64.0) | 0.66 [0.47, 0.92] | 0.02 | 0.68 [0.49, 0.94] | 0.03 | |
| G | 378 (68.0) | 334 (60.1) | 1.00 | – | 1.00 | – | |
| A | 178 (32.0) | 222 (39.9) | 0.71 [0.55, 0.91] | 0.006 | 0.73 [0.57, 0.93] | 0.01 |
Distributions of the rs895819 (χ2=2.55, p=0.11) and rs11671784 (χ2=0.006, p=0.94) genotypes were in Hardy-Weinberg equilibrium.
Adjusted for age, sex, residence, family history of gastric cancer, smoking and drinking status.
CI – confidence interval; OR – odds ratio.
For rs11671784, the frequency of A allele in the cases was 32.0%, which was significantly lower than 39.9% in controls (χ2=7.56; p=0.006) (Table 2). The frequencies of rs11671784 genotypes (GG/GA/AA) in cases and controls were significantly different (χ2=7.56; p=0.02). The observed rs11671784 genotype frequency in cases and controls were in agreement with Hardy-Weinberg equilibrium (cases: χ2=0.018, p=0.89; controls: χ2=0.006, p=0.94), suggesting no population stratification. Compared with GG homozygotes, the OR after adjustment of potential covariates in the AA group was 0.52 (95% CI 0.31–0.87, p=0.02) and in the GA group it was 0.72 (95% CI 0.51–1.03, p=0.06) (Table 2). Generally, individuals with variant genotypes (GA + AA) had only a 0.68-fold risk of gastric cancer compared with GG homozygotes carriers (adjusted OR=0.68, 95% CI 0.49–0.94, p=0.03) (Table 2). These results suggest individuals with rs11671784 A allele had decreased risk of gastric cancer risk relative to GG carriers.
Stratified analysis of rs895819 A/G and rs11671784 G/A polymorphism by clinic-pathologic features
Table 3 shows association of rs895819 A/G and rs11671784 G/A polymorphisms with clinic-pathological features among the 59 gastric cancer cases that received resection. For rs895819, there were 23 cases with AA, 25 with AG, and 11 with GG. For rs11671784, 25 cases were GG genotype, 21 were GA, and 13 were AA. By stratifying clinic-pathologic features, we observed rs895819 G variants were moderately associated with both lymphatic invasion (OR=2.95, 95% CI, 0.99–8.75, p=0.05) and lymph node metastasis (OR=2.95, 95% CI 1.00–8.77, p=0.05) (Table 3). For rs11671784, the A variants were associated with significantly reduced risk of lymphatic invasion (OR=0.20; 95% CI 0.06–0.65; p=0.0008) (Table 3).
Table 3.
The association between rs895819/rs11671784 polymorphism and clinic-pathological features.
| SNP | Variable | Genotype | *Adjusted OR [95% CI] | *P-value | |
|---|---|---|---|---|---|
| GG+AG (11+25) | AA (23) | ||||
| rs895819 | Age (median) | ||||
| <58 | 7+14 | 13 | 1.08 [0.37, 3.10] | 0.89 | |
| ≥58 | 4+11 | 10 | 0.93 [0.32, 2.67] | 0.89 | |
| Tumor size | |||||
| ≤3 cm | 6+12 | 9 | 1.56 [0.54, 4.50] | 0.41 | |
| >3 cm | 5+13 | 14 | 0.64 [0.22, 1.86] | 0.41 | |
| Lymph node metastasis | |||||
| Absent | 4+10 | 15 | 0.34 [0.11, 1.01] | 0.05 | |
| Present | 7+15 | 8 | 2.95 [0.99, 8.75] | 0.05 | |
| Lymphatic invasion | |||||
| Absent | 3+8 | 13 | 0.34 [0.11, 1.00] | 0.05 | |
| Present | 8+17 | 10 | 2.95 [1.00, 8.77] | 0.05 | |
| H. pylori infection | |||||
| H. pylori (−) | 3+8 | 4 | 2.09 [0.58, 7.60] | 0.26 | |
