Abstract
In 2011, SCOEL classified RCF as a secondary genotoxic carcinogen and supported a practical threshold. Inflammation was considered the predominant manifestation of RCF toxicity. Intrapleural and intraperitoneal implantation induced mesotheliomas and sarcomas in laboratory animals. Chronic nose-only inhalation bioassays indicated that RCF exposure in rats increased the incidence of lung cancer and similar exposures resulted in mesothelioma in hamsters, but these studies may have been compromised by overload. Epidemiological studies in the US and Europe showed an association between exposure and prevalence of respiratory symptoms and pleural plaques, but no interstitial fibrosis, mesotheliomas, or increased numbers of lung tumors were observed. As the latency of asbestos induced mesotheliomas can be up to 50 years, the relationship between RCF exposure and respiratory malignances has not been fully determined. Nonetheless, it is possible to offer useful perspectives. RCF and rock wool have similar airborne fiber dimensions and biopersistence. Therefore, it is likely that these fibers have similar toxicology. Traditional rock wool has been the subject of numerous cohort and case control studies. For rock wool, IARC (2002) concluded that the epidemiological studies did not provide evidence of carcinogenicity. Based on analogies with rock wool (read across), it is reasonable to believe that increases in lung cancer or any mesotheliomas are unlikely to be found in the RCF-exposed cohort. RCF producers have developed a product stewardship program to measure and control fiber concentrations and to further understand the health status of their workers.
Keywords: Biopersistence, epidemiology, fiber dimensions, mesothelioma, pleural plaques, refractory ceramic fiber, rock wool
Introduction
This article offers some fresh perspectives on the possible carcinogenicity of refractory ceramic fiber (RCF) with relevance to setting occupational exposure limits (OELs). It builds upon earlier work (Brown et al., 2005; Mast et al., 2000a; Utell & Maxim, 2010) seeking to understand the relevant toxicological and epidemiological data on this fiber. Briefly, we conclude that there are many similarities between RCF and traditional rock wool.1 These fibers have similar dimensions (when airborne in the workplace), similar breakage mechanisms, and similar biopersistence. The results of intraperitoneal (IP) injection studies are similar for both fibers and a nose-only inhalation bioassay resulted in fibrosis and tumors for RCF and fibrosis, but no tumors for rock wool. Both fibers are included in epidemiological studies; to date the ongoing RCF study has not resulted in interstitial fibrosis, incremental lung cancer, or any mesothelioma. The RCF study is limited in terms of the size and exposure duration of the cohort, although the duration (and, therefore, ability to detect effects with greater latency) will increase in the future. However, the rock wool studies are much more powerful statistically and do not reveal any elevated SMRs for lung cancer or mesothelioma.
Background
RCFs (CAS no. 142844-00-6), also termed aluminosilicate wools (ASW) are amorphous fibers that belong to a class of materials termed synthetic vitreous fibers (SVFs), which also includes glass wool, rock (stone) wool, slag wool and special purpose glass fibers. Details on RCF composition and production methods are available in several sources (e.g. AFSSET, 2007; ATSDR, 2004; IARC 2002; National Research Council, 2000; NIOSH, 2006; SCOEL, 2011).
RCFs have several desirable properties as high-temperature insulating materials, including low thermal conductivity, low heat storage (low volumetric heat capacity), excellent thermal shock resistance, light weight, good corrosion resistance and ease of installation (ERM, 1995). Depending upon the formulation, the maximum use temperature can be as high as 1430 °C (ERM, 1995; NIOSH 2006; TIMA, 1993). For this reason, RCFs (and certain other fibers) are also termed high temperature insulating wools (HTIWs).
As produced or processed some RCF is respirable and RCF is relatively biopersistent compared to many other SVFs (but very much less biopersistent than amphibole asbestos). The combination of respirability and biopersistence raises concern over possible adverse health effects (including carcinogenicity) resulting from inhalation of RCF.
Carcinogen classification
In 1988, an IARC Working Group reviewed the available evidence for RCF and placed RCF in Group 2B (possibly carcinogenic to humans). This classification was reaffirmed by a subsequent Working Group meeting in 2001 (IARC, 2002), which concluded that there was sufficient evidence in experimental animals but inadequate evidence in humans for the carcinogenicity of refractory ceramic fibers.
In Europe, the 1997 Dangerous Substances Directive, RCF was listed as a Category 2 carcinogen based only on animal studies. The Dangerous Substances Directive is being phased out in favor of the new globally harmonized system. The phase-out process began with EC Regulation No. 1272/2008 of the European Parliament and the Council of 16 December 2008 on classification, labeling and packaging (CLP) of substances and mixtures. As part of the transition to the new CLP regulation, substance category labels under the Dangerous Substance Directive were “translated” into the CLP globally harmonized system, a new but equivalent scheme for classification and labeling in 2009. As a result, the old Category 2 Carcinogens are now automatically classified as Category 1B carcinogens which are substances “presumed to have carcinogenic potential for humans, classification is largely based on animal evidence”.
The Scientific Committee on Occupational Exposure Limits (SCOEL, 2011) classified refractory ceramic fibers as a secondary genotoxic carcinogen and supported a practical threshold. Inflammation was considered the predominant manifestation of RCF toxicity.
Potential health effects associated with RCF exposure have been assessed using both experiments on laboratory animals and epidemiological studies (morbidity and mortality) of cohorts occupationally exposed to RCF. Other relevant information includes studies on the dimensions of airborne fibers in the workplace and in vitro and in vivo studies on dissolution/biopersistence.
Results of the animal studies are widely regarded as evidence of RCF carcinogenicity. Utell & Maxim (2010) provide a short history of the results of the animal studies conducted from the late 1950s until the present. Perhaps of greatest potential relevance to carcinogen classification for RCF are the results of chronic nose-only inhalation bioassays on rats and hamsters conducted by RCC in Geneva (Mast et al., 1995a,b; McConnell et al., 1995), which indicated RCF-exposed rats and hamsters developed fibrosis and tumors. As noted above, an IARC Working Group has reviewed the available evidence and concluded on two occasions that, on balance, there was sufficient evidence of carcinogenicity in experimental animals. Nonetheless, interpretation of the available animal studies is not straightforward. The RCC studies were believed to be the “state of the art” at the time. However, subsequent analysis of these studies concluded that overload was likely (Mast et al., 2000a,b) – chiefly due to a high and non-representative amount of particles in the exposure aerosol (Maxim et al., 1997) – and it was not possible to assess the relative contribution of these particles to the observed response (Brown et al., 2005, references therein). The IARC Working Group acknowledged possible confounding (IARC, 2002, p. 233):
“The Working Group noted that the greater particulate fraction of RCF 1 could have influenced the development of inflammation and subsequent carcinogenic response in the chronic inhalation studies of RCF 1. The extent of this influence is difficult to assess quantitatively”.
As noted above, several epidemiological studies of occupational exposure to RCF have been conducted in both Europe (at the Institute of Occupational Medicine [IOM]) and the United States (at the University of Cincinnati) as part of a comprehensive product stewardship program [PSP] (Maxim et al., 2008 for details) designed to detect, measure and control risks associated with occupational exposure to RCF. These morbidity and mortality studies have been extensively reported in the peer-reviewed literature (Burge et al., 1995; Cowie et al., 2001; LeMasters et al., 1998, 2003; Lockey et al., 1996, 1998, 2002; McKay et al., 2010; Trethowan et al., 1995; Utell & Maxim, 2010; Walker et al., 2002, 2012a,b). Collectively, these studies indicate that occupational exposure to RCF results in:
Respiratory symptoms (LeMasters et al., 1998) similar to those reported in other dust-exposed populations,
A statistically, but not clinically, significant decrease in certain measures of respiratory function in one cross-sectional study (LeMasters et al., 1998) for certain subgroups (e.g. male current or former smokers). A further longitudinal study (Lockey et al., 1998) revealed no excessive decline in lung function.
A statistically significant increase in the prevalence of pleural plaques (Lockey et al., 1996, 2002), but no evidence of parenchymal disease and
No evidence of increased deaths from lung cancer or any cases of mesothelioma (LeMasters et al., 2003).
In sum, the available epidemiological data indicate that symptoms are similar to other dust- exposed populations; there is some evidence for decreases in certain measures of lung function, a dose-related increase in pleural plaques, but no interstitial fibrosis, no elevated lung cancer rates and no mesotheliomas.
When IARC reviewed the available epidemiological data on RCF in 2002, they concluded that these data were “inadequate”, in part based on the limited size and (at the time) relatively short exposure duration of the cohort in the mortality study. The number of persons in the cohort places limits on the statistical power of the results and the exposure duration needs to be considered in terms of the latency for various lung diseases (see below). The mortality study is ongoing, however, and will become more powerful in the future.
SCOEL (2011) addressed the question of whether or not RCF was genotoxic to assess whether or not it might have a threshold. This Committee concluded that genotoxic effects noted in some studies were secondary and used some of the epidemiology data (lung function data) to derive a no observed adverse effects level based on cumulative exposure over a 45-year working lifetime. Based on these calculations, SCOEL recommended an OEL of 0.3 f/ml. The Health Council of the Netherlands (DECOS, 2011) also reviewed the available data on RCF and concluded:
“Overall, the Committee considers the induction of chronic inflammation as the most plausible mechanism of carcinogenic action of RCFs. This would imply a threshold mechanism of action. In addition, it is unlikely that RCFs possess stochastic genotoxic properties via direct production or reactive oxygen species, due to the very low iron content. However, the Committee emphasizes that the relevance of genotoxicity testing for fibers is limited due to a lack of in vitro assays suitable for fibres”.
Although the epidemiological data on RCF are limited, other SVFs have been the subject of much more powerful studies. In particular, rock wool has been extensively studied and these studies (see discussion below) are properly viewed as negative, so it is of interest to make some comparisons between RCF and rock wool.
Other relevant fiber properties
Most scientists subscribe to the so-called 3Ds (dose-durability-dimension) theory of fiber toxicity (Dement, 1990; Donaldson & Tran, 2004; Maxim et al., 2006; Oberdörster, 2000; Oberdörster et al., 2005). The importance of dose is obvious. Fiber dimensions are relevant for two reasons:
Fiber diameters are relevant because diameters affect the respirability of fibers. Broadly, fibers with diameters greater than 3 microns (μm) do not penetrate the deep lung. What is relevant here is the distribution of fiber diameters as found in or near the breaching zone of those exposed, not the diameters of the bulk fibers.
- Fiber lengths are potentially relevant because there is evidence that longer fibers (at least those longer than approximately 20 μm)2 are potentially more toxic because these are too large to be fully engulfed by macrophages. As noted by ATSDR (2004):“Fibers with diameters greater than about 3 μm are not inhaled into the deepest regions of the lungs. Fibers with lengths greater than about 15–20 μm are not engulfed by macrophages, and are more likely to lead to lung injury than shorter fibers that are more readily removed by macrophages”.
There is a substantial body of literature from both animal experiments and epidemiological studies with asbestos and other fibers that supports the idea that longer fibers are likely to be more toxic than shorter fibers (Berman & Crump, 2008; Berman et al., 1995; Bernstein, 2007; Bernstein et al., 2001a,b; Bolton et al., 1982, 1984; Davis & Jones, 1988; Davis et al., 1986; Dodson et al., 2003; Heintz et al., 2010; Lippmann, 1990; Miller et al., 1999; Pott et al., 1974; Schinwald et al., 2012; Stanton et al., 1977; Stayner et al., 2007; and references therein). There may or may not be a unique “bright line” separating more from less toxic fibers, but the evidence suggests that longer than around 10–20 μm (Schinwald et al., 2012, estimate a shorter critical length) are more toxic if they are also respirable and biopersistent.
The ability of the fiber to remain in the lung, termed durability if measured in in vitro studies, or biopersistence if measured in in vivo studies has also been found to be a key determinant of fiber toxicity (ATSDR, 2004; Bernstein et al., 2001a,b; Eastes & Hadley, 1994; Eastes et al., 1996; Hesterberg et al., 1994, 1998; ILSI Working Group, 2005; McConnell, 2000; Maxim et al., 2006; Moolgavkar et al., 2000, 2001a,b,c; Oberdörster, 2000).
Dissolution rates are measured in in vitro studies in simulated lung fluid. These rates are quantified by a kinetic constant for dissolution, K dis, typically measured in units of nanograms per square centimeter per hour (ng/cm2/h).
Biopersistence is measured in vivo in studies of laboratory animals. The recommended standardized in vivo protocol uses short term (5-day, 6 h/day) inhalation exposures of Fischer 344 rats to a well characterized (length and diameter distribution) fiber aerosol with at least 100 fibers per milliliter (100 f/ml) greater than 20 microns (μm) long, followed by a post-exposure period during which animals are sacrificed at intervals of (at least) 1 day, 2 or 3 days, 14 days, 4 weeks and 3 months and fiber lung burdens determined. Alternatively, repeated intra-tracheal applications of small doses can be used. The weighted halftime (WT1/2) for fibers ≧20 μm long (calculated from one- or two-compartment models) is taken as the relevant measure of biopersistence. Studies show (Maxim et al., 2006, references therein) that there is very good correlation between K dis and WT1/2 for various synthetic vitreous fibers (SVFs) and, as or more important, excellent correlation between either of these measures and the results of chronic animal bioassays.
Fiber chemistry is also relevant, particularly as chemistry affects biopersistence. Based on the 3D model, it is likely that fibers with similar airborne fiber dimensions and similar biopersistence are likely to have similar toxicological properties. The next sections compare dimensions and biopersistence of RCF with rock wool fibers.
Fiber dimensions
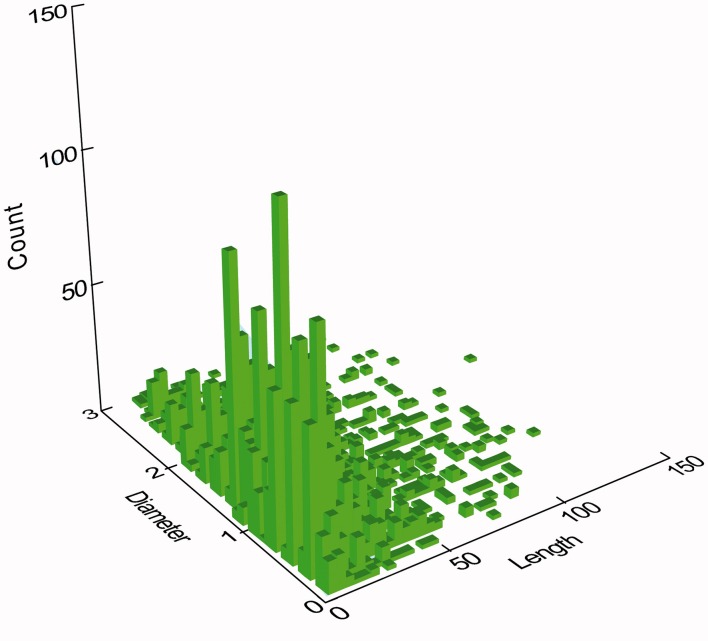
Critical fiber dimensions are fiber diameter and length. As part of the product stewardship program for RCF, manufacturers collect fiber concentrations at plants that produce RCF and facilities operated by their customers. Most of these are analyzed using phase contrast optical microscopy (PCOM) and concentrations determined using either NIOSH 7400 B or WHO counting rules. For various research purposes these personal monitoring samples are analyzed using transmission electron microscopy (TEM) and the fiber lengths and diameters are measured. Figure 1 shows a histogram of the joint distribution of lengths and diameters of 4031 airborne respirable RCF fibers using NIOSH 7400 B counting rules.3 As can be seen, the combination of the fiberization process and subsequent dispersion (settling) of fibers in the workplace leads to a very broad distribution of airborne respirable fiber diameters and lengths.
