Abstract
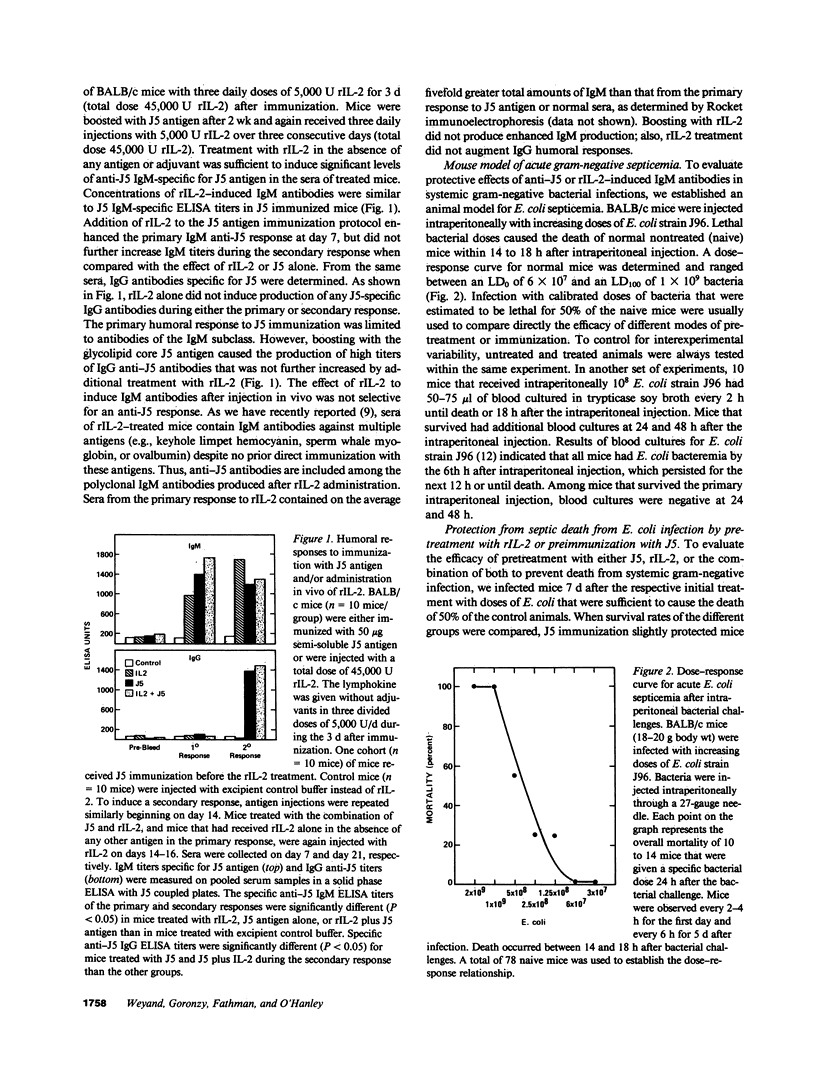
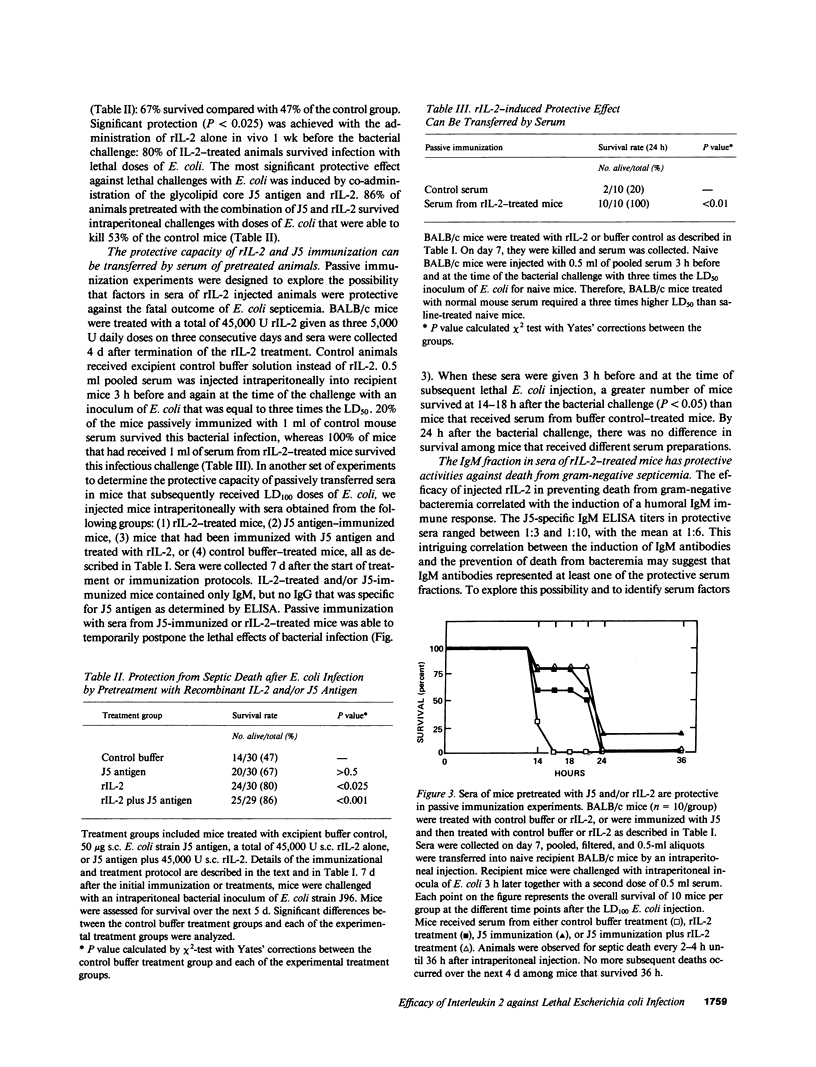
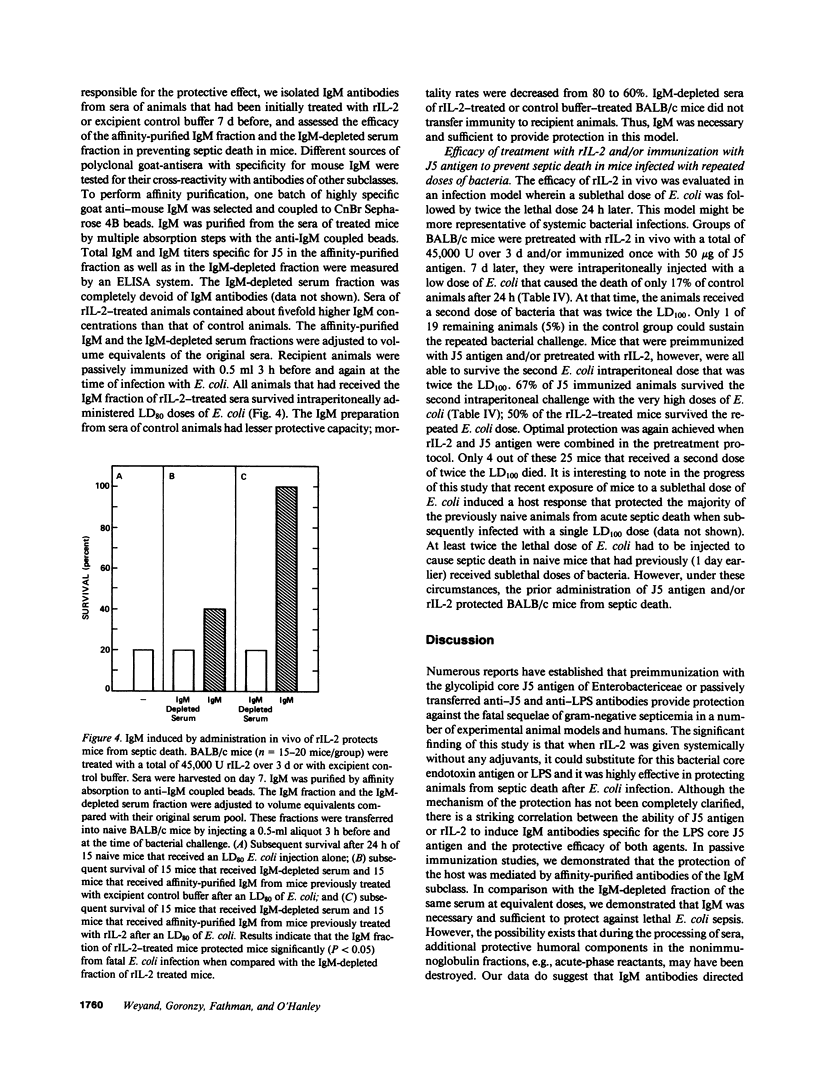
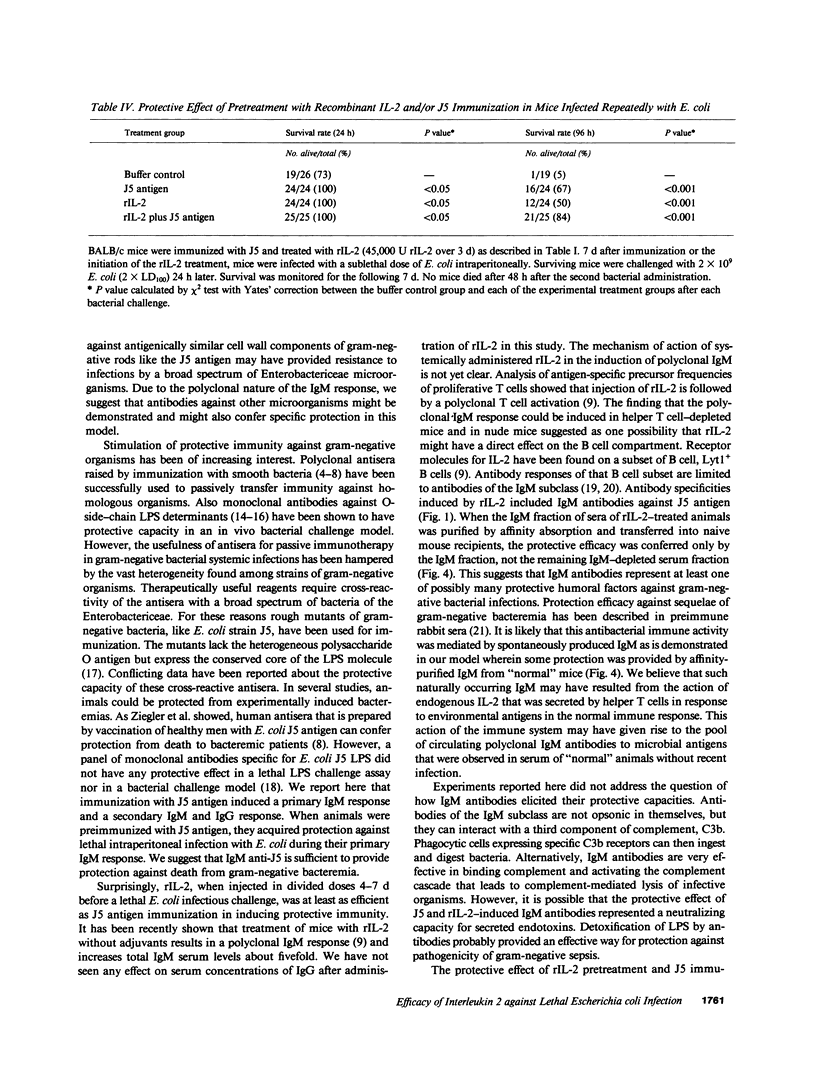
Administration in vivo of recombinant interleukin 2 (rIL-2) to mice induces a polyclonal IgM response. When co-administered with a specific antigen, rIL-2 can enhance concentrations of murine IgM antibodies specific for the antigen by fivefold within 7 d of initial treatment. IgM antibodies that are induced after injection of rIL-2 include antibodies specific for J5, a cell wall core lipopolysaccharide (LPS) antigen that is shared by the different members of the Enterobactericeae family. We report here that mice pretreated with rIL-2 or immunized with J5 antigen 7 d before bacterial challenge were protected from septic death that is caused by intraperitoneal challenges with Escherichia coli. Optimal protection was provided by a combined J5 antigen and rIL-2 treatment. Acquisition of the rIL-2 and J5 antigen-induced protection against lethal bacterial infection coincided temporally with maximal serum IgM titers that also contained IgM antibodies specific for the J5 antigen. In passive immunization experiments, the affinity-purified IgM fraction in sera of rIL-2-treated animals was identified as necessary and sufficient for protection. The IgM-depleted serum had no protective effect. The nonspecific augmentation of host-defense mechanisms without the induction of endotoxin manifestations makes rIL-2 a potential candidate to any alternative LPS-containing vaccines for the prevention of bacterial infections by gram-negative organisms since the core LPS antigen is shared among gram-negative bacteria.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berry L. J. Bacterial toxins. CRC Crit Rev Toxicol. 1977 Nov;5(3):239–318. doi: 10.3109/10408447709082601. [DOI] [PubMed] [Google Scholar]
