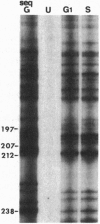
Abstract
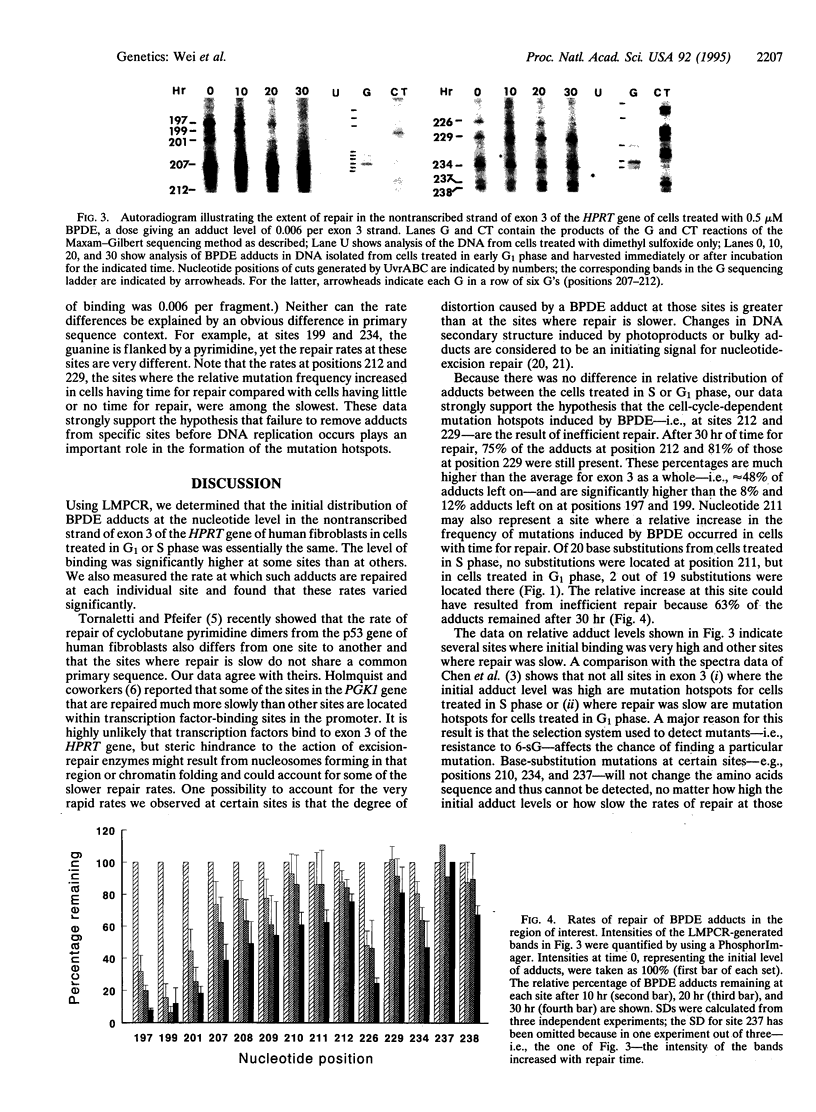
When populations of repair-proficient diploid human fibroblasts were treated with (+/-)-7 beta, 8 alpha-dihydroxy-9 alpha, 10 alpha-epoxy-7,8,9,10-tetrahydrobenzo[a]pyrene (BPDE) during early S phase, just as the hypoxanthine phosphoribosyltransferase gene (HPRT) was being replicated, 5% of the induced base substitutions were found at nt 212, and 5% of the substitutions were found at nt 229 in exon 3. However, when the population was treated in early G1 phase to allow at least 12 hr for repair before the onset of S phase, 21% of the substitutions were found at nt 212, and 10% were found at nt 229. No such cell-cycle-dependent difference in distribution of base substitutions occurred in excision-repair-deficient cells. To test whether the increase in the relative frequency of mutations resulted from inefficient repair at these sites, we adapted ligation-mediated PCR to measure the rates of removal of BPDE adducts from individual sites in exon 3 of the HPRT gene. Cells were treated with 0.5 microM BPDE in early G1 phase and harvested immediately or after 10, 20, and 30 hr for repair. the nontranscribed strand of exon 3 was analyzed for the original distribution of adducts and those remaining after repair, using Escherichia coli UvrABC excinuclease to excise the adducts and annealing a 5' biotinylated gene-specific primer to the DNA and extending it with Sequenase 2.0 to generate a blunt end at the site of each cut. A linker was ligated to the blunt end, and the desired fragments were isolated from the rest of the genomic DNA by using magnetic beads, amplified by PCR, and analyzed on a sequencing gel. The distribution of fragments of particular lengths indicated the relative number of BPDE adducts initially formed or remaining at specific sites. The rates of repair at individual sites varied widely along exon 3 of the HPRT gene and were very slow at nt 212 and 229, strongly supporting the hypothesis that inefficient DNA repair plays an important role in the formation of mutation hotspots.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brash D. E., Rudolph J. A., Simon J. A., Lin A., McKenna G. J., Baden H. P., Halperin A. J., Pontén J. A role for sunlight in skin cancer: UV-induced p53 mutations in squamous cell carcinoma. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10124–10128. doi: 10.1073/pnas.88.22.10124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brash D. E., Seetharam S., Kraemer K. H., Seidman M. M., Bredberg A. Photoproduct frequency is not the major determinant of UV base substitution hot spots or cold spots in human cells. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3782–3786. doi: 10.1073/pnas.84.11.3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R. H., Maher V. M., Brouwer J., van de Putte P., McCormick J. J. Preferential repair and strand-specific repair of benzo[a]pyrene diol epoxide adducts in the HPRT gene of diploid human fibroblasts. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5413–5417. doi: 10.1073/pnas.89.12.5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R. H., Maher V. M., McCormick J. J. Effect of excision repair by diploid human fibroblasts on the kinds and locations of mutations induced by (+/-)-7 beta,8 alpha-dihydroxy-9 alpha,10 alpha-epoxy-7,8,9,10- tetrahydrobenzo[a]pyrene in the coding region of the HPRT gene. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8680–8684. doi: 10.1073/pnas.87.21.8680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R. H., Maher V. M., McCormick J. J. Lack of a cell cycle-dependent strand bias for mutations induced in the HPRT gene by (+/-)-7 beta,8 alpha-dihydroxy-9 alpha,10 alpha-epoxy-7,8,9,10-tetrahydrobenzo(a)pyrene in excision repair-deficient human cells. Cancer Res. 1991 May 15;51(10):2587–2592. [PubMed] [Google Scholar]
- Conney A. H. Induction of microsomal enzymes by foreign chemicals and carcinogenesis by polycyclic aromatic hydrocarbons: G. H. A. Clowes Memorial Lecture. Cancer Res. 1982 Dec;42(12):4875–4917. [PubMed] [Google Scholar]
- Gao S., Drouin R., Holmquist G. P. DNA repair rates mapped along the human PGK1 gene at nucleotide resolution. Science. 1994 Mar 11;263(5152):1438–1440. doi: 10.1126/science.8128226. [DOI] [PubMed] [Google Scholar]
- Kalinowski D. P., Illenye S., Van Houten B. Analysis of DNA damage and repair in murine leukemia L1210 cells using a quantitative polymerase chain reaction assay. Nucleic Acids Res. 1992 Jul 11;20(13):3485–3494. doi: 10.1093/nar/20.13.3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Mueller P. R., Wold B. In vivo footprinting of a muscle specific enhancer by ligation mediated PCR. Science. 1989 Nov 10;246(4931):780–786. doi: 10.1126/science.2814500. [DOI] [PubMed] [Google Scholar]
- Sancar A., Rupp W. D. A novel repair enzyme: UVRABC excision nuclease of Escherichia coli cuts a DNA strand on both sides of the damaged region. Cell. 1983 May;33(1):249–260. doi: 10.1016/0092-8674(83)90354-9. [DOI] [PubMed] [Google Scholar]
- Sancar A., Sancar G. B. DNA repair enzymes. Annu Rev Biochem. 1988;57:29–67. doi: 10.1146/annurev.bi.57.070188.000333. [DOI] [PubMed] [Google Scholar]
- Seeberg E., Steinum A. L., Nordenskjöld M., Söderhäll S., Jernström B. Strand-break formation in DNA modified by benzo[alpha]pyrene diolepoxide. Quantitative cleavage by Escherichia coli uvrABC endonuclease. Mutat Res. 1983 Jun;112(3):139–145. doi: 10.1016/0167-8817(83)90036-6. [DOI] [PubMed] [Google Scholar]