Figure 1.
Diameter and length distribution of 4031 airborne respirable RCF fibers from workplace samples.
Measured airborne respirable RCF fiber diameters range from 0.07 to 3.0 microns (μm) and lengths that range from 5.0 (the threshold length requirement for both NIOSH 7400 B and WHO counting rules) to 138 μm.
Table 1 provides summary statistics on airborne respirable RCF fiber lengths and diameters. Note that the respirable fiber diameters are ≤3 μm, the limit of respirability, and lengths all ≧5 μm in accordance with NIOSH 7400 B counting rules.
Table 1.
RCF fiber diameter and length data.
| Number of fibers | 4031 Diameters (µm) | 4031 Lengths (µm) |
|---|---|---|
| Minimum | 0.070 | 5.000 |
| Maximum | 3.000 | 138.000 |
| Range | 2.930 | 133.000 |
| Geometric mean | 0.911 | 17.406 |
| Median | 1.000 | 16.670 |
| Arithmetic Mean | 1.091 | 22.428 |
| Std. Error | 0.010 | 0.285 |
| Standard Dev | 0.616 | 18.088 |
| Variance | 0.379 | 327.189 |
| Coefficient of variation (CV) | 0.565 | 0.807 |
| Skewness (G1) | 0.755 | 2.071 |
| SE Skewness | 0.039 | 0.039 |
| Kurtosis (G2) | 0.011 | 5.131 |
| SE Kurtosis | 0.077 | 0.077 |
The statistics shown in Figure 1 and Table 1 are airborne (from personal monitoring samplers) respirable fibers, not bulk fibers. Airborne fibers are relevant in terms of human exposure. Size distributions of bulk and airborne fibers differ. Diameters of airborne fibers are typically smaller than the bulk fiber (Schneider et al., 1983) due to the effects of settling and ventilation. As noted by Schneider et al. (1983):
“When the fibers are dispersed into the air during handling, cutting, etc. only thin fibers will remain airborne. It is a general experience that the measured median diameter in an air-sample is consistently and substantially smaller than the nominal diameter of the product”.
Christensen et al. (1993) reported the diameters of bulk RCF ranged from 2.4 to 3.8 μm (as measured by scanning electron microscopy), depending upon product (higher for spun than blown blanket). However, once airborne the structures with greater diameters settle out preferentially, leaving the size distribution of the airborne structures shifted downwards towards fibers with smaller diameters as shown in Table 1. The diameter distribution data shown here are for a mixture of RCF products as produced and used in the workplace and include both blown and spun fibers. The sections provided below provide comparable data for rock wool fibers.
Fiber diameter comparisons
Comparisons of airborne respirable RCF and rock wool fiber dimensions as reported in several studies are given below. Generally speaking, the diameters of bulk RSW (rock/slag wool) products are greater than those for RCF (IARC, 2002), but as noted above, the relevant comparison is between airborne fiber dimensions.
Corn et al. (1976) measured fiber concentrations in two rock wool plants using the spinning process. They did not report the mean or median diameters of the airborne fibers, but from the data presented on the percentage of fibers with diameters <1 μm and <3 μm, it is clear that this interval included the median diameters. The true median diameters of the respirable fibers are likely to be significantly less than 3 μm as the samples included fibers with diameters of as much as 7 μm, which would not be respirable.
Esman et al. (1979) measured fiber concentrations and dimensions in 16 facilities producing manmade mineral fibers. Among these plants 2, 5, 7, 11 and 13 produced RSW with nominal diameters ranging from 5 to 8 μm. The median diameters of the airborne fibers were less than the nominal diameters and ranged from ∼0.5 to ∼1.5 μm, a range that brackets that for RCF shown in Table 1.
Robinson et al. (1982) conducted an epidemiological and environmental study of rock and slag mineral wool production workers was undertaken at a plant that has been in operation since the early 1900s (using the spinning process). These investigators measured fiber diameters and lengths using PCOM. (Because the practical limit of detection for PCOM is ∼ 0.2 μm, this will tend to overstate diameters.) The reported median diameter was 2.2 μm. Although this is larger than that shown above for RCF, the discrepancy is partially accounted for by the fact that the rock wool was spun (not blown) and PCOM was used.
Schneider et al. (1985) measured respirable airborne fiber dimensions of rock and glass wool. Table 2 shows the relevant diameter measurements for rock wool.
Table 2.
Parameters of the size distribution of rock wool reported by Schneider et al. (1985).
| Site | Number of samples | Geometric mean diameter (µm) | Geometric standard deviation | Geometric mean length (µm) | Geometric standard deviation |
|---|---|---|---|---|---|
| Rock wool Plant A | 6 | 0.95 | 3.1 | 13 | 3.4 |
| Rock wool Plant B | 38 | 0.99 | 3.3 | 14 | 3.6 |
| Use of Rock wool | 21 | 1.20 | 2.7 | 22 | 4.0 |
These diameters (and lengths) are generally comparable to those for RCF (Table 1). For example, the geometric mean diameter and length from the RCF data set are 0.91 and 17 μm, respectively.
Cherrie et al. (1986) reported results of fiber monitoring at European glass and rock wool plants. Among other things, this article presents data relevant to the distribution of fiber diameters and lengths. Median diameters of rock wool samples (p 21) ranged from 1.2 to 2.0 μm.
In a 1988 study sponsored by the Swedish Work Environmental Fund, Krantz provided exposure measurements of manmade mineral fibers (both glass wool and rock wool) from ten production plants in Sweden. In addition to fiber concentration measurements, Krantz (1988) used scanning electron microscopy (SEM) data on the lengths and diameters of glass and rock wool fibers. With respect to fiber diameters, Krantz (1988) reported the mean diameter of airborne respirable rock wool insulation fibers to be 1.0 μm, with a range from 0.57 to 1.77 μm. Note from Table 1 that the median and arithmetic mean diameters for RCF are 1.0 and 1.09 μm, respectively, which closely matches the Krantz (1988) measurements for rock wool. In accordance with expectation, the arithmetic mean and median diameters for airborne respirable rock wool fibers were substantially smaller than the nominal diameter (5–7 μm) of the bulk rock wool fibers.
The IARC (2002) Working Group that reviewed various mineral fibers summarized data on the dimensions of airborne respirable rock wool fibers from several papers. Geometric mean diameters for rock wool varied among the papers cited from 0.3 to 1.9 μm, depending upon the study.
Kauffer et al. (2003) reported on a study of various instrumental techniques for measuring airborne fiber concentrations. They used a dust-generating device to generate clouds of various types of fibers, including rock wool. The measured geometric diameter of the rock wool fiber in this study was 0.34 μm, which is considerable smaller than that for RCF. This study is of limited utility for comparative purposes because the fiber clouds were artificially generated and are not necessarily representative of those in the workplace.
More recently, Campopiano et al. (2012) studied personal monitoring samples of airborne fiber concentrations of pressed mineral panels employed as false ceilings. Four workers were investigated for eight working days. As part of this study, SEM was used to measure the diameters and lengths of these fibers. Airborne respirable mineral wool fiber diameters measured in this study ranged from 0.1 to 2.7 μm, with reported (sample size not specified) arithmetic mean and median diameters of 1.27 and 1.12 μm, respectively. These diameters are not materially different from those given above for RCF.
Table 3 summarizes the studies reported above in terms of RCF and rock wool diameters. Collectively, these indicate that, notwithstanding differences in the diameters of bulk RCF and rock wool, the airborne diameters are quite similar. For comparison, fiber diameters of various types of asbestos are very much smaller (typically very much thinner than 0.5 μm; ATSDR, 2001; Cheng, 1986; Gibbs & Hwang, 1980; Hwang, 1983; Rood & Streeter, 1984; Verma & Clark, 1995).
Table 3.
Comparison of fiber diameters (microns) for RCF and rock wool from different sources.
| Fiber | Geometric mean diameter | Median diameter | Arithmetic mean diameter | Source | Comments |
|---|---|---|---|---|---|
| RCF | 0.91 | 1.00 | 1.09 | Table 1 | |
| Rock/Slag wool | <3 | Corn et al. (1976) | Median overstated because non-respirable fibers counted. | ||
| 0.5–1.5 | Esmen et al. (1979) | Median values vary with nominal diameter of bulk fibers. | |||
| 2.2 | Robinson et al. (1982) | Single plant using spinning process measured by PCOM. | |||
| 0.95–1.2 | Schneider et al. (1985) | Reported values vary with plant and process. | |||
| 1.2–2 | Cherrie et al. (1986) | Median diameters varied with plant. | |||
| 1.0 | Krantz (1988) | Mean diameters ranged from 0.57 to 1.77 µm among ten plants. | |||
| 0.3–1.9 | IARC (2002) | Range from various studies cited in this publication. | |||
| 1.12 | 1.27 | Campopiano et al. (2012) | SEM measurements on workers installing ceiling panels. |
Fiber length comparisons
Lengths of airborne respirable rock wool fibers have also been measured by several investigators. Table 4 provides a summary in similar format to Table 3. As can be seen, RCF fiber lengths are broadly similar to those reported for RSW. Rock wool fiber lengths reported by Campopiano et al. (2012) are longer than those measured for RCF, but this difference may not be material because fiber potency may not increase materially beyond a certain length. If this conjecture is not correct, then the possible potency of might be greater than that for RCF based on length.
Table 4.
Comparison of fiber lengths for RCF and rock wool from different sources.
| Fiber | Geometric mean length | Median length | Arithmetic mean length | Source | Comments |
|---|---|---|---|---|---|
| RCF | 17.4 | 16.7 | 22.4 | This study | See Table 1. |
| Rock/Slag wool | ∼20 | Corn et al. (1976) | Values shown are approximate. | ||
| 12–30 | Esmen et al. (1979) | Median values are approximate as read from graph. | |||
| 16 | Robinson et al. (1982) | Single plant using spinning process measured by PCOM. | |||
| 13–22 | Schneider et al. (1985) | Reported values vary with plant and process. | |||
| 10–20 | Cherrie et al. (1986) | Median lengths varied with plant. | |||
| 10–30 | Krantz (1988) | Range is approximate as read from graph. | |||
| 7–22 | IARC (2002) | Range from various studies cited in this publication. | |||
| 27.77 | 28.05 | 37.52 | Campopiano et al. (2012) | SEM measurements on workers installing ceiling panels. |
Fiber concentrations
Workplace fiber concentrations vary inter alia with the fiber type, plant, type of work being done, the engineering (e.g. general and local exhaust ventilation) and workplace controls in use, and whether or not respirators are worn and, if so, the assigned protection factor). Several authors have reported on fiber concentrations at plants producing rock or slag wool (Cherrie et al., 1986; Corn et al., 1976; Esman et al., 1979, 1982). For the most part, fiber concentrations at MMVF plants were reportedly <0.5 f/ml (Esman et al., 1979) and more recently (Cherrie et al., 1986) perhaps <0.1 f/ml. Fiber concentrations at RCF plants have been systematically monitored at both manufacturing plants and customer locations as part of a product stewardship program for more than 20 years (Maxim et al., 2008). Weighted average (by number of workers in each functional job category) fiber concentrations at manufacturing facilities in recent years are approximately 0.2 f/ml (0.3 f/ml at customer facilities).
Biopersistence
As noted above, biopersistence is a key determinant of fiber toxicology. Deposited fibers are cleared from the deep lung by dissolution, breakage, and clearance by macrophages. In vitro studies measure rates of dissolution, whereas in vivo measurements capture all three ways that fibers are removed. Although studies show that in vitro and in vivo measurements are correlated, in vivo measurements (particularly those taken using standard protocols, such as short-term inhalation studies on rats) are preferred. The most relevant measure of biopersistence is the weighted half time, WT1/2 of fibers ≧20 µm in length as calculated from a one compartment or two compartment model. Measured values of WT1/2 range from just a few days for very low biopersistence fibers, such as wollastonite and certain glass wools, to 1000 or more days for some forms of amphibole asbestos.
The measured in vitro durability (K dis) constant for rock wool is larger than that measured for RCF and also that there is good correlation between in vitro and in vivo measures. For example, data provided in Guldberg et al., 1998, report values for K dis of 47 and 24 ng/cm2 h for MMVF21 and RCF, respectively, at a pH of 4.5 and 23 and 8 ng/cm2 h for MMVF21 and RCF, respectively, at a pH of 7.4. These data alone would argue that RCF is more durable than MMVF21, although the difference in K dis values is relatively small when compared to the likely error of the measurement.
However, where available, in vivo measures of biopersistence (WT1/2 values) are preferable as these more closely mimic the various processes by which fibers are removed (removal by macrophages, dissolution and breakage). Therefore, for example, the EU Directive 97/69/EC, dated 5 December 1997, provides a system, through Nota Q, for demonstrating that mineral wool fibers can be exonerated from carcinogenicity and not be classified as a hazardous substance. Nota Q allows for exoneration by any one of four methods. The four methods are: short-term biopersistence test by inhalation, short-term biopersistance test by intra-tracheal instillation, an appropriate intra-peritoneal test or a long term inhalation test. These criteria are all based on in vivo, rather than in vitro data. Because we have extensive data on WT1/2 values for both fibers, we have used these for comparison.
Rock wool
Traditional rock wool (referred to as MMVF21 or in one study, “fiber L”) WT1/2 values have been measured in several studies (see, e.g. results reported in Bernstein et al., 1996, 2001a,b; Hesterberg et al., 1996, 1998; HVGB, 1998; Kudo & Aizawa, 2008; Musselman et al., 1994). Measured WT1/2 values vary among the studies, with an arithmetic mean value of approximately 59 days. [This is slightly lower than the value cited in Maxim et al. (2006) 62.5 days, because additional and more recent measurements are included.]
The specific chemical composition of MMVF21 is given in Hesterberg et al. (1998); the five largest components in terms of weight percent are SiO2, 45.9%; CaO, 17%; Al2O3, 13.75%; MgO, 9.5%; and Fe2O3, 6.9%. The corresponding percentages for the similar fiber L are given in Bernstein et al. (1996) as SiO2, 46.3%; CaO, 10.04%; Al2O3, 13.5%; MgO, 9.1%; and Fe2O3, 13.2%.
RCF
RCF WT1/2 values have also been measured in several studies (see reported results in Bernstein, 1997a,b; Bernstein et al., 1997, 2001a,b; Hesterberg et al., 1998; HVGB, 1998). These WT1/2 values range from 41 to 64 days with an arithmetic mean of approximately 50 days.
Similarities in fiber dimension and biopersistence
The difference between the WT1/2 values of 50 and 59 days for RCF and traditional rock wool is neither statistically significant nor material. Therefore, for practical purposes, rock wool and RCF can be regarded as having similar biopersistence. And, although both fibers have half times greater than several other SVFs, both fibers are very much less biopersistent than various types of amphibole asbestos including amosite (WT1/2 = 418 days, Hesterberg et al., 1998), crocidolite (WT1/2 = 817 days, Hesterberg et al., 1998) or tremolite (WT1/2 ≅ ∞; Bernstein & Hoskins, 2006; Bernstein et al., 2003). In addition, as noted above, breathing zone samples of these two fibers have similar dimensions. These comparisons suggest that RCF and rock wool are likely to have similar toxicological properties as well.
Fiber breakage mechanism similarities
As noted above, fibers undergo various changes when deposited in the lower regions of the lung, including breakage, dissolution and removal by macrophages. The mechanism of fiber breakage is a potentially relevant property because some fibers, such as chrysotile asbestos break along the longitudinal axis, creating additional fibers of smaller diameter. However, because they are amorphous (i.e. non-crystalline), neither rock wool nor RCF have cleavage planes that cause them to split lengthwise into fibers with smaller diameters. Rather, these break transversely (across the fiber), resulting in fibers which are of the same diameter as the original fiber but shorter (thus more easily removed by macrophages), together with a small amount of dust (Assuncao & Corn, 1975; IARC, 2002; NIOSH, 2006).