| H. pylori (+) | 8+17 | 19 | 0.48 [0.13, 1.74] | 0.26 | |
| Clinical stage | |||||
| I,II | 0+3 | 6 | 0.26 [0.06, 1.16] | 0.08 | |
| III, IV | 11+22 | 17 | 3.88 [0.86, 17.48] | 0.08 | |
| SNP | Variables | Genotype | *Adjusted OR [95% CI] | *P-value | |
| AA+GA (13+21) | GG (25) | ||||
| rs11671784 | Age (median) | ||||
| <58 | 8+14 | 12 | 1.99 [0.69, 5.70] | 0.2 | |
| ≥58 | 5+7 | 13 | 0.50 [0.18, 1.44] | 0.2 | |
| Tumor size | |||||
| ≤3 cm | 6+7 | 14 | 0.49 [0.17, 1.39] | 0.18 | |
| >3 cm | 7+14 | 11 | 2.06 [0.72, 5.87] | 0.18 | |
| Lymph node metastasis | |||||
| Absent | 8+12 | 9 | 2.54 [0.88, 7.36] | 0.09 | |
| Present | 5+9 | 16 | 0.39 [0.14, 1.14] | 0.09 | |
| Lymphatic invasion | |||||
| Absent | 8+11 | 5 | 5.07 [1.54, 16.67] | 0.008 | |
| Present | 5+10 | 20 | 0.20 [0.06, 0.65] | 0.008 | |
| H. pylori infection | |||||
| H. pylori (−) | 9 | 6 | 1.14 [0.35, 3.76] | 0.83 | |
| H. pylori (+) | 25 | 19 | 0.88 [0.27, 2.89] | 0.83 | |
| Clinical stage | |||||
| I,II | 2+4 | 3 | 1.57 [0.35, 7.00] | 0.55 | |
| III, IV | 11+17 | 22 | 0.64 [0.14, 2.84] | 0.55 | |
Adjusted for age, sex, residence, family history of gastric cancer, smoking and drinking status.
CI – confidence interval; OR – odds ratio.
Effect of rs895819 A/G and rs11671784 G/A polymorphism on miR-27a expression
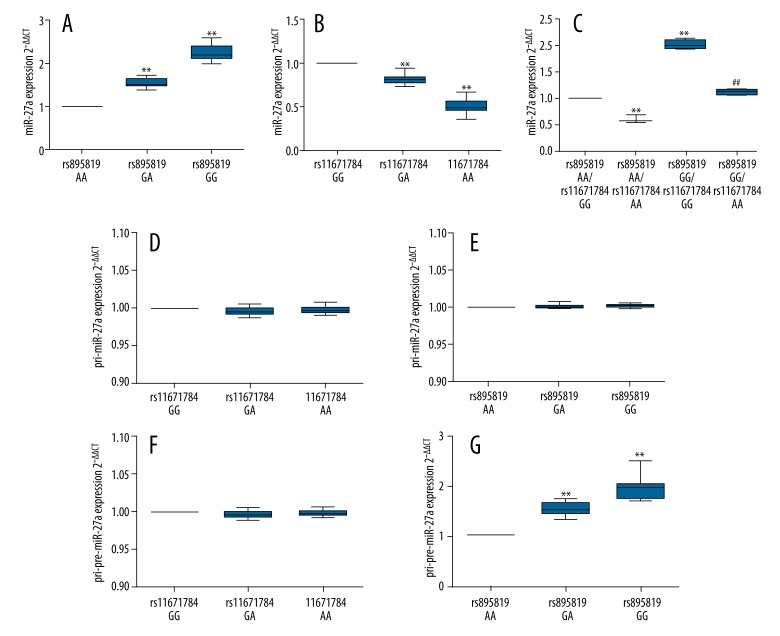
miR-27a expression of the 59 gastric tumor tissues was quantified to assess how rs895819 and rs11671784 influenced miR-27a expression. In both groups, expression of miR-27a was significantly affected by genotypes. In rs895819 AA/AG/GG groups compared with AA, miR-27a expression in AG and GG group were approximately 155% and 222% higher (p<0.01) (Figure 1A). In rs11671784 GG/GA/AA groups, miR-27a expression in GA and AA group were only 81% and 51% of that in GG group (p<0.01) (Figure 1B). Interestingly, when cases of rs895819 AA/rs11671784 GG (n=5) were set as control, mir-27a expression in rs895819 AA/rs11671784 AA (n=3), rs895819 GG/rs11671784 GG (n=4), and rs895819 GG/rs11671784 AA (n=4) were 66%, 202%, and 112%, respectively (Figure 1C). rs895819 GG/rs11671784 GG group had the highest miR-27a expression, which was significantly higher than in the other 3 groups (Figure 1C). miR-27a expression in rs895819 GG/rs11671784 AA group was significantly lower than that in rs895819 GG/rs11671784 GG (p<0.01), suggesting the miR-27 increase due to rs895819 G variant was partly offset by rs11671784 A variant (Figure 1C). When examining the expression of total pri-miR27a in rs895819 and rs11671784 variant groups, no difference was observed (Figure 1D, 1E). However, when detecting the expression of total pri- and pre-miR27a, significantly higher level of expression was observed in rs895819 GA and AA groups compared with GG group (p<0.01) (Figure 1G), but no significant difference was observed among three rs11671784 variant groups (Figure 1F). This means that the rs895819 A/G and rs11671784 G/A polymorphism-related epitomic miR-27a expression happens in different stages – the former is during the pri- to pre-miRNA stage, while the later happens during the pre-miRNA to mature miRNA stage.
