Abstract
Aims
Type 1 diabetes has been associated with an elevated relative risk (RR) of mortality compared to the general population. To review published studies on the RR of mortality of Type 1 diabetes patients compared to the general population, we conducted a meta-analysis and examined the temporal changes in the RR of mortality over time.
Methods
Systematic review of studies reporting RR of mortality for Type 1 diabetes compared to the general population. We conducted meta-analyses using a DerSimonian and Laird random effects model to obtain the average effect and the distribution of RR estimates. Sub-group meta-analyses and multivariate meta-regression analysis was performed to examine heterogeneity. Summary RR with 95% CIs was calculated using a random-effects model.
Results
26 studies with a total of 88 subpopulations were included in the meta-analysis and overall RR of mortality was 3.82 (95% CI 3.41, 3.4.29) compared to the general population. Observations using data prior to 1971 had a much larger estimated RR (5.80 (95% CI 4.20, 8.01)) when compared to: data between; 1971 and 1980 (5.06 (95% CI 3.44, 7.45)); 1981–90 (3.59 (95% CI 3.15, 4.09)); and those after 1990 (3.11 (95% CI 2.47, 3.91)); suggesting mortality of Type 1 diabetes patients when compared to the general population have been improving over time. Similarly, females (4.54 (95% CI 3.79–5.45)) had a larger RR estimate when compared to males (3.25 (95% CI 2.82–3.73) and the meta-regression found evidence for temporal trends and sex (p<0.01) accounting for heterogeneity between studies.
Conclusions
Type 1 diabetes patients’ mortality has declined at a faster rate than the general population. However, the largest relative improvements have occurred prior to 1990. Emphasis on intensive blood glucose control alongside blood pressure control and statin therapy may translate into further reductions in mortality in coming years.
Introduction
Prior to the availability of insulin, people who developed diabetes before the age of 30 died within an average of 1.4 years of disease onset [1]. The commercial production of insulin in the 1920 s saw a dramatic decrease in mortality for Type 1 diabetes mellitus patients. Whilst the secular downward trend in mortality among the general population over the twentieth century is well known [2], it has not been as well documented in the type 1 diabetes population. One small study in Bucharest reported the decline in mortality over 6 decades [3], whilst other studies only examine this trend in people with type 1 diabetes over the past decade [3]–[5].
The introduction of blood glucose self-monitoring and the increased awareness of the importance of controlling blood glucose to reduce the onset and progression of diabetic complications following the publication of the Diabetes Complications and Complications Trial (DCCT) [6] have led to an intensification of treatment. This has translated into improved life expectancy for patients with type 1 diabetes [3], [4], however it is unclear whether mortality for Type 1 diabetes patients relative to the general population has reduced over time.
Studies have quantified the increased relative risk (RR) of mortality for people with Type 1 diabetes compared with the general population, but there has been no systematic review to estimate pooled measures of RR or to explore the degree to which these vary across regions and over time. A recent large scale study [7] involving individual participant data from 97 prospective studies was unable to separate people with Type 1 and Type 2 diabetes, and so could only provide overall estimates of relative mortality. Type 1 diabetes is typically diagnosed at young ages, and patients are susceptible over a longer period of time [8] to diabetic complications (e.g. renal disease, neuropathy, macrovascular disease); all of which may elevate mortality.
The purpose of this study was to undertake a systematic review of RR of mortality among Type 1 diabetes patients compared to the general population and to conduct a meta-analysis of the findings. Sub-group meta-analyses and meta-regression were also conducted to explore the extent to which the heterogeneity between studies could be explained by temporal changes in the relative risk of mortality for type 1 diabetes patients relative to the general population.
Materials and Methods
Data Sources and Searches
Our systematic review aimed to identify studies that compared mortality of Type 1 diabetes patients to the general population and that reported RRs of mortality or standardised mortality ratios (SMR).
We identified studies for inclusion using a systematic literature search of the electronic databases: MEDLINE, EMBASE, CINAHL, PubMed and Cochrane’s systematic reviews. Other databases that were used: The Health Economic Evaluation Database (HEED); the Digital Theses Database; Google Scholar; the TUFTS CEA register; NHS Economic Evaluation Database and the Health Technology Assessment website. We used Google Scholar and Pubmed to conduct a citation search of identified studies and we also examined the citations list of reported articles to identify further potential studies.
Study Selection
Inclusion criteria included articles published before April 2012 in English in peer reviewed journals in which study subjects have Type 1 diabetes and the overall mortality (as opposed to complication specific studies) among Type 1 diabetes patients is compared to the general population. The review was limited to human studies. Review articles were excluded after references lists had been searched. Where multiple papers used the same cohort in estimating a RR of mortality, only the one that used the longest follow-up was included into the analysis. Studies generally explicitly identified Type 1 diabetes populations. However, we identified Type 1 diabetes populations if patients were diagnosed under 30 years of age and/or classified as insulin only if the study was not clear in its description of diabetes patients.
Data Extraction and Quality Assessment
The reported RR estimates and their standard errors were extracted from each study for the meta-analysis. Studies that did not report uncertainty around their RR estimate (through standard error or confidence intervals) were excluded from both the meta-analysis and meta-regression. Multiple observations from some studies [9]–[16] were only included if the estimates used different populations to estimate these observations.
We assessed the quality of studies by completing the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) checklist for cohort studies.
Two independent reviewers examined the abstracts and full texts of all articles to determine whether the inclusion criteria were fulfilled. The reviewers also independently extracted the data for the meta-analysis and meta-regression. A third reviewer was assigned to re-examine all full text articles to ensure all data extracted was correct and finalise any discrepancies between the first two reviewers.