Animal studies
Both RCF and rock wool have been included in several animal (intraperitoneal injection and inhalation) studies. Similarities and differences are reviewed below.
Similarities in animal studies
Animal tests results also indicate that effects of exposure to RCF and rock wool are similar. Although intraperitoneal (IP) injection studies have been criticized because they are not a normal physiological route of exposure, IP studies are sometimes used to provide an indication of potential hazard. IP injection studies on rats and hamsters indicated that RCF was capable of inducing tumors (Davis et al., 1984; Miller et al., 1999; Pott et al., 1987; Smith et al., 1987). Similarly, IP studies of various rock wool fibers, including MMVF21, have produced tumors in experimental animals (Pott et al., 1987; Roller et al., 1996). The Roller et al. (1996) study estimated the dose (in 109 fibers of l/d > 5 and d < 2 μm) necessary to produce a 25% tumor risk as 0.032 × 109 fibers and, on the basis of this measure, concluded that MMVF21 was intermediate in toxicity between crocidolite (0.012 × 109 fibers) and tremolite (0.064 × 109 fibers) asbestos – a result inconsistent with available epidemiological evidence on rock wool. Roller et al. (1996) did not estimate a corresponding value for RCF.
Rödelsperger (2004) used a model devised by Berry (1999) to explain how results might differ between animals and humans. Specifically, Rödelsperger (2004) noted:
“The carcinogenic potency of crocidolite and ceramic fibers from inhalation and intraperitoneal injection in rats is similar. However, it cannot be predicted, that this similarity likewise exists for humans, despite of differences in fiber size and bio-persistency. Rather the consequences of the dissolution rates may be quite different for humans and rats”.
Differences in animal studies
Well-designed chronic inhalation studies are thought preferable to other animal studies for risk assessment and both RCF and MMVF21 have been evaluated in these studies. The RCF studies (Mast et al., 1995a,b) resulted in the development of both fibrosis and tumors, whereas the MMVF21 study (McConnell et al., 1994) resulted in fibrosis, but no tumors – a potentially relevant difference. The RCF and MMVF21 rat studies were conducted using a similar protocol and at the same laboratory (Research and Consulting Company then of Geneva, Switzerland) and used similar experimental conditions.4 For example, similar gravimetric doses were used (nominal values of 0 through 30 mg/m3) and similar aerosol fiber concentrations (ranging from 0 to approximately 200 f/cc of WHO fibers5 ). After 24 months exposure, lung burdens (in fibers per milligram of dry lung) at the high dose (30 mg/m3) were approximately 242 × 103, 177 × 103 and 275 × 103 for rock wool, slag wool and RCF, respectively.
However (Brown et al., 2005) the RCF study may have been compromised by overload resulting from use of a test substance that was not representative of that found in the workplace.
RCF was also the subject of a chronic inhalation study in Syrian golden hamsters (McConnell et al., 1995). This chronic, single-dose, nose-only inhalation study resulted in a significant incidence (41%) of pleural mesotheliomas in hamsters exposed to 30 mg/m3 (215 WHO f/ml) RCF for 18 months. No similar inhalation study on hamsters has been reported for MMVF21, however, so there is no basis for comparison with RCF. Moreover (Morrow et al., 1996; Warheit & Hartsky, 1994) the relevance of the hamster model for assessments of mesothelioma in humans has been questioned.
Epidemiological studies
As noted above, both RCF and rock wool have been the subjects of epidemiological studies. Similarities and differences are highlighted below. For epidemiological studies in the United States, the rock wool cohorts were (and are) chiefly exposed to traditional rock wool. For studies in Europe, workers were exposed to traditional rock wool through approximately the year 2002 and afterwards to a newly developed (and less biopersistent) material. As the effects of interest have relatively long latency periods, it is appropriate to regard both cohorts as consisting of traditional rock wool.
Mortality studies (RCF)
The RCF mortality study (LeMasters et al., 2003) followed current and former male workers employed in two manufacturing plants between 1952 and 2000 to investigate any possible excess mortality. This ongoing study found no excess mortality related to all deaths, all cancers, malignancies or diseases of the respiratory system, including mesothelioma. The study also employed Cox’s proportional hazards model (adjusted for age and race), which did not show elevated total risk with cumulative RCF exposure. The study found an unexpected, but statistically significant association with cancers of the urinary organs, which will continue to be investigated. Although negative with respect to lung disease, the study is limited by sample size (942 workers) and by the limited time since first exposure (mean latency period of 21 years at time of publication). The study had sufficient time since first exposure to address lung cancer (most studies conclude that lung cancer has a latency ∼20 or more years).
However, as shown in Table A1 (at end of the report due to its length), reported mesothelioma latencies range from a minimum of approximately 6 years (McDonald & McDonald, 1979) to as much as 75 years (Bianchi & Bianchi, 2007), with a median in the range of 20–50 years. The most recent study (Frost, 2013) from the Great Britain asbestos survey offers the following conclusions:
“After excluding missing data, there were 614 workers who died with mesothelioma between 1978 and 2005. Total follow-up time was 9280 person-years, with a median latency of 22.8 years (95% confidence interval (CI) 16.0–27.2 years). In the fully adjusted model, latency was around 29% longer for females compared with males (TR (time ratio) = 1.29, 95% CI = 1.18–1.42), and 5% shorter for those who died with asbestosis compared with those who did not (TR = 0.95, 95% CI = 0.91–0.99)”.
In reviewing Table A1, note that the estimated/measured mesothelioma latency is not constant even for a specific cohort; latency is a random variable. Although 20–50 years may be a reasonable range for the median latency, the observed range of reported latencies in any sample of mesotheliomas cases is very broad. Thus, among those persons who have developed mesothelioma, some will develop it in a much shorter or much longer time period than the estimated mean or median latency.
The RCF mortality study, therefore, has limited power to address mesothelioma. Nonetheless, as mesothelioma, latency is a random variable if there was any appreciable incidence of mesothelioma in the RCF-exposed cohort, then we might have expected to have seen some cases with latencies less than the mean/median latency. The fact that none have been seen to date is beginning to be relevant. As these cohort ages, the strength of the strength of the evidence will increase if no mesotheliomas are observed subsequently.
Mortality studies (rock wool)
IARC conducted a careful review of various SVFs (including rock wool) in 2001 (IARC, 2002). The IARC Working Group reviewed all the available evidence – particularly the available epidemiological evidence, including all the studies then published by Marsh and others at the University of Pittsburgh. Based on this comprehensive review, IARC changed the carcinogen classification for rock wool (and fiberglass) from Group 2B (possible human carcinogen) to group 3 (not classifiable as to its carcinogenicity to humans). Table A2 (first part, shown at the end because of its length) summarizes the studies cited in the IARC Monograph.
In summarizing the results of human studies, IARC (2002) concluded:
“The present evaluation relies mainly on cohort and nested case–control studies, in which exposure to rock (stone) wool and exposure to slag wool were not considered separately. The extended follow-up of the rock (stone)/slag wool cohort from the USA indicated an overall elevated risk of respiratory cancer when either national or local comparison rates were used. However, no association was found with duration of exposure or with time since first exposure. Standardized mortality ratios were no longer elevated when indirect adjustment for smoking was made. The nested case–control study showed no association between respiratory cancer and estimated cumulative exposure to respirable fibers, with or without adjustment for possible confounding by smoking and other sources of occupational exposure. Another nested case–control study partially overlapping with the study in the USA showed no increased risk for respiratory cancer in association with exposure to slag wool. The extended follow-up of the European cohort study indicated an overall elevated risk for lung cancer when national comparison rates were used. This study showed an increasing risk with years since first exposure. The highest standardized mortality ratio was found among workers with the longest time since first employment and among those first employed in the ‘early technological phase’, i.e. before the introduction of oil and binders and use of the batch-processing method. However, in a case–control study that included detailed information on exposure to fibers, individual smoking habits and potential occupational confounders, no increased risk of lung cancer with increasing fiber exposure was reported. The results from these studies provide no evidence of an increased risk for pleural mesotheliomas or any other tumours”. [Emphasis added]
The available evidence that exposures to rock or slag wool did not lead to significant increases in lung cancer or mesothelioma was substantial as of 2002. Since the 2002 IARC Monograph was published, additional studies have appeared in the literature covering both fiberglass and rock wool that are broadly consistent with IARC’s 2002 decision. These additional studies are also summarized in Table A2.
With respect to rock wool, for example, Kjaerheim et al. (2002)6 analyzed data on rock and slag wool (RSW) workers in plants in Denmark, Germany, Norway and Sweden and found:
“For cumulative exposure to RSW assessed with a 15-year lag, the smoking-adjusted odds ratios in the second, third, and fourth quartiles of exposure were 1.3 (95% confidence interval [CI] = 0.7–2.3), 1.0 (CI = 0.5–1.9) and 0.7 (CI = 0.3–1.3). Similar results were obtained when we included only those workers employed for more than 1 year, when we included other indicators of RSW exposure, and after control for co-exposures”.
The authors concluded:
“This study provides no evidence of a carcinogenic effect on the lung of rock and slag wool under exposure circumstances in the production industry during the last 4–5 decades”.
Baccarelli et al. (2006) conducted a study to examine the risk of lung cancer from exposure to dusts and fibers (including MMVF) in Leningrad Province, Russia. The study is not fully informative because fiberglass and rock/slag (mineral) wool exposures were pooled in the analysis. To investigate lung cancer risk in relation to exposure to various dusts and fibers, the authors identified 540 pathologically diagnosed lung cancer cases and 582 controls from the 1993–1998 autopsy records of the 88 hospitals of Leningrad Province, Russia. Lifetime job-specific exposure measurements were available for 15 organic, 15 manmade and 28 natural-inorganic agents. Results of this study were described by the authors as follows:
“In male workers, increased risks were found for linen dust (OR = 3.68, 95% CI 1.00–13.6, adjusted for age, smoking and residence), and unspecified DFs (OR = 1.44, 95% CI 1.07–1.94). Small non-significant excess risks were observed for quartz dust (OR = 1.27; 95% CI 0.83–1.93) and manmade vitreous fibers (MMVFs; OR = 1.82, 95% CI 0.88–3.75). In female subjects, risks were non-significantly associated with paper dust (OR = 1.77, 95% CI 0.74–4.20), and unspecified DFs (OR = 1.52, 95% CI 0.77–3.03)”.
Carel et al. (2007) performed a multicenter case-control study of exposure to asbestos and manmade vitreous fibers and risk of lung cancer in Europe and concluded that the odds ratio (OR) for exposure to MMVF was elevated (1.23) but not significantly so (95% CI = 0.88–1.71). These investigators did not distinguish between fiberglass and mineral wool; these were lumped into a general category of exposure to MMVFs.
Pintos et al. (2008) performed two case-control studies on cohorts from Montreal, Canada. They found increased risks of lung cancer for substantial exposure to asbestos, but a non-significant odds ratio (1.1, 95% CI = 0.37–3.22) for exposure to manmade vitreous fibers. The authors grouped fiberglass and mineral wool together because they were not able to distinguish between exposures to the two fiber types from interviews. Pintos et al. (2009) performed a similar analysis for mesothelioma and computed the mesothelioma OR for exposure to any asbestos type as 3.7 (95% CI = 1.7–7.8), but were not able to disentangle the effects of MMVF.
Lipworth et al. (2009) performed a meta-analysis of 16 studies relative to rock wool specifically and reported:
“Sixteen estimates of lung cancer risk yielded a summary relative risk (RR) of 1.21 (95% CI = 1.11–1.32, based on 1662 exposed cases). Corresponding RRs were 1.26 (95% CI = 1.10–1.44) in studies of production workers (with similar risk for RW and GW workers), 1.06 (95% CI = 0.77–1.48) in studies of end users and 1.18 (95% CI = 0.98–1.42) in community-based studies. The summary RR for [lung and head and neck] HN cancer was 1.36 (95% CI = 1.13–1.63, 414 exposed cases). With a few exceptions, all studies that assessed the risk of lung or HN cancer according to various indices of MMVF exposure failed to detect a dose-risk relation. There was limited evidence of a confounding effect of tobacco smoking. No clear excess of pleural mesothelioma has been reported in MMVF-exposed workers”. [Material in square brackets added for clarity.]
Lipworth et al. (2009) concluded:
“Despite a small elevation in RR [relative risk] for lung cancer among MMVF [man made vitreous fibers] production workers, the lack of excess risk among end users, the absence of any dose-risk relation, the likelihood of detection bias, and the potential for residual confounding by smoking and asbestos exposure argue against a carcinogenic effect of MMVF, RW [rock wool], or GW [glass wool] at this time. Similar conclusions apply to HN cancer risk among workers exposed to MMVF”. [Material in square brackets added for clarity.]
Marsh et al. (2011) reexamined the available evidence on the relation between respiratory system cancer risk and MMVF exposure. This article was focused on exposure to fiberglass, but covered other MMVFs as well. Specifically Marsh et al. (2011) cites work of Pintos et al. (2008) from two population-based case-control studies in Montreal, Canada. Exposures to MMVF (fiberglass and RSW combined) were categorized as non-exposed, non-substantial and substantial. Neither study revealed a statistically significant elevated risk of lung cancer among those exposed to MMVF. Marsh et al. (2011) also cited a review and meta-analysis by Lipworth et al. (2009) and concluded:
“…despite a small elevation in the risk for lung cancer in the industry and community-based studies, the absence of consistent evidence of an exposure–response relationship, the likelihood of detection bias, and the potential for residual confounding by smoking and asbestos exposure argue against a carcinogenic effect of MMVF, GW, or RSW at this time”.
SCOEL (2012) examined the available evidence for carcinogenicity of rock and glass wool fibers and concluded:
“The life time studies in rats on rock wool and slag wool as well as insulation fiber glass (and of TISMO7 ) did not reveal carcinogenic effects. Recent evaluations of the epidemiological studies of workers exposed to respirable rock wool and glass wool fibers (Lipworth et al., 2010) and glass wool fibers (NTP, 2010) support these data”.8
Lacourt et al. (2013) reported results of a French pooled case–control study of persons occupationally co-exposed to asbestos, mineral wool and silica. The authors claim that a significant association between mesothelioma and mineral wool exposure was observed after adjustment for occupational asbestos exposure. Bonde (2013) criticized this study because the selection of the control group was inappropriate and that exposures to asbestos, mineral wool and silica were highly correlated.
Pleural plaques
As noted above, the epidemiology studies on RCF have shown a dose-related increase in the prevalence of pleural plaques (Lockey et al., 1996, 2002, 2012). Pleural plaques are usually taken as a measure of fiber (particularly asbestos) exposure, but do not cause impairment of lung function, are not precursors of lung cancer or mesothelioma, and are not an independent risk factor in the development of cancer (ACC Review, 2004; ATSDR, 2000; Ameille, 2012; Ameille et al., 2011; Banks & Dedhia, 2011; British Lung Foundation, 2011; Crapo, 2005; Downer et al., 2013; Edelman, 1988; Federal Register, 2005; Gevenois & de Vuyst, 2006; Harber et al., 1987; Hillerdal, 1997, 2001; IIAC, 2008; Jones et al., 1996; Letourneux, 1999; Newman & Rose, 1989; Partanen et al., 1992; Reid et al., 2005; Reinhartz, 2004; Robinson & Lake, 2005; Rubin, 1986; Smith, 1994).
