Abstract
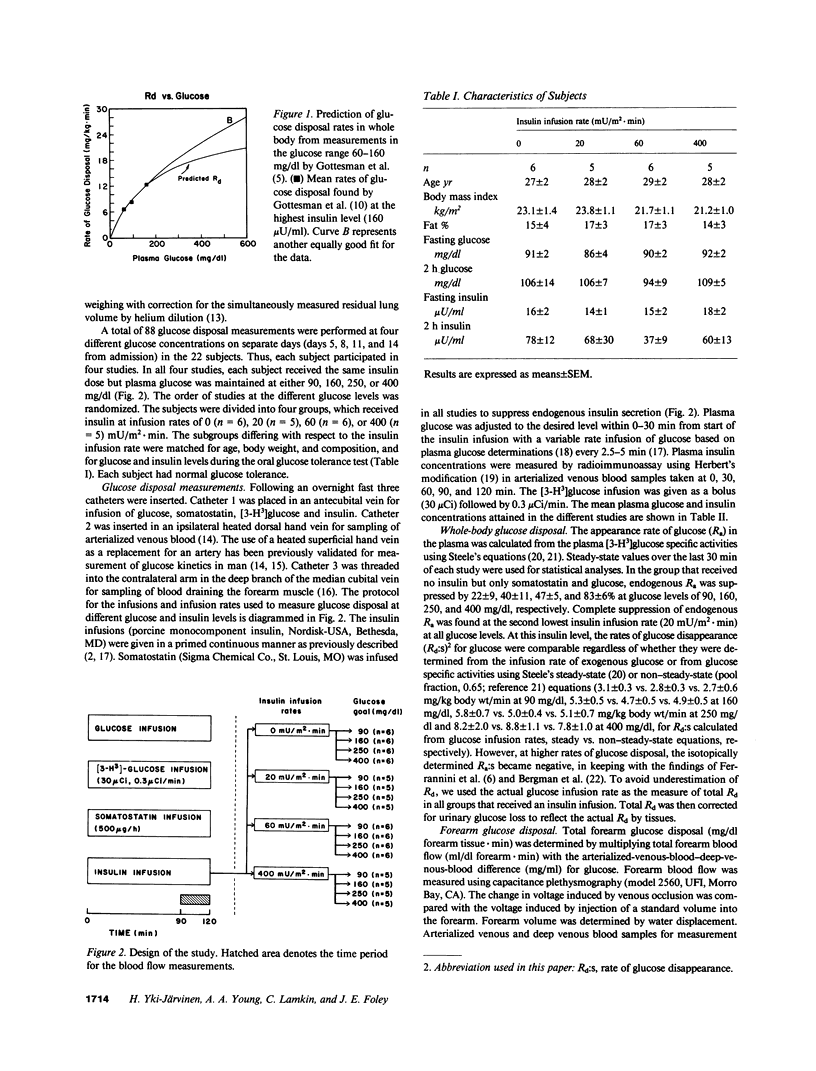
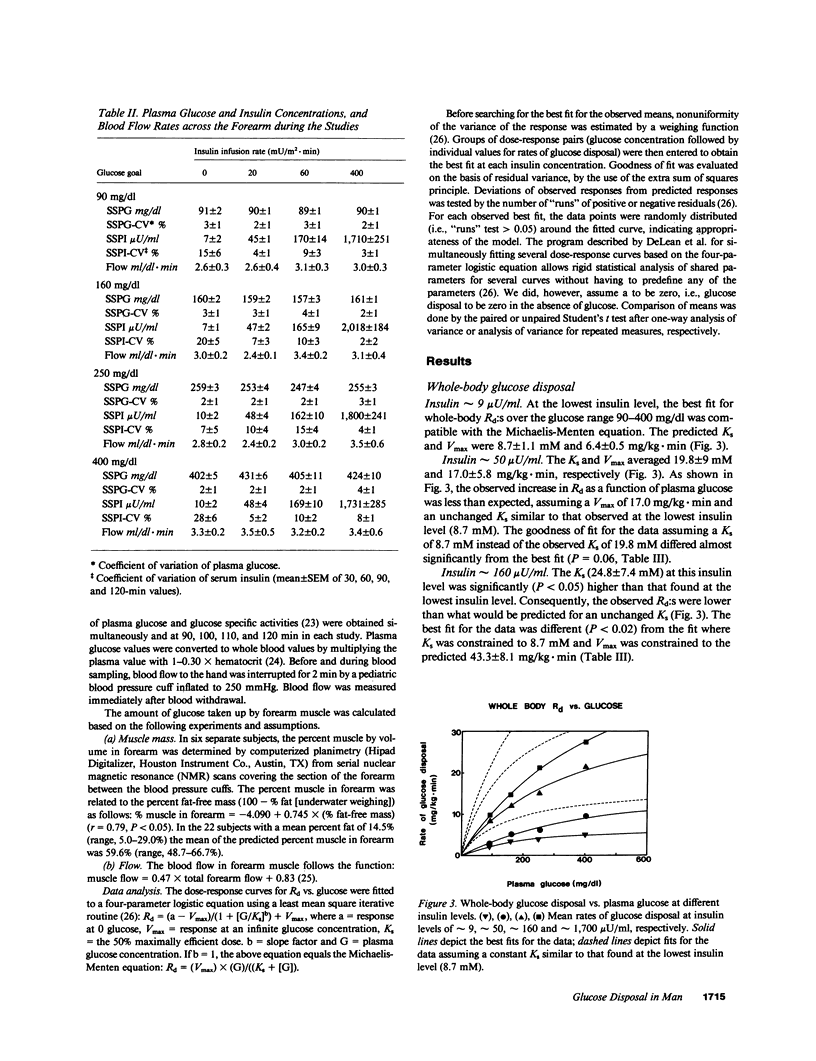
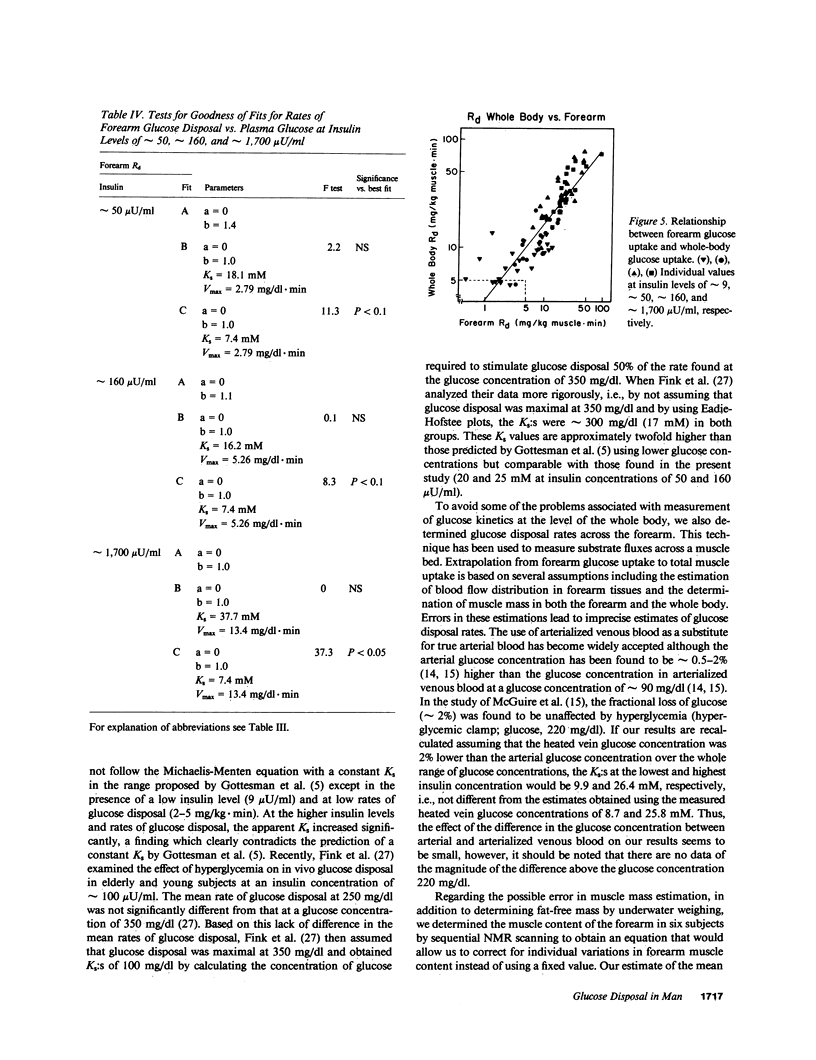
We reevaluated the concept that the in vivo glucose disposal rate in man is determined by the activity of the glucose transport system. Rates of glucose disposal were determined in whole body and across forearm at four insulin levels (approximately 9, approximately 50, approximately 160, and approximately 1700 microU/ml) and at each insulin level at four glucose levels (approximately 90, approximately 160, approximately 250, and approximately 400 mg/dl). At the lowest insulin level, the Michaelis constants (Ks:s) for glucose disposal in whole body (8.7 +/- 1.1 mM) and across forearm (7.4 +/- 1.4) mM) were compatible with a Ks determined in vitro for the transport system. At higher insulin levels, the apparent Ks increased significantly in whole body (16.2-37.7 mM) and across forearm (20.7-31.2 mM). We interpret the apparent increase of Ks by insulin to reflect a shift in the rate-limiting step from glucose transport to some step beyond transport.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abumrad N. N., Rabin D., Diamond M. P., Lacy W. W. Use of a heated superficial hand vein as an alternative site for the measurement of amino acid concentrations and for the study of glucose and alanine kinetics in man. Metabolism. 1981 Sep;30(9):936–940. doi: 10.1016/0026-0495(81)90074-3. [DOI] [PubMed] [Google Scholar]
- Bergman R. N., Finegood D. T., Ader M. Assessment of insulin sensitivity in vivo. Endocr Rev. 1985 Winter;6(1):45–86. doi: 10.1210/edrv-6-1-45. [DOI] [PubMed] [Google Scholar]
- Best J. D., Judzewitsch R. G., Pfeifer M. A., Beard J. C., Halter J. B., Porte D., Jr The effect of chronic sulfonylurea therapy on hepatic glucose production in non-insulin-dependent diabetes. Diabetes. 1982 Apr;31(4 Pt 1):333–338. doi: 10.2337/diab.31.4.333. [DOI] [PubMed] [Google Scholar]
