Abstract
Rho GTPases are molecular switches that elicit distinct effects on the actomyosin cytoskeleton to accurately promote cytokinesis. Although they represent less than 1% of the human genome, Rho GTPases exert disproportionate control over cell division. Crucial to this master regulatory role is their localized occupation of specific domains of the cell to ensure the assembly of a contractile ring at the proper time and place. RhoA occupies the division plane and is the central positive Rho family regulator of cytokinesis. Rac1 is a negative regulator of cytokinesis and is inactivated within the division plane while active Rac1 occupies the cell poles. Cdc42 regulation during cytokinesis is less studied, but thus far a clear role has only been shown during polar body emission. Here we review what is known about the function of Rho family GTPases during cell division, as well as their upstream regulators and known downstream cytokinetic effectors.
Rho family guanosine triphosphatases or GTPases (including RhoA, Rac1, and Cdc42) act as molecular switches that choreograph complex cellular behaviors in eukaryotes. RhoA, the founding member of the Rho family, was originally isolated from Aplysia ganglia as a Ras homolog [1]. Soon after, Cdc42 was identified in budding yeast [2, 3], and then Rac1 was discovered as a target of botulinum toxins [4]. Similar to Ras, Rho GTPase signaling is misregulated in a multitude of human cancers (for review see [5–9]). Rho family small GTPases exert their control over the cell by each regulating distinct functions of the actin cytoskeleton [10, 11], as well as microtubule dynamics [12–15]. Recently, it has become clear that RhoA, Rac1, and Cdc42 act in concert to spatially and temporally regulate actin dynamics and orchestrate complex cellular processes [16]. These processes include cell motility, cell polarization, cell adhesion, chromosome inheritance, and, most importantly for this review, cytokinesis.
Rho GTPases are lipid-modified enzymes that function as master regulators in many signal transduction cascades. Lipid modification allows association with the plasma membrane, and GTP hydrolysis confers a binary molecular “switch” activity on Rho family proteins. When bound to GTP they are active and can interact with downstream effectors; when bound to GDP, however, they are inactive. Because the innate GTP hydrolysis activity of small GTPases is quite low, regulatory proteins further modulate Rho GTPases by catalyzing GTP hydrolysis and exchange of GDP for GTP. Three main classes of upstream regulatory proteins modulate small GTPases. Guanosine nucleotide dissociation inhibitors (GDIs) maintain GTPases in their inactive state by binding to their GDP-bound form, sequestering them in the cytosol, as well as protecting them from proteolysis [17]. Guanine nucleotide exchange factors (GEFs) catalyze the exchange of GDP for GTP, thus activating GTPases [18]. GTPase activating proteins (GAPs) catalyze the intrinsic hydrolase activity of the GTPase, thus turning ‘off’ the GTPase [18]. Many of these regulators control the localized activity of Rho GTPases by themselves localizing to the sub-cellular domain of interest, often via transport by/or association with the microtubule and actin cytoskeletons. Thus spatial and temporal regulation of Rho GTPases and their regulators leads to local modulation of downstream effectors and directed alterations in actin dynamics.
Cytokinesis, the physical division of one cell into two daughter cells, is a quintessential example of Rho family GTPase coordination that requires a great deal of spatial and temporal synchronization driven by upstream regulators to ensure accuracy. Separation of the chromosomes must be coordinated with ingression of the plasma membrane in order to generate two distinct daughter cells, each with a single genomic complement. To position the division plane, the microtubule-rich mitotic spindle communicates with the cell cortex via Rho GTPase-mediated signal transduction pathways to locally activate the assembly and constriction of the actomyosin contractile ring at the cell equator [19]. The spindle thus coordinates nuclear and cytoplasmic division by localizing a number of Rho regulators to the division site.
This review provides a summary of what is currently known about the function and regulation of Rho family GTPases during cytokinesis in animal cells. We will focus mainly on the most widely studied positive regulator of cytokinesis, RhoA [19], but we will also cover what is known about the less well-studied members of the Rho family, Rac1 and Cdc42. In each section, we will discuss any GEFs and GAPs required for cytokinesis. RhoGDIs will not be discussed here in detail. While it is likely that RhoGDIs participate in cytokinesis (see [20, 21]), specific cytokinetic RhoGDIs per se have not yet been identified in metazoa. Nevertheless, RhoGDIs are shared by Rho family members and these regulators function to protect Rho GTPases from degradation [22]; thus overexpression or even local enrichment of one Rho family member could easily affect the activation state and stability of other Rho family members through competition for RhoGDIs. As many of the landmark studies on the role of Rho GTPases during cytokinesis rely heavily on the expression of constitutively active or dominant negative mutant forms of RhoA, Rac1, and/or Cdc42, it should be taken into consideration that some of the results produced by these studies may be due to indirect effects on other Rho family members [22].
Importantly, the story of Rho GTPase regulation during cytokinesis is not complete; there are many unknowns and confusing results, which we also attempt to cover here. Finally, although we emphasize cytokinesis, the same general paradigms may underlie Rho GTPase control of a multitude of complex cell behaviors.
A Conserved Positive Regulatory Role for RhoA in Cytokinesis
RhoA positively regulates cytokinesis in dividing embryos, meiotically dividing cells, and in many somatic divisions. An essential role for RhoA in cytokinesis was first shown in dividing Xenopus embryos [20]. Embryos injected with a dominant negative or constitutively active form of RhoA or C3 exotransferase, a bacterial toxin that specifically ADP ribosylates and inactivates RhoA [23], fail to properly form a contractile ring and do not divide (see also [24]). Cytokinesis in Drosophila melanogaster embryos is also dependent on RhoA. Fly embryos either isolated from RhoA-null mutants, injected with C3, or expressing a dominant-negative RhoA, all show defects in cytokinesis [25, 26]. Further, RhoA is required for cytokinesis in the nematode Caenorhabditis elegans, as depletion of the worm RhoA ortholog (RHO-1) in embryos by RNA interference blocks cytokinesis with little to no cleavage furrow ingression [27, 28]. Microinjection of C3 into early zebrafish embryos or mouse eggs also blocks early cleavages [29, 30]. Thus RhoA plays a conserved role in embryonic cytokinesis across metazoa.
RhoA also plays a conserved role in meiotic cytokinesis in a multitude of animal systems (for review see also [31–33]). Injection of mouse eggs with C3 or anti-RhoA antibodies leads to a failure of polar body emission during meiosis II [30, 34]. In Xenopus eggs, C3 expression prevents meiotic cytokinesis [35, 36]. Worm eggs likewise require RhoA activity for polar body emission (our unpublished results; [37]). Thus meiotic cytokinesis in animal systems is also dependent on RhoA.
In mammalian somatic cells, it is less clear whether there is an absolute requirement for RhoA during cytokinesis. In some cases, RhoA is obviously required for cell division. For example, treatment of rat kidney epithelial cells or Mouse T lymphoma cells with C3 completely blocked cytokinesis [38, 39]. In addition, one study found that an isolate of NIH 3T3 cells became binucleate following treatment with C3 [40], and RhoAf/f LoxP flanked homozygous mouse embryonic fibroblasts also fail in cytokinesis when RhoA deletion is induced [41]. In other cases, however, RhoA activity may be dispensable for division depending on the degree of cell adhesion (see more below). For example, poorly adherent cells, such as HeLa cells and some NRK cells, display major defects in cytokinesis and contractile ring constriction when treated with C3, while more adherent cells treated with C3, including a separate NIH 3T3 isolate and Rat1A cells can divide successfully [38, 42]. Many of the cells that successfully divided following C3 treatment nevertheless displayed abnormalities during constriction and/or had some minor rate of cytokinesis failure, so cytokinesis likely is not “normal” without RhoA activity [38]. In tissue specific knockout mice, a role for RhoA in cytokinesis is equally confusing. That is, the deletion of RhoA does not perturb keratinocyte division in vivo, but leads to multi-nucleation when deleted in primary cultured keratinocytes from the same mice [43]. Thus a clear role for RhoA activity in mammalian somatic cell cytokinesis is debatable.
How can the differences in a requirement for RhoA in somatic cell cytokinesis be explained? One possibility is that mammalian RhoA paralogs RhoB and RhoC function redundantly with RhoA during cytokinesis. However, this would not explain the C3 results as this toxin inhibits RhoA, RhoB, and RhoC [23]. It is also possible that cytokinesis in some mammalian cell types depends more heavily on regulation by other Rho family small GTPases (see more below).
RhoA localizes to equatorial membrane before cell division occurs
The spatial and temporal localization of active (GTP-bound) RhoA suggests that it specifies the division plane. Immuno-localization studies have shown that RhoA protein accumulates at the division plane before the cell begins to divide in a number of mammalian cultured cell lines as well as in developing embryos across multiple phyla [44–49]. A zone of RhoA also predicts the division plane in monopolar cells dividing to produce anucleate cytoblasts [50]. The use of in vivo RhoA activity probes has confirmed an equatorial zone of active RhoA in the division plane precedes furrow formation in multiple cultured cell lines, echinoderm and Xenopus embryos [35, 51], and during polar body emission in Xenopus eggs [35, 52].
