Abstract
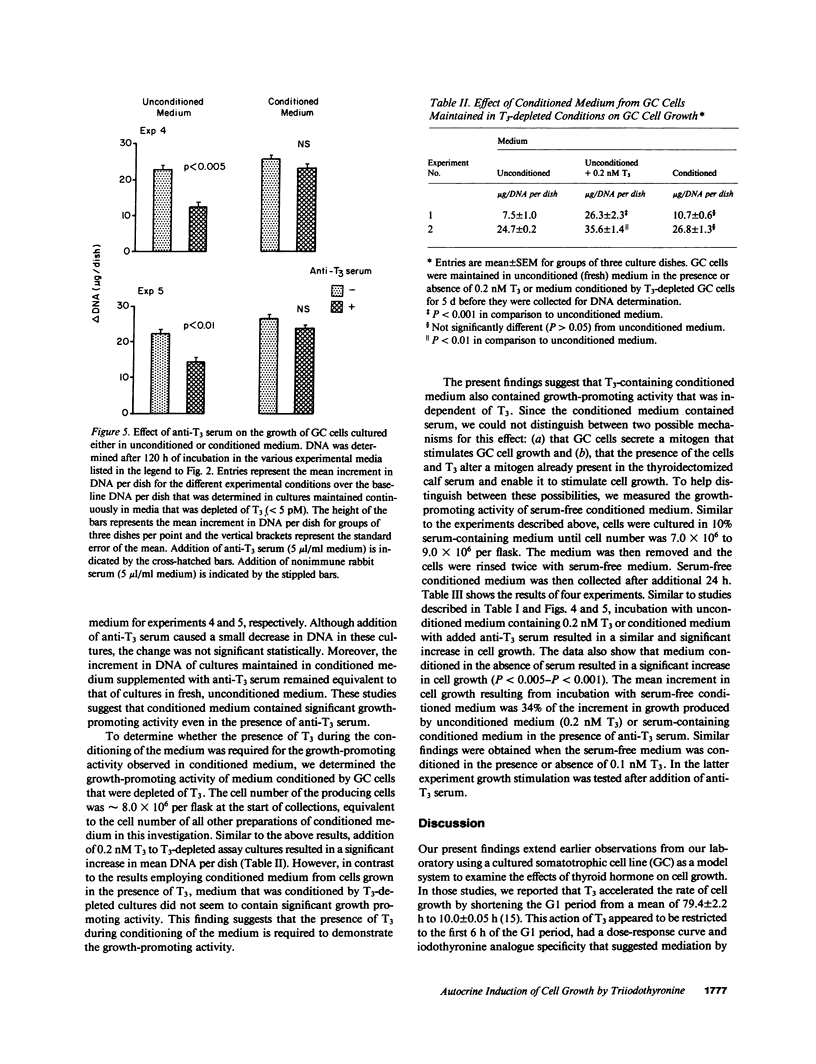
L-Triiodothyronine (T3) stimulates DNA synthesis and replication of cultured GC cells, a T3-responsive growth hormone (GH)-secreting cell line. To determine whether T3 stimulates secretion of an autocrine growth factor, we compared the growth-promoting activity of medium conditioned by T3-stimulated and T3-depleted cells to that of unconditioned medium. Addition of polyclonal rabbit anti-T3 serum to T3-containing media decreased cellular T3 content by 50-70%. In unconditioned medium, anti-T3 serum decreased T3-induced cell growth and GH production by 40-70%. In conditioned medium, anti-T3 serum also effected a 45-70% decrease in induction of GH secretion but did not attenuate the growth-promoting activity. Growth-promoting activity was not detected in medium conditioned by T3-depleted cells. Thus, conditioned medium from T3-containing GC cell cultures contains growth-promoting activity that is independent of T3. Further, the induction of GC cel growth by T3 may occur, at least in part, by induction of an autocrine growth factor.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Astier H. S., DeFesi C. R., Surks M. I. Kinetics of deoxyribonucleic acid synthesis and replication of thyrotrophs and somatotrophs during development of hypothyroidism and L-triiodothyronine treatment of hypothyroid rats. Endocrinology. 1980 May;106(5):1537–1548. doi: 10.1210/endo-106-5-1537. [DOI] [PubMed] [Google Scholar]
- Beach D. H., Jacobson M. Influences of thyroxine on cell proliferation in the retina of the clawed frog at different ages. J Comp Neurol. 1979 Feb 1;183(3):615–623. doi: 10.1002/cne.901830309. [DOI] [PubMed] [Google Scholar]
- Betsholtz C., Westermark B., Ek B., Heldin C. H. Coexpression of a PDGF-like growth factor and PDGF receptors in a human osteosarcoma cell line: implications for autocrine receptor activation. Cell. 1984 Dec;39(3 Pt 2):447–457. doi: 10.1016/0092-8674(84)90452-5. [DOI] [PubMed] [Google Scholar]
- Binoux M., Faivre-Bauman A., Lassarre C., Barret A., Tixier-Vidal A. Triiodothyronine stimulates the production of insulin-like growth factor (IGF) by fetal hypothalamus cells cultured in serum-free medium. Brain Res. 1985 Aug;353(2):319–321. doi: 10.1016/0165-3806(85)90222-6. [DOI] [PubMed] [Google Scholar]
- Carpenter G., Cohen S. Epidermal growth factor. Annu Rev Biochem. 1979;48:193–216. doi: 10.1146/annurev.bi.48.070179.001205. [DOI] [PubMed] [Google Scholar]
- Chernausek S. D., Underwood L. E., Utiger R. D., Van Wyk J. J. Growth hormone secretion and plasma somatomedin-C in primary hypothyroidism. Clin Endocrinol (Oxf) 1983 Sep;19(3):337–344. doi: 10.1111/j.1365-2265.1983.tb00007.x. [DOI] [PubMed] [Google Scholar]
- Clemmons D. R. Multiple hormones stimulate the production of somatomedin by cultured human fibroblasts. J Clin Endocrinol Metab. 1984 May;58(5):850–856. doi: 10.1210/jcem-58-5-850. [DOI] [PubMed] [Google Scholar]