- Tang M. S., Pierce J. R., Doisy R. P., Nazimiec M. E., MacLeod M. C. Differences and similarities in the repair of two benzo[a]pyrene diol epoxide isomers induced DNA adducts by uvrA, uvrB, and uvrC gene products. Biochemistry. 1992 Sep 15;31(36):8429–8436. doi: 10.1021/bi00151a006. [DOI] [PubMed] [Google Scholar]
- Tornaletti S., Pfeifer G. P. Slow repair of pyrimidine dimers at p53 mutation hotspots in skin cancer. Science. 1994 Mar 11;263(5152):1436–1438. doi: 10.1126/science.8128225. [DOI] [PubMed] [Google Scholar]
- Van Houten B., Masker W. E., Carrier W. L., Regan J. D. Quantitation of carcinogen-induced DNA damage and repair in human cells with the UVR ABC excision nuclease from Escherichia coli. Carcinogenesis. 1986 Jan;7(1):83–87. doi: 10.1093/carcin/7.1.83. [DOI] [PubMed] [Google Scholar]
- Van Houten B. Nucleotide excision repair in Escherichia coli. Microbiol Rev. 1990 Mar;54(1):18–51. doi: 10.1128/mr.54.1.18-51.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M., Maher V. M., McCormick J. J. Excision repair of UV- or benzo[a]pyrene diol epoxide-induced lesions in xeroderma pigmentosum variant cells is 'error free'. Mutat Res. 1985 Nov;146(3):285–294. doi: 10.1016/0167-8817(85)90070-7. [DOI] [PubMed] [Google Scholar]
- Wei S. J., Chang R. L., Bhachech N., Cui X. X., Merkler K. A., Wong C. Q., Hennig E., Yagi H., Jerina D. M., Conney A. H. Dose-dependent differences in the profile of mutations induced by (+)-7R,8S-dihydroxy-9S,10R-epoxy-7,8,9,10-tetrahydrobenzo(a)pyrene in the coding region of the hypoxanthine (guanine) phosphoribosyltransferase gene in Chinese hamster V-79 cells. Cancer Res. 1993 Jul 15;53(14):3294–3301. [PubMed] [Google Scholar]
- Yang J. L., Chen R. H., Maher V. M., McCormick J. J. Kinds and location of mutations induced by (+/-)-7 beta,8 alpha-dihydroxy-9 alpha,10 alpha-epoxy-7,8,9,10-tetrahydrobenzo[a]pyrene in the coding region of the hypoxanthine (guanine) phosphoribosyltransferase gene in diploid human fibroblasts. Carcinogenesis. 1991 Jan;12(1):71–75. doi: 10.1093/carcin/12.1.71. [DOI] [PubMed] [Google Scholar]
- Yang L. L., Maher V. M., McCormick J. J. Relationship between excision repair and the cytotoxic and mutagenic effect of the 'anti' 7,8-diol-9,10-epoxide of benzo[a]pyrene in human cells. Mutat Res. 1982 Jun;94(2):435–447. doi: 10.1016/0027-5107(82)90306-2. [DOI] [PubMed] [Google Scholar]
- Ziegler A., Leffell D. J., Kunala S., Sharma H. W., Gailani M., Simon J. A., Halperin A. J., Baden H. P., Shapiro P. E., Bale A. E. Mutation hotspots due to sunlight in the p53 gene of nonmelanoma skin cancers. Proc Natl Acad Sci U S A. 1993 May 1;90(9):4216–4220. doi: 10.1073/pnas.90.9.4216. [DOI] [PMC free article] [PubMed] [Google Scholar]