It is hypothesized that spindle microtubules dictate the site of cell division by specifying RhoA activation. Indeed, following microtubule depolymerization, GTP-bound RhoA does not properly localize to the cell equator [35, 53]. RhoA activity dynamics can also respond to the anaphase mitotic spindle. This was shown most elegantly with a micromanipulation assay akin to those done by Ray Rappaport [54], where the mitotic spindle was pushed with a small glass rod to displace it from the cell center leading to a corresponding displacement of the contractile ring [55]. Simultaneous monitoring of RhoA activity via a fluorescently tagged RhoA activity probe (GFP-Rhotekin Rho Binding Domain) revealed that the zone of RhoA activation moved in concert to the new position of the spindle and thus established a new division plane [35].
There are two sets of anaphase spindle microtubules that might activate RhoA in the equatorial plane: central spindle (or midzone) microtubules and astral microtubules. The central spindle is an anti-parallel array of highly stable microtubules that forms between the separating chromosomes in anaphase. Astral microtubules are dynamic microtubules that emanate circumferentially from the centrosomes. A subset of these astral microtubules grow from the chromosomal regions of the spindle towards the division plane and contact the equatorial cortex directly, and are more stable than most astral microtubules [56, 57]. It has been proposed that these more stable microtubules promote efficient cytokinesis via motor dependent delivery of a furrow-stimulating factor to the cell cortex at the division plane [53, 56–63]. Experiments by von Dassow et al., however, rule out the necessity for direct contact via astral microtubules, at least in cleavage stage echinoderm embryos. They used the tubulin deacetylase inhibitor Trichostatin A (TSA) to specifically disrupt dynamic astral microtubules while affecting neither anaphase onset nor central spindle assembly [64]. Without astral microtubule-mediated contact between the cortex and the spindle, cytokinesis was able to proceed, as did the assembly of an equatorial zone of RhoA activity, though the zone of RhoA activity was broader than in controls [64]. This experiment suggests the zone of active RhoA in this system can be specified without microtubules contacting the cell cortex. The molecular mechanisms that permit the spindle to activate RhoA from a distance will be an exciting area of study in the future.
RhoA GEFs and GAPs Occupy the Division Plane during Cytokinesis
Theoretical and in vivo studies have found that RhoA GTPase “flux” or cycling between GTP-RhoA and GDP-RhoA is critical to tightly focus a zone of active RhoA at the site of cell division [21, 65]. This spatial regulation of RhoA activity during cytokinesis is thought to be driven by targeted RhoA GEFs and GAPs, many of which associate with the central spindle and cell cortex within the division plane (Figure 1). The oncogenic Rho family GEF Ect2 (epithelial cell transforming sequence 2) is required for cytokinesis in a multitude of species across phyla. Ect2 localizes to the central spindle in Drosophila [58, 66, 67], Xenopus eggs undergoing polar body extrusion [36], and in mammalian cultured cells [48, 49, 60]. Ect2 associates with the central spindle by binding to MgcRacGAP, which forms a complex with the plus end directed kinesin MKLP1 (called Centralspindlin, see more below) [48, 49, 60, 67–69]. In C. elegans, RNAi-mediated depletion of ECT-2 disrupts the first embryonic cleavage [37, 70], and a hypomorphic ect-2 allele was shown to block cytokinesis in epidermal P cells [71]. Mutations in the Drosophila ortholog of Ect2 (Pebble) result in a failure to form a contractile ring and subsequent failure of cytokinesis [25]. Although in vitro Ect2 can function as a GEF for Cdc42, Rac1, and RhoA [72], disrupting Ect2 function phenotypically resembles RhoA disruption with a loss of contractile ring constriction. Therefore Ect2 is thought to predominantly function upstream of RhoA.
Figure 1. Rho GTPase signaling at the central spindle.

Active Rho GTPases form spatially distinct activity zones within dividing animal cells (upper). GTP-bound RhoA localizes to the division plane. GTP-bound Rac*, on the other hand, has been found to be enriched in the polar regions of the cell and excluded from the division plane. Equatorial Rho family GTPase activity is regulated by GEFs and GAPs, many of which localize to the microtubule-rich central spindle (lower, close-up view). Together, MKLP1 and MgcRacGAP form a complex (called Centralspindlin) that participates in bundling of central spindle microtubules. *The polar enrichment of active Rac during cytokinesis has only been shown in cultured mammalian cells. **Cdc42 activity has been shown to be enriched in the polar region of one side of the cell during asymmetric cell divisions but has not been closely examined during symmetric divisions.
While Ect2 appears to be the primary regulator of RhoA activation during cytokinesis, other GEFs are likely to assist with RhoA activation to ensure efficient division, at least in cultured human cells. Like Ect2, MyoGEF also localizes to the central spindle, is required for cytokinesis, and promotes activation of RhoA in U2OS and HeLa cell lines [73, 74]. Another central spindle associated GEF, GEF-H1, has also been shown to participate in RhoA activation. Disruption of GEF-H1 leads to ectopic blebbing and furrowing, and increased cytokinesis failure in HeLa cells [75]. The RhoA GEF Vav3 is transiently upregulated during mitosis, and over-expression of Vav3 disrupts cytokinesis in a RhoA dependent manner [76]. How all of these RhoA GEFs work together to promote efficient cytokinesis, however, is not well understood.
Two GAPs have been implicated in regulating RhoA activity during cytokinesis to date: p190RhoGAP and MgcRacGAP. p190RhoGAP localizes to the contractile ring in a breast cancer cell line [77]. In vitro, p190RhoGAP can inactivate RhoA, Rac1, and Cdc42 [78]; however, during cytokinesis p190RhoGAP has been proposed to predominantly regulate RhoA [79, 80]. In support of this model, experiments with FRET-based activity probes for RhoA have shown that dominant negative p190RhoGAP expression increases the levels of active RhoA, while expression of control p190RhoGAP reduces the levels of active RhoA [80]. As would be expected with a RhoA GAP, the overexpression of p190RhoGAP blocks cytokinesis [77]. Further, endogenous p190RhoGAP degradation is required for cytokinesis [81], and this reduction in p190RhoGAP levels may also help promote activation of RhoA during cell division.
Although MgcRacGAP (male germ cell Rac GAP) is clearly essential for cytokinesis [27, 28, 48, 49, 60, 65, 68, 69, 82–89], a role for MgcRacGAP in directly regulating RhoA is highly controversial. MgcRacGAP is targeted to the central spindle via association with the plus-end directed kinesin ZEN-4/MKLP1 (Figure 1) [90]. Disrupting this association leads to cytokinesis failure in most metazoan systems [28, 49, 58, 90]. Further, artificially targeting the fly ortholog of MgcRacGAP (RacGAP50C) to the cell membrane in S2 cells by fusing it to an integral membrane protein leads to ectopic furrowing at sites of enrichment [84]. Thus MgcRacGAP is not only required but also sufficient in some systems for cytokinetic furrow formation.
The controversy over a role for MgcRacGAP in regulating RhoA during cytokinesis stems from extensive in vitro data that shows MgcRacGAP has strong GAP activity for Cdc42 and Rac1, but not for RhoA [27, 87, 91, 92]. Because of the importance of RhoA during cytokinesis, however, MgcRacGAP was first proposed to predominantly control RhoA activity [27, 58, 82], perhaps by promoting GTP turnover [21, 65]. One study in HeLa cells suggested that phosphorylation of the GAP domain of MgcRacGAP at S387 in anaphase by Aurora-B kinase can switch the specificity of the GAP activity from Rac1 to RhoA [93]. This model is contentious, however, as S387 is not conserved across phyla. Recently, the Aurora-B specificity switch model has been directly challenged, as MgcRacGAP was found to maintain higher specificity towards Rac1 and Cdc42 with and without inhibition of Aurora-B, CDK1, or Polo kinase activity in HeLa cells [87]. Moreover, the S387D mutant proposed to mimic the Aurora-B driven switch [93], was found to eliminate all GAP activity rather than increase the specificity for RhoA [87].
In support of a role for MgcRacGAP in RhoA regulation, expression of CYK-4/MgcRacGAP bearing mutations in, or deletion of, the GAP domain broadens the equatorially associated zone of RhoA activity and blocks cytokinesis in dividing Xenopus embryos and cultured mammalian cells [42, 65]. However, genetically in both flies and worms, RhoA disruption enhances GAP dead MgcRacGAP mutants, indicating RhoA activation probably functions in parallel to MgcRacGAP activity [28, 87, 94] (see more below). In other cellular contexts RhoA activity is highly interdependent upon both Rac1 and Cdc42 activity (e.g. [16]). Therefore the effect of disrupting MgcRacGAP activity on RhoA during cytokinesis may not be direct. A recent publication proposed a complicated role for MgcRacGAP in activating RhoA via regulation of the GEF Ect2 [86], but there is currently no evidence that the GAP domain of MgcRacGAP can regulate Ect2 in vivo or in vitro. Undoubtedly the search for a clear and specific RhoA GAP with a conserved role in cytokinesis will be the subject of future research.