Data Synthesis and Analysis
A meta-analysis was conducted for the RR of mortality for Type 1 diabetes mellitus patients, which pools the results gathered from multiple studies into an overall average effect size. We used the DerSimonian and Laird random effects model for the meta-analysis, which accounts for both the within study variation and expected between study heterogeneity and was conducted in Stata version 12.0 (Stata Corporation, College Station, TX, USA). Studies that measured multiple RRs for different population groups were used as separate observations. We conducted a trim-and-fill analysis to explore the potential of publication bias from our studies.
To explore the possible reasons for heterogeneity among studies numerous sub-group meta-analyses were conducted along with a multivariate meta-regression. Fixed-effects inverse variance meta-analyses were conducted to explore heterogeneity across the categorical groups of studies. Study characteristics extracted for sub-group analysis and meta-regression included:
A binary variable comparing studies conducted in European countries to non-European countries
A continuous variable for the year of the median study date in estimating the relative risk of mortality
A binary variable for sex
A continuous variable for the sample size of the study
A binary variable comparing patients’ age at diagnosis to be less than 18 years to patients older than 18 years.
The sub-group meta-analyses were conducted for:
Sex
Studies conducted in Europe, in non-European countries and in the United Kingdom
Studies which used a dataset commencing before 1971, between 1971–80, between 1981–1990, and after 1990
Studies including patients who were diagnosed before 18 years of age only and studies including patients who were diagnosed after 18 years of age
Results and Discussion
Literature search and study characteristics
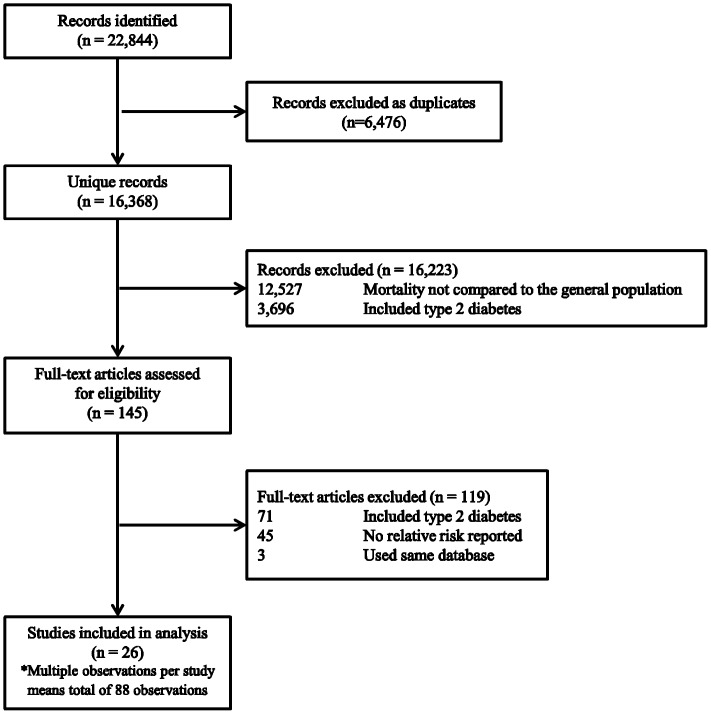
A flow chart of the literature search and its results are reported in Figure 1. Initially, the search identified 22,844 titles and abstracts from all databases. 145 studies were reviewed in full, of which 39 studies were initially included. However, some studies provided more than one RR estimate. Studies [10], [17], [18] and certain observations of studies [19] were excluded if they used the same database as other studies, with a shorter follow-up period. Only 2 studies were different between the first two reviewers, which the third reviewer finalised the discrepancies after discussing the issue between the reviewers.
Figure 1. Flow chart of study selection process.
The meta-analysis and meta-regression used 88 observations from 26 different studies [5], [9], [12], [14], [16], [19]–[40]. Only 1 study estimated RR using hazard ratios [37] and all other studies used SMRs. Details of the studies used, summary statistics and meta-analysis results are shown in Table 1. Studies were conducted in 20 different countries, and the number of patients ranged from 75 to 12,684 per study, with a median of 382 patients. Patient age ranged between 1–79 years, with the majority diagnosed before 18 years. Full search terms of the database MEDLINE and the STROBE checklist for all studies included in the analysis can be found in the electronic supplementary material (Table S1 & Table S2 in File S1).
Table 1. List of studies used in meta-analysis, country of analysis, date of conducted studies, age at diagnosis, mean age patients, number of patients in the study, percentage of males in study, years of follow-up, mortality rates and measurement of risk of mortality.