- Clemmons D. R., Underwood L. E., Van Wyk J. J. Hormonal control of immunoreactive somatomedin production by cultured human fibroblasts. J Clin Invest. 1981 Jan;67(1):10–19. doi: 10.1172/JCI110001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemmons D. R., Van Wyk J. J. Evidence for a functional role of endogenously produced somatomedinlike peptides in the regulation of DNA synthesis in cultured human fibroblasts and porcine smooth muscle cells. J Clin Invest. 1985 Jun;75(6):1914–1918. doi: 10.1172/JCI111906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dainat J., Rebiére A. In vivo action of a single injection of thyroxine on the cerebellar protein synthesis and cellular multiplications, in normal and hypothyroid young rats. Study of the respective effects of the dose and time action of the hormone. Acta Neurol Scand. 1980 Feb;61(2):65–77. doi: 10.1111/j.1600-0404.1980.tb01469.x. [DOI] [PubMed] [Google Scholar]
- DeFesi C. R., Astier H. S., Surks M. I. Kinetics of thyrotrophs and somatotrophs during development of hypothyroidism and L-triiodothyronine treatment of hypothyroid rats. Endocrinology. 1979 Apr;104(4):1172–1180. doi: 10.1210/endo-104-4-1172. [DOI] [PubMed] [Google Scholar]
- DeFesi C. R., Fels E. C., Surks M. I. L-Triiodothyronine (T3) stimulates growth of cultured GC cells by action early in the G1 period: evidence for mediation by the nuclear T3 receptor. Endocrinology. 1985 May;116(5):2062–2069. doi: 10.1210/endo-116-5-2062. [DOI] [PubMed] [Google Scholar]
- DeFesi C. R., Fels E. C., Surks M. I. Triiodothyronine stimulates growth of cultured GC cells by action early in the G1 period. Endocrinology. 1984 Jan;114(1):293–295. doi: 10.1210/endo-114-1-293. [DOI] [PubMed] [Google Scholar]
- DeFesi C. R., Surks M. I. 3,5,3'-Triiodothyronine effects on the growth rate and cell cycle of cultured GC cells. Endocrinology. 1981 Jan;108(1):259–267. doi: 10.1210/endo-108-1-259. [DOI] [PubMed] [Google Scholar]
- Diamond D. J., Goodman H. M. Regulation of growth hormone messenger RNA synthesis by dexamethasone and triiodothyronine. Transcriptional rate and mRNA stability changes in pituitary tumor cells. J Mol Biol. 1985 Jan 5;181(1):41–62. doi: 10.1016/0022-2836(85)90323-7. [DOI] [PubMed] [Google Scholar]
- Dickson R. B., Huff K. K., Spencer E. M., Lippman M. E. Induction of epidermal growth factor-related polypeptides by 17 beta-estradiol in MCF-7 human breast cancer cells. Endocrinology. 1986 Jan;118(1):138–142. doi: 10.1210/endo-118-1-138. [DOI] [PubMed] [Google Scholar]
- Duprez V., Lenoir G., Dautry-Varsat A. Autocrine growth stimulation of a human T-cell lymphoma line by interleukin 2. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6932–6936. doi: 10.1073/pnas.82.20.6932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingrich S. A., Smith P. J., Shapiro L. E., Surks M. I. 5,5'-Diphenylhydantoin (phenytoin) attenuates the action of 3,5,3'-triiodo-L-thyronine in cultured GC cells. Endocrinology. 1985 Jun;116(6):2306–2313. doi: 10.1210/endo-116-6-2306. [DOI] [PubMed] [Google Scholar]