RhoA Effectors during Cytokinesis
During cytokinesis, RhoA functions upstream of both the assembly and constriction of an actomyosin contractile ring (Figure 2). RhoA activity elicits these events by simultaneously triggering both filamentous actin assembly and myosin-II motor activation [19]. RhoA promotes filamentous actin assembly via activation of the diaphanous-related formins, releasing these proteins from an auto-inhibited state [95, 96]. Once activated, diaphanous-related formins nucleate and elongate the linear actin filaments required for the formation of a contractile ring [97–99]. To trigger myosin-II activation, RhoA is upstream of two key kinases: 1) Rho-associated protein kinase (ROCK) and 2) Citron kinase. ROCK and Citron [100] are related serine-threonine kinases that modulate actomyosin-based contractility by phosphorylating myosin light chain (MLC) and activating myosin-II filament assembly and thus the motor activity of myosin-II [101, 102]. Active myosin-II binds to diaphanous-related formin-generated actin filaments and drives constriction of these filaments, much like the sliding filament model defining the contraction of muscle cells. ROCK also inhibits myosin light chain phosphatase, which would otherwise dephosphorylate MLC and inhibit myosin-II motor activity [103]. In addition, ROCK activates LIM kinase, which phosphorylates and inactivates the actin depolymerizing factor cofilin prior to mitosis, thus stabilizing actin filaments [104, 105]. In this way, RhoA is thought to stimulate both the assembly and constriction of an actomyosin contractile ring.
Figure 2. Rho GTPase signaling transduction pathways during cytokinesis.
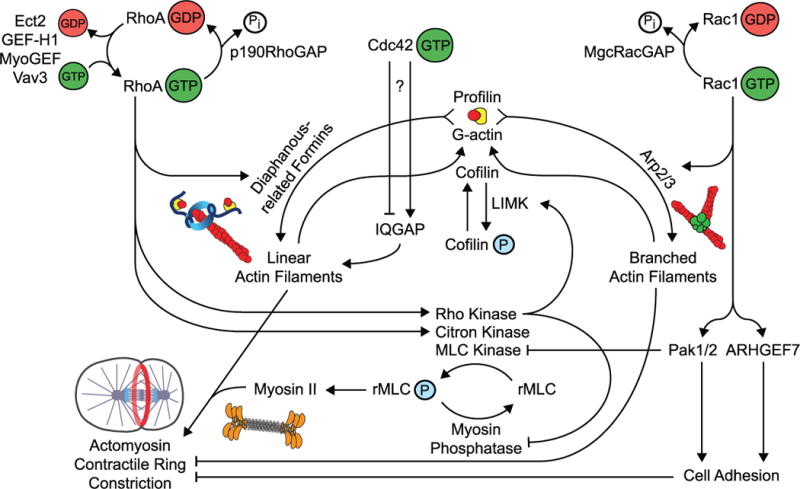
Rho GTPase regulators drive the accumulation of active, GTP-bound RhoA and inactive GDP-bound Rac1. Active RhoA promotes cytokinesis by coordinating diaphanous related formin (DRF)-mediated formation of linear filamentous actin with contractile force driven by myosin-II motor activity. Active Rac1 inhibits cytokinesis by promoting Arp2/3 complex-mediated assembly of branched actin and preventing the disassembly of equatorial cell adhesion. Thus MgcRacGAP inactivates Rac1 within the division plane. The only potential downstream effector of Cdc42 during cytokinesis described thus far is IQGAP, but the role of Cdc42 during cytokinesis is still unclear.
The role of Citron Kinase during cytokinesis is less well conserved, and binding of Citron to RhoA does not seem to depend on GTP association [106]. Disruption of Citron in cultured mammalian cells or Drosophila S2 cells and neuroblasts results in a late cytokinesis defect with reduced levels of RhoA at the midbody [106], but does not block assembly or constriction of the contractile ring [46, 106–109]. Throughout Drosophila development, Citron kinase is required for cytokinesis in many tissues [94, 110]. However, Citron knockout mice and rats develop normally, and cytokinesis defects are predominantly observed in neuronal cells [111–114] and developing spermatocytes [115]. Thus, outside of flies, Citron likely plays a more specialized role or functions redundantly with other myosin-II activators during cytokinesis in a tissue specific manner.
Rac1: A Negative Regulator of Cytokinesis
Unlike RhoA, Rac1 has been proposed to negatively regulate contractile ring constriction during cytokinesis (Figure 2) [116]. During interphase, Rac1 activity stimulates membrane protrusion at the leading edge of the cell [10] by promoting actin nucleation through WAVE-mediated activation of the Arp2/3 complex, leading to the formation of a dynamic branched actin network [117]. During cytokinesis, ectopic activation of Rac1 disrupts division. In dividing mammalian cultured HeLa, Rat1A, NIH3T3, and NRK cell lines, expression of constitutively active Rac1 induces multinucleation [42]. Further, a FRET reporter for Rac1 activity in HeLa cells showed a reduction in activity at the division plane with increased signal in the polar cortical regions [51]. As expected for a negative regulator, the expression of dominant negative Rac1 does not block cytokinesis [42], nor does disrupting Rac1 activity by RNAi or mutation [27, 28, 86, 94]. Thus, Rac1 likely functions as a negative regulator during cell division.
Rac1 activity during cytokinesis is thought to be kept in check by the central spindle localized GAP MgcRacGAP (see also above). In C. elegans, the suppression of Rac1 activity by mutation or RNAi can rescue cytokinesis failure caused by reduced GAP activity alleles of MgcRacGAP (cyk-4 in worms) [28, 86]. Similarly, in Drosophila, a tissue specific mutant lacking all three fly Rac isoforms can also suppress the rough eye phenotype caused by a loss of RacGAP50C (fly MgcRacGAP ortholog) or Sticky (Citron Kinase ortholog) function [94]. Further, in HeLa cells expressing a GAP dead version of MgcRacGAP, Rac1 depletion can rescue the cytokinesis failure [87]. Lastly, in HeLa cells expressing GAP-dead MgcRacGAP, Rac1 activity remained high at the division plane during cytokinesis failure [42] (Figure 1). Thus, in contrast to RhoA, where inactivation of RhoA leads to inhibition of cytokinesis, the inactivation of Rac1 seems to be essential for cytokinesis to properly occur (Figure 2).
What are the Rac1 effectors targeted for inactivation during cytokinesis? Genetic analysis in C. elegans embryos suggests that Rac1 must be inactivated to prevent activation of the Arp2/3 complex, which nucleates branched actin filaments [28]. In humans, a dominant activating mutation in WASp (another activator of the Arp2/3 complex) leads to neutropenia due to cytokinesis failure during neutrophil proliferation, further suggesting that Arp2/3 complex activation is inhibitory to division [118]. It is possible that this branched actin competes with diaphanous-related formins for the “ingredients” required to assemble filamentous actin (such as profilin, G-actin, actin binding proteins). Equally possible is that branched actin is simply a poor substrate for myosin-II mediated constriction and therefore the Arp2/3 complex must be held inactive [119]. Rac1 inactivation by MgcRacGAP may also function to keep the activity of p21-activated kinases (PAKs) low during cytokinesis. Expression of constitutively active Pak1, a Rac1 effector that phosphorylates and suppresses myosin light chain kinase (MLCK), blocks cytokinesis when expressed in HeLa cells [42]. MLCK, an activator of myosin-II, localizes to the division plane, and participates in cytokinesis [120]. Thus Rac1 is a critical negative regulator of cytokinesis as multiple effectors act in opposition to efficient actomyosin ring constriction, perhaps by inhibiting contractility in the polar regions of the cell where Rac1 activity is enriched [42, 51].
Rac1 inactivation is likely also required to locally inhibit cell adhesion at the division plane in adherent HeLa cells [87, 121]. In a recent paper, ectopic GAP-dead MgcRacGAP expression resulted in cytokinesis failure, and division could be rescued by depleting Rac1 or the downstream effectors Pak1/2 and ARHGEF7, two regulators of cell adhesion [87]. Further, expression of constitutively active Rac1 or GAP-dead MgcRacGAP led to ectopic cell adhesions in the division plane [87], suggesting that MgcRacGAP-dependent Rac1 inhibition also facilitates contractile ring constriction by blocking equatorial cell adhesion [87, 121]. It is possible that different species or cell types use Rac1 inhibition to disable specific pathways during cytokinesis, and that other Rac1 activated pathways yet to be discovered are also negatively regulated during division.