| Study | Country | Study period | Age at diagnosis | n | Mortality Rates (95% CI) | Type of Study | Relative Risk Type | Sex |
| Alleman et al. 2009 [22] | Switzerland | 1974–2006 | 35–54 | 104 | 3.80 (3.00–4.80) | Cohort | SMR | Male |
| Alleman et al. 2009 [22] | Switzerland | 1974–2006 | 35–54 | 174 | 5.40 (4.30–6.80) | Cohort | SMR | Female |
| Asao et al. 2003 [9] | Finland | 1965–1979 | <18 | 2817 | 3.20 (2.80–3.70) | Cohort | SMR | Male |
| Asao et al. 2003 [9] | Finland | 1965–1979 | <18 | 2309 | 5.20 (4.20–6.30) | Cohort | SMR | Female |
| Asao et al. 2003 [9] | Japan | 1965–1979 | <18 | 566 | 9.00 (6.80–11.70) | Cohort | SMR | Male |
| Asao et al. 2003 [9] | Japan | 1965–1979 | <18 | 842 | 18.50 (14.70–23.00) | Cohort | SMR | Female |
| Barcelo et al. 2007 [23] | Cuba | 1965–1970 | <10 | 259 | 8.58 (6.18–12.28) | Cohort | SMR | Male |
| Barcelo et al. 2007 [23] | Cuba | 1965–1970 | <10 | 245 | 8.82 (6.70–12.74) | Cohort | SMR | Female |
| Barcelo et al. 2007 [23] | United States | 1965–1970 | <10 | 449 | 3.42 (2.36–5.13) | Cohort | SMR | Male |
| Barcelo et al. 2007 [23] | United States | 1965–1970 | <10 | 438 | 3.78 (2.66–5.56) | Cohort | SMR | Female |
| Botha et al. 1992 [24] | UK and Ireland | 1972–1981 | <2 | 179 | 2.30 (0.60–9.50) | Cohort | SMR | Male |
| Botha et al. 1992 [24] | UK and Ireland | 1972–1981 | <2 | 131 | 9.90 (4.10–24.20) | Cohort | SMR | Female |
| Bruno et al. 2009 [25] | Italy | 1974–2000 | <30 | 688 | 1.71 (0.99–2.95) | Cohort | SMR | Male |
| Bruno et al. 2009 [25] | Italy | 1974–2000 | <30 | 522 | 2.86 (1.29–6.37) | Cohort | SMR | Female |
| Collado-mesa et al. 1997 [26] | Cuba | 1965–1991 | <15 | 259 | 7.50 (5.30–10.30) | Cohort | SMR | Male |
| Collado-mesa et al. 1997 [26] | Cuba | 1965–1991 | <15 | 245 | 10.00 (6.90–14.60) | Cohort | SMR | Female |
| Feltbower et al. 2008 [27] | England | 1978–2004 | 15–29 | 568 | 5.80 (3.80–8.60) | Cohort | SMR | Male |
| Feltbower et al. 2008 [27] | England | 1978–2004 | < = 14 | 1742 | 4.20 (3.20–5.50) | Cohort | SMR | Male |
| Feltbower et al. 2008 [27] | England | 1978–2004 | 15–29 | 329 | 7.50 (3.40–14.30) | Cohort | SMR | Female |
| Feltbower et al. 2008 [27] | England | 1978–2004 | < = 14 | 1607 | 4.10 (2.60–6.30) | Cohort | SMR | Female |
| Florkowski et al. 2003 [12] | New Zealand | 1984–1999 | <30 | 206 | 3.00 (2.20–4.00) | Cohort | SMR | Male |
| Florkowski et al. 2003 [12] | New Zealand | 1984–1999 | > = 30 | NA | 1.60 (1.30–1.90) | Cohort | SMR | Male |
| Florkowski et al. 2003 [12] | New Zealand | 1984–1999 | <30 | 229 | 2.70 (1.90–3.90) | Cohort | SMR | Female |
| Florkowski et al. 2003 [12] | New Zealand | 1984–1999 | > = 30 | NA | 1.70 (1.40–2.00) | Cohort | SMR | Female |
| Gnavi et al. 2004 [28] | Italy | 1991–1999 | > = 20 | NA | 1.98 (1.56–2.47) | Cohort | SMR | Male |
| Gnavi et al. 2004 [28] | Italy | 1991–1999 | > = 20 | NA | 3.36 (2.59–4.28) | Cohort | SMR | Female |
| Grausland 2010 [29] | Denmark | 1973–2006 | NA | NA | 3.20 (2.80–3.70) | Cohort | SMR | Male |
| Grausland 2010 [29] | Denmark | 1973–2006 | NA | NA | 3.50 (3.00–4.10) | Cohort | SMR | Female |
| Harjutsalo et al. 2011 [30] | Finland | 1970–1999 | < = 14 | NA | 3.00 (2.70–3.40) | Cohort | SMR | Male |
| Harjutsalo et al. 2011 [30] | Finland | 1970–1999 | 15–29 | 93,559 | 2.60 (2.04–2.80) | Cohort | SMR | Male |
| Harjutsalo et al. 2011 [30] | Finland | 1970–1999 | < = 14 | NA | 5.50 (4.80–6.30) | Cohort | SMR | Female |
| Harjutsalo et al. 2011 [30] | Finland | 1970–1999 | 15–29 | 56,345 | 3.70 (3.20–4.30) | Cohort | SMR | Female |
| Joner & Patrick 1991 [17] | Norway | 1973–1988 | < = 14 | 1,040 | 2.15 (1.20–3.55) | Cohort | SMR | Male |
| Joner & Patrick 1991 [17] | Norway | 1973–1988 | < = 14 | 874 | 1.86 (0.60–4.34) | Cohort | SMR | Female |