- Greene L. A., Shooter E. M. The nerve growth factor: biochemistry, synthesis, and mechanism of action. Annu Rev Neurosci. 1980;3:353–402. doi: 10.1146/annurev.ne.03.030180.002033. [DOI] [PubMed] [Google Scholar]
- Gresik E. W., Schenkein I., van der Noen H., Barka T. Hormonal regulation of epidermal growth factor and protease in the submandibular gland of the adult mouse. Endocrinology. 1981 Sep;109(3):924–929. doi: 10.1210/endo-109-3-924. [DOI] [PubMed] [Google Scholar]
- Hinkle P. M., Kinsella P. A. Thyroid hormone induction of an autocrine growth factor secreted by pituitary tumor cells. Science. 1986 Dec 19;234(4783):1549–1552. doi: 10.1126/science.3097825. [DOI] [PubMed] [Google Scholar]
- Hoffman C. W., Dent J. N. Hormonal regulation of cellular proliferation in the epidermis of the red-spotted newt. Gen Comp Endocrinol. 1977 Aug;32(4):522–530. doi: 10.1016/0016-6480(77)90236-2. [DOI] [PubMed] [Google Scholar]
- Kumegawa M., Ikeda E., Hosoda S., Takuma T. In vitro effects of thyroxine and insulin on myoblasts from chick embryo skeletal muscle. Dev Biol. 1980 Oct;79(2):493–499. doi: 10.1016/0012-1606(80)90134-7. [DOI] [PubMed] [Google Scholar]
- LEBLOND C. P., CARRIERE R. The effect of growth hormone and thyroxine on the mitotic rate of the intestinal mucosa of the rat. Endocrinology. 1955 Mar;56(3):261–266. doi: 10.1210/endo-56-3-261. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lauder J. M. The effects of early hypo- and hyperthyroidism on the development of rat cerebellar cortex. III. Kinetics of cell proliferation in the external granular layer. Brain Res. 1977 Apr 22;126(1):31–51. doi: 10.1016/0006-8993(77)90213-x. [DOI] [PubMed] [Google Scholar]
- Manni A., Pontari M., Wright C. Autocrine stimulation by prolactin of hormone-responsive breast cancer growth in culture. Endocrinology. 1985 Nov;117(5):2040–2043. doi: 10.1210/endo-117-5-2040. [DOI] [PubMed] [Google Scholar]
- Marek J., Schüllerova M., Schreiberova O., Límanová Z. Effect of thyroid function on serum somatomedin activity. Acta Endocrinol (Copenh) 1981 Apr;96(4):491–497. doi: 10.1530/acta.0.0960491. [DOI] [PubMed] [Google Scholar]
- Masterson E., Edelhauser H. F., Van Horn D. L. Development of corneal transparency in embryonic chick: influence of exogenous thyroxine and thiouracil on structure, water and electrolyte content. Dev Biol. 1975 Apr;43(2):233–239. doi: 10.1016/0012-1606(75)90023-8. [DOI] [PubMed] [Google Scholar]
- Meuer S. C., Hussey R. E., Cantrell D. A., Hodgdon J. C., Schlossman S. F., Smith K. A., Reinherz E. L. Triggering of the T3-Ti antigen-receptor complex results in clonal T-cell proliferation through an interleukin 2-dependent autocrine pathway. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1509–1513. doi: 10.1073/pnas.81.5.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses H. L., Branum E. L., Proper J. A., Robinson R. A. Transforming growth factor production by chemically transformed cells. Cancer Res. 1981 Jul;41(7):2842–2848. [PubMed] [Google Scholar]