- Bogardus C., Lillioja S., Stone K., Mott D. Correlation between muscle glycogen synthase activity and in vivo insulin action in man. J Clin Invest. 1984 Apr;73(4):1185–1190. doi: 10.1172/JCI111304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLES D. R., COOPER K. E., MOTTRAM R. F., OCCLESHAW J. V. The source of blood samples withdrawn from deep forearm veins via catheters passed upstream from the median cubital vein. J Physiol. 1958 Jul 14;142(2):323–328. doi: 10.1113/jphysiol.1958.sp006019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOPER K. E., EDHOLM O. G., MOTTRAM R. F. The blood flow in skin and muscle of the human forearm. J Physiol. 1955 May 27;128(2):258–267. doi: 10.1113/jphysiol.1955.sp005304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudry I. H., Gould M. K. Kinetics of glucose uptake in isolated soleus muscle. Biochim Biophys Acta. 1969 May 6;177(3):527–536. doi: 10.1016/0304-4165(69)90315-8. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Jacot E., Jequier E., Maeder E., Wahren J., Felber J. P. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes. 1981 Dec;30(12):1000–1007. doi: 10.2337/diab.30.12.1000. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Tobin J. D., Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979 Sep;237(3):E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- DeLean A., Munson P. J., Rodbard D. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose-response curves. Am J Physiol. 1978 Aug;235(2):E97–102. doi: 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- Dillon R. S. Importance of the hematocrit in interpretation of blood sugar. Diabetes. 1965 Oct;14(10):672–674. doi: 10.2337/diab.14.10.672. [DOI] [PubMed] [Google Scholar]
- Ferrannini E., Smith J. D., Cobelli C., Toffolo G., Pilo A., DeFronzo R. A. Effect of insulin on the distribution and disposition of glucose in man. J Clin Invest. 1985 Jul;76(1):357–364. doi: 10.1172/JCI111969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink R. I., Wallace P., Olefsky J. M. Effects of aging on glucose-mediated glucose disposal and glucose transport. J Clin Invest. 1986 Jun;77(6):2034–2041. doi: 10.1172/JCI112533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley J. E., Gliemann J. Accumulation of 2-deoxyglucose against its concentration gradient in rat adipocytes. Biochim Biophys Acta. 1981 Oct 20;648(1):100–106. doi: 10.1016/0005-2736(81)90129-2. [DOI] [PubMed] [Google Scholar]