| Laing et al. 1999 [31] | United Kingdom | 1972–1997 | <30 | 12,684 | 2.70 (2.50–2.90) | Cohort | SMR | Male |
| Laing et al. 1999 [31] | United Kingdom | 1972–1997 | <30 | 11,047 | 4.00 (3.60–4.40) | Cohort | SMR | Female |
| McNally et al. 1995 [14] | England | 1940–1949 | <17 | 463 | 9.44 (4.91–18.14) | Cohort | SMR | Male |
| McNally et al. 1995 [14] | England | 1950–1959 | <17 | 463 | 6.12 (3.18–11.75) | Cohort | SMR | Male |
| McNally et al. 1995 [14] | England | 1960–1969 | <17 | 463 | 2.99 (1.34–6.65) | Cohort | SMR | Male |
| McNally et al. 1995 [14] | England | 1970–1979 | <17 | 463 | 2.30 (0.87–6.10) | Cohort | SMR | Male |
| McNally et al. 1995 [14] | England | 1980–1989 | <17 | 463 | 1.69 (0.24–11.96) | Cohort | SMR | Male |
| McNally et al. 1995 [14] | England | 1940–1949 | <17 | 382 | 10.55 (4.41–25.22) | Cohort | SMR | Female |
| McNally et al. 1995 [14] | England | 1950–1959 | <17 | 382 | 2.86 (0.71–11.41) | Cohort | SMR | Female |
| McNally et al. 1995 [14] | England | 1960–1969 | <17 | 382 | 5.91 (1.91–18.31) | Cohort | SMR | Female |
| McNally et al. 1995 [14] | England | 1970–1979 | <17 | 382 | 4.78 (1.82–12.60) | Cohort | SMR | Female |
| McNally et al. 1995 [14] | England | 1980–1989 | <17 | 382 | 4.05 (0.57–28.54) | Cohort | SMR | Female |
| Morrish et al. 2001 [21] | Croatia | 1975–1988 | NA | 222 | 3.46 (2.14–5.28) | Cohort | SMR | Male |
| Morrish et al. 2001 [21] | Croatia | 1975–1988 | NA | 180 | 3.36 (1.09–7.84) | Cohort | SMR | Female |
| Morrish et al. 2001 [21] | Cuba | 1975–1988 | NA | 258 | 6.85 (4.06–10.82) | Cohort | SMR | Male |
| Morrish et al. 2001 [21] | Cuba | 1975–1988 | NA | 257 | 7.90 (3.79–14.53) | Cohort | SMR | Female |
| Morrish et al. 2001 [21] | Germany | 1975–1988 | NA | 285 | 6.82 (4.95–9.15) | Cohort | SMR | Male |
| Morrish et al. 2001 [21] | Germany | 1975–1988 | NA | 275 | 6.55 (4.35–9.46) | Cohort | SMR | Female |
| Morrish et al. 2001 [21] | Hong Kong | 1975–1988 | NA | 198 | 3.44 (1.65–6.32) | Cohort | SMR | Male |
| Morrish et al. 2001 [21] | Hong Kong | 1975–1988 | NA | 224 | 6.37 (2.75–12.55) | Cohort | SMR | Female |
| Morrish et al. 2001 [21] | Poland | 1975–1988 | NA | 241 | 4.27 (3.17–5.62) | Cohort | SMR | Male |
| Morrish et al. 2001 [21] | Poland | 1975–1988 | NA | 245 | 7.00 (4.93–9.64) | Cohort | SMR | Female |
| Morrish et al. 2001 [21] | Switzerland | 1975–1988 | NA | 278 | 3.92 (2.53–5.78) | Cohort | SMR | Male |
| Morrish et al. 2001 [21] | Switzerland | 1975–1988 | NA | 256 | 7.42 (4.89–10.80) | Cohort | SMR | Female |
| Morrish et al. 2001 [21] | United Kingdom | 1975–1988 | NA | 254 | 1.88 (1.13–2.94) | Cohort | SMR | Male |
| Morrish et al. 2001 [21] | United Kingdom | 1975–1988 | NA | 243 | 3.38 (2.14–5.07) | Cohort | SMR | Female |
| Muggeo et al. 1995 [32] | Italy | 1986–1991 | <18 | 95 | 2.70 (0.54–7.90) | Cohort | SMR | Male |
| Muggeo et al. 1995 [32] | Italy | 1986–1991 | <18 | 75 | 5.08 (1.02–14.86) | Cohort | SMR | Female |
| Nishimura et al. 2001 [5] | United States | 1965–1999 | <18 | 558 | 3.25 (2.55–3.98) | RCT | SMR | Male |
| Nishimura et al. 2001 [5] | United States | 1965–1999 | <18 | 517 | 6.90 (5.51–8.46) | RCT | SMR | Female |
| Podar et al. 2000 [16] | Estonia | 1980–1995 | <30 | 269 | 4.41 (1.79–8.15) | Cohort | SMR | Male |
| Podar et al. 2000 [16] | Estonia | 1980–1995 | <30 | 249 | 4.86 (1.32–12.44) | Cohort | SMR | Female |
| Podar et al. 2000 [16] | Finland | 1980–1995 | <30 | 2,798 | 1.38 (0.84–2.13) | Cohort | SMR | Male |
| Podar et al. 2000 [16] | Finland | 1980–1995 | <30 | 2,358 | 2.26 (1.17–3.95) | Cohort | SMR | Female |
| Podar et al. 2000 [16] | Lithuania | 1983–1995 | <30 | 360 | 5.28 (2.73–9.22) | Cohort | SMR | Male |
| Podar et al. 2000 [16] | Lithuania | 1983–1995 | <30 | 338 | 12.56 (6.69–21.47) | Cohort | SMR | Female |