Much less has been published regarding the relationship between rock wool exposure and pleural plaques in exposed populations. Table 5 summarizes results from a study by Järvholm et al. (1995) of workers in a factory producing rock wool as compared to the occurrence of pleural plaques in non-exposed referents. These data are subdivided by age group because of the likelihood that the appearance of plaques is correlated with age. Although a higher proportion of the exposed group (1.59%) than referents (0.55%) were found to have plaques, the association fails to reach statistical significance (p = 0.09 computed by the authors).
Table 5.
Occurrence of pleural plaques among males exposed to mineral wool.
| Exposed to mineral wool |
Not exposed |
|||||
|---|---|---|---|---|---|---|
| Age group (years) | Workers | Workers with plaques | Frequency (%) | Referents | Referents with plaques | Frequency (%) |
| 20–29 | 202 | 0 | 0.00 | 159 | 0 | 0.00 |
| 30–39 | 215 | 1 | 0.47 | 136 | 0 | 0.00 |
| 40–49 | 138 | 2 | 1.45 | 107 | 0 | 0.00 |
| 50–59 | 122 | 6 | 4.92 | 86 | 2 | 2.33 |
| 60–69 | 77 | 3 | 3.90 | 53 | 1 | 1.89 |
| All | 754 | 12 | 1.59 | 541 | 3 | 0.55 |
Summary of data from Järvholm et al. (1995).
Interstitial fibrosis
Lockey et al. (2012) studied the relationship between RCF exposure and possible interstitial fibrosis. They used three possible measures of RCF exposure, production duration, production latency and cumulative RCF exposure. They concluded:
“There was no association between any exposure metric and interstitial radiographic changes for either profusion > 1/0 or > 0/1 group”.
A comprehensive review on the relationship between occupational exposure to rock wool and the development of interstitial fibrosis was conducted by de Vuyst et al. (1995). These investigators concluded:
“There is no firm evidence that exposure to glass-, rock- and slag-wool is associated with lung fibrosis, pleural lesions or non-specific respiratory disease in humans”.
There have been isolated case reports of interstitial fibrosis among those exposed to rock or glass wool (Guber et al., 2006; Yamaya et al., 2000), however.
Similarities and differences between RCF and rock wool based on epidemiological studies
The RCF studies have shown that exposed workers have a significantly greater likelihood of developing pleural plaques than non-exposed referents. Moreover, the frequency of pleural plaques increases with dose. Rock wool has been less well studied in this regard. The limited available data suggest that rock wool exposure might be associated with the development of pleural plaques, but in the only study available the differences in frequency of plaques among exposed and non-exposed populations was not statistically significant.
There is no evidence that occupational exposure to RCF results in the development of interstitial fibrosis. Epidemiological studies on rock wool lead to a similar conclusion.
Mortality studies on both RCF and rock wool fail to demonstrate any statistically significant increase in lung cancer or mesothelioma in fiber-exposed populations. For rock wool, there is a very large database of studies that support this conclusion. The ongoing RCF studies have less statistical power and shorter exposure duration. Over time, the ability to detect differences in disease frequency for effects with greater latency will increase.
Overall summary
Table 6 summarizes the key similarities and differences between RCF and rock wool in terms of relevant studies.
Table 6.
Similarities and differences between RCF and rock wool.
| Comparison | ||
|---|---|---|
| Physical properties | Biopersistence | RCF and rock wool have nearly identical biopersistence as measured by WT1/2 values from short term inhalation exposures with rats. |
| Airborne fiber dimensions | Though bulk fiber diameters differ, diameters of airborne fibers from personal monitoring samples are quite similar. Fiber lengths of RCF and rock wool are similar. | |
| Breakage mechanism | Both RCF and rock wool break transversely rather than longitudinally. Thus, any breakage results in fibers that are more easily removed by macrophages. | |
| Animal studies | IP studies | IP studies of rats show that exposure to both RCF and rock wool result in the development of tumors. |
| Inhalation studies | A well-done chronic bioassay of rats exposed (nose-only) to rock wool (MMVF21) resulted in mild fibrosis, but no tumors. A similar study on RCF resulted in both fibrosis and tumors, although the result may have been undermined by overload resulting from exposure to a test article with a non-representative ratio of particles to fibers. | |
| Epidemiological studies | Pleural plaques | Exposure to RCF results in a dose-dependent statistically significant increase in the frequency of pleural plaques. Limited data on rock wool exposure resulted in an increase in the frequency of pleural plaques in exposed workers that was not statistically significant. |
| Interstitial Fibrosis | No interstitial fibrosis seen in cohorts exposed to either rock wool or RCF. | |
| Increased lung cancer or mesothelioma | Mortality studies fail to indicate any increase in either lung cancer or mesothelioma among cohorts exposed to either RCF or mesothelioma. The statistical power of the rock wool studies is much larger, however. |
Conclusions
This analysis summarizes some key similarities between rock wool, which is appropriately regarded as non-carcinogenic, and RCF, which is classified as a potential human carcinogen. Key physical similarities include comparable fiber dimensions, fiber breakage mechanism and biopersistence. Animal IP studies have similar outcomes. A chronic nose-only inhalation bioassay resulted in fibrosis, but no tumors, in laboratory animals exposed to rock wool. A similar study on RCF resulted in both fibrosis and tumors, although interpretation of this study is not straightforward as the test substance used was not representative of that found in the workplace and overload may have resulted. Epidemiological studies with cohorts occupationally exposed to RCF and rock wool show that neither substance has resulted in interstitial fibrosis, increased rates of lung cancer, or any mesotheliomas. Exposure to RCF results in a statistically significant dose-related increase in pleural plaques. Limited data on rock wool suggests that there might be a similar increase, but this result is not statistically significant. As a class, the epidemiological studies on rock wool are substantially more powerful because the sizes of the exposed cohorts are much larger and the exposure duration longer, which permits improved assessment of effects with long latencies, such as mesothelioma. For RCF, the mortality study continues and the duration of exposure of members of the cohort will increase, permitting more robust conclusions to be drawn in the future.
These similarities suggest that possible risks associated with occupational exposure to RCF have been overstated. Our study further supports the assumption that mesothelioma is specifically related to asbestos and erionite exposure:
DECOS (2010): “It is likely that in the Netherlands almost all mesotheliomas are attributable to asbestos”, especially amphiboles.
Murphy et al. (2011): “Mesothelioma is almost exclusively found after asbestos exposure and is a particle response unique to fibrous particles”.
Lacourt et al. (2013): “Except asbestos, only erionite fibers are recognized as an etiologic factor for pleural mesothelioma”.
Boffetta et al. (2014): “The combined evidence from epidemiology and toxicology provide little evidence that exposure to SVF increases the risk of mesothelioma”.
However, risks of exposure to any respirable and relatively durable fiber need to be managed. Manufacturers of both RCF and rock wool have developed product stewardship programs, which seek to assess and control possible risks.
Based on analogies with rock wool (read across), it is reasonable to believe that increases in lung cancer or any mesotheliomas are unlikely to be found in the RCF-exposed cohort. However, despite several attempts to find predictors with reasonable sensitivity, specificity, positive prediction value, and “lead” time, there are no published studies reporting success. For example, Sandén & Järvholm (1991) examined a cohort study of 3893 shipyard workers exposed to asbestos and assessed the value of medical monitoring, asbestos exposure, pleural plaques and respiratory symptoms, and found that all were of low value as predictors of risk of mesothelioma. Nor have various biomarkers proven useful in predicting mesothelioma (Filiberti et al., 2014; Gube et al., 2011; Imperatori et al., 2013), although some may have promise (Hirohashi et al., 2014). Robinson & Lake (2005) discuss various indicators with diagnostic relevance but these present no useful lead time as a predictor. Given the present state of the art, therefore, it will be necessary to continue the ongoing RCF mortality study to provide definitive evidence regarding the development of mesothelioma.
Acknowledgements
The responsibility for errors and omissions rests with the authors, who are solely responsible for the findings and conclusions expressed herein. It is also appropriate to acknowledge the constructive comments of the anonymous reviewers of this manuscript in draft. Their comments were relevant and improved the clarity and comprehensiveness of this work.
Appendix
Table A1.
Mesothelioma latency as reported in various studies and review articles listed in chronological order.
| Mean (Years) | Median (Years) | Range | Cohort | Source |
|---|---|---|---|---|
| NR | NR | NR | Article claims that latency period is approximately 35 years. | Selikoff et al. (1965, as cited in Banaei et al., 2000) |
| 24–36 | Canadian gas mask assembly workers. Study reports ranges from other studies as 20–35 years and 6–44 years. | McDonald & McDonald (1979) | ||
| 32.3 | 14–57 | 144 workers with mesothelioma in UK factories using asbestos in manufacturing and insulation. | Browne & Smither (1983) | |
| 37 | 19–68 | Cancer registry of Norway. | Mowé et al. (1984) | |
| 35–40 | Study of mesothelioma in Great Britain in 1968–1983 concludes that the median latency is 35–40 years. | Jones et al. (1988) | ||
| 32 | 11 to >50 | 1105 workers in 24 cohorts summarized in this review; latency exceed 20 years in 96% of all cases. | Lanphear & Buncher (1992); see also Weill et al. (2004) | |
| 33 | 24–43 | Shipyard workers in Sweden | Sandén et al. (1992) | |
| 48.1 | Cohort from Uppsala, Sweden | Hillerdal (1994) | ||
| 30–40 | Inhabitants of the Metsovo area, north-west Greece | Sakellariou et al. (1996) | ||
| 41.4 | <30 | 15–67 | 168 mesothelioma cases in south east England | Yates et al. (1997) |
| NR | NR | NR | Review article, claims that “extensive research revealed a latency period of 30–45 years in most cases”. | Baas et al. (1998) |
| 37.4 | 4–66 | Different groups in Australia | Yeung et al. (1999) | |
| 39.7 | 42 | 17–60 | Three cases in UK | Attanoos et al. (2000) |
| 48.8 | 51.0 | 14–75 | 380 cases of malignant pleural mesothelioma in the Trieste–Monfalcone area, 1968–2000. | Bianchi et al. (2001) |
| >40 | Mean latency times greater than 40 years among subjects occupationally exposed to asbestos were reported by the French Mesothelioma Registry. | Desoubeaux (2001, as cited in Marinaccio et al., 2005) | ||
| Latency reported to be 40–50 years for asbestos related mesothelioma in Japan. | Morinaga et al. (2001) | |||
| NR 37.6 | 43.0 NR | 20–49 NR | Review article covers two small occupationally exposed Japanese cohorts. | Morinaga et al. (2001) |
| 37.8 | 11–68 | 821 cases from German mesothelioma register. | Neumann et al. (2001) | |
| ∼39 | 11 to >50 | 800 among 1517 cases of mesothelioma from various cohorts. | Suzuki (2001) | |
| 44.9 51.0 12.3 | Workers in South African mines Crocidolite miners Amosite miners Miners exposed to both crocidolite and amosite | Carbone et al. (2002) | ||
| 56 both sexes | Turkish cohort living in a rural area. | Metintas et al. (2002) | ||
| 48.5 | 301 cases of mesothelioma between 1979 and 1999 in workers from the Devonport Naval Dockyard; the mean was lower (42 years) among more heavily exposed trades. | Hilliard et al. (2003) | ||
| 46 SD 11 years | 22 mesothelioma cases in Hong Kong. | Chang et al. (2006) | ||
| 10 cases of mesothelioma among patients <40 years old | Kane et al. (2006) | |||
| 48.8 29.6 | 19 51 29 | 14–75 28–32 | Review article covers several cohorts 400 pleural mesotheliomas in Italy Insulation workers in Italian cohort | Bianchi & Bianchi (2007, and references therein) |
| 52.6 | 53.0 | 32–64 | 215 cases of malignant pleural mesothelioma were diagnosed at the Hospital of Monfalcone, Italy. | Bianchi et al. (2007) |
| 43.7 men 42.8 women | 1941 cases of mesothelioma (pleural and peritoneal) in New South Wales, Australia. | Hyland et al. (2007) | ||
| 44.6 | Italian register | Marinaccio et al. (2007) | ||
| 34.8 48.7 55.3 37.1 46.0 50.8 44.9 | 33.0 51.0 56.0 33.0 47.5 55.0 46.0 | 27–49 13–73 35–71 25–60 28–69 27–62 25–64 | 801 pleural mesotheliomas diagnosed in hospitals in the Trieste and Monfalcone districts of Italy. Latency estimates varied with cohort as shown below: Insulation Shipbuilding Maritime trades Port activities Other industries Domestic exposure Other | Bianchi & Bianchi (2009) |
| 8.5 | 8.5 | Case report of single bystander exposed to a site at which asbestos-containing materials were being dismantled | Bitchatchi et al. (2010) | |
| 36.9 | <10 to >60 | 679 cases from GB Asbestosis and Mesothelioma Registers | Harding & Darnton (2010) | |
| 36.8 | Former workers and residents exposed to crocidolite at Wittenoom, Western Australia. | Aboagye-Sarfo et al. (2011) | ||
| 48.5 | 18–70 | 238 cases of malignant mesothelioma for which latency was estimated for 191 cases. | Haber & Haber (2011) | |
| 36.9 39.8 43.7 39.7 33.1 | Estimates given for various cohorts: Wittenoom workers 95% CI (31.4–42.3) Other asbestos workers 95% CI (34.3–45.2) Wittenoom residents 95% CI (38.0–49.5) Other non-occupational 95% CI (33.9–45.6) Home renovators 95% CI (27.5–38.8) | Olsen et al. (2011) | ||
| 48.3 | 25–68 | Shipyard workers in Monfalcone | Bianchi & Bianchi (2012) | |
| NR | 43 | 13–81 | 929 clinically confirmed deaths due to mesothelioma | Gemba et al. (2012) |
| NR | 22.8 | NR | 614 mesotheliomas deaths (between 1978 and 2005) among asbestos workers in the UK; latency approximately 29% longer for females compared to males. Median latencies vary among other groups from 8.2 to 34 years | Frost (2013) |
NR = not reported.
Table A2.