The exception to the rule that Rac1 is inhibitory to cytokinesis may be during meiosis in mouse oocytes (for review see [33]). In mouse oocytes undergoing meiosis, expression of a dominant negative Rac1 construct blocks cytokinesis and polar body emission [122]. However, this dominant negative Rac1 also disrupts the asymmetric meiotic spindle anchoring to the cortex, a step required for polar body emission. Therefore, the effect on meiotic cytokinesis may be indirect [122]. In fact disrupting Rac1 activity after spindle anchoring does not affect cytokinesis in Xenopus eggs [52]; hence it is not clear if Rac1 activation is required for cytokinesis in maternal meiosis.
Cdc42 during cytokinesis
Whether Cdc42 functions as a clear positive or negative regulator during all metazoan cytokinetic events is debatable, but there is evidence supporting a positive role for Cdc42 during polar body emission. In most animal systems, simply disrupting Cdc42 activity (by RNAi for example) does not block cytokinesis [26–28, 123]. However, in many systems, constitutive activation of Cdc42 prevents division. In HeLa cells and early Drosophila embryos, constitutive activation of Cdc42 leads to giant, multinucleated cells indicative of multiple rounds of cytokinesis failure [26, 124]. In Xenopus embryos, injection of either dominant negative or constitutively active forms of Cdc42 block cytokinesis. However the dominant negative injection is less penetrant, and very high protein levels are required for the effect (>10μM in the embryo) [24]. Constitutive activation of Cdc42 in rat kidney epithelial cells also results in cytokinesis failure [125]. On the other hand, Rac1 and Cdc42 have some overlap in effectors (e.g. Pak1), thus disrupting the balance of Cdc42 within the cell could lead to non-specific activation of Rac1 effectors and induce cytokinesis failure. In HeLa cells, Cdc42 depletion leads to a reduction of f-actin and a broader zone of RhoA at the cell equator; however, even in this case cytokinesis still proceeds [125].
The localization and activity of Cdc42 during cytokinesis has not been studied in detail in animal cells. In asymmetrically dividing cells, Cdc42 is enriched in a polar cortical cap on one side of the cell where it functions in maintaining cell polarity [126–131], and this polar cap of CDC-42 is active, at least in C. elegans embryos [132]. Immuno-staining of cleaving mouse embryos revealed that Cdc42 localizes to the cortex during interphase, the mitotic spindle during mitosis, and the central spindle and midbody during cytokinesis [133]. A FRET-based activity probe for Cdc42 in dividing HeLa cells showed that Cdc42 activity is high at the plasma membrane during interphase, decreases upon entry into mitosis, and upon anaphase is suppressed at the membrane and increases at intracellular membrane compartments [51]. Taken together, a clear role for Cdc42 in regulating mitotic cytokinesis has not been shown.
On the other hand, Cdc42 activity is essential for polar body emission during animal cell meiosis [31–33]. In meiotic Xenopus eggs, expression of a dominant negative Cdc42 prevents polar body emission [36, 52, 134]. In this system, a cap of active Cdc42 is encircled by a zone of active RhoA, which marks the site of contractile ring assembly, and the proper localization of both of these GTPases is required for meiotic divisions in the oocyte [36]. The cap of active Cdc42 is thought to promote local activation of the Arp2/3 complex above the extruding polar body [134]. Cdc42 is also essential for polar body emission in mouse oocytes [36, 133, 135].
Polar body emission is one of the most extreme examples of asymmetric division throughout animal development, producing one large oocyte and a very small polar body in both meiosis I and II. Perhaps this cortical cap of Cdc42 activity at the top of the polar body functions to minimize the forces required for these highly polarized divisions. Indeed, Cdc42 is required to maintain cell polarity during asymmetric cell division in a multitude of animal systems (see above). It may be that Cdc42 plays a more specified role in positively regulating asymmetric cytokinetic events.
IQGAP, a scaffolding protein with actin stabilizing activity named for IQ motifs and a GAP-like domain (which lacks enzymatic activity), has been proposed to be downstream of Cdc42 activity during mouse meiotic cytokinesis (for review on IQGAP proteins see [136]). In worm zygotes, IQGAP is required for polar body emission [137]. However, in mouse oocytes undergoing meiosis, IQGAP localizes to a contractile ring around the base of the polar body during cytokinesis where RhoA is active [133], and is not enriched at the polar body cortex where active Cdc42 was shown to be enriched in Xenopus eggs [36]. In mitotic cytokinesis, IQGAP also localizes to the contractile ring [133, 138] where RhoA is likely the predominantly active Rho family small GTPase. Therefore, it is unclear if IQGAP is a Cdc42 effector during cytokinesis in animal cells.
Summary
Though Rho GTPases represent less than 1% of the human genome, these small proteins exert disproportionate control over cytokinesis [18]. Crucial to this master regulatory role is their occupation of specific regions within the cell at specific times during cytokinesis to ensure the assembly of a contractile ring at the proper time and place. RhoA occupies the division plane and is the main positive Rho family regulator of cytokinesis. At the division plane, RhoA stimulates both linear actin assembly and myosin-II motor activity to promote the assembly and constriction of a contractile ring. In contrast, Rac1 is a negative regulator of cytokinesis in most metazoan systems, and preliminary studies have shown active Rac1 is excluded from the division plane but occupies the cell poles. Rac1 inhibition at the division plane is important to prevent Arp2/3 complex activation and to disassemble cell adhesions to allow for contractile ring constriction. The function and spatial regulation of Cdc42 activity is less well understood, but thus far, it seems Cdc42 may contribute positively to highly asymmetric meiotic divisions. The intricate and highly spatially regulated interplay among all Rho family GTPases is essential for a cell to efficiently execute a multitude of behaviors, and it seems cytokinesis is no exception.
The future of understanding how Rho family small GTPases contribute to the spatial regulation of cytokinesis will be to: 1) determine the localization of active Rac1 and Cdc42 during cytokinesis at high resolution in multiple model systems, 2) identify the GEFs that activate Cdc42 and Rac1 and the GAPs that inactivate Cdc42 and RhoA activity during division, 3) understand the functional coordination among Rho family GTPases throughout cytokinesis and 4) discover the cytokinetic downstream effectors that mediate Rho GTPase family function, especially for Rac1 and Cdc42. It is undoubtedly an exciting time in the field.
Dedication
We dedicate this review to the late Ray Rappaport and his wife Barbara Rappaport, the godparents of cytokinesis. Their inspirational work has served as the foundation for all of us who appreciate the beauty and complexity of studying cell division.
Acknowledgments
We thank all members of the Canman lab for their support, and are grateful to Tim Davies, Mimi Shirasu-Hiza, Amy Maddox, Bill Bement, and the anonymous reviewers for critical comments on this manuscript.