- Goodman M. N., Berger M., Ruderman N. B. Glucose metabolism in rat skeletal muscle at rest. Effect of starvation, diabetes, ketone bodies and free fatty acids. Diabetes. 1974 Nov;23(11):881–888. doi: 10.2337/diab.23.11.881. [DOI] [PubMed] [Google Scholar]
- Gottesman I., Mandarino L., Verdonk C., Rizza R., Gerich J. Insulin increases the maximum velocity for glucose uptake without altering the Michaelis constant in man. Evidence that insulin increases glucose uptake merely by providing additional transport sites. J Clin Invest. 1982 Dec;70(6):1310–1314. doi: 10.1172/JCI110731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert V., Lau K. S., Gottlieb C. W., Bleicher S. J. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965 Oct;25(10):1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- KIPNIS D. M., HELMREICH E., CORI C. F. Studies of tissue permeability. IV. The distribution of glucose between plasma and muscle. J Biol Chem. 1959 Jan;234(1):165–170. [PubMed] [Google Scholar]
- Kubo K., Foley J. E. Rate-limiting steps for insulin-mediated glucose uptake into perfused rat hindlimb. Am J Physiol. 1986 Jan;250(1 Pt 1):E100–E102. doi: 10.1152/ajpendo.1986.250.1.E100. [DOI] [PubMed] [Google Scholar]
- Lynch R. M., Paul R. J. Compartmentation of carbohydrate metabolism in vascular smooth muscle: evidence for at least two functionally independent pools of glucose 6-phosphate. Biochim Biophys Acta. 1986 Aug 1;887(3):315–318. doi: 10.1016/0167-4889(86)90159-x. [DOI] [PubMed] [Google Scholar]
- MORGAN H. E., HENDERSON M. J., REGEN D. M., PARK C. R. Regulation of glucose uptake in muscle. I. The effects of insulin and anoxia on glucose transport and phosphorylation in the isolated, perfused heart of normal rats. J Biol Chem. 1961 Feb;236:253–261. [PubMed] [Google Scholar]
- MORGAN H. E., REGEN D. M., PARK C. R. IDENTIFICATION OF A MOBILE CARRIER-MEDIATED SUGAR TRANSPORT SYSTEM IN MUSCLE. J Biol Chem. 1964 Feb;239:369–374. [PubMed] [Google Scholar]
- McGuire E. A., Helderman J. H., Tobin J. D., Andres R., Berman M. Effects of arterial versus venous sampling on analysis of glucose kinetics in man. J Appl Physiol. 1976 Oct;41(4):565–573. doi: 10.1152/jappl.1976.41.4.565. [DOI] [PubMed] [Google Scholar]