| Raymond et al. 1995 [33] | United Kingdom | 1983–1992 | Dec-94 | 2,482 | 1.49 (1.29–1.71) | Cohort | SMR | Male |
| Raymond et al. 1995 [33] | United Kingdom | 1983–1992 | Dec-94 | 2,088 | 1.77 (1.51–2.06) | Cohort | SMR | Female |
| Riley et al. 1995 [34] | Australia | 1984–1993 | <30 | 263 | 3.20 (2.10–4.70) | Cohort | SMR | Male |
| Riley et al. 1995 [34] | Australia | 1984–1993 | > = 30 | 186 | 1.40 (1.10–1.80) | Cohort | SMR | Male |
| Riley et al. 1995 [34] | Australia | 1984–1993 | <30 | 217 | 7.30 (4.60–10.90) | Cohort | SMR | Female |
| Riley et al. 1995 [34] | Australia | 1984–1993 | > = 30 | 169 | 2.20 (1.70–2.80) | Cohort | SMR | Female |
| Roberts et al. 2004 [35] | United Kingdom | 1968–1996 | <30 | 2,603 | 6.50 (4.40–9.00) | Cohort | SMR | Male |
| Roberts et al. 2004 [35] | United Kingdom | 1968–1996 | <30 | 2,389 | 12.80 (8.50–17.90) | Cohort | SMR | Female |
| Sartor & Dahlquist 1995 [36] | Sweden | 1977–1990 | <15 | 2,653 | 2.62 (1.72–4.00) | Cohort | SMR | Male |
| Sartor & Dahlquist 1995 [36] | Sweden | 1977–1990 | <15 | 2,341 | 3.84 (2.32–6.35) | Cohort | SMR | Female |
| Skrivarhaug et al. 2006 [37] | Norway | 1973–2002 | <15 | 1,034 | 3.90 (3.10–4.90) | Cohort | SMR | Male |
| Skrivarhaug et al. 2006 [37] | Norway | 1973–2002 | <15 | 872 | 4.00 (2.70–5.60) | Cohort | SMR | Female |
| Soedamah-Muthu et al. 2006 [38] | United Kingdom | 1992–1999 | < = 35 | 4,216 | 3.30 (2.70–4.00) | Cohort | HR | Male |
| Soedamah-Muthu et al. 2006 [38] | United Kingdom | 1992–1999 | < = 35 | 3,497 | 4.50 (3.50–5.60) | Cohort | HR | Female |
| Swerdlow & Jones 1995 [39] | United Kingdom | 1966–1992 | NA | 2,907 | 1.58 (1.50–1.66) | Cohort | SMR | Male |
| Swerdlow & Jones 1995 [39] | United Kingdom | 1966–1992 | NA | 2,874 | 2.31 (2.20–2.43) | Cohort | SMR | Female |
| Waernbaum et al. 2006 [40] | Sweden | 1983–1999 | 15–34 | NA | 1.90 (1.50–2.40) | Cohort | SMR | Male |
| Waernbaum et al. 2006 [40] | Sweden | 1983–1999 | 15–34 | NA | 1.60 (1.00–2.60) | Cohort | SMR | Female |
| Wibell et al. 2001 [41] | Sweden | 1983–1992 | 15–34 | NA | 2.10 (1.30–3.10) | Cohort | SMR | Male |
| Wibell et al. 2001 [41] | Sweden | 1983–1999 | 15–34 | NA | 1.50 (0.70–3.60) | Cohort | SMR | Female |
Overall meta-analysis
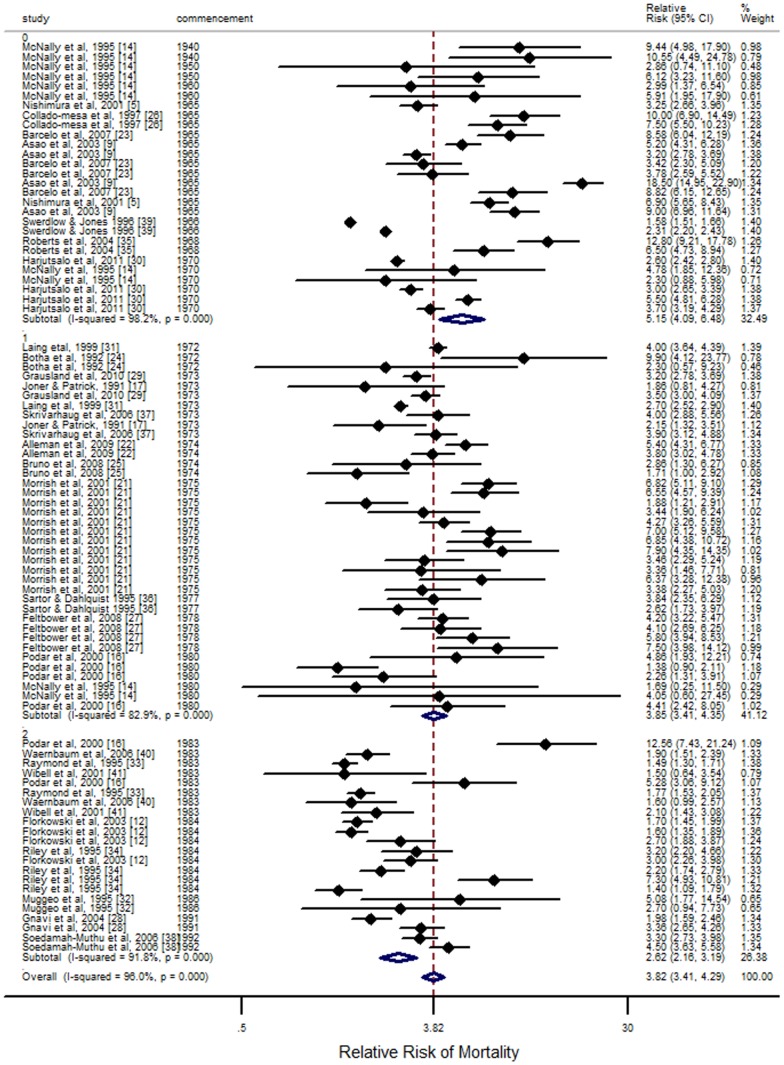
Results of the overall meta-analysis of 88 observations are shown in Table 2, and a meta-analysis forest plot is depicted in Figure 2, stratified by a studies commencement date. Overall RR of mortality was estimated at 3.82 (95% CI 3.41, 4.29) for Type 1 diabetes mellitus patients compared to the general population. The I-squared value was estimated at 96.0%, indicating the high degree of heterogeneity across studies.
Table 2. Meta-analysis results by different categories, showing the number observations used, the pooled estimate and 95% confidence intervals and I2 estimate for heterogeneity.
| No. of studies | Pooled Estimate (95% CI) | I2 estimate | |
| All studies | 88 | 3.82 (3.41–4.29) | 96.0% |
| Studies commenced before 1970 | 10 | 5.80 (4.20–8.01) | 69.5% |
| Studies commenced between 1971–1980 | 12 | 5.06 (3.44–7.45) | 98.8% |
| Studies commenced between 1981–1990 | 50 | 3.59 (3.15–4.09) | 93.1% |
| Studies commenced after 1990 | 16 | 3.11 (2.47–3.91) | 92.7% |
| Studies with patients age at diagnosisbefore 18 years | 41 | 4.93 (4.13–5.88) | 90.8% |
| Studies with patients age at diagnosisafter 18 years | 8 | 2.41 (1.75–3.32) | 94.7% |
| Male | 44 | 3.25 (2.82–3.73) | 95.3% |
| Female | 44 | 4.54 (3.79–5.45) | 95.8% |
| United Kingdom studies | 28 | 3.78 (3.13–4.57) | 96.4% |
| European studies | 66 | 3.56 (3.16–4.00) | 95.2% |
| Non-European studies | 22 | 4.63 (3.28–6.55) | 97.0% |
Figure 2. Random effects meta-analysis by median study date (< = 1970, 1971–1980, 1981–1990, >1990).