Figure 1.
Effect of rs895819 A/G and rs11671784 G/A polymorphism on miR-27a expression (A) Relative miR-27a expression in rs895819 variants; (B) Relative miR-27a expression in rs11671784 variants; (C) Relative miR-27a expression in rs895819 and rs11671784 homozygote carriers; (D) Relative pri-miR-27a expression in rs11671784 variants; (E) Relative pri-miR-27a expression in rs895819 variants; (F) Relative total pri- and pre-miR-27a expression in rs11671784 variants; G. Relative total pri- and pre-miR-27a expression in rs895819 variants. ** P<0.01, ## P<0.01 vs. rs895819 GG/rs11671784 GG. Error bars depict min to max.
Meta-analysis of SNPs and gastric cancer
Characteristics of the Studies involved
A total of 3 studies were identified through searching PubMed, CNKI, and Web of Science – 2 for rs895819 [17,18] and 1 for rs11671784 [19]. The search process was not given in this context. First-hand data obtained from this study was integrated in subsequent meta-analysis of miR-27a and gastric cancer risk. The final meta-analysis involved 1769 cancer cases and 1973 cancer-free controls. The key characteristics of studies involved are given in Table 4.
Table 4.
The key characteristics of studies involved.
| SNP & study | Country | Ethnicity | Genotyping method | Source of cases | Sample size | Case | Control | p of HWE | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | Control | AA | AG | GG | AA | AG | GG | ||||||
| rs895819 | |||||||||||||
| Sun et al. 2010 | China | Asian | PCR-RFLP | HB | 304 | 304 | 115 | 135 | 54 | 145 | 119 | 40 | 0.05 |
| Zhou et al. 2012 | China | Asian | MassARRAY | HB | 295 | 413 | 166 | 122 | 7 | 214 | 167 | 32 | 0.94 |
| Song et al. 2014 | China | Asian | Direct Sequencing | HB | 278 | 278 | 105 | 124 | 49 | 131 | 111 | 36 | 0.11 |
| GG | AG | AA | GG | AG | AA | ||||||||
| rs11671784 | |||||||||||||
| Song et al. 2014 | China | Asian | Direct Sequencing | HB | 278 | 278 | 128 | 122 | 28 | 100 | 134 | 44 | 0.93 |
| Yang et al. 2014 | China | Asian | TaqMan | HB | 892 | 978 | 398 | 414 | 80 | 371 | 487 | 120 | 0.12 |
HWE – Hardy-Weinberg equilibrium; PCR-RFLP – polymerase chain reaction-restriction fragment length polymorphism; HB – hospital based of control.
Quantitative synthesis
All eligible studies were pooled and stratified to estimate the association of these 2 SNPs with overall gastric cancer risk (Table 5). Although the rs895819 variant G allele might slightly increase the risk of gastric cancer, it was not statistically significant (OR=1.12, 95% CI 0.78–1.60, p=0.55). However, significant heterogeneity was observed among the 3 studies involved (p-H=0.001, I2=85%). Subsequent sensitivity analyses showed the exclusion of the Zhou et al. study [18] eliminated heterogeneity (p-H=0.95, I2=0%). Under this situation, meta-analysis of the other 2 studies showed variant G allele could significantly increase the risk of gastric cancer (OR=1.42, 95% CI 1.10–1.83, p=0.006). For rs11671784 G/A polymorphism, variant A allele could significantly decrease the risk of gastric cancer, regardless of allelic contrast (G vs. A), homozygote comparison (AA vs. GG), heterozygote comparison (AG vs. GG), dominant model (AA vs. GG+GA), and recessive model (AA+AG vs. GG) (Table 5). No heterogeneity was found within these groups.