References
- 1.Madaule P, Axel R. A novel ras-related gene family. Cell. 1985;41:31–40. doi: 10.1016/0092-8674(85)90058-3. [DOI] [PubMed] [Google Scholar]
- 2.Bender A, Pringle JR. Multicopy suppression of the cdc24 budding defect in yeast by CDC42 and three newly identified genes including the ras-related gene RSR1. Proc Natl Acad Sci U S A. 1989;86:9976–9980. doi: 10.1073/pnas.86.24.9976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson DI, Pringle JR. Molecular characterization of CDC42, a Saccharomyces cerevisiae gene involved in the development of cell polarity. J Cell Biol. 1990;111:143–152. doi: 10.1083/jcb.111.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Didsbury J, Weber RF, Bokoch GM, Evans T, Snyderman R. rac, a novel ras-related family of proteins that are botulinum toxin substrates. J Biol Chem. 1989;264:16378–16382. [PubMed] [Google Scholar]
- 5.Rathinam R, Berrier A, Alahari SK. Role of Rho GTPases and their regulators in cancer progression. Front Biosci. 2012;17:2561–2571. doi: 10.2741/3872. [DOI] [PubMed] [Google Scholar]
- 6.Ellenbroek SI, Collard JG. Rho GTPases: functions and association with cancer. Clinical & experimental metastasis. 2007;24:657–672. doi: 10.1007/s10585-007-9119-1. [DOI] [PubMed] [Google Scholar]
- 7.Tang Y, Olufemi L, Wang MT, Nie D. Role of Rho GTPases in breast cancer. Front Biosci. 2008;13:759–776. doi: 10.2741/2718. [DOI] [PubMed] [Google Scholar]
- 8.Buongiorno P, Bapat B. Rho GTPases and cancer. Prog Mol Subcell Biol. 2005;40:29–53. doi: 10.1007/3-540-27671-8_2. [DOI] [PubMed] [Google Scholar]
- 9.Mardilovich K, Olson MF, Baugh M. Targeting Rho GTPase signaling for cancer therapy. Future Oncol. 2012;8:165–177. doi: 10.2217/fon.11.143. [DOI] [PubMed] [Google Scholar]
- 10.Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- 11.Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 12.Gundersen GG, Wen Y, Eng CH, Schmoranzer J, Cabrera-Poch N, Morris EJ, Chen M, Gomes ER. Regulation of microtubules by Rho GTPases in migrating cells. Novartis Found Symp. 2005;269:106–116. discussion 116–126, 223–130. [PubMed] [Google Scholar]
- 13.Grigoriev I, Borisy G, Vorobjev I. Regulation of microtubule dynamics in 3T3 fibroblasts by Rho family GTPases. Cell Motil Cytoskeleton. 2006;63:29–40. doi: 10.1002/cm.20107. [DOI] [PubMed] [Google Scholar]
- 14.Waterman-Storer CM, Worthylake RA, Liu BP, Burridge K, Salmon ED. Microtubule growth activates Rac1 to promote lamellipodial protrusion in fibroblasts. Nat Cell Biol. 1999;1:45–50. doi: 10.1038/9018. [DOI] [PubMed] [Google Scholar]
- 15.Wittmann T, Waterman-Storer CM. Cell motility: can Rho GTPases and microtubules point the way? J Cell Sci. 2001;114:3795–3803. doi: 10.1242/jcs.114.21.3795. [DOI] [PubMed] [Google Scholar]
- 16.Machacek M, Hodgson L, Welch C, Elliott H, Pertz O, Nalbant P, Abell A, Johnson GL, Hahn KM, Danuser G. Coordination of Rho GTPase activities during cell protrusion. Nature. 2009;461:99–103. doi: 10.1038/nature08242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia-Mata R, Boulter E, Burridge K. The ‘invisible hand’:regulation of RHO GTPases by RHOGDIs. Nat Rev Mol Cell Biol. 2011;12:493–504. doi: 10.1038/nrm3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- 19.Piekny A, Werner M, Glotzer M. Cytokinesis: welcome to the Rho zone. Trends Cell Biol. 2005;15:651–658. doi: 10.1016/j.tcb.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 20.Kishi K, Sasaki T, Kuroda S, Itoh T, Takai Y. Regulation of cytoplasmic division of Xenopus embryo by rho p21 and its inhibitory GDP/GTP exchange protein (rho GDI) J Cell Biol. 1993;120:1187–1195. doi: 10.1083/jcb.120.5.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bement WM, Miller AL, Von Dassow G. Rho GTPase activity zones and transient contractile arrays. Bioessays. 2006;28:983–993. doi: 10.1002/bies.20477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boulter E, Garcia-Mata R, Guilluy C, Dubash A, Rossi G, Brennwald PJ, Burridge K. Regulation of Rho GTPase crosstalk, degradation and activity by RhoGDI1. Nat Cell Biol. 2010;12:477–483. doi: 10.1038/ncb2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilde C, Aktories K. The Rho-ADP-ribosylating C3 exoenzyme from Clostridium botulinum and related C3-like transferases. Toxicon: official journal of the International Society on Toxinology. 2001;39:1647–1660. doi: 10.1016/s0041-0101(01)00152-0. [DOI] [PubMed] [Google Scholar]
- 24.Drechsel DN, Hyman AA, Hall A, Glotzer M. A requirement for Rho and Cdc42 during cytokinesis in Xenopus embryos. Curr Biol. 1997;7:12–23. doi: 10.1016/s0960-9822(06)00023-6. [DOI] [PubMed] [Google Scholar]
- 25.Prokopenko SN, Brumby A, O’keefe L, Prior L, He Y, Saint R, Bellen HJ. A putative exchange factor for Rho1 GTPase is required for initiation of cytokinesis in Drosophila. Genes & Development. 1999;13:2301–2314. doi: 10.1101/gad.13.17.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crawford JM, Harden N, Leung T, Lim L, Kiehart DP. Cellularization in Drosophila melanogaster is disrupted by the inhibition of rho activity and the activation of Cdc42 function. Dev Biol. 1998;204:151–164. doi: 10.1006/dbio.1998.9061. [DOI] [PubMed] [Google Scholar]
- 27.Jantsch-Plunger V, Gönczy P, Romano A, Schnabel H, Hamill D, Schnabel R, Hyman AA, Glotzer M. CYK-4: A Rho family gtpase activating protein (GAP) required for central spindle formation and cytokinesis. The Journal of Cell Biology. 2000;149:1391–1404. doi: 10.1083/jcb.149.7.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Canman JC, Lewellyn L, Laband K, Smerdon SJ, Desai A, Bowerman B, Oegema K. Inhibition of Rac by the GAP activity of centralspindlin is essential for cytokinesis. Science. 2008;322:1543–1546. doi: 10.1126/science.1163086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lai SL, Chang CN, Wang PJ, Lee SJ. Rho mediates cytokinesis and epiboly via ROCK in zebrafish. Mol Reprod Dev. 2005;71:186–196. doi: 10.1002/mrd.20290. [DOI] [PubMed] [Google Scholar]
- 30.Moore GD, Ayabe T, Visconti PE, Schultz RM, Kopf GS. Roles of heterotrimeric and monomeric G proteins in sperm-induced activation of mouse eggs. Development. 1994;120:3313–3323. doi: 10.1242/dev.120.11.3313. [DOI] [PubMed] [Google Scholar]
- 31.Maddox AS, Azoury J, Dumont J. Polar body cytokinesis. Cytoskeleton (Hoboken) 2012 doi: 10.1002/cm.21064. [DOI] [PubMed] [Google Scholar]
- 32.Liu XJ. Polar body emission. Cytoskeleton (Hoboken) 2012 doi: 10.1002/cm.21041. [DOI] [PubMed] [Google Scholar]
- 33.Verlhac MH, Dumont J. Interactions between chromosomes, microfilaments and microtubules revealed by the study of small GTPases in a big cell, the vertebrate oocyte. Molecular and cellular endocrinology. 2008;282:12–17. doi: 10.1016/j.mce.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 34.Zhong ZS, Huo LJ, Liang CG, Chen DY, Sun QY. Small GTPase RhoA is required for ooplasmic segregation and spindle rotation, but not for spindle organization and chromosome separation during mouse oocyte maturation, fertilization, and early cleavage. Mol Reprod Dev. 2005;71:256–261. doi: 10.1002/mrd.20253. [DOI] [PubMed] [Google Scholar]
- 35.Bement WM, Benink HA, Von Dassow G. A microtubule-dependent zone of active RhoA during cleavage plane specification. The Journal of Cell Biology. 2005;170:91–101. doi: 10.1083/jcb.200501131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang X, Ma C, Miller AL, Katbi HA, Bement WM, Liu XJ. Polar body emission requires a RhoA contractile ring and Cdc42-mediated membrane protrusion. Dev Cell. 2008;15:386–400. doi: 10.1016/j.devcel.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sönnichsen B, Koski LB, Walsh A, Marschall P, Neumann B, Brehm M, Alleaume AM, Artelt J, Bettencourt P, Cassin E, et al. Full-genome RNAi profiling of early embryogenesis in Caenorhabditis elegans. Nature. 2005;434:462–469. doi: 10.1038/nature03353. [DOI] [PubMed] [Google Scholar]
- 38.O’Connell CB, Wheatley SP, Ahmed S, Wang YL. The small GTP-binding protein rho regulates cortical activities in cultured cells during division. J Cell Biol. 1999;144:305–313. doi: 10.1083/jcb.144.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moorman JP, Bobak DA, Hahn CS. Inactivation of the small GTP binding protein Rho induces multinucleate cell formation and apoptosis in murine T lymphoma EL4. J Immunol. 1996;156:4146–4153. [PubMed] [Google Scholar]