- NARAHARA H. T., OZAND P., CORI C. F. Studies of tissue permeability. VII. The effect of insulin on glucose penetration and phosphorylation in frog muscle. J Biol Chem. 1960 Dec;235:3370–3378. [PubMed] [Google Scholar]
- NARAHARA H. T., OZAND P. Studies of tissue permeability. IX. The effect of insulin on the penetration of 3-methylglucose-H3 in frog muscle. J Biol Chem. 1963 Jan;238:40–49. [PubMed] [Google Scholar]
- PARK C. R., REINWEIN D., HENDERSON M. J., CADENAS E., MORGAN H. E. The action of insulin on the transport of glucose through the cell membrane. Am J Med. 1959 May;26(5):674–684. doi: 10.1016/0002-9343(59)90227-x. [DOI] [PubMed] [Google Scholar]
- POST R. L., MORGAN H. E., PARK C. R. Regulation of glucose uptake in muscle. III. The interaction of membrane transport and phosphorylation in the control of glucose uptake. J Biol Chem. 1961 Feb;236:269–272. [PubMed] [Google Scholar]
- Radziuk J., Norwich K. H., Vranic M. Experimental validation of measurements of glucose turnover in nonsteady state. Am J Physiol. 1978 Jan;234(1):E84–E93. doi: 10.1152/ajpendo.1978.234.1.E84. [DOI] [PubMed] [Google Scholar]
- STEELE R., WALL J. S., DE BODO R. C., ALTSZULER N. Measurement of size and turnover rate of body glucose pool by the isotope dilution method. Am J Physiol. 1956 Sep;187(1):15–24. doi: 10.1152/ajplegacy.1956.187.1.15. [DOI] [PubMed] [Google Scholar]
- Simpson I. A., Cushman S. W. Hormonal regulation of mammalian glucose transport. Annu Rev Biochem. 1986;55:1059–1089. doi: 10.1146/annurev.bi.55.070186.005211. [DOI] [PubMed] [Google Scholar]
- Wardzala L. J., Jeanrenaud B. Potential mechanism of insulin action on glucose transport in the isolated rat diaphragm. Apparent translocation of intracellular transport units to the plasma membrane. J Biol Chem. 1981 Jul 25;256(14):7090–7093. [PubMed] [Google Scholar]
- Whitesell R. R., Abumrad N. A. Increased affinity predominates in insulin stimulation of glucose transport in the adipocyte. J Biol Chem. 1985 Mar 10;260(5):2894–2899. [PubMed] [Google Scholar]