Table 5.
Pooled ORs and 95% CIs of the SNPs and gastric cancer risk.
| SNP | No. studies | G vs. A | GG vs. AA | GA vs. AA | (GA+GG) vs. AA | GG vs. (GA+AA) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| rs895819 | 3 | OR (95% CI) | P | P-H | I2 | OR (95% CI) | P | P-H | I2 | OR (95% CI) | P | P-H | I2 | OR (95% CI) | P | P-H | I2 | OR (95% CI) | P | P-H | I2 |
| 1.12 [0.78, 1.60] | 0.55 | 0.001 | 85% | 0.97 [0.40, 2.40] | 0.95 | 0.0007 | 86% | 1.22 [0.92, 1.62] | 0.16 | 0.13 | 51% | 1.21 [0.83, 1.78] | 0.32 | 0.01 | 77% | 0.29 [0.04, 2.19] | 0.23 | <0.00001 | 96% | ||
| A vs. G | AA vs. GG | AG vs. GG | (AA+AG) vs. GG | AA vs. (AG+GG) | |||||||||||||||||
| rs11671784 | 2 | OR (95% CI) | P | P-H | I2 | OR (95% CI) | P | P-H | I2 | OR (95% CI) | P | P-H | I2 | OR (95% CI) | P | P-H | I2 | OR (95% CI) | P | P-H | I2 |
| 0.78 [0.69, 0.88] | <0.00001 | 0.39 | 0% | 0.59 [0.45, 0.77] | 0.0001 | 0.49 | 0% | 0.77 [0.65, 0.92] | 0.003 | 0.6 | 0 | 0.73 [0.62, 0.86] | 0.0002 | 0.47 | 0% | 0.67 [0.52, 0.87] | 0.03 | 0.58 | 0% | ||
P – p value; P-H – P value of Q for heterogeneity test; I2 >50%, high heterogeneity; Random effects model was used when P value of Q for heterogeneity test P-H >0.1 or I2 >50%; otherwise, fixed effect model was used.
Discussion
As it has gradually become recognized as an oncogene, the oncogenic activity of miR-27a has been reported in gastric cancer [10,11]. Previous studies observed it could regulate ZBTB10 and lead to overexpression of Sp proteins and Sp-dependent genes, which are 2 important factors in gastric cancer cell survival and angiogenesis [17,23]. In addition, expression of miR-27a is also negatively associated with expression of HOXA10, which is a member of the HOX gene family that encodes transcription factors and is involved in carcinoma development [19]. rs11671784 G/A polymorphism could directly affect the expression of HOXA10 [19]. In this study, it was observed that individuals with rs895819 A/G polymorphism exhibited significantly increased risk of gastric cancer, while subjects with rs11671784 G/A polymorphism were associated with significantly reduced gastric cancer risk.
Preliminary functional assays showed that rs895819 A/G polymorphism increased miR-27a expression, while rs11671784 G/A polymorphism reduced the expression. qRT-PCR results demonstrated that rs11671784 A variant might offset the upregulating effect of rs895819 G variant on miR-27 expression. Variations in pre-miRNA were found to be associated with aberrant maturation process of miRNAs due to their influence on transcription or mutation process [24,25]. Theoretically, altered integrity and secondary structure of pre-miRNAs might significantly affect processing and maturation of miRNAs [26]. In this study, the 3 variant groups of rs11671784 had no significant difference in pri-miR-27a or total pri- and pre-miR27a level, suggesting normal processing from mRNA to pri-miRNA and to pre-miRNA. Therefore, rs11671784 A variants associated with miR-27a downregulation might be caused by a block from pre-miR-27a to the mature form. This finding is consistent a report by Yang et al. [19] that rs11671784 G/A polymorphism did not affect transcription or post-transcription processes of the miR-27a gene. However, for rs895819, this study observed increased total pri-miR-27a and pre-miR-27a levels in AG and GG groups compared with the AA group, showing an aberrant process from pri-miR-27a to pre-miR-27a. Since rs895819 is on the terminal loop, its variation might impair DGCR8 binding and subsequent processing of pri-miRNA according to the Junction Anchoring model [27]. Thus, it is hypothesized in this study that rs895819 A/G polymorphism might lead to faster processing of pri-miR-27a to pre-miR-27a. In addition, this study also observed that rs895819 G variants were moderately associated with lymphatic invasion and lymph node metastasis, while rs11671784 A variants were associated with significantly reduced risk of lymphatic invasion. These observations might be closely related to ectopic miR-27a expression. However, further detailed study is required to confirm their association. These findings show that a single-nucleotide change on the non-mature miRNA sequence could also affect biogenesis of the mature form, thus exhibiting profound biological effects. Therefore, measurement of miRNA-27a rs11671784 G/A and rs895819 A/G polymorphism based on serum samples might be used as a strategy to predict gastric cancer susceptibility.