Studies of cancer in workers exposed to rock (stone) and wool and slag wool.
| Reference, plants | Description, employment, follow-up | No. of deaths, cases (controls), type of cancer | Exposure categories | No. of cases | Relative risks (95% CI) | Comments |
|---|---|---|---|---|---|---|
| As reported in IARC (2002). USA (University of Pittsburgh) | ||||||
| Cohort studies | ||||||
| Marsh et al. (1990) 6 plants | 1846 male workersa employed 1945–1963, follow-up 1946–1985 | 73 deaths from respiratory cancer | Time since first employment <10 years 10–19 years 20–29 years ≥30 years Duration of employment <10 years 10–19 years 20–29 years ≥30 years | 2 13 24 34 38 15 11 9 | SMR 1.36 [1.06–1.71] 0.89 1.56 1.37 1.32 1.43 [1.01–1.96] 1.46 1.18 1.18 | Local rates Local rates |
| Marsh et al. (1996) 5 plants 1 plant | N-cohort (cohort participating in the new program): 3035 male and female workersa employed 1945–1978 O-cohort (cohort from the original plant): 443 male workersa employed 1945–1963 | 71 deaths from respiratory cancer (68 in men) 32 deaths from respiratory cancer | Time since first employment N-cohort (men only) <10 years 10–19 years 20–29 years ≥30 years O-cohort <20 years 20–29 years ≥30 years | 2 13 23 30 3 8 21 | SMR 0.58 1.22 1.35 1.06 0.95 [0.20–2.78] 1.41 [0.61–2.78] 1.71 [1.06–2.61] | Local rates Asbestos exposure |
| Marsh et al. (1996) 1 plant | Follow-up until 1989 | Duration of employment N-cohort (men only) <10 years 10–19 years 20–29 years ≥30 years O-cohort <10 years 10–19 years ≥20 years | 39 15 8 6 15 7 10 | SMR 1.14 1.34 1.07 0.89 1.32 2.02 1.61 | ||
| Nested case–control study | ||||||
| Marsh et al. (1996) 5 plants | N-cohort 54 deathsa from respiratory cancer (men) 54 deaths from respiratory cancer (men) | 107 male controls 101 male controls | Cumulative exposure to respiratory fibers <3 fibers/cm3-months 3–14 fibers/cm3-months 15–39 fibers/cm3-months ≥40 fibers/cm3-months <3 fibers/cm3-months 3–14 fibers/cm3-months 15–39 fibers/cm3-months ≥40 fibers/cm3-months | Odds ratio 1.0 0.70 0.59 0.71 1.0 0.64 0.55 0.58 | Unadjusted for smoking p for linear trend = 0.76 Adjusted for smoking p for linear trend = 0.64 | |
| USA | ||||||
| Nested case–control study | ||||||
| Duration of employment | Odds ratio | |||||
| 1 plant | O-cohort 24 deathsa from respiratory cancer (men) 1970–1989 18 deaths from respiratory cancer | 47 controls 31 controls | <2 years 2–4 years 5–19 years ≥20 years <2 years 2–4 years 5–19 years ≥20 years | 1.0 1.62 0.23 0.85 1.0 1.82 0.33 0.73 | Smokers only p for linear trend = 0.21 Smokers only p for linear trend = 0.47 | |
| Odds ratio | NIOSH exposure classification | |||||
| Wong et al. (1991) 9 plants slag wool workers (4 plants also in Marsh et al., 1990, 1996) | 55 men who died from lung cancera 1970–1989 | 98 male controls who had died from other causes | Exposed/unexposed Exposed ≥ 7 fibers/cm3-months Exposed < 7 fibers/cm3-months | 50 27 | 0.90 (0.23–3.49) 0.94 (0.23–3.78) 0.86 (0.42–1.79) 0.98 (0.47–2.04) | Unadjusted for smoking Adjusted for smoking Unadjusted for smoking Adjusted for smoking |
| European study | ||||||
| Cohort study | ||||||
| SMR | ||||||
| Plato et al. (1995) Sweden 2 plants (included in Boffetta et al., 1997) | 1569 male and female workers employeda before 1978, follow-up 1952–1990 for mortality | 13 deaths from lung cancer | Duration of employment with 20-year lag: <2 years 2–9 years 10–19 years ≥20 years Plant-specific cumulative fiber exposure (fibers/cm3-years): <1 1–2 >2 | 1 5 1 2 7 4 2 | [1.57 (0.83–2.68)] [1.02 (0.55–1.75)] 1.10 (0.28–6.12) 2.69 (0.87–6.27) 0.87 (0.02–4.89) 1.43 (0.17–5.16) SMR 2.01 (0.81–4.13) 2.45 (0.67–6.21) 0.62 (0.08–2.24) | Local rates National rates Local rates |
| Plato et al. (1995) Sweden 2 plants (included in Boffetta et al., 1997) | Follow-up 1958–1989 for incidence | 13 cases of lung cancer 13 cases of stomach cancer | Duration of employment: <2 years 2–9 years 10–19 years ≥20 years | 1 7 3 2 | SIR 0.69 (0.02–3.84) 2.12 (0.85–4.37) 1.63 (0.34–4.76) 1.61 (0.20–5.83) SIR 1.71 (0.91–2.93) | Local rates Local rates |
| Boffetta et al. (1997) 7 plants Denmark, Germany, Norway and Sweden Mortality study | 4912 male and female workersa employed 1933–1977, follow-up until 1990–1991 | 97 deaths from lung cancer | Time since first employment: ≤9 years 10–19 years 20–29 years ≥30 years Duration of employment 1–4 years 5–9 years 10–19 years ≥20 years Technological phase Late Intermediate Early | 10 26 29 32 31 21 21 24 76 12 9 | SMR 1.34 (1.08–1.63) Relative riskb 1.0 1.3 (0.6–3.0) 1.2 (0.5–3.1) 1.4 (0.4–4.6) 1.0 1.4 (0.8–2.4) 1.0 (0.5–1.8) 1.6 (0.8–3.1) 1.0 1.0 (0.5–2.3) 1.1 (0.4–2.8) | National rates Adjusted for age, calendar year, country, technological phase and duration of employment. p for linear trend = 0.67 Adjusted for age, calendar year, country, technological phase and time since first employment. p for linear trend = 0.27 Adjusted for age, calendar year, country, duration of employment and time since first employment. |
| Boffetta et al. (1997) 7 plants Denmark, Germany, Norway and Sweden Mortality study | 8 deaths from oral cancer +cancer of the pharynx; 6 deaths from cancer of the larynx; 8 deaths from cancer of the oesophagu | SMR 1.33 (0.57–2.61) 1.96 (0.72–4.27) 1.25 (0.54–2.46) | National rates | |||
| Consonni et al. (1998) Denmark, Germany, Norway and Sweden 7 plants | 9603 male workers employed until 1977 follow-up until 1990–1991 | 159 deaths from lung cancer 97 deaths from lung cancer in workers with ≥1 year of employment | Cumulative exposure ≤0.007 fiber/cm3-years 0.008–0.136 fiber/cm3-years 0.137–1.367 fiber/cm3-years >1.368 fibers/cm3-years ≤0.139 fiber/cm3-years 0.140–0.729 fiber/cm3-years 0.730–2.622 fibers/cm3-years >2.622 fibers/cm3-years | 39 40 40 40 25 24 24 24 | Relative riskb 1.0 1.3 (0.8–2.4) 1.2 (0.7–2.1) 1.5 (0.7–3.0) 1.0 0.9 (0.4–2.0) 0.8 (0.3–1.9) 1.0 (0.4–2.7) | Adjusted for age, calendar period, country, time since first employment and employment status. p for linear trend = 0.4 p for linear trend = 1.0 |
| Boffetta et al. (1999) | 3685 male and female workersa employed 1933–1977 follow-up 1994–1995 | 73 cases of lung cancer 31 cases of cancer of the oral cavity, pharynx or larynx | Time since first employment: ≤9 years 10–19 years 20–29 years ≥30 years Duration of employment (15-year lag) 1–4 years 5–9 years 10–19 years ≥20 years Technological phase Late Intermediate Early | 7 21 25 20 33 11 10 5 50 14 9 | SIR 1.08 (0.85–1.36) Relative riskb 1.0 1.8 (0.7–4.7) 2.4 (0.9–6.8) 3.0 (0.8–10.5) 1.0 1.0 (0.5–2.1) 1.2 (0.5–2.6) 2.0 (0.7–6.2) 1.0 0.8 (0.4–1.7) 0.8 (0.3–2.0) SIR 1.46 (0.99–2.07) | National rates Adjusted for ages, gender, country and technological phase p for linear trend = 0.1 Adjusted for age, gender, country, technological phase and time since first employment p for linear trend = 0.4 Adjusted for age, gender, country and time since first employment p for linear trend = 0.5 National rates |
| Post-IARC (2002). | ||||||
| Case–control study | ||||||
| Kjaerheim et al. (2002) 7 plants | 133 cases of lung cancer, rock (stone) wool/slag wool male workers employed 1937–1976, follow-up 1971–1996 | 513 male controls | Cumulative fiber exposure in quartiles All workers quartile 1 quartile 2 quartile 3 quartile 4 Workers employed >1 year quartile 1 quartile 2 quartile 3 quartile 4 Cumulative fiber exposure, in quartiles, lagged 15 years All workers quartile 1 quartile 2 quartile 3 quartile 4 Workers employed >1 year quartile 1 quartile 2 quartile 3 quartile 4 | 33 32 33 34 12 3 26 34 36 36 30 30 23 5 18 29 | Odds ratio 1.0 0.86 (0.47–1.56) 0.91 (0.51–1.63) 0.51 (0.28–0.93) 1.0 2.08 (0.36–11.91) 0.85 (0.34–2.15) 0.52 (0.21–1.30) 1.0 1.25 (0.66–2.34) 1.02 (0.54–1.93) 0.67 (0.35–1.27) 1.0 2.00 (0.41–9.83) 0.76 (0.27–2.17)\⊂ 0.63 (0.28–1.42) | Although after the IARC meeting, this was included in the IARC Monograph. Adjusted for age, country and tobacco smoking. p for linear trend = 0.04 p for linear trend = 0.11 p for linear trend = 0.17 p for linear trend = 0.19 |
| Kjaerheim et al. (2002) | Duration of exposure in rock (stone)/slag wool industry All workers Unexposed 1 year 2–6 years 7–40 years Workers employed >1 year Unexposed 1 year 2–6 years 7–40 years Duration of exposure in rock (stone)/slag wool industry, lagged 15 years All workers Unexposed 1 year 2–5 years 6–39 years Workers employed >1year Unexpected 1 year 2–5 years 6–39 years | 7 58 32 35 6 2 32 35 28 51 28 25 18 4 28 25 | Odds ratio 1.0 1.24 (0.47–3.26) 0.86 (0.32–2.31) 0.85 (0.32–2.26) 1.0 0.51 (0.05–5.50) 0.60 (0.17–2.08) 0.65 (0.20–2.12) 1.0 1.57 (0.82–2.99) 1.20 (0.61–2.34) 0.97 (0.48–2.00) 1.0 1.06 (0.21–5.30) 1.01 (0.44–2.34) 0.98 (0.39–2.47) | p for linear trend = 0.23 p for linear trend = 0.63 p for linear trend = 0.15 p for linear trend = 0.96 | ||
| Baccarelli et al. (2006) | Lifetime job-specific exposure measurements were available for 15 organic, 15 man-made and 28 natural-inorganic agents | 540 lung cancer cases and 582 controls from the 1993–1998 autopsy records. | Various exposure categories including those exposed to MMVFs | 1.82 (0.88–3.75) | All MMVFs pooled, RSW not included as specific category. | |
| Carel et al. (2007) | Multi-center case-control study of lung cancer and exposure to asbestos and MMVFs | 16 centers in six Central and Eastern European countries and the UK during the period 1998–2002, 115 cases and 89 controls exposed to MMVF | Low exposure intensity Medium High Year of first exposure ∼1960 1961–1970 1971–1980 1981–1990 1991– Overall | 73 34 8 45 30 25 11 4 | 1.23 (0.82–1.84 1.28 (0.70–2.34) 1.02 (0.31–3.33) 1.36 (0.80–2.31) 1.09 (0.60–1.97) 1.05 (0.54–2.03) 1.64 (0.54–4.96) 1.57 (0.25–9.88) 1.23 (0.88–1.71) | All MMVFs pooled. |
| Pintos et al. (2008) | Two case control studies in Montreal, Canada studying effects of exposure on lung cancer | 1144 controls/922 cases in cohort 1; 978 controls/809 cases in study 2 | Non-exposed Any exposure Non-substantial level Substantial level | 1425 153 129 24 | 1.00 1.05 (0.80–1.40) 1.10 (0.81–1.49) 0.86 (0.45–1.63) | All MMVFs pooled. Figures at left refer to both cohorts pooled. |
| Lacourt et al. (2013) | Cases came from hospital-based case-control study performed between 1987 and 1996 or identified through the French National Mesothelioma Surveillance Program. | Cases and controls varied with type of exposure studied; table at right is one of several related to MMVF exposure | Not exposed Exposed Exposure duration (years) 0–7 7–18 18–32 >32 | 474 725 144 173 164 244 | 1.00 1.8 (1.5–2.3) 1.6 (1.2–2.1) 1.8 (1.3–2.4) 1.3 (1.0–1.9) 3.5 (2.5–4.9) | Study considered exposure to asbestos, MMVF and silica. Exposure to all MMVFs pooled. See critical comments by Bonde (2013). |
SMR, standardized mortality ratio; SIR, standardized incidence ratio; respiratory cancer, ICD-8, 160–163.
Employed for ≥ 1 year.
Poisson regression analysis.
Footnotes
Declaration of interest The research for this work was sponsored by Unifrax 1 LLC, a company that produces a range of high temperature insulating wool materials.
1When administered in various animal tests this traditional rock wool was referred to as MMVF21. In the early 2000s, Rockwool International developed a less biopersistent fiber called HT wool (Kamstrup et al., 2002). Lower biopersistence rock wool has replaced traditional rock wool in Europe and Japan and is displacing traditional rock wool elsewhere. Rock/stone wool (included under CAS # 65997-17-3) also included under mineral wool is produced/sold in the United States according to various extant safety data sheets that specifically reference this CAS # and is also produced elsewhere in the world (e.g. China, Colombia and Pakistan) according to manufacturers’ literature. HT-stone wool, the more soluble product has a different chemical abstracts service number (CAS # 287922-11-6).
2Krombach et al. (1997) estimate the average size of a human macrophage is approximately 21.2 ± 0.3 µm.
3These rules count all structures with length ≥5 microns, diameter ≤3 microns and aspect ratio (ratio of length to diameter) ≥5:1 as respirable fibers. WHO conventions use an aspect ratio cutoff of 3:1.
4A different positive control was used in these studies. The RCF study used chrysotile asbestos as a positive control, whereas the MMVF21 study used crocidolite asbestos.
5At the high dose, WHO aerosol concentrations were 243, 213 and 187 f/cc for rock wool, stone wool and RCF, respectively.
6This study was actually published after the IARC Working Group meeting, but was available to the authors of the IARC Monograph and was included in the 2002 Monograph.
7These are potassium octatitanate fibres.