- 40.Rubin EJ, Gill DM, Boquet P, Popoff MR. Functional modification of a 21-kilodalton G protein when ADP-ribosylated by exoenzyme C3 of Clostridium botulinum. Mol Cell Biol. 1988;8:418–426. doi: 10.1128/mcb.8.1.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Melendez J, Stengel K, Zhou X, Chauhan BK, Debidda M, Andreassen P, Lang RA, Zheng Y. RhoA GTPase is dispensable for actomyosin regulation but is essential for mitosis in primary mouse embryonic fibroblasts. J Biol Chem. 2011;286:15132–15137. doi: 10.1074/jbc.C111.229336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoshizaki H. Cell Type-specific Regulation of RhoA Activity during Cytokinesis. Journal of Biological Chemistry. 2004;279:44756–44762. doi: 10.1074/jbc.M402292200. [DOI] [PubMed] [Google Scholar]
- 43.Jackson B, Peyrollier K, Pedersen E, Basse A, Karlsson R, Wang Z, Lefever T, Ochsenbein AM, Schmidt G, Aktories K, et al. RhoA is dispensable for skin development, but crucial for contraction and directed migration of keratinocytes. Mol Biol Cell. 2011;22:593–605. doi: 10.1091/mbc.E09-10-0859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takaishi K, Sasaki T, Kameyama T, Tsukita S, Takai Y. Translocation of activated Rho from the cytoplasm to membrane ruffling area, cell-cell adhesion sites and cleavage furrows. Oncogene. 1995;11:39–48. [PubMed] [Google Scholar]
- 45.Yonemura S, Hirao-Minakuchi K, Nishimura Y. Rho localization in cells and tissues. Exp Cell Res. 2004;295:300–314. doi: 10.1016/j.yexcr.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 46.Madaule P, Eda M, Watanabe N, Fujisawa K, Matsuoka T, Bito H, Ishizaki T, Narumiya S. Role of citron kinase as a target of the small GTPase Rho in cytokinesis. Nature. 1998;394:491–494. doi: 10.1038/28873. [DOI] [PubMed] [Google Scholar]
- 47.Nishimura Y, Nakano K, Mabuchi I. Localization of Rho GTPase in sea urchin eggs. FEBS Lett. 1998;441:121–126. doi: 10.1016/s0014-5793(98)01531-2. [DOI] [PubMed] [Google Scholar]
- 48.Kamijo K, Ohara N, Abe M, Uchimura T, Hosoya H, Lee JS, Miki T. Dissecting the role of Rho-mediated signaling in contractile ring formation. Mol Biol Cell. 2006;17:43–55. doi: 10.1091/mbc.E05-06-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yüce O, Piekny A, Glotzer M. An ECT2-centralspindlin complex regulates the localization and function of RhoA. The Journal of Cell Biology. 2005;170:571–582. doi: 10.1083/jcb.200501097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hu CK, Coughlin M, Field CM, Mitchison TJ. Cell polarization during monopolar cytokinesis. The Journal of Cell Biology. 2008;181:195–202. doi: 10.1083/jcb.200711105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yoshizaki H. Activity of Rho-family GTPases during cell division as visualized with FRET-based probes. The Journal of Cell Biology. 2003;162:223–232. doi: 10.1083/jcb.200212049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ma C, Benink HA, Cheng D, Montplaisir V, Wang L, Xi Y, Zheng PP, Bement WM, Liu XJ. Cdc42 activation couples spindle positioning to first polar body formation in oocyte maturation. Curr Biol. 2006;16:214–220. doi: 10.1016/j.cub.2005.11.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Murthy K, Wadsworth P. Dual role for microtubules in regulating cortical contractility during cytokinesis. Journal of Cell Science. 2008;121:2350–2359. doi: 10.1242/jcs.027052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rappaport R. Cytokinesis in Animal Cells. Cambridge University Press; Cambridge, UK: 1996. [Google Scholar]
- 55.Rappaport R. Repeated furrow formation from a single mitotic apparatus in cylindrical sand dollar eggs. J Exp Zool. 1985;234:167–171. doi: 10.1002/jez.1402340120. [DOI] [PubMed] [Google Scholar]
- 56.Canman JC, Cameron LA, Maddox PS, Straight A, Tirnauer JS, Mitchison TJ, Fang G, Kapoor TM, Salmon ED. Determining the position of the cell division plane. Nature. 2003;424:1074–1078. doi: 10.1038/nature01860. [DOI] [PubMed] [Google Scholar]
- 57.Foe VE, von Dassow G. Stable and dynamic microtubules coordinately shape the myosin activation zone during cytokinetic furrow formation. J Cell Biol. 2008;183:457–470. doi: 10.1083/jcb.200807128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Somers WG, Saint R. A RhoGEF and Rho family GTPase-activating protein complex links the contractile ring to cortical microtubules at the onset of cytokinesis. Dev Cell. 2003;4:29–39. doi: 10.1016/s1534-5807(02)00402-1. [DOI] [PubMed] [Google Scholar]
- 59.Rappaport R. Cytokinesis in animal cells. Int Rev Cytol. 1971;31:169–213. doi: 10.1016/s0074-7696(08)60059-5. [DOI] [PubMed] [Google Scholar]
- 60.Nishimura Y, Yonemura S. Centralspindlin regulates ECT2 and RhoA accumulation at the equatorial cortex during cytokinesis. Journal of Cell Science. 2006;119:104–114. doi: 10.1242/jcs.02737. [DOI] [PubMed] [Google Scholar]
- 61.Shannon KB, Canman JC, Ben Moree C, Tirnauer JS, Salmon ED. Taxol-stabilized microtubules can position the cytokinetic furrow in mammalian cells. Molecular Biology of the Cell. 2005;16:4423–4436. doi: 10.1091/mbc.E04-11-0974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vale RD, Spudich JA, Griffis ER. Dynamics of myosin, microtubules, and Kinesin-6 at the cortex during cytokinesis in Drosophila S2 cells. The Journal of Cell Biology. 2009;186:727–738. doi: 10.1083/jcb.200902083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Odell GM, Foe VE. An agent-based model contrasts opposite effects of dynamic and stable microtubules on cleavage furrow positioning. The Journal of Cell Biology. 2008;183:471–483. doi: 10.1083/jcb.200807129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dassow GV, Verbrugghe KJC, Miller AL, Sider JR, Bement WM. Action at a distance during cytokinesis. The Journal of Cell Biology. 2009 doi: 10.1083/jcb.200907090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Miller AL, Bement WM. Regulation of cytokinesis by Rho GTPase flux. Nat Cell Biol. 2009;11:71–77. doi: 10.1038/ncb1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Somma MP, Fasulo B, Cenci G, Cundari E, Gatti M. Molecular dissection of cytokinesis by RNA interference in Drosophila cultured cells. Molecular Biology of the Cell. 2002;13:2448–2460. doi: 10.1091/mbc.01-12-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zavortink M, Contreras N, Addy T, Bejsovec A, Saint R. Tum/RacGAP50C provides a critical link between anaphase microtubules and the assembly of the contractile ring in Drosophila melanogaster. Journal of Cell Science. 2005;118:5381–5392. doi: 10.1242/jcs.02652. [DOI] [PubMed] [Google Scholar]
- 68.Wolfe BA, Takaki T, Petronczki M, Glotzer M. Polo-like kinase 1 directs assembly of the HsCyk-4 RhoGAP/Ect2 RhoGEF complex to initiate cleavage furrow formation. PLoS Biol. 2009;7:e1000110. doi: 10.1371/journal.pbio.1000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Burkard ME, Maciejowski J, Rodriguez-Bravo V, Repka M, Lowery DM, Clauser KR, Zhang C, Shokat KM, Carr SA, Yaffe MB, et al. Plk1 self-organization and priming phosphorylation of HsCYK-4 at the spindle midzone regulate the onset of division in human cells. PLoS Biol. 2009;7:e1000111. doi: 10.1371/journal.pbio.1000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schonegg S, Constantinescu AT, Hoege C, Hyman AA. The Rho GTPase-activating proteins RGA-3 and RGA-4 are required to set the initial size of PAR domains in Caenorhabditis elegans one-cell embryos. Proc Natl Acad Sci U S A. 2007;104:14976–14981. doi: 10.1073/pnas.0706941104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Morita K, Hirono K, Han M. The Caenorhabditis elegans ect-2 RhoGEF gene regulates cytokinesis and migration of epidermal P cells. EMBO Rep. 2005;6:1163–1168. doi: 10.1038/sj.embor.7400533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tatsumoto T, Xie X, Blumenthal R, Okamoto I, Miki T. Human ECT2 is an exchange factor for Rho GTPases, phosphorylated in G2/M phases, and involved in cytokinesis. J Cell Biol. 1999;147:921–928. doi: 10.1083/jcb.147.5.