Meta-analysis of previous studies of rs895819 A/G and rs11671784 G/A polymorphism on gastric cancer risk also confirmed the reverse risk-increase and risk-decrease effect of these 2 SNPs. However, significant heterogeneity was also observed in rs895819 studies. Due to the limited number of studies on these 2 SNPs and gastric cancer risk, more clinical data need to be collected and the underlying mechanism should be further explored.
This study had several limitations. First, the sample size is relatively small, especially for the stratified analysis among the cancer patients for assessing the association between miR-27a variants and clinic-pathologic features. However, the meta-analysis partly offset the influence on estimation of gastric cancer risks. Second, this was a hospital-based association study. Therefore, there might be selection bias. However, the potential influence of this limitation was minimized through matching cases and controls in age and sex and adjusting the putative confounding factors in statistical analysis. Third, the influence of SNPs on disease development and the frequency of risky variants often vary from population to population. The primary study and the meta-analysis were also based on an Asian population. Thus, it is necessary to detect the effects these 2 SNPs in other populations. Fourth, the number of studies included in the meta-analysis is small. More case-control studies are required to confirm the findings. Fifth, the methods used to detect SNP polymorphism were not consistent in the studies of the meta-analysis. Direct sequencing, PCR- RFLP, TaqMan method, and MassARRAY were used in different studies. Since different methods have different sensitivity in detecting the mutations, the possible bias associated with detection methods may directly affect the accuracy of pooled results.
Conclusions
rs895819 and rs11671784 can inversely affect gastric cancer risk and the influence was closely related to their effects on miR-27a expression. These findings provide novel insight into molecular mechanisms of miR-27a in gastric cancer development and support better development of early diagnosis for gastric cancer. Measurement of miRNA-27a rs11671784 G/A and rs895819 A/G polymorphism based on serum samples might be used as a strategy to predict gastric cancer susceptibility.
Footnotes
Source of support: Departmental sources
References
- 1.Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Brenner H, Rothenbacher D, Arndt V. Epidemiology of stomach cancer. Methods Mol Biol. 2009;472:467–77. doi: 10.1007/978-1-60327-492-0_23. [DOI] [PubMed] [Google Scholar]
- 3.Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 4.Hartgrink HH, Jansen EP, van Grieken NC, et al. Gastric cancer. Lancet. 2009;374:477–90. doi: 10.1016/S0140-6736(09)60617-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruan K, Fang X, Ouyang G. MicroRNAs: novel regulators in the hallmarks of human cancer. Cancer Lett. 2009;285:116–26. doi: 10.1016/j.canlet.2009.04.031. [DOI] [PubMed] [Google Scholar]
- 6.Xiao C, Rajewsky K. MicroRNA control in the immune system: basic principles. Cell. 2009;136:26–36. doi: 10.1016/j.cell.2008.12.027. [DOI] [PubMed] [Google Scholar]
- 7.Calin GA, Croce CM. MicroRNA-cancer connection: the beginning of a new tale. Cancer Res. 2006;66:7390–94. doi: 10.1158/0008-5472.CAN-06-0800. [DOI] [PubMed] [Google Scholar]
- 8.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–66. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 9.Lu J, Getz G, Miska EA, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–38. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 10.Liu T, Tang H, Lang Y, et al. MicroRNA-27a functions as an oncogene in gastric adenocarcinoma by targeting prohibitin. Cancer Lett. 2009;273:233–42. doi: 10.1016/j.canlet.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Lerner M, Lundgren J, Akhoondi S, et al. MiRNA-27a controls FBW7/hCDC4-dependent cyclin E degradation and cell cycle progression. Cell Cycle. 2011;10:2172–83. doi: 10.4161/cc.10.13.16248. [DOI] [PubMed] [Google Scholar]