Horizontal bars and circles widths denote 95% CIs, and box sizes indicate relative weight in the analysis.
Subgroup analyses and meta-regression analysis to explore cross-study heterogeneity
All sub-group meta-analyses results are also shown in Table 2. Fixed-effects inverse variance meta-analysis for all sub-groups found strong evidence of heterogeneity within sub-groups, and heterogeneity between sub-groups (p<0.01). When conducting separate meta-analyses for men and women, RRs of death were 3.25 (95% CI 2.82, 3.73) and 4.54 (95% CI 3.79, 5.45), respectively. I-squared estimates of 95.3% (men) and 95.8% (women) showed similar heterogeneity as the overall meta-analysis results which suggest that heterogeneity between studies is not explained by gender differences. Similar results were shown for studies conducted in Europe and in the United Kingdom, with RR of mortality of 3.56 (95% 3.16, 4.00) and 3.78 (95% CI 3.13, 4.57), respectively. When stratified by the median study date, observations that used data prior to 1971 had a much higher estimated RR (5.80 (95% CI 4.20, 8.01)) than studies that used data after 1990 (3.11 (95% CI 2.47, 3.92)) with a clear temporal improvement in relative mortality over time. A large amount of heterogeneity can be explained from observations that used data prior to 1971, with an I-squared estimated at 69.5%, compared to 92.7% for studies after 1990.
Further investigation of the possible sources of between-study heterogeneity was performed through multivariate meta-regression analyses and reported in Table 3. 24 studies and 76 observations were included into the primary meta-regression. We decided a linear temporal trend was a better fit than non-linear trends, based on adjusted R-squared values. The assumed linear temporal trend captured by the standardized median date of study was significant, suggesting an improving trend in relative mortality over time for studies whose median date was after 1982. By way of interpretation for every year increase in the year of the data used after 1982, the average RR of mortality decreased by 2%. Similarly, males had a lower RR of mortality on average by 30% than females. Studies conducted in Europe and the numbers of patients in studies were found to have an insignificant effect on the average RR of mortality in the meta-regression. When we included a binary variable to determine whether age at diagnosis was at childhood (<18 years) in a separate meta-regression, it reduced our analysis to 20 studies and 56 observations shown in Table 3. The newly introduced variable was found to have an insignificant effect. However, the adjusted R-squared estimates which explain the proportion of between-study variance improved from 21.8% to 25.74%, respectively.
Table 3. Meta-regression results, determining the factors that account for the heterogeneity between different studies’ relative risk of mortality estimates.
| 24 studies, 74 observations | 20 studies, 56 observations | ||||||||
| Variable | Coefficient | P value | 95%CI Lower | 95%CI Upper | Variable | Coefficient | P value | 95%CI Lower | 95%CI Upper |
| Intercept | 5.09 | <0.01 | 3.81 | 6.80 | Intercept | 6.05 | <0.01 | 4.45 | 8.21 |
| Median date of study* | 0.98 | <0.01 | 0.97 | 0.99 | Median date of study* | 0.98 | 0.03 | 0.97 | 0.99 |
| Sex (Male = 1) | 0.70 | 0.01 | 0.54 | 0.90 | Sex (Male = 1) | 0.68 | 0.01 | 0.50 | 0.91 |
| Region (Europe** = 1) | 0.80 | 0.13 | 0.60 | 1.07 | Region (Europe** = 1) | 0.88 | 0.43 | 0.63 | 1.22 |
| Number of patients in study*** | 0.99 | 0.34 | 0.99 | 1.00 | Number of patients in study*** | 0.99 | 0.35 | 0.99 | 1.00 |
| Age at diagnosis (>18 years old = 1) | 0.66 | 0.13 | 0.38 | 1.13 | |||||
*Median date of study standardized by deducting the median date of studies of 1982.
**66 observations conducted in Europe.
***Number of patients in study standardized by deducting the mean number of patients of 1163.
The trim-and-fill analysis plot is reported in Figure S2 in File S1. No trimming of data has been performed, suggesting there is no evidence of publication bias.
Discussion
This study represents the first meta-analysis of the RRs of mortality for Type 1 diabetes patients compared to the general population. Although the meta-analysis showed on average over three-fold RR in mortality compared to the general population, there is a clear reduction in the risk of mortality when examining populations over time. Our sub-group analyses showed studies examining databases prior to 1971 had almost double the RR of mortality relative to the general population when compared to studies using databases after 1990. Whilst the general population’s mortality has reduced during this period, it is clear that for type 1 diabetes patients, their mortality has been declining at an even faster rate than the general population.
To put this into perspective, a recent study estimated SMR in the months following an acute myocardial infarction to be four times greater which is similar to the overall relative mortality in our analysis across all studies [41]. As we have demonstrated above there is a significant decline in relative mortality. The pooled standardised mortality of studies initiated since 1990 suggests around a three-fold all-cause mortality risk. While this suggests significant improvements in relative survival of people with Type 1 diabetes, they are still at a greater mortality risk than people with Type 2 diabetes [42], or long-term survivors of a myocardial infarction [41].