- Nunez J. Effects of thyroid hormones during brain differentiation. Mol Cell Endocrinol. 1984 Sep;37(2):125–132. doi: 10.1016/0303-7207(84)90043-1. [DOI] [PubMed] [Google Scholar]
- Nyborg J. K., Nguyen A. P., Spindler S. R. Relationship between thyroid and glucocorticoid hormone receptor occupancy, growth hormone gene transcription, and mRNA accumulation. J Biol Chem. 1984 Oct 25;259(20):12377–12381. [PubMed] [Google Scholar]
- Patel A. J., Lewis P. D., Balázs R., Bailey P., Lai M. Effects of thyroxine on postnatal cell acquisition in the rat brain. Brain Res. 1979 Aug 17;172(1):57–72. doi: 10.1016/0006-8993(79)90895-3. [DOI] [PubMed] [Google Scholar]
- Roberts A. B., Anzano M. A., Lamb L. C., Smith J. M., Sporn M. B. New class of transforming growth factors potentiated by epidermal growth factor: isolation from non-neoplastic tissues. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5339–5343. doi: 10.1073/pnas.78.9.5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels H. H., Stanley F., Casanova J. Depletion of L-3,5,3'-triiodothyronine and L-thyroxine in euthyroid calf serum for use in cell culture studies of the action of thyroid hormone. Endocrinology. 1979 Jul;105(1):80–85. doi: 10.1210/endo-105-1-80. [DOI] [PubMed] [Google Scholar]
- Short J., Wedmore R., Kibert L., Zemel R. Triidothyronine: on its role as a specific hepatomitogen. Cytobios. 1980;28(111-112):165–177. [PubMed] [Google Scholar]
- Siminoski K., Bernanke J., Kay C., Murphy R. A. Steroids and triiodothyronine reduce nerve growth factor concentrations in medium conditioned by L-929 fibroblasts. Endocrinology. 1986 Apr;118(4):1417–1425. doi: 10.1210/endo-118-4-1417. [DOI] [PubMed] [Google Scholar]
- Smith P. J., Surks M. I. 5,5'-Diphenylhydantoin (dilantin) decreases cytosol and specific nuclear 3,5,3'-triiodothyronine binding in rat anterior pituitary in vivo and in cultured GC cells. Endocrinology. 1984 Jul;115(1):283–290. doi: 10.1210/endo-115-1-283. [DOI] [PubMed] [Google Scholar]
- Spindler S. R., Mellon S. H., Baxter J. D. Growth hormone gene transcription is regulated by thyroid and glucocorticoid hormones in cultured rat pituitary tumor cells. J Biol Chem. 1982 Oct 10;257(19):11627–11632. [PubMed] [Google Scholar]
- Surks M. I., DeFesi C. R. Determination of the cell number of each cell type in the anterior pituitary of euthyroid and hypothyroid rats. Endocrinology. 1977 Sep;101(3):946–958. doi: 10.1210/endo-101-3-946. [DOI] [PubMed] [Google Scholar]
- Surks M. I., Kumara-Siri M. H. Increase in nuclear thyroid and glucocorticoid receptors and growth hormone production during the deoxyribonucleic acid synthesis phase of the cell growth cycle. Endocrinology. 1984 Mar;114(3):873–879. doi: 10.1210/endo-114-3-873. [DOI] [PubMed] [Google Scholar]