- 12.Katada T, Ishiguro H, Kuwabara Y, et al. microRNA expression profile in undifferentiated gastric cancer. Int J Oncol. 2009;34:537–42. [PubMed] [Google Scholar]
- 13.Landi D, Gemignani F, Barale R, et al. A catalog of polymorphisms falling in microRNA-binding regions of cancer genes. DNA Cell Biol. 2008;27:35–43. doi: 10.1089/dna.2007.0650. [DOI] [PubMed] [Google Scholar]
- 14.Ryan BM, Robles AI, Harris CC. Genetic variation in microRNA networks: the implications for cancer research. Nat Rev Cancer. 2010;10:389–402. doi: 10.1038/nrc2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flego V, Ristic S, Devic Pavlic S, et al. Tumor necrosis factor-alpha gene promoter -308 and -238 polymorphisms in patients with lung cancer as a second primary tumor. Med Sci Monit. 2013;19:846–51. doi: 10.12659/MSM.889554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hua HB, Yan TT, Sun QM. miRNA polymorphisms and risk of gastric cancer in Asian population. World J Gastroenterol. 2014;20:5700–7. doi: 10.3748/wjg.v20.i19.5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun Q, Gu H, Zeng Y, et al. Hsa-mir-27a genetic variant contributes to gastric cancer susceptibility through affecting miR-27a and target gene expression. Cancer Sci. 2010;101:2241–47. doi: 10.1111/j.1349-7006.2010.01667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou Y, Du WD, Chen G, et al. Association analysis of genetic variants in microRNA networks and gastric cancer risk in a Chinese Han population. J Cancer Res Clin Oncol. 2012;138:939–45. doi: 10.1007/s00432-012-1164-8. [DOI] [PubMed] [Google Scholar]
- 19.Yang Q, Jie Z, Ye S, et al. Genetic variations in miR-27a gene decrease mature miR-27a level and reduce gastric cancer susceptibility. Oncogene. 2014;33:193–202. doi: 10.1038/onc.2012.569. [DOI] [PubMed] [Google Scholar]
- 20.Hao W, Su Z, Liu B, et al. Drinking and drinking patterns and health status in the general population of five areas of China. Alcohol Alcohol. 2004;39:43–52. doi: 10.1093/alcalc/agh018. [DOI] [PubMed] [Google Scholar]
- 21.Yang R, Burwinkel B. A bias in genotyping the miR-27a rs895819 and rs11671784 variants. Breast Cancer Res Treat. 2012;134:899–901. doi: 10.1007/s10549-012-2140-3. [DOI] [PubMed] [Google Scholar]
- 22.Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanai M, Wei D, Li Q, et al. Loss of Kruppel-like factor 4 expression contributes to Sp1 overexpression and human gastric cancer development and progression. Clin Cancer Res. 2006;12:6395–402. doi: 10.1158/1078-0432.CCR-06-1034. [DOI] [PubMed] [Google Scholar]
- 24.Jazdzewski K, Murray EL, Franssila K, et al. Common SNP in pre-miR-146a decreases mature miR expression and predisposes to papillary thyroid carcinoma. Proc Nat Acad Sci USA. 2008;105:7269–74. doi: 10.1073/pnas.0802682105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu Y, Liu CM, Qi L, et al. Two common SNPs in pri-miR-125a alter the mature miRNA expression and associate with recurrent pregnancy loss in a Han-Chinese population. RNA Biol. 2011;8:861–72. doi: 10.4161/rna.8.5.16034. [DOI] [PubMed] [Google Scholar]
- 26.Zeng Y, Cullen BR. Sequence requirements for micro RNA processing and function in human cells. RNA. 2003;9:112–23. doi: 10.1261/rna.2780503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han J, Lee Y, Yeom KH, et al. Molecular basis for the recognition of primary microRNAs by the Drosha-DGCR8 complex. Cell. 2006;125:887–901. doi: 10.1016/j.cell.2006.03.043. [DOI] [PubMed] [Google Scholar]