Our results also place in context two large studies estimating relative mortality of people with Type 1 diabetes based on registries in Denmark [43] and Scotland [44]. While Jorgensen et al. [43] reported improvements in mortality rates in type 1 diabetes patients over the study period between 2002 and 2011, the relative risks of 2.58 (95% CI 2.23, 2.98) for men and 2.71 (95% CI 2.18, 3.38) reported in the Scottish study based on data collected between 2005 and 2007 [44] are not significantly different from our estimates of all studies initiated since 1980.
While we have observed secular improvement in relative mortality for people with type 1 diabetes for over 50 years, the largest relative improvements occurred prior to 1980. However, it is important to continue to monitor the relative mortality of people with type 1 diabetes, particularly in light of published results from the DCCT [6] which indicate metabolic memory and legacy effects from intensive blood glucose control on complications of diabetes [45], [46] which may translate into further improvements in mortality in coming years.
As expected there was a large amount of between-study heterogeneity. Differences between the composition of Type 1 diabetes cohorts in the study and the associated background population with which to estimate RR of mortality from along with studies being conducted at different time periods leads one to expect a certain level of heterogeneity between studies. We conducted a number of sub-group meta-analyses (European studies, non-European studies, stratification by commencement date of studies and sex) and still found high levels of heterogeneity, as represented by the large I-squared values in Table 2. A random-effects meta-regression was conducted to combine the results of studies to account for the variation. The meta-regression shows a negative linear trend, with a coefficient of 0.98 (95% CI 0.97–0.99) suggesting decreasing relative mortality over time. The only other significant factor that explained the variation between studies was by sex as women were estimated to have a higher RR of mortality when compared to men, 4.54 (95% CI 3.79, 5.45) and 3.25 (95% CI 2.82, 3.73) respectively. This is indicative of the improving level of treatments for patients with Type 1 diabetes. However, there were still unobserved factors which attributed to heterogeneity after conducting a meta-regression and sub-group meta-analyses. Differences in the duration of studies and in the baseline characteristics of the type 1 diabetes patients, the background mortality of the general population to which the RR are calculated and the inconsistent reporting of patient characteristics across studies meant the influence of other confounders (diabetes-specific complications, duration of diabetes, median age of diagnosis, body weight, smoking, type of treatment regimen, HbA1c levels, etc.) that potentially could be associated with heterogeneity surrounding RR in mortality of Type 1 diabetes patients could not be assessed. Previous studies examining SMR’s in a meta-analysis framework also reported high levels of between-study heterogeneity [47], [48].
There was a considerable range of estimated RR of mortality, with the lowest estimated at 1.38 (95% CI 0.84–2.13) by a study in Finland [16] and the highest RR of mortality estimated at 18.5 (95% CI 14.7–23.0) in Japan [9]. Whilst this meta-analysis shows considerable heterogeneity in RR of mortality between different developed countries, there is a lack of information reported in less developed countries, and studies reporting mortality values were often based on a small number of cases [49]. Further research needs to be conducted in order to determine the RR of mortality in developed countries compared to developing countries.
Conclusions
In conclusion, this study has estimated people with type 1 diabetes have an elevated risk of mortality when compared to the general population, although the gap between the two populations have been decreasing. However, the sub-group meta-analyses suggests that the largest reductions in relative mortality have been achieved prior to 1980, although improvements in treatments following the DCCT imply metabolic memory and legacy effects of intensive glucose control along with statin treatment and blood pressure control could potentially reduce relative mortality further in the future.
Supporting Information
Tables S1 and S2 and Figures S1 and S2. Table S1. Summary of terms used in the Medline search strategy. Table S2. STROBE checklist for cohort studies. Figure S1. Cumulative meta-analysis by median study date (< = 1970, 1971–1980, 1981–1990,>1990). Horizontal bars and circles widths denote 95% CIs, and box sizes indicate relative weight in the analysis. Figure S2. Trim-and-fill analysis of the included estimates. No trimming of data was performed, suggesting no evidence of publication bias.
(DOCX)
Acknowledgments
We thank Edmund Fitzgerald of the University of Sydney for being an independent reviewer of studies for the meta-analysis. Alex James Avery (Centre for Health Policy, University of Melbourne, Australia) was involved in assessing the quality of studies and assembling the STROBE checklist.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.
Funding Statement
PMC was funded from an Australian NHMRC Career Development Award (571122) and partially funded from the NHMRC project grant (1028335). TWCL was partially funded from the NHMRC Capacity Building Grant (571372) and the NHMRC project grant (1028335). AJH was supported by NHMRC Capacity Building Grant (571372). AJP and WHH were partially supported by an Australian NHMRC project grant (1028335). WHH was supported by grant numbers P60DK020572 (MDRTC) and P30DK092926 (MCDTR) both from the National Institute of Diabetes and Digestive and Kidney Diseases. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Joslin EP (1917) The Treatment of Diabetes Mellitus, with Observations upon 1300 cases. California: Philadelphia: Lea & Febiger.
- 2. Kesteloot H, Sans S, Kromhout D (2006) Dynamics of cardiovascular and all-cause mortality in Western and Eastern Europe between 1970 and 2000. . Eur Heart J 27:107–113. [DOI] [PubMed] [Google Scholar]
- 3. Ioacara S, Lichiardopol R, Ionescu-Tirgoviste C, Cheta D, Sabau S, et al. (2009) Improvements in life expectancy in type 1 diabetes patients in the last six decades. . Diabetes Res Clin Pract 86:146–151. [DOI] [PubMed] [Google Scholar]
- 4.Miller RG, Secrest AM, Sharma RK, Songer TJ, Orchard TJ (2012) Improvements in the life expectancy of type 1 diabetes: The Pittsburgh Epidemiology of Diabetes Complications Study cohort. Diabetes. [DOI] [PMC free article] [PubMed]
- 5. Nishimura R, LaPorte RE, Dorman JS, Tajima N, Becker D, et al. (2001) Mortality trends in type 1 diabetes - The Allegheny County (Pennsylvania) Registry 1965–1999. . Diabetes Care 24:823–827. [DOI] [PubMed] [Google Scholar]
- 6.DCCT and EDIC: The Diabetes Control and Complications Trial and Follow-up Study.