- Braude A. I., Douglas H. Passive immunization against the local Shwartzman reaction. J Immunol. 1972 Feb;108(2):505–512. [PubMed] [Google Scholar]
- Colwell D. E., Michalek S. M., Briles D. E., Jirillo E., McGhee J. R. Monoclonal antibodies to Salmonella lipopolysaccharide: anti-O-polysaccharide antibodies protect C3H mice against challenge with virulent Salmonella typhimurium. J Immunol. 1984 Aug;133(2):950–957. [PubMed] [Google Scholar]
- Davis C. E., Ziegler E. J., Arnold K. F. Neutralization of meningococcal endotoxin by antibody to core glycolipid. J Exp Med. 1978 Apr 1;147(4):1007–1017. doi: 10.1084/jem.147.4.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELBEIN A. D., HEATH E. C. THE BIOSYNTHESIS OF CELL WALL LIPOPOLYSACCHARIDE IN ESCHERICHIA COLI. I. THE BIOCHEMICAL PROPERTIES OF A URIDINE DIPHOSPHATE GALACTOSE 4-EPIMERASELESS MUTANT. J Biol Chem. 1965 May;240:1919–1925. [PubMed] [Google Scholar]
- Finland M. Changing ecology of bacterial infections as related to antibacterial therapy. J Infect Dis. 1970 Nov;122(5):419–431. doi: 10.1093/infdis/122.5.419. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Greisman S. E., Young E. J., DuBuy B. Mechanisms of endotoxin tolerance. 8. Specificity of serum transfer. J Immunol. 1973 Nov;111(5):1349–1360. [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R., Herzenberg L. A., Herzenberg L. A. Progenitors for Ly-1 B cells are distinct from progenitors for other B cells. J Exp Med. 1985 Jun 1;161(6):1554–1568. doi: 10.1084/jem.161.6.