Supplementary material available online
References
- Aboagye-Sarfo P, Reid A, de Klerk N, et al. Determinants of latency periods of lung cancer (LC) and malignant mesothelioma (MM) in former workers and residents exposed to crocidolite at Wittenoom Western Australia. Abstract from the ATS International Conference published in AJRCCM, C53. Lung disease due to asbestos. 2011;2011:A4812. [Google Scholar]
- ACC. ACC Review: Asbestos-related disease. 2004. http://hazelarmstronglaw.co.nz/beta/wp-content/uploads/2012/10/ACC-Review-Asbestos-related-disease.pdf [Google Scholar]
- Agence Française de Sécurité Sanitaire de l’Environment et du Travail (AFSSET) Saisine 2004/012 Rapport Final Relatif aux Fibres. 2007. Les fibres minérales artificielles, evaluation de l’exposition de la population générale et des travailleurs; p. 290.http://www.afssa.fr/ET/DocumentsET/fibres_minerales_artificielles_siliceuses_2007.pdf [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological Profile for Synthetic Vitreous Fibers. Atlanta, Georgia: 2004. p. 332.http://www.atsdr.cdc.gov/toxprofiles/tp161.pdf [PubMed] [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological Profile for Asbestos. Atlanta, Georgia: 2001. p. 441.http://www.atsdr.cdc.gov/toxprofiles/tp61.pdf [PubMed] [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR) Asbestos toxicity physiologic effects. 2000. http://www.atsdr.cdc.gov/asbestos/asbestos/health_effects/ [Google Scholar]
- Ameille J. The different pleuro-pulmonary pathologies related to asbestos: definitions, epidemiology and evolution. Rev Mal Respir. 2012;29:1035–46. doi: 10.1016/j.rmr.2012.02.012. [DOI] [PubMed] [Google Scholar]
- Ameille J, Brochard P, Letourneux M. Asbestos-related cancer risk in patients with asbestosis or pleural plaques. Rev Mal Respir. 2011;28:e11–17. doi: 10.1016/j.rmr.2011.04.008. [DOI] [PubMed] [Google Scholar]
- Assuncao J, Corn M. The effects of milling on diameters and lengths of fibrous glass and chrysotile asbestos fibers. Am Ind Hyg Assoc J. 1975;36:811–19. doi: 10.1080/0002889758507347. [DOI] [PubMed] [Google Scholar]
- Attanoos RL, Suvarna SK, Rhead E, et al. Malignant vascular tumours of the pleura in “asbestos” workers and endothelial differentiation in malignant mesothelioma. Thorax. 2000;55:860–3. doi: 10.1136/thorax.55.10.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas P, Schouwink H, Zoetmulder FAN. Malignant pleural mesothelioma. Ann Oncol. 1998;9:139–49. doi: 10.1023/a:1008239116237. [DOI] [PubMed] [Google Scholar]
- Baccarelli A, Khmelnitskii O, Tretiakova M, et al. Risk of lung cancer from exposure to dusts and fibers in Leningrad Province, Russia. Am J Ind Med. 2006;49:460–7. doi: 10.1002/ajim.20316. [DOI] [PubMed] [Google Scholar]
- Banaei A, Auvert B, Goldberg M, et al. Future trends in mortality of French men from mesothelioma. Occup Environ Med. 2000;57:488–94. doi: 10.1136/oem.57.7.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks DE, Dedhia HV. Jindal SK, Shankar P, Raoof S, Gupta D, Aggarwal A. Textbook of pulmonary and critical care medicine. 1st. New Delhi, India: Jaypee Brothers Medical Publishers; 2011. Chapter 110 – the health risks of asbestos exposure inhalation; pp. 1352–66. [Google Scholar]
- Berman DW, Crump KS. A meta-analysis of asbestos-related cancer risk that addresses fiber size and mineral type. Crit Rev Toxicol. 2008;38:49–73. doi: 10.1080/10408440802273156. [DOI] [PubMed] [Google Scholar]
- Berman DW, Crump KS, Chatfield EJ, et al. The sizes, shapes, and mineralogy of asbestos structures that induce lung tumors or mesothelioma in AF/HAN rats following inhalation. Risk Anal. 1995;15:181–95. doi: 10.1111/j.1539-6924.1995.tb00312.x. [DOI] [PubMed] [Google Scholar]
- Bernstein DM. Synthetic vitreous fibers: a review toxicology, epidemiology and regulations. Crit Rev Toxicol. 2007;37:839–86. doi: 10.1080/10408440701524592. [DOI] [PubMed] [Google Scholar]
- Bernstein DM, Hoskins JA. The health effects of chrysotile: current perspective bases upon recent data. Reg Toxicol Pharm. 2006;45:252–64. doi: 10.1016/j.yrtph.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Bernstein DM, Chevalier J, Smith P. Comparison of Calidria chrysotile asbestos to pure tremolite: inhalation biopersistence and histopathology following short-term exposure. Inhal Toxicol. 2003;15:1387–419. doi: 10.1080/08958370390248888. [DOI] [PubMed] [Google Scholar]
- Bernstein DM, Sintes JMR, Ersboell BK, Kunert J. Biopersistence of synthetic mineral fibers as a predictor of chronic inhalation toxicity in rats. Inhal Toxicol. 2001a;13:823–49. doi: 10.1080/089583701752378133. [DOI] [PubMed] [Google Scholar]
- Bernstein DM, Sintes JMR, Ersboell BK, Kunert J. Biopersistence of synthetic mineral fibers as a predictor of chronic intraperitoneal injection tumor response in rats. Inhal Toxicol. 2001b;13:851–75. doi: 10.1080/089583701752378142. [DOI] [PubMed] [Google Scholar]
- Bernstein DM. Data analysis of IT and INH biopersistence data. Joint Research Centre, Environment Institute, European Chemicals Bureau; Ispra, Italy: 1997a. p. 15. [Google Scholar]
- Bernstein DM. Correlation between short term biopersistence and chronic toxicity studies. Joint Research Centre, Environment Institute, European Chemicals Bureau; Ispra, Italy: 1997b. p. 67. [Google Scholar]
- Bernstein DM, Morscheidt C, de Meringo A, et al. The biopersistence of fibres following inhalation and intratracheal instillation exposure. Ann Occup Hyg. 1997;41:224–30. [Google Scholar]
- Bernstein DM, Morschedit C, Grimm H-G, et al. Evaluation of soluble fibers using the inhalation biopersistence model, a nine-fiber comparison. Inhal Toxicol. 1996;8:345–85. [Google Scholar]
- Berry C. Models for mesothelioma incidence following exposure to fibers in terms of timing and duration of exposure and the biopersistence of fibers. Inhal Toxicol. 1999;11:111–30. doi: 10.1080/089583799197203. [DOI] [PubMed] [Google Scholar]
- Bianchi C, Bianchi T. Mesothelioma among shipyard workers in Monfalcone, Italy. Indian J Occup Environ Med. 2012;16:119–23. doi: 10.4103/0019-5278.111753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi C, Bianchi T. Malignant pleural mesothelioma in Italy. Indian J Occup Environ Med. 2009;13:80–3. doi: 10.4103/0019-5278.55124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi C, Bianchi T. Malignant mesothelioma: global incidence and relationship with asbestos. Ind Health. 2007;45:379–87. doi: 10.2486/indhealth.45.379. [DOI] [PubMed] [Google Scholar]
- Bianchi C, Bianchi T, Ramani L. Malignant mesothelioma of the pleura and other malignancies in the same patient. Tumori. 2007;93:19–22. doi: 10.1177/030089160709300104. [DOI] [PubMed] [Google Scholar]
- Bianchi C, Brollo A, Ramani L, et al. Asbestos exposure in malignant mesothelioma of the pleura: a survey of 557 cases. Ind Health. 2001;39:161–7. doi: 10.2486/indhealth.39.161. [DOI] [PubMed] [Google Scholar]
- Bitchatchi E, Kayser K, Perelman M, Richter ED. Mesothelioma and asbestos in a young woman following occupational asbestos exposure: short latency and long survival: case report. Diag Pathol. 2010;5:81–4. doi: 10.1186/1746-1596-5-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boffetta P, Donaldson K, Moolgavkar S, Mandel JS. A systematic review of occupational exposure to synthetic vitreous fibers and mesothelioma. Crit Rev Toxicol. 2014;44:436–49. doi: 10.3109/10408444.2014.899558. [DOI] [PubMed] [Google Scholar]
- Boffetta P, Andersen A, Hansen J, et al. Cancer incidence among European man-made vitreous fibre production workers. Scand J Work Environ Health. 1999;25:222–6. doi: 10.5271/sjweh.427. [DOI] [PubMed] [Google Scholar]
- Boffetta P, Saracci R, Andersen A, et al. Cancer mortality among man-made vitreous fibre production workers. Epidemiology. 1997;8:259–68. doi: 10.1097/00001648-199705000-00006. [DOI] [PubMed] [Google Scholar]
- Bolton RE, Davis JMG, Miller B, et al. The effect of dose of asbestos on mesothelioma production in the laboratory rat. Proceedings of the 6th International Pneumonoconiosis Conference. 1984;2:1028–35. [Google Scholar]
- Bolton RE, Davis JMG, Donaldson K, Wright A. Variation in the carcinogenicity of mineral fibres. Ann Occup Hyg. 1982;26:569–82. [PubMed] [Google Scholar]
- Bonde JP. No indication that mineral wool causes mesothelioma. Am J Respir Crit Care Med. 2013;188:873. doi: 10.1164/rccm.201304-0655LE. [DOI] [PubMed] [Google Scholar]
- British Lung Foundation. (2011). Pleural Plaques., Online report, version 2.2, May 2011. Hosted on the British Lung Foundation. Available at http://www.blf.org.uk/Conditions/Detail/Pleural-plaques .
- Brown RC, Bellmann B, Muhle H, et al. Survey of the biological effects of refractory ceramic fibres: overload and its possible consequences. Ann Occup Hyg. 2005;49:295–307. doi: 10.1093/annhyg/meh098. [DOI] [PubMed] [Google Scholar]
- Browne K, Smither WJ. Asbestos-related mesothelioma: factors discriminating between pleural and peritoneal sites. Br J Ind Med. 1983;40:145–52. doi: 10.1136/oem.40.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burge PS, Calvert IA, Trethowan WN, Harrington JM. Are the respiratory health effects found in manufacturers of ceramic fibers due to the dust rather than the exposure to fibers? Occup Environ Med. 1995;52:105–9. doi: 10.1136/oem.52.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campopiano A, Zakrewska A, Olori A, et al. Glass fiber exposure assessment during ceiling installation by European Standard EN 689: study of airborne fiber distribution. Atmos Pollut Res. 2012;3:192–8. [Google Scholar]
- Carbone M, Kratzke RA, Testa JR. The pathogenesis of mesothelioma. Semin Oncol. 2002;29:2–17. doi: 10.1053/sonc.2002.30227. [DOI] [PubMed] [Google Scholar]
- Carel R, Olsson AC, Zarisze D, et al. Occupational exposure to asbestos and man-made vitreous fibres and risk of lung cancer: a multicenter case-control study in Europe. Occup Environ Med. 2007;64:502–8. doi: 10.1136/oem.2006.027748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang KC, Leung CC, Tam CM, et al. Malignant mesothelioma in Hong Kong. Respir Med. 2006;100:75–82. doi: 10.1016/j.rmed.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Cheng Y-S. Bivariate lognormal distribution for characterizing asbestos fiber aerosols. Aerosol Sci Tech. 1986;5:359–68. [Google Scholar]
- Cherrie J, Dodgson J, Groat S, Maclaren W. Environmental surveys in the European man-made mineral fiber production industry. Scand J Work Environ Health. 1986;12:18–25. [PubMed] [Google Scholar]
- Christensen VR, Eastes W, Hamilton RD, Struss AW. Fiber diameter distributions in typical MMVF wool insulation products. Am Ind Hyg Assoc J. 1993;54:232–8. [Google Scholar]
- Consonni D, Boffetta P, Andersen A, et al. Lung cancer mortality among European rock/slag wool workers: exposure-response analysis. Cancer Causes Control. 1998;9:411–16. doi: 10.1023/a:1008871718323. [DOI] [PubMed] [Google Scholar]
- Corn M, Hammad Y, Whittier D, Kotsko N. Employee exposure to airborne fiber and total particulate matter in two mineral wool facilities. Environ Res. 1976;12:59–74. doi: 10.1016/0013-9351(76)90009-8. [DOI] [PubMed] [Google Scholar]
- Cowie HA, Wild P, Beck J, et al. An epidemiological study of the respiratory health of workers in the European refractory ceramic fibre (RCF) industry. Occup Environ Med. 2001;58:800–10. doi: 10.1136/oem.58.12.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crapo J. (2005). Testimony: A fair and efficient system to resolve claims of victims for bodily injury caused by asbestos, and for other purposes. Written Statement of Dr. James D. Crapo, Professor of Medicine, National Jewish Medical and Research Center and University of Colorado Health Sciences Center Before the Senate Committee on the Judiciary Concerning S.852, “FAIR Act of 2005”. April 26, 2006. 10 p.
- Davis JM, Jones JD. Comparison of the pathogenicity of long and short fibres of chrysotile asbestos in rats. Br J Exp Pathol. 1988;69:717–37. [PMC free article] [PubMed] [Google Scholar]
- Davis JMG, Addison J, Bolton RE, et al. The pathogenicity of long versus short fiber samples of amosite asbestos administered to rats by inhalation and intraperitoneal injection. Br J Exp Pathol. 1986;67:415–30. [PMC free article] [PubMed] [Google Scholar]
- Davis JMG, Addison J, Bolton RE, et al. Biological effects of man-made mineral fibers. Vol. 2. Copenhagen: World Health Organization, Regional Office for Europe; 1984. The pathogenicity of fibrous ceramic aluminum silicate glass administered to rats by inhalation or peritoneal injection; pp. 303–22. [Google Scholar]
- Dement JM. Overview: workshop on fiber toxicology research needs. Environ Health Perspect. 1990;88:261–8. doi: 10.1289/ehp.9088261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desoubeaux N, Bouvier V, Gervais R, et al. Mesotheliomes malins en Basse-Normandie: analyse descriptive, facteurs pronostiques et survie – une et́ude de population. Rev Epidemiol Sante Publ. 2001;49:523–9. [PubMed] [Google Scholar]
- de Vuyst P, Dumortier P, Swaen GMH, et al. Respiratory health effects of man-made vitreous (mineral) fibres. Eur Respir J. 1995;8:2149–73. doi: 10.1183/09031936.95.08122149. [DOI] [PubMed] [Google Scholar]
- Dodson RF, Atkinson MA, Levin JL. Asbestos fiber length as related to potential pathogenicity: a critical review. Am J Ind Med. 2003;44:291–7. doi: 10.1002/ajim.10263. [DOI] [PubMed] [Google Scholar]
- Donaldson K, Tran CL. An introduction to the short-term toxicity of respirable industrial fibres. Mut Res Fund Mol Mech Mut. 2004;553:5–9. doi: 10.1016/j.mrfmmm.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Downer NJ, Ali NJ, Au-Yong IT. Investigating pleural thickening. BMJ. 2013;346:e8376. doi: 10.1136/bmj.e8376. [DOI] [PubMed] [Google Scholar]
- Eastes W, Hadley JG. Role of fiber dissolution in biological activity in rats. Reg Toxicol Pharm. 1994;20:S104–12. [PubMed] [Google Scholar]
- Eastes W, Hadley JG, Bender JR. Assessing the biological activity of fibers: insights into the role of fiber durability. J Occup Health Safety Aust NZ. 1996;12:381–5. [Google Scholar]
- Edelman DA. Asbestos exposure, pleural plaques and the risk of lung cancer. Int Arch Occup Environ Health. 1988;60:389–93. doi: 10.1007/BF00381385. [DOI] [PubMed] [Google Scholar]
- Environmental Resources Management (ERM) Description and characterization of the ceramic fibres industry of the European Union. Environmental Resources Limited; Brussels, Belgium: 1995. [Google Scholar]
- Esman N, Corn M, Hammad Y, et al. Summary of measurements of employee exposures to airborne dust and fiber in sixteen facilities producing MMMFs. Am Ind Hyg Assoc J. 1979;40:106–17. doi: 10.1080/15298667991429408. [DOI] [PubMed] [Google Scholar]