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wu D, Asiedu M, Adelstein RS, Wei Q. A novel guanine nucleotide exchange factor MyoGEF is required for cytokinesis. Cell Cycle. 2006;5:1234–1239. doi: 10.4161/cc.5.11.2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Asiedu M, Wu D, Matsumura F, Wei Q. Centrosome/spindle pole-associated protein regulates cytokinesis via promoting the recruitment of MyoGEF to the central spindle. Mol Biol Cell. 2009;20:1428–1440. doi: 10.1091/mbc.E08-01-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Birkenfeld J, Nalbant P, Bohl BP, Pertz O, Hahn KM, Bokoch GM. GEF-H1 modulates localized RhoA activation during cytokinesis under the control of mitotic kinases. Dev Cell. 2007;12:699–712. doi: 10.1016/j.devcel.2007.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fujikawa K, Inoue Y, Sakai M, Koyama Y, Nishi S, Funada R, Alt FW, Swat W. Vav3 is regulated during the cell cycle and effects cell division. Proc Natl Acad Sci U S A. 2002;99:4313–4318. doi: 10.1073/pnas.052715699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Su L. p190RhoGAP is cell cycle regulated and affects cytokinesis. The Journal of Cell Biology. 2003;163:571–582. doi: 10.1083/jcb.200308007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Settleman J, Albright CF, Foster LC, Weinberg RA. Association between GTPase activators for Rho and Ras families. Nature. 1992;359:153–154. doi: 10.1038/359153a0. [DOI] [PubMed] [Google Scholar]
- 79.Mikawa M, Su L, Parsons SJ. Opposing roles of p190RhoGAP and Ect2 RhoGEF in regulating cytokinesis. Cell Cycle. 2008;7:2003–2012. doi: 10.4161/cc.7.13.6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Su L, Pertz O, Mikawa M, Hahn K, Parsons SJ. p190RhoGAP negatively regulates Rho activity at the cleavage furrow of mitotic cells. Experimental Cell Research. 2009;315:1347–1359. doi: 10.1016/j.yexcr.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Manchinelly SA, Miller JA, Su L, Miyake T, Palmer L, Mikawa M, Parsons SJ. Mitotic down-regulation of p190RhoGAP is required for the successful completion of cytokinesis. J Biol Chem. 2010;285:26923–26932. doi: 10.1074/jbc.M110.103804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee JS, Kamijo K, Ohara N, Kitamura T, Miki T. MgcRacGAP regulates cortical activity through RhoA during cytokinesis. Exp Cell Res. 2004;293:275–282. doi: 10.1016/j.yexcr.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 83.Zhao W-m, Fang G. MgcRacGAP controls the assembly of the contractile ring and the initiation of cytokinesis. Proc Natl Acad Sci USA. 2005;102:13158–13163. doi: 10.1073/pnas.0504145102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.D’Avino PP, Savoian MS, Capalbo L, Glover DM. RacGAP50C is sufficient to signal cleavage furrow formation during cytokinesis. Journal of Cell Science. 2006;119:4402–4408. doi: 10.1242/jcs.03210. [DOI] [PubMed] [Google Scholar]
- 85.Maddox AS, Oegema K. Closing the GAP: a role for a RhoA GAP in cytokinesis. Mol Cell. 2003;11:846–848. doi: 10.1016/s1097-2765(03)00151-5. [DOI] [PubMed] [Google Scholar]
- 86.Loria A, Longhini KM, Glotzer M. The RhoGAP domain of CYK-4 has an essential role in RhoA activation. Curr Biol. 2012;22:213–219. doi: 10.1016/j.cub.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bastos RN, Penate X, Bates M, Hammond D, Barr FA. CYK4 inhibits Rac1-dependent PAK1 and ARHGEF7 effector pathways during cytokinesis. The Journal of Cell Biology. 2012;198:865–880. doi: 10.1083/jcb.201204107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yamada T, Hikida M, Kurosaki T. Regulation of cytokinesis by mgcRacGAP in B lymphocytes is independent of GAP activity. Exp Cell Res. 2006;312:3517–3525. doi: 10.1016/j.yexcr.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 89.Hirose K, Kawashima T, Iwamoto I, Nosaka T, Kitamura T. MgcRacGAP is involved in cytokinesis through associating with mitotic spindle and midbody. J Biol Chem. 2001;276:5821–5828. doi: 10.1074/jbc.M007252200. [DOI] [PubMed] [Google Scholar]
- 90.Mishima M, Kaitna S, Glotzer M. Central spindle assembly and cytokinesis require a kinesin-like protein/RhoGAP complex with microtubule bundling activity. Dev Cell. 2002;2:41–54. doi: 10.1016/s1534-5807(01)00110-1. [DOI] [PubMed] [Google Scholar]
- 91.Toure A, Dorseuil O, Morin L, Timmons P, Jegou B, Reibel L, Gacon G. MgcRacGAP, a new human GTPase-activating protein for Rac and Cdc42 similar to Drosophila rotundRacGAP gene product, is expressed in male germ cells. J Biol Chem. 1998;273:6019–6023. doi: 10.1074/jbc.273.11.6019. [DOI] [PubMed] [Google Scholar]
- 92.Kawashima T, Hirose K, Satoh T, Kaneko A, Ikeda Y, Kaziro Y, Nosaka T, Kitamura T. MgcRacGAP is involved in the control of growth and differentiation of hematopoietic cells. Blood. 2000;96:2116–2124. [PubMed] [Google Scholar]
- 93.Minoshima Y, Kawashima T, Hirose K, Tonozuka Y, Kawajiri A, Bao YC, Deng X, Tatsuka M, Narumiya S, May WS, Jr, et al. Phosphorylation by aurora B converts MgcRacGAP to a RhoGAP during cytokinesis. Dev Cell. 2003;4:549–560. doi: 10.1016/s1534-5807(03)00089-3. [DOI] [PubMed] [Google Scholar]
- 94.D’avino PP. Mutations in sticky lead to defective organization of the contractile ring during cytokinesis and are enhanced by Rho and suppressed by Rac. The Journal of Cell Biology. 2004;166:61–71. doi: 10.1083/jcb.200402157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Watanabe N, Madaule P, Reid T, Ishizaki T, Watanabe G, Kakizuka A, Saito Y, Nakao K, Jockusch BM, Narumiya S. p140mDia, a mammalian homolog of Drosophila diaphanous, is a target protein for Rho small GTPase and is a ligand for profilin. EMBO J. 1997;16:3044–3056. doi: 10.1093/emboj/16.11.3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Otomo T, Otomo C, Tomchick DR, Machius M, Rosen MK. Structural basis of Rho GTPase-mediated activation of the formin mDia1. Mol Cell. 2005;18:273–281. doi: 10.1016/j.molcel.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 97.Severson AF, Baillie DL, Bowerman B. A Formin Homology protein and a profilin are required for cytokinesis and Arp2/3-independent assembly of cortical microfilaments in C. elegans. Curr Biol. 2002;12:2066–2075. doi: 10.1016/s0960-9822(02)01355-6. [DOI] [PubMed] [Google Scholar]
- 98.Afshar K, Stuart B, Wasserman SA. Functional analysis of the Drosophila diaphanous FH protein in early embryonic development. Development. 2000;127:1887–1897. doi: 10.1242/dev.127.9.1887. [DOI] [PubMed] [Google Scholar]
- 99.Watanabe S, Okawa K, Miki T, Sakamoto S, Morinaga T, Segawa K, Arakawa T, Kinoshita M, Ishizaki T, Narumiya S. Rho and anillin-dependent control of mDia2 localization and function in cytokinesis. Mol Biol Cell. 2010;21:3193–3204. doi: 10.1091/mbc.E10-04-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yamashiro S, Totsukawa G, Yamakita Y, Sasaki Y, Madaule P, Ishizaki T, Narumiya S, Matsumura F. Citron kinase, a Rho-dependent kinase, induces di-phosphorylation of regulatory light chain of myosin II. Mol Biol Cell. 2003;14:1745–1756. doi: 10.1091/mbc.E02-07-0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kosako H, Yoshida T, Matsumura F, Ishizaki T, Narumiya S, Inagaki M. Rho-kinase/ROCK is involved in cytokinesis through the phosphorylation of myosin light chain and not ezrin/radixin/moesin proteins at the cleavage furrow. Oncogene. 2000;19:6059–6064. doi: 10.1038/sj.onc.1203987. [DOI] [PubMed] [Google Scholar]
- 102.Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J Biol Chem. 1996;271:20246–20249. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- 103.Piekny AJ, Mains PE. Rho-binding kinase (LET-502) and myosin phosphatase (MEL-11) regulate cytokinesis in the early Caenorhabditis elegans embryo. Journal of Cell Science. 2002;115:2271–2282. doi: 10.1242/jcs.115.11.2271. [DOI] [PubMed] [Google Scholar]
- 104.Geneste O, Copeland JW, Treisman R. LIM kinase and Diaphanous cooperate to regulate serum response factor and actin dynamics. The Journal of Cell Biology. 2002;157:831–838. doi: 10.1083/jcb.200203126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Amano T, Kaji N, Ohashi K, Mizuno K. Mitosis-specific activation of LIM motif-containing protein kinase and roles of cofilin phosphorylation and dephosphorylation in mitosis. J Biol Chem. 2002;277:22093–22102. doi: 10.1074/jbc.M201444200. [DOI] [PubMed] [Google Scholar]