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R., Honda M., Herzenberg L. A., Steinberg A. D., Herzenberg L. A. Ly-1 B cells: functionally distinct lymphocytes that secrete IgM autoantibodies. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2494–2498. doi: 10.1073/pnas.81.8.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman B. M., Cross A. S., Futrovsky S. L., Sidberry H. F., Sadoff J. C. Monoclonal antibodies reactive with K1-encapsulated Escherichia coli lipopolysaccharide are opsonic and protect mice against lethal challenge. Infect Immun. 1986 May;52(2):617–619. doi: 10.1128/iai.52.2.617-619.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland T. N., Ziegler E. J. An immunoprotective monoclonal antibody to lipopolysaccharide. J Immunol. 1984 May;132(5):2590–2592. [PubMed] [Google Scholar]
- Lotze M. T., Matory Y. L., Ettinghausen S. E., Rayner A. A., Sharrow S. O., Seipp C. A., Custer M. C., Rosenberg S. A. In vivo administration of purified human interleukin 2. II. Half life, immunologic effects, and expansion of peripheral lymphoid cells in vivo with recombinant IL 2. J Immunol. 1985 Oct;135(4):2865–2875. [PubMed] [Google Scholar]
- Madonna G. S., Peterson J. E., Ribi E. E., Vogel S. N. Early-phase endotoxin tolerance: induction by a detoxified lipid A derivative, monophosphoryl lipid A. Infect Immun. 1986 Apr;52(1):6–11. doi: 10.1128/iai.52.1.6-11.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madonna G. S., Vogel S. N. Early endotoxin tolerance is associated with alterations in bone marrow-derived macrophage precursor pools. J Immunol. 1985 Dec;135(6):3763–3771. [PubMed] [Google Scholar]