- Esman N, Sheehan MJ, Corn M, et al. Exposure of employees to manmade vitreous fibers: installation of insulation materials. Environ Res. 1982;28:386–98. doi: 10.1016/0013-9351(82)90137-2. [DOI] [PubMed] [Google Scholar]
- Federal Register. Part II, Department of Labor, Mine Safety and Health Administration, 30 CFR Parts 56, 57, and 71. Asbestos Exposure Limit; Proposed Rule. 2005;70:43950–89. [Google Scholar]
- Filiberti R, Marroni P, Spigno F, et al. Is soluble mesothelin-related protein an upfront predictive marker of pleural mesothelioma? A prospective study on Italian workers exposed to asbestos. Oncology. 2014;86:33–43. doi: 10.1159/000355687. [DOI] [PubMed] [Google Scholar]
- Frost G. The latency period of mesothelioma among a cohort of British asbestos workers (1978–2005) Br J Cancer. 2013;109:1965–73. doi: 10.1038/bjc.2013.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemba K, Fujimoto N, Kato K, et al. National survey of malignant mesothelioma and asbestos exposure in Japan. Cancer Sci. 2012;103:483–90. doi: 10.1111/j.1349-7006.2011.02165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gevenois PA, de Vuyst P. Baert AL, Sartor K. Part of the medical radiology, diagnostic imaging and radiation oncology series. New York: Springer; 2006. Imaging of occupational and environmental disorders of the chest; p. 315. [Google Scholar]
- Gibbs GW, Hwang CY. Wagner JC. Biological effects of mineral fibers. Vol. 30. Copenhagen: IARC Scientific Publications No; 1980. Dimensions of airborne asbestos fibres; pp. 69–78. [PubMed] [Google Scholar]
- Gube M, Taegger D, Weber DG, et al. Performance of biomarkers SMRP, CA125, and CYFRA 21-1 as a potential tumor markers for malignant mesothelioma and lung cancer in a cohort of workers formerly exposed to asbestos. Arch Toxicol. 2011;85:185–92. doi: 10.1007/s00204-010-0580-2. [DOI] [PubMed] [Google Scholar]
- Guber A, Lerman S, Lerman Y, et al. Pulmonary fibrosis in a patient with exposure to glass wool fibers. Am J Ind Med. 2006;49:1066–9. doi: 10.1002/ajim.20394. [DOI] [PubMed] [Google Scholar]
- Guldberg M, Christensen VR, Perander M, et al. Measurement of in-vitro fibre dissolution rate at acidic pH. Ann Occup Hyg. 1998;42:233–43. [Google Scholar]
- Haber SE, Haber JM. Malignant mesothelioma: a clinical study of 238 cases. Ind Health. 2011;49:166–72. doi: 10.2486/indhealth.ms1147. [DOI] [PubMed] [Google Scholar]
- Harber P, Moshenifar Z, Oren A, Lew M. Pleural plaques and asbestos-associated malignancy. J Occup Med. 1987;29:641–4. [PubMed] [Google Scholar]
- Harding A-H, Darnton AJ. Asbestosis and mesothelioma among British asbestos workers (1971–2005) Am J Ind Med. 2010;53:1070–80. doi: 10.1002/ajim.20844. [DOI] [PubMed] [Google Scholar]
- Hauptverband der gewerblichen Berufsgenossenschaften (HVGB) Fasern—Test zur Abschätzung der Biobeständigkeit und zum Verstaubungsverhalten. 1998. p. 369.http://www.dguv.de/ifa/Publikationen/Reports-Download/BIA-Reports-1997-bis-1998/BIA-Report-2-98/index.jsp [Google Scholar]
- Health Council of the Netherlands (DECOS) Refractory ceramic fibres, evaluation of the carcinogenicity and genotoxicity. The Hague: Health Council of the Netherlands; 2011. http://www.gezondheidsraad.nl/sites/default/files/201129_1.pdf [Google Scholar]
- Health Council of the Netherlands (DECOS) Asbestos, risks of environmental and occupational exposure. The Hague: Health Council of the Netherlands; 2010. http://www.gezondheidsraad.nl/sites/default/files/201010E.pdf [Google Scholar]
- Heintz NH, Janssen-Heininger YM, Mossman BT. Asbestos, lung cancers, and mesotheliomas: from molecular approaches to targeting tumor survival pathways. Am J Respir Cell Mol Biol. 2010;42:133–9. doi: 10.1165/rcmb.2009-0206TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesterberg TW, Chase G, Axten C, et al. Biopersistence of synthetic vitreous fibers and amosite asbestos in the rat lung following inhalation. Toxicol Appl Pharmacol. 1998;151:262–75. doi: 10.1006/taap.1998.8472. [DOI] [PubMed] [Google Scholar]
- Hesterberg TW, Miller WC, Musselman RP, et al. Biopersistence of man-made vitreous fibers and crocidolite asbestos in the rat lung following inhalation. Fund Appl Toxicol. 1996;29:267–79. doi: 10.1006/faat.1996.0031. [DOI] [PubMed] [Google Scholar]
- Hesterberg TW, Miiller WC, Mast R, et al. Relationship between lung biopersistence and biological effects of man-made vitreous fibers after chronic inhalation in rats. Environ Health Perspect. 1994;102:133–7. doi: 10.1289/ehp.94102s5133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillerdal G. Presented at the USEPA 2001 Asbestos Health Effects Conference. Oakland, CA: 2001. Radiological changes as markers of environmental exposure and environmental risk of lung cancer and mesothelioma. [Google Scholar]
- Hillerdal G. Pleural plaques: incidence and epidemiology, exposed workers and the general population. Indoor-Built Environ. 1997;6:86–95. [Google Scholar]
- Hillerdal G. Pleural plaques and risk for bronchial carcinoma and mesothelioma. A prospective study. Chest. 1994;105:144–50. doi: 10.1378/chest.105.1.144. [DOI] [PubMed] [Google Scholar]
- Hilliard AK, Lovett JK, McGavin CR. The rise and fall in incidence of malignant mesothelioma from a British Naval Dockyard, 1979–1999. Occup Med. 2003;53:209–12. doi: 10.1093/occmed/kqg051. [DOI] [PubMed] [Google Scholar]
- Hirohashi T, Igarashi K, Abe M, et al. Retrospective analysis of large-scale research screening of construction workers for the early diagnosis of mesothelioma. Mol Clin Oncol. 2014;2:26–30. doi: 10.3892/mco.2013.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang C. Size and shape of airborne asbestos fibres in mines and mills. Br J Ind Med. 1983;40:273–9. doi: 10.1136/oem.40.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyland RA, Ware S, Johnson AR, Yates DH. Incidence trends and gender differences in malignant mesothelioma in New South Wales, Australia. Scand J Work Environ Health. 2007;33:286–92. doi: 10.5271/sjweh.1145. [DOI] [PubMed] [Google Scholar]
- ILSI Working Group. Testing of fibrous particles: short-term assays and strategies. lnhal Toxicol. 2005;17:497–537. doi: 10.1080/08958370591001121. [DOI] [PubMed] [Google Scholar]
- Imperatori A, Castiglioni M, Mortara L, et al. The challenge of prognostic markers in pleural mesothelioma. J Thorac Dis. 2013;5:205–6. doi: 10.3978/j.issn.2072-1439.2013.06.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Industrial Industries Advisory Council (IIAC) Position Paper 23 Pleural Plaques. United Kingdom: 2008. p. 60.http://www.iiac.org.uk/pdf/pos_papers/pp23.pdf [Google Scholar]
- International Agency for Research on Cancer (IARC) Man-made vitreous fibres. 2002;81 http://monographs.iarc.fr/ENG/Monographs/vol81/ [Google Scholar]
- Järvholm B, Hillerdal G, Järliden A-K, et al. Occurrence of pleural plaques in workers with exposure to mineral wool. Int Arch Occup Environ Health. 1995;67:343–6. doi: 10.1007/BF00385650. [DOI] [PubMed] [Google Scholar]
- Jones RN, Hughes JM, Weill H. Asbestos exposure, asbestosis, and asbestos-attributable lung cancer. Thorax. 1996;51:S9–15. doi: 10.1136/thx.51.suppl_2.s9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RD, Smith DM, Thomas PG. Mesothelioma in Great Britain in 1968–1983. Scand J Work Environ Health. 1988;14:145–52. doi: 10.5271/sjweh.1938. [DOI] [PubMed] [Google Scholar]
- Kamstrup O, Ellehauge A, Collier CG, Davis JMG. Carcinogenicity studies after intraperitoneal injection of two types of stone wool fibres in rats. Ann Occup Hyg. 2002;46:135–42. doi: 10.1093/annhyg/mef034. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Chahinian AP, Holland JF. Malignant mesothelioma in young adults. Cancer. 2006;65:1449–55. doi: 10.1002/1097-0142(19900315)65:6<1449::aid-cncr2820650633>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Kauffer E, Martin P, Grzebyk M, et al. Comparison of two direct-reading instruments (FM-7400 and Fibrecheck FC-2) with phase contrast optical microscopy to measure to airborne fibre number concentration. Ann Occup Hyg. 2003;47:413–26. doi: 10.1093/annhyg/meg055. [DOI] [PubMed] [Google Scholar]
- Kjaerheim K, Boffetta P, Hansen J, et al. Lung cancer among rock and slag wool production workers. Epidemiology. 2002;13:445–53. doi: 10.1097/00001648-200207000-00013. [DOI] [PubMed] [Google Scholar]
- Krantz S. Exposure to man-made mineral fibers at ten production plants in Sweden. Scand J Work Environ Health. 1988;14:49–51. [PubMed] [Google Scholar]
- Krombach F, Muncing S, Allmeling AM, et al. Cell size of alveolar machrophages: an interspecies comparison. Environ Health Perspect. 1997;105:1261–3. doi: 10.1289/ehp.97105s51261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo M, Aizawa Y. Biopersistence of rock wool in lungs after short-term inhalation in rats. Inhal Toxicol. 2008;20:137–47. doi: 10.1080/08958370701821375. [DOI] [PubMed] [Google Scholar]
- Lacourt A, Gramond C, Audignon S, et al. Pleural mesothelioma and occupational coexposure to asbestos, mineral wool, and silica. Am J Respir Crit Care Med. 2013;187:977–82. doi: 10.1164/rccm.201210-1911OC. [DOI] [PubMed] [Google Scholar]
- Lanphear BP, Buncher CR. Latent period for malignant mesothelioma of occupational origin. J Occup Med. 1992;7:718–21. [PubMed] [Google Scholar]
- LeMasters GK, Lockey JE, Yiin JH, et al. Mortality of workers occupationally exposed to refractory ceramic fibers. J Occup Environ Med. 2003;45:440–50. doi: 10.1097/01.jom.0000052968.43131.b5. [DOI] [PubMed] [Google Scholar]
- LeMasters GK, Lockey JE, Levin LS, et al. An industry-wide pulmonary study of men and women manufacturing refractory ceramic fibers. Am J Epidemiol. 1998;148:910–19. doi: 10.1093/oxfordjournals.aje.a009717. [DOI] [PubMed] [Google Scholar]
- Letourneux M. Risk assessment of benign asbestosis (dose-effect relationship, time-effect relationship, co-factors) Rev Mal Respir. 1999;16:1270–7. [PubMed] [Google Scholar]
- Lippmann M. Effects of fiber characteristics on lung deposition, retention, and disease. Environ Health Perspect. 1990;88:311–17. doi: 10.1289/ehp.9088311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipworth L, La Vecchia C, Bosetti C, McLaughlin JK. Occupational exposure to rock wool and glass wool and risk of cancers of the lung and the head and neck: a systematic review and meta-analysis. JOEM. 2009;51:1057–87. doi: 10.1097/JOM.0b013e3181b35125. [DOI] [PubMed] [Google Scholar]
- Lockey JE, Roggli VL, Hilbert TJ, et al. Biopersistence of refractory ceramic fiber (RCF) in human lung tissue and a 20-year follow-up of radiographic pleural changes in workers. J Occup Environ Med. 2012;54:781–8. doi: 10.1097/JOM.0b013e31825296fd. [DOI] [PubMed] [Google Scholar]
- Lockey JE, LeMasters GK, Levin L, et al. A longitudinal study of chest radiographic changes of workers in the refractory ceramic fiber industry. Chest. 2002;121:2044–51. doi: 10.1378/chest.121.6.2044. [DOI] [PubMed] [Google Scholar]
- Lockey JE, Levin L, LeMasters GK, et al. Longitudinal estimates of pulmonary function in refractory ceramic fiber manufacturing workers. Am J Respir Crit Care Med. 1998;157:1226–33. doi: 10.1164/ajrccm.157.4.9707131. [DOI] [PubMed] [Google Scholar]
- Lockey J, LeMasters G, Rice C, et al. Refractory ceramic fiber exposure and pleural plaques. Am J Respir Crit Care Med. 1996;154:1405–10. doi: 10.1164/ajrccm.154.5.8912756. [DOI] [PubMed] [Google Scholar]
- Marinaccio A, Binazzi A, Cauzillo G, et al. Analysis of latency time and its determinants in asbestos related malignant mesothelioma cases of the Italian register. Eur J Cancer. 2007;43:2722–8. doi: 10.1016/j.ejca.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Marinaccio A, Montanaro F, Mastrantonio M, et al. Predictions of mortality from pleural mesothelioma in Italy: a model based on asbestos consumption figures supports results from age-period-cohort models. Int J Cancer. 2005;115:142–7. doi: 10.1002/ijc.20820. [DOI] [PubMed] [Google Scholar]
- Marsh GM, Buchanich JM, Youk AO. Fiber glass exposure and human respiratory system cancer risk: lack of evidence persists since 2001 IARC re-evaluation. Regul Toxicol Pharm. 2011;60:84–92. doi: 10.1016/j.yrtph.2011.02.009. [DOI] [PubMed] [Google Scholar]
- Marsh G, Stone R, Youk A, et al. Mortality among United States rock wool and slag wool workers: 1989 Update. J Occup Health Saf Aust NZ. 1996;12:297–312. [Google Scholar]
- Marsh GM, Enterline PE, Stone RA, Henderson VL. Mortality among a cohort of US man-made mineral fiber workers: 1985 Follow-up. J Occup Med. 1990;32:594–604. doi: 10.1097/00043764-199007000-00009. [DOI] [PubMed] [Google Scholar]
- Mast RW, McConnell EE, Anderson R, et al. Studies on the chronic toxicity (inhalation) of four types of refractory ceramic fiber in male Fischer 344 rats. Inhal Toxicol. 1995a;7:425–67. doi: 10.3109/08958379509015208. [DOI] [PubMed] [Google Scholar]
- Mast RW, McConnell EE, Hesterberg TW, et al. Multiple dose chronic inhalation study of size-separated kaolin refractory fiber in male Fischer 344 rats. Inhal Toxicol. 1995b;7:469–502. doi: 10.3109/08958379509015209. [DOI] [PubMed] [Google Scholar]
- Mast RW, Maxim LD, Utell MJ, Walker AM. Refractory ceramic fiber: toxicology, epidemiology, and risk analyses – a review. Inhal Toxicol. 2000a;12:359–99. doi: 10.1080/089583700196103. [DOI] [PubMed] [Google Scholar]
- Mast RW, Yu CP, Oberdörster G, et al. A retrospective review of the carcinogenicity of refractory ceramic fiber in two chronic Fischer 344 rat inhalation studies: an assessment of the MTD and implications for risk assessment. Inhal Toxicol. 2000b;12:1141–72. doi: 10.1080/08958370050198511. [DOI] [PubMed] [Google Scholar]
- Maxim LD, Allshouse J, Fairfax RF, et al. Workplace monitoring of occupational exposure to refractory ceramic fiber – a 17-year retrospective. Inhal Toxicol. 2008;20:289–309. doi: 10.1080/08958370701866040. [DOI] [PubMed] [Google Scholar]
- Maxim LD, Hadley JG, Potter RM, Niebo R. The role of fiber durability/ biopersistence of silica-based synthetic vitreous fibers and their influence on toxicology. Regul Toxicol Pharm. 2006;46:42–62. doi: 10.1016/j.yrtph.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Maxim LD, Kelly WP, Walters T, Waugh R. A multi-year workplace-monitoring program for refractory ceramic fibers. Regul Toxicol Pharm. 1997;26:156–71. doi: 10.1006/rtph.1997.1153. [DOI] [PubMed] [Google Scholar]
- McConnell EE. A science-based paradigm for the classification of synthetic vitreous fibers. Regul Toxicol Pharm. 2000;32:14–21. doi: 10.1006/rtph.2000.1409. [DOI] [PubMed] [Google Scholar]