- 106.Bassi ZI, Verbrugghe KJ, Capalbo L, Gregory S, Montembault E, Glover DM, D’Avino PP. Sticky/Citron kinase maintains proper RhoA localization at the cleavage site during cytokinesis. J Cell Biol. 2011;195:595–603. doi: 10.1083/jcb.201105136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Echard A, Hickson GRX, Foley E, O’Farrell PH. Terminal cytokinesis events uncovered after an RNAi screen. Curr Biol. 2004;14:1685–1693. doi: 10.1016/j.cub.2004.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Naim V, Imarisio S, Di Cunto F, Gatti M, Bonaccorsi S. Drosophila citron kinase is required for the final steps of cytokinesis. Molecular Biology of the Cell. 2004;15:5053–5063. doi: 10.1091/mbc.E04-06-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gai M, Camera P, Dema A, Bianchi F, Berto G, Scarpa E, Germena G, Di Cunto F. Citron kinase controls abscission through RhoA and anillin. Mol Biol Cell. 2011;22:3768–3778. doi: 10.1091/mbc.E10-12-0952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shandala T, Gregory SL, Dalton HE, Smallhorn M, Saint R. Citron kinase is an essential effector of the Pbl-activated Rho signalling pathway in Drosophila melanogaster. Development. 2004;131:5053–5063. doi: 10.1242/dev.01382. [DOI] [PubMed] [Google Scholar]
- 111.Di Cunto F, Imarisio S, Hirsch E, Broccoli V, Bulfone A, Migheli A, Atzori C, Turco E, Triolo R, Dotto GP, et al. Defective neurogenesis in citron kinase knockout mice by altered cytokinesis and massive apoptosis. Neuron. 2000;28:115–127. doi: 10.1016/s0896-6273(00)00090-8. [DOI] [PubMed] [Google Scholar]
- 112.LoTurco JJ, Sarkisian MR, Cosker L, Bai J. Citron kinase is a regulator of mitosis and neurogenic cytokinesis in the neocortical ventricular zone. Cereb Cortex. 2003;13:588–591. doi: 10.1093/cercor/13.6.588. [DOI] [PubMed] [Google Scholar]
- 113.Ackman JB, Ramos RL, Sarkisian MR, Loturco JJ. Citron kinase is required for postnatal neurogenesis in the hippocampus. Dev Neurosci. 2007;29:113–123. doi: 10.1159/000096216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Anastas SB, Mueller D, Semple-Rowland SL, Breunig JJ, Sarkisian MR. Failed cytokinesis of neural progenitors in citron kinase-deficient rats leads to multiciliated neurons. Cereb Cortex. 2011;21:338–344. doi: 10.1093/cercor/bhq099. [DOI] [PubMed] [Google Scholar]
- 115.Cunto FD, Imarisio S, Camera P, Boitani C, Altruda F, Silengo L. Essential role of citron kinase in cytokinesis of spermatogenic precursors. J Cell Sci. 2002;115:4819–4826. doi: 10.1242/jcs.00163. [DOI] [PubMed] [Google Scholar]
- 116.D’Avino PP, Glover DM. Cytokinesis: mind the GAP. Nat Cell Biol. 2009;11:112–114. doi: 10.1038/ncb0209-112. [DOI] [PubMed] [Google Scholar]
- 117.Pollard TD. Regulation of actin filament assembly by Arp2/3 complex and formins. Annu Rev Biophys Biomol Struct. 2007;36:451–477. doi: 10.1146/annurev.biophys.35.040405.101936. [DOI] [PubMed] [Google Scholar]
- 118.Moulding DA, Blundell MP, Spiller DG, White MR, Cory GO, Calle Y, Kempski H, Sinclair J, Ancliff PJ, Kinnon C, et al. Unregulated actin polymerization by WASp causes defects of mitosis and cytokinesis in X-linked neutropenia. J Exp Med. 2007;204:2213–2224. doi: 10.1084/jem.20062324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yang Q, Zhang XF, Pollard TD, Forscher P. Arp2/3 complex-dependent actin networks constrain myosin II function in driving retrograde actin flow. J Cell Biol. 2012;197:939–956. doi: 10.1083/jcb.201111052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dulyaninova NG, Patskovsky YV, Bresnick AR. The N-terminus of the long MLCK induces a disruption in normal spindle morphology and metaphase arrest. J Cell Sci. 2004;117:1481–1493. doi: 10.1242/jcs.00993. [DOI] [PubMed] [Google Scholar]
- 121.Davies T, Canman JC. Stuck in the middle: Rac, adhesion, and cytokinesis. The Journal of Cell Biology. 2012;198:769–771. doi: 10.1083/jcb.201207197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Halet G, Carroll J. Rac activity is polarized and regulates meiotic spindle stability and anchoring in mammalian oocytes. Dev Cell. 2007;12:309–317. doi: 10.1016/j.devcel.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 123.Gotta M, Abraham MC, Ahringer J. CDC-42 controls early cell polarity and spindle orientation in C. elegans. Curr Biol. 2001;11:482–488. doi: 10.1016/s0960-9822(01)00142-7. [DOI] [PubMed] [Google Scholar]
- 124.Dutartre H, Davoust J, Gorvel JP, Chavrier P. Cytokinesis arrest and redistribution of actin-cytoskeleton regulatory components in cells expressing the Rho GTPase CDC42Hs. J Cell Sci. 1996;109(Pt 2):367–377. doi: 10.1242/jcs.109.2.367. [DOI] [PubMed] [Google Scholar]
- 125.Zhu X, Wang J, Moriguchi K, Liow LT, Ahmed S, Kaverina I, Murata-Hori M. Proper regulation of Cdc42 activity is required for tight actin concentration at the equator during cytokinesis in adherent mammalian cells. Exp Cell Res. 2011;317:2384–2389. doi: 10.1016/j.yexcr.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Prehoda KE. Polarization of Drosophila neuroblasts during asymmetric division. Cold Spring Harb Perspect Biol. 2009;1:a001388. doi: 10.1101/cshperspect.a001388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Aceto D, Beers M, Kemphues KJ. Interaction of PAR-6 with CDC-42 is required for maintenance but not establishment of PAR asymmetry in C. elegans. Developmental Biology. 2006;299:386–397. doi: 10.1016/j.ydbio.2006.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Motegi F, Sugimoto A. Sequential functioning of the ECT-2 RhoGEF, RHO-1 and CDC-42 establishes cell polarity in Caenorhabditis elegans embryos. Nat Cell Biol. 2006;8:978–985. doi: 10.1038/ncb1459. [DOI] [PubMed] [Google Scholar]
- 129.Etienne-Manneville S. Cdc42–the centre of polarity. J Cell Sci. 2004;117:1291–1300. doi: 10.1242/jcs.01115. [DOI] [PubMed] [Google Scholar]
- 130.Joberty G, Petersen C, Gao L, Macara IG. The cell-polarity protein Par6 links Par3 and atypical protein kinase C to Cdc42. Nat Cell Biol. 2000;2:531–539. doi: 10.1038/35019573. [DOI] [PubMed] [Google Scholar]
- 131.Lin D, Edwards AS, Fawcett JP, Mbamalu G, Scott JD, Pawson T. A mammalian PAR-3-PAR-6 complex implicated in Cdc42/Rac1 and aPKC signalling and cell polarity. Nat Cell Biol. 2000;2:540–547. doi: 10.1038/35019582. [DOI] [PubMed] [Google Scholar]
- 132.Kumfer KT, Cook SJ, Squirrell JM, Eliceiri KW, Peel N, O’Connell KF, White JG. CGEF-1 and CHIN-1 regulate CDC-42 activity during asymmetric division in the Caenorhabditis elegans embryo. Mol Biol Cell. 2010;21:266–277. doi: 10.1091/mbc.E09-01-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bielak-Zmijewska A, Kolano A, Szczepanska K, Maleszewski M, Borsuk E. Cdc42 protein acts upstream of IQGAP1 and regulates cytokinesis in mouse oocytes and embryos. Developmental Biology. 2008;322:21–32. doi: 10.1016/j.ydbio.2008.06.039. [DOI] [PubMed] [Google Scholar]
- 134.Leblanc J, Zhang X, McKee D, Wang ZB, Li R, Ma C, Sun QY, Liu XJ. The Small GTPase Cdc42 Promotes Membrane Protrusion during Polar Body Emission via ARP2-Nucleated Actin Polymerization. Mol Hum Reprod. 2011 doi: 10.1093/molehr/gar026. [DOI] [PubMed] [Google Scholar]
- 135.Na J, Zernicka-Goetz M. Asymmetric positioning and organization of the meiotic spindle of mouse oocytes requires CDC42 function. Curr Biol. 2006;16:1249–1254. doi: 10.1016/j.cub.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 136.Shannon KB. IQGAP Family Members in Yeast, Dictyostelium, and Mammalian Cells. International journal of cell biology. 2012;2012:894817. doi: 10.1155/2012/894817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Skop AR, Liu H, Yates J, Meyer BJ, Heald R. Dissection of the mammalian midbody proteome reveals conserved cytokinesis mechanisms. Science. 2004;305:61–66. doi: 10.1126/science.1097931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nishimura Y, Mabuchi I. An IQGAP-like protein is involved in actin assembly together with Cdc42 in the sea urchin egg. Cell Motil Cytoskeleton. 2003;56:207–218. doi: 10.1002/cm.10146. [DOI] [PubMed] [Google Scholar]