- Marks M. I., Ziegler E. J., Douglas H., Corbeil L. B., Braude A. I. Induction of immunity against lethal Haemophilus influenzae type b infection by Escherichia coli core lipopolysaccharide. J Clin Invest. 1982 Apr;69(4):742–749. doi: 10.1172/JCI110512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner K. M., Manyak C. L., Williams E., Jackson J., Jewell M., Gammon M. T., Ehrenfreund C., Hayes E., Callahan L. T., 3rd, Zweerink H. Characterization of murine monoclonal antibodies to Escherichia coli J5. Infect Immun. 1986 Apr;52(1):56–62. doi: 10.1128/iai.52.1.56-62.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myerowitz R. L., Medeiros A. A., O'Brien T. F. Recent experience with bacillemia due to gram-negative organisms. J Infect Dis. 1971 Sep;124(3):239–246. doi: 10.1093/infdis/124.3.239. [DOI] [PubMed] [Google Scholar]
- O'Hanley P., Lark D., Falkow S., Schoolnik G. Molecular basis of Escherichia coli colonization of the upper urinary tract in BALB/c mice. Gal-Gal pili immunization prevents Escherichia coli pyelonephritis in the BALB/c mouse model of human pyelonephritis. J Clin Invest. 1985 Feb;75(2):347–360. doi: 10.1172/JCI111707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg S. A., Grimm E. A., McGrogan M., Doyle M., Kawasaki E., Koths K., Mark D. F. Biological activity of recombinant human interleukin-2 produced in Escherichia coli. Science. 1984 Mar 30;223(4643):1412–1414. doi: 10.1126/science.6367046. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A., Lotze M. T., Muul L. M., Leitman S., Chang A. E., Ettinghausen S. E., Matory Y. L., Skibber J. M., Shiloni E., Vetto J. T. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl J Med. 1985 Dec 5;313(23):1485–1492. doi: 10.1056/NEJM198512053132327. [DOI] [PubMed] [Google Scholar]
- Shigeta M., Takahara S., Knox S. J., Ishihara T., Vitetta E. S., Fathman C. G. Two independent pathways of helper activity provided by a single T cell clone. J Immunol. 1986 Jan;136(1):34–38. [PubMed] [Google Scholar]
- Weyand C. M., Goronzy J., Dallman M. J., Fathman C. G. Administration of recombinant interleukin 2 in vivo induces a polyclonal IgM response. J Exp Med. 1986 Jun 1;163(6):1607–1612. doi: 10.1084/jem.163.6.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams Z., Hertogs C. F., Pluznik D. H. Use of mice tolerant to lipopolysaccharide to demonstrate requirement of cooperation between macrophages and lymphocytes to generate lipopolysaccharide-induced colony-stimulating factor in vivo. Infect Immun. 1983 Jul;41(1):1–5. doi: 10.1128/iai.41.1.1-5.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler E. J., Douglas H., Sherman J. E., Davis C. E., Braude A. I. Treatment of E. coli and klebsiella bacteremia in agranulocytic animals with antiserum to a UDP-gal epimerase-deficient mutant. J Immunol. 1973 Aug;111(2):433–438. [PubMed] [Google Scholar]
- Ziegler E. J., McCutchan J. A., Fierer J., Glauser M. P., Sadoff J. C., Douglas H., Braude A. I. Treatment of gram-negative bacteremia and shock with human antiserum to a mutant Escherichia coli. N Engl J Med. 1982 Nov 11;307(20):1225–1230. doi: 10.1056/NEJM198211113072001. [DOI] [PubMed] [Google Scholar]