- McConnell EE, Mast RW, Hesterberg TW, et al. Chronic inhalation toxicity of a kaolin-based refractory ceramic fiber in Syrian golden hamsters. Inhal Toxicol. 1995;7:503–32. doi: 10.3109/08958379509015209. [DOI] [PubMed] [Google Scholar]
- McConnell EE, Kamstrup O, Musselman R, et al. Chronic inhalation study of size-separated rock and slag wool insulation fibers in Fischer 344/N rats. Inhal Toxicol. 1994;6:571–61. [Google Scholar]
- McDonald JC, McDonald AD. Age and latency in mesothelioma. Lancet. 1979;314:1074. doi: 10.1016/s0140-6736(79)92468-1. [DOI] [PubMed] [Google Scholar]
- McKay RT, LeMasters GK, Hilbert TJ, et al. A long term study of pulmonary function among US refractory ceramic fibre workers. Occup Environ Med. 2010;68:89–95. doi: 10.1136/oem.2009.048033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metintas S, Metintas M, Ucgun I, Oner U. Malignant mesothelioma due to environmental exposure to asbestos. Chest. 2002;122:2224–9. doi: 10.1378/chest.122.6.2224. [DOI] [PubMed] [Google Scholar]
- Miller BG, Searl A, Davis JMG, et al. Influence of fibre length, dissolution and biopersistence on the production of mesothelioma in the rat peritoneal cavity. Ann Occup Hyg. 1999;43:155–66. [PubMed] [Google Scholar]
- Moolgavkar SH, Brown RC, Turim J. Biopersistence, fiber length, and cancer risk assessment for inhaled fibers. Inhal Toxicol. 2001a;13:755–72. doi: 10.1080/089583701316941294. [DOI] [PubMed] [Google Scholar]
- Moolgavkar SH, Turim J, Brown RC. The power of the European Union protocol to test for carcinogenicity of inhaled fibers. Regul Toxicol Pharmacol. 2001b;33:350–5. doi: 10.1006/rtph.2001.1474. [DOI] [PubMed] [Google Scholar]
- Moolgavkar SH, Turim J, Brown RC, Luebeck EG. Long man-made fibers and lung cancer risk. Regul Toxicol Pharmacol. 2001c;33:138–46. doi: 10.1006/rtph.2000.1443. [DOI] [PubMed] [Google Scholar]
- Moolgavkar SH, Luebeck EG, Turim J, Brown RC. Lung cancer risk associated with exposure to man-made fibers. Drug Chem Toxicol. 2000;23:223–42. doi: 10.1081/dct-100100112. [DOI] [PubMed] [Google Scholar]
- Morinaga K, Kishimoto T, Sakatani M, et al. Asbestos-related lung cancer and mesothelioma in Japan. Ind Health. 2001;39:65–74. doi: 10.2486/indhealth.39.65. [DOI] [PubMed] [Google Scholar]
- Morrow PE, Haseman JK, Hobbs CH, et al. Workshop overview - the maximum tolerated dose for inhalation bioassays: toxicity vs. overload. Fund Appl Toxicol. 1996;29:155–67. doi: 10.1006/faat.1996.0017. [DOI] [PubMed] [Google Scholar]
- Mowé G, Gylseth B, Hartveit F, Skaug V. Occupational asbestos exposure, lung-fiber concentration and latency time in malignant mesothelioma. Scand J Work Environ Health. 1984;10:293–8. doi: 10.5271/sjweh.2326. [DOI] [PubMed] [Google Scholar]
- Murphy FA, Poland CA, Duffin R, et al. Length-dependent retention of carbon nanotubes in the pleral space of mice initiates sustained inflammation and progressive fibrosis on the parietal pleura. Am J Pathol. 2011;178:2587–600. doi: 10.1016/j.ajpath.2011.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musselman RP, Miiller WC, Eastes W, et al. Biopersistence of man-made vitreous fibers and crocidolite fibers in rat lungs following short-term exposures. Environ Health Perspect. 1994;102:139–43. doi: 10.1289/ehp.94102s5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute for Occupational Safety and Health (NIOSH) Criteria for a recommended standard, occupational exposure to refractory ceramic fibers. Department of Health and Human Services, Centers for Disease Control Prevention, National Institute for Occupational Safety and Health; Atlanta, Georgia: 2006. p. 203. [Google Scholar]
- National Research Council (NRC) Review of the U.S. Navy’s exposure standard for manufactured vitreous fibers, subcommittee on manufactured vitreous fibers, Committee on Toxicology, Board on Environmental Studies and Toxicology, Commission on Life Sciences, National Research Council. Washington, DC; National Academy Press: 2000. [Google Scholar]
- National Toxicology Program (NTP) Draft Report on Carcinogens. Substance Profile for Glass Wool Fibers (Respirable) as a Class. 2010. National Toxicology Program.http://ntp.niehs.nih.gov/ntp/roc/twelfth/2010/drftsubprofiles/gwf20100421.pdf [Google Scholar]
- Neumann V, Günther S, Müller K-M, Fischer M. Malignant mesothelioma - German mesothelioma register 1987–1999. Int Arch Occup Environ Health. 2001;74:383–95. doi: 10.1007/s004200100240. [DOI] [PubMed] [Google Scholar]
- Newman LS, Rose CS. Occupational asbestosis and related diseases. Medical/Scientific Update. National Jewish Center for Immunology and Respiratory Medicine; 1989. http://www.mutnicklaw.com/occupational.pdf [Google Scholar]
- Oberdörster G, Maynard A, Donaldson K, et al. Principles for characterizing the potential human health effects from exposure to nanomaterials: elements of a screening strategy. Part Fibre Toxicol. 2005;2:35. doi: 10.1186/1743-8977-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdörster G. Determinants of the pathogenicity of man-made vitreous fibers (MMVF) Int Arch Occup Environ Health. 2000;73:S60–8. doi: 10.1007/pl00014628. [DOI] [PubMed] [Google Scholar]
- Olsen NJ, Franklin PJ, Reid A, et al. Increasing incidence of malignant mesothelioma after exposure to asbestos during home maintenance and renovation. Med J Aust. 2011;195:271–4. doi: 10.5694/mja11.10125. [DOI] [PubMed] [Google Scholar]
- Partanen T, Nurminen M, Zitting A, et al. Localized pleural plaques and lung cancer. Am J Ind Med. 1992;22:185–92. doi: 10.1002/ajim.4700220205. [DOI] [PubMed] [Google Scholar]
- Pintos J, Parent M-E, Case BW, et al. Risk of mesothelioma and occupational exposure to asbestos and man-made vitreous fibers: evidence from two case-control studies in Montreal, Canada. J Occup Environ Med. 2009;51:1177–84. doi: 10.1097/JOM.0b013e3181b68cef. [DOI] [PubMed] [Google Scholar]
- Pintos J, Parent M-E, Rousseau M-C, et al. Occupational exposure to asbestos and man-made vitreous fibers, and risk of lung cancer: evidence from two case-control studies in Montreal, Canada. J Occup Environ Med. 2008;50:1273–81. doi: 10.1097/JOM.0b013e31818345bb. [DOI] [PubMed] [Google Scholar]
- Plato N, Westerholm P, Gustavsson P, et al. Cancer incidence, mortality, and exposure-response calculations among Swedish man-made vitreous fiber production workers. Scand J Work Environ Health. 1995;21:353–61. doi: 10.5271/sjweh.49. [DOI] [PubMed] [Google Scholar]
- Pott F, Hoth F, Friedrichs KH. Tumorigenic effect of fibrous dusts in experimental animals. Environ Health Perspect. 1974;9:313–15. doi: 10.1289/ehp.749313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pott F, Ziem U, Reiffer F-J, et al. Carcinogenicity studies on fibres, metal compounds, and some other dusts in rats. Exp Pathol. 1987;32:129–52. doi: 10.1016/s0232-1513(87)80044-0. [DOI] [PubMed] [Google Scholar]
- Reid A, de Klerk N, Ambrosini G, et al. The additional risk of malignant mesothelioma in former workers and residents of Wittenoom with benign pleural disease or asbestosis. Occup Environ Med. 2005;62:665–9. doi: 10.1136/oem.2004.018531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhartz A. Fact sheet on pleural plaques. 2004. http://ibasecretariat.org/ar_pleural_plaque_faq.php [Google Scholar]
- Robinson BWS, Lake RA. Advances in malignant mesothelioma. N Engl J Med. 2005;353:1591–603. doi: 10.1056/NEJMra050152. [DOI] [PubMed] [Google Scholar]
- Robinson CF, Dement JM, Ness GO, Waxweiler RJ. Mortality patterns of rock and slag mineral wool production workers: an epidemiological and environmental study. Br J Ind Med. 1982;39:45–53. doi: 10.1136/oem.39.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rödelsperger K. Extrapolation of the carcinogenic potency of fibers from rats to humans. Inhal Toxicol. 2004;16:801–7. doi: 10.1080/08958370490505016. [DOI] [PubMed] [Google Scholar]
- Roller MF, Pott K, Kamino G-H, et al. Results of current intraperitoneal carcinogenicity studies with mineral and vitreous fibres. Exp Toxic Pathol. 1996;48:3–12. doi: 10.1016/S0940-2993(96)80084-4. [DOI] [PubMed] [Google Scholar]
- Rood AP, Streeter RR. Size distributions of occupational airborne asbestos textile fibres as determined by transmission electron microscopy. Ann Occup Hyg. 1984;28:333–9. doi: 10.1093/annhyg/28.3.333. [DOI] [PubMed] [Google Scholar]
- Rubin A-H. Common problems in asbestos –related pulmonary diseases. Am J Ind Health. 1986;10:555–62. doi: 10.1002/ajim.4700100512. [DOI] [PubMed] [Google Scholar]
- Sakellariou K, Malamou-Mitso V, Haritou A, et al. Malignant pleural mesothelioma from nonoccupational asbestos exposure in Metsovo north-west Greece): slow end of an epidemic? Eur Respir J. 1996;9:1206–10. doi: 10.1183/09031936.96.09061206. [DOI] [PubMed] [Google Scholar]
- Sandén Å, Järvholm B, Larsson S, Thiringer G. The risk of lung cancer and mesothelioma after cessation of asbestos exposure: a prospective cohort study of shipyard workers. Eur Respir J. 1992;5:281–5. [PubMed] [Google Scholar]
- Sandén Å, Järvholm B. A study of possible predictors of mesothelioma in shipyard workers exposed to asbestos. J Occup Med. 1991;33:770–3. [PubMed] [Google Scholar]
- Schinwald A, Murphy F, Prina-Mello A, et al. The threshold length for fibre-induced acute pleural inflammation: shedding light on the early events in asbestos-induced mesothelioma. Toxicol Sci. 2012;128:461–70. doi: 10.1093/toxsci/kfs171. [DOI] [PubMed] [Google Scholar]
- Schneider T, Skotte J, Nissen P. Man-made mineral fiber size fractions and their interrelation. Scand J Work Environ Health. 1985;11:117–22. doi: 10.5271/sjweh.2244. [DOI] [PubMed] [Google Scholar]
- Schneider T, Holst E, Skotte J. Size distributions of airborne fibres generated from man-made mineral fibre products. Ann Occup Hyg. 1983;27:157–71. doi: 10.1093/annhyg/27.2.157. [DOI] [PubMed] [Google Scholar]
- Scientific Committee on Occupational Exposure Limits (SCOEL) Recommendation from the Scientific Committee on Occupational Exposure Limits for manmade-mineral fibres (MMMF) with no indication for carcinogenicity and not specified elsewhere. 2012. p. 17. [Google Scholar]
- Scientific Committee on Occupational Exposure Limits (SCOEL) Recommendation from the Scientific Committee on Occupational Exposure Limits for Refractory Ceramic fibres. 2011. http://ec.europa.eu/social/BlobServlet?docId=7371&langId=en [Google Scholar]
- Selikoff IJ, Churg J, Hammond EC. Relation between exposure to asbestos and mesothelioma. N Engl J Med. 1965;272:560–5. doi: 10.1056/NEJM196503182721104. [DOI] [PubMed] [Google Scholar]
- Smith D. Plaques, cancer, and confusion. Chest. 1994;105:8–9. doi: 10.1378/chest.105.1.8. [DOI] [PubMed] [Google Scholar]
- Smith DM, Ortiz LW, Archuleta RF, Johnson NF. Long term health effects in hamsters and rats exposed chronically to manmade vitreous fibers. Ann Occup Hyg. 1987;31:731–43. doi: 10.1093/annhyg/31.4b.731. [DOI] [PubMed] [Google Scholar]
- Stanton M, Layard M, Tegeris A, et al. Carcinogenicity of fibrous glass: pleural response in the rat in relation to fiber dimension. J Natl Cancer Inst. 1977;58:587–97. doi: 10.1093/jnci/58.3.587. [DOI] [PubMed] [Google Scholar]
- Stayner LT, Kuempel E, Gilbert S, et al. An epidemiologic study of the roll of chrysotile asbestos fiber dimensions in determining respiratory disease risk in exposed workers. Occup Environ Med. 2007;65:613–19. doi: 10.1136/oem.2007.035584. [DOI] [PubMed] [Google Scholar]
- Suzuki Y. Pathology of human malignant mesothelioma. Ind Health. 2001;39:183–5. doi: 10.2486/indhealth.39.183. [DOI] [PubMed] [Google Scholar]
- Thermal Insulation Manufacturers Association (TIMA) Nomenclature Committee. Refractory Ceramic Fibers Coalition (RCFC) Washington, DC: 1993. Man-made vitreous fibers: nomenclature, chemical and physical properties. [Google Scholar]
- Trethowan WN, Burge PS, Rossiter CE, et al. Study of the respiratory health of employees in seven European plants that manufacture ceramic fibers. Occup Environ Med. 1995;52:97–104. doi: 10.1136/oem.52.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utell MJ, Maxim LD. Refractory ceramic fiber (RCF) toxicity and epidemiology: a review. Inhal Toxicol. 2010;22:500–21. doi: 10.3109/08958370903521224. [DOI] [PubMed] [Google Scholar]
- Verma DK, Clark NE. Relationship between phase contrast microscopy and transmission electron microscopy results of samples from occupational exposure to airborne chrysotile asbestos. Am Ind Hyg J. 1995;56:866–73. [Google Scholar]
- Walker AM, Maxim LD, Utell M. Are airborne refractory ceramic fibers similar to asbestos in their carcinogenicity? Inhal Toxicol. 2012a;24:416–24. doi: 10.3109/08958378.2012.683892. [DOI] [PubMed] [Google Scholar]
- Walker AM, Maxim LD, Utell M. Corigendum. Are airborne refractory ceramic fibers similar to asbestos in their carcinogenicity? Inhal Toxicol. 2012b;24:928–9. doi: 10.3109/08958378.2012.736736. [DOI] [PubMed] [Google Scholar]
- Walker AM, Maxim LD, Utell M. Risk analysis for mortality from respiratory tumors in a cohort of refractory ceramic fiber workers. Regul Toxicol Pharm. 2002;35:95–104. doi: 10.1006/rtph.2001.1513. [DOI] [PubMed] [Google Scholar]
- Warheit DB, Hartsky MA. Mohr U, Dungworth DL, Mauderly JL, Oberdörster G. Toxic and carcinogenic effects of solid particles in the respiratory tract. Washington, DC: International Life Sciences Institute (ILSI) Press; 1994. Influences of gender, species, and strain differences in pulmonary toxicological assessments of inhaled particles and/or fibers; pp. 253–65. [Google Scholar]
- Weill H, Hughes AM, Churg AM. Changing trends in US mesothelioma incidence. Occup Environ Med. 2004;61:438–41. doi: 10.1136/oem.2003.010165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong O, Foliart D, Trent LS. A case–control study of lung cancer in a cohort of workers potentially exposed to slag wool fibres. Br J Ind Med. 1991;48:818–24. doi: 10.1136/oem.48.12.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaya M, Nakayama K, Hosoda M, et al. A rockwool fibre worker with lung fibrosis. Lancet. 2000;355:1723–4. doi: 10.1016/S0140-6736(05)73126-3. [DOI] [PubMed] [Google Scholar]
- Yates DH, Corrin B, Stidolph PN, Browne K. Malignant mesothelioma in south east England: clinicopathological experience of 272 cases. Thorax. 1997;52:507–12. doi: 10.1136/thx.52.6.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung P, Rogers A, Johnson A. Distribution of mesothelioma cases in different occupational groups and industries in Australia, 1979–1995. Appl Occup Environ Hyg. 1999;14:759–67. doi: 10.1080/104732299302189. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.