- Surks M. I., Schadlow A. R., Oppenheimer J. H. A new radioimmunoassay for plasma L-triiodothyronine: measurements in thyroid disease and in patients maintained on hormonal replacement. J Clin Invest. 1972 Dec;51(12):3104–3113. doi: 10.1172/JCI107137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignon F., Capony F., Chambon M., Freiss G., Garcia M., Rochefort H. Autocrine growth stimulation of the MCF 7 breast cancer cells by the estrogen-regulated 52 K protein. Endocrinology. 1986 Apr;118(4):1537–1545. doi: 10.1210/endo-118-4-1537. [DOI] [PubMed] [Google Scholar]
- Walker P., Coulombe P., Dussault J. H. Time- and dose-dependent effect of triiodothyronine on submaxillary gland epidermal growth factor concentration in adult female mice. Endocrinology. 1982 Oct;111(4):1133–1139. doi: 10.1210/endo-111-4-1133. [DOI] [PubMed] [Google Scholar]
- Walker P. Thyroxine increases neonatal mouse submandibular gland mRNA-directed synthesis of epidermal growth factor. Biochem Cell Biol. 1986 Apr;64(4):290–296. doi: 10.1139/o86-040. [DOI] [PubMed] [Google Scholar]
- Walker P., Weichsel M. E., Jr, Eveleth D., Fisher D. A. Ontogenesis of nerve growth factor and epidermal growth factor in submaxillary glands and nerve growth factor in brains of immature male mice: correlation with ontogenesis of serum levels of thyroid hormones. Pediatr Res. 1982 Jul;16(7):520–524. doi: 10.1203/00006450-198207000-00004. [DOI] [PubMed] [Google Scholar]
- Walker P., Weichsel M. E., Jr, Guo S. M., Fisher D. A., Fisher D. A. Radioimmunoassay for mouse nerve growth factor (NGF). Effects of thyroxine administration on tissue NGF levels. Brain Res. 1980 Mar 31;186(2):331–341. doi: 10.1016/0006-8993(80)90979-8. [DOI] [PubMed] [Google Scholar]
- Walker P., Weichsel M. E., Jr, Hoath S. B., Poland R. E., Fisher D. A. Effect of thyroxine, testosterone, and corticosterone on nerve growth factor (NGF) and epidermal growth factor (EGF) concentrations in adult female mouse submaxillary gland: dissociation of NGF and EGF responses. Endocrinology. 1981 Aug;109(2):582–587. doi: 10.1210/endo-109-2-582. [DOI] [PubMed] [Google Scholar]
- Wion D., Barrand P., Dicou E., Scott J., Brachet P. Serum and thyroid hormones T3 and T4 regulate nerve growth factor mRNA levels in mouse L cells. FEBS Lett. 1985 Sep 9;189(1):37–41. doi: 10.1016/0014-5793(85)80837-1. [DOI] [PubMed] [Google Scholar]
- Wright M. L., Sicbaldi E. M., Loveridge K. M., Pike P. A., Majerowski M. A. Cell population kinetics in tadpole limb epidermis during thyroxine-induced, spontaneous, and prolactin-inhibited metamorphosis. Gen Comp Endocrinol. 1981 Apr;43(4):451–461. doi: 10.1016/0016-6480(81)90229-x. [DOI] [PubMed] [Google Scholar]
- Yaffe B. M., Samuels H. H. Hormonal regulation of the growth hormone gene. Relationship of the rate of transcription to the level of nuclear thyroid hormone-receptor complexes. J Biol Chem. 1984 May 25;259(10):6284–6291. [PubMed] [Google Scholar]