- 7. The Emerging Risk Factors Collaboration (2011) Diabetes Mellitus, Fasting Glucose, and Risk of Cause-Specific Death. . New England Journal of Medicine 364:829–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Daneman D (2006) Type 1 diabetes. The Lancet. 367:847–858. [DOI] [PubMed] [Google Scholar]
- 9. Asao K, Sarti C, Forsen T, Hyttinen V, Nishimura R, et al. (2003) Long-term mortality in nationwide cohorts of childhood-onset type 1 diabetes in Japan and Finland. . Diabetes Care 26:2037–2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brown LJ, Scott RS, Moir CL (2001) All-cause mortality in the Canterbury (New Zealand) insulin-treated Diabetic Registry population. . Diabetes Care 24:56–63. [DOI] [PubMed] [Google Scholar]
- 11.(1995) International analysis of insulin-dependent diabetes mellitus mortality: a preventable mortality perspective. The Diabetes Epidemiology Research International (DERI) Study. . American Journal of Epidemiology 142:612–618. [PubMed] [Google Scholar]
- 12. Florkowski CM, Scott RS, Graham PJ, Han DY, Moir CL (2003) Cause-specific and total mortality in the Canterbury (New Zealand) insulin-treated Diabetic Registry population: a 15-year follow-up study. . Diabetic Medicine 20:191–197. [DOI] [PubMed] [Google Scholar]
- 13. Lipton R, Good G, Mikhailov T, Freels S, Donoghue E (1999) Ethnic differences in mortality from insulin-dependent diabetes mellitus among people less than 25 years of age. . Pediatrics 103:952–956. [DOI] [PubMed] [Google Scholar]
- 14. McNally PG, Raymond NT, Burden ML, Burton PR, Botha JL, et al. (1995) Trends in mortality of childhood-onset insulin-dependent diabetes mellitus in Leicestershire:1940–1991. . Diabetic Medicine 12:961–966. [DOI] [PubMed] [Google Scholar]
- 15. Patterson CC, Dahlquist G, Harjutsalo V, Joner G, Feltbower RG, et al. (2007) Early mortality in EURODIAB population-based cohorts of type 1 diabetes diagnosed in childhood since 1989. . Diabetologia 50:2439–2442. [DOI] [PubMed] [Google Scholar]
- 16. Podar T, Solntsev A, Reunanen A, Urbonaite B, Zalinkevicius R, et al. (2000) Mortality in patients with childhood-onset type 1 diabetes in Finland, Estonia, and Lithuania - Follow-up of nationwide cohorts. . Diabetes Care 23:290–294. [DOI] [PubMed] [Google Scholar]
- 17. Nystrom L, Ostman J, Wall S, Wibell L, Arnqvist H, et al. (1992) Mortality of All Incident Cases of Diabetes-Mellitus in Sweden Diagnosed 1983–1987 at Age 15–34 Years. . Diabetic Medicine 9:422–427. [DOI] [PubMed] [Google Scholar]
- 18. Sartor G, Nystrom L, Dahlquist G (1991) The Swedish Childhood Diabetes Study: a seven-fold decrease in short-term mortality? Diabetic Medicine. 8:18–21. [DOI] [PubMed] [Google Scholar]
- 19. Morrish NJ, Wang SL, Stevens LK, Fuller JH, Keen H (2001) Mortality and causes of death in the WHO Multinational Study of Vascular Disease in Diabetes. Diabetologia 44 Suppl. 2: S14–21. [DOI] [PubMed] [Google Scholar]
- 20. Allemann S, Saner C, Zwahlen M, Christ ER, Diem P, et al. (2009) Long-term cardiovascular and non-cardiovascular mortality in women and men with type 1 and type 2 diabetes mellitus: a 30-year follow-up in Switzerland. . Swiss Medical Weekly 139:576–583. [DOI] [PubMed] [Google Scholar]
- 21. Barcelo A, Bosnyak Z, Orchard T (2007) A cohort analysis of type 1 diabetes mortality in Havana and Allegheny County, Pittsburgh, PA. . Diabetes Research & Clinical Practice 75:214–219. [DOI] [PubMed] [Google Scholar]
- 22. Botha JL, Parker H, Raymond NT, Swift PG (1992) Diabetes diagnosed before the age of 2 years: mortality in a British cohort 8–17 years after onset. . Int J Epidemiol 21:1132–1137. [DOI] [PubMed] [Google Scholar]
- 23. Bruno G, Cerutti F, Merletti F, Novelli G, Panero F, et al. (2009) Short-term mortality risk in children and young adults with type 1 diabetes: the population-based Registry of the Province of Turin, Italy. . Nutrition Metabolism & Cardiovascular Diseases 19:340–344. [DOI] [PubMed] [Google Scholar]
- 24. Collado-Mesa F, Diaz-Diaz O, Melian-Torres R, Suarez-Perez R, Vera-Gonzalez M, et al. (1997) Mortality of childhood-onset IDDM patients. A cohort study in Havana City Province, Cuba. . Diabetes Care 20:1237–1241. [DOI] [PubMed] [Google Scholar]
- 25. Feltbower RG, Bodansky HJ, Patterson CC, Parslow RC, Stephenson CR, et al. (2008) Acute complications and drug misuse are important causes of death for children and young adults with type 1 diabetes: results from the Yorkshire Register of diabetes in children and young adults. . Diabetes Care 31:922–926. [DOI] [PubMed] [Google Scholar]
- 27. Grauslund J, Jorgensen TMM, Nybo M, Green A, Rasmussen LM, et al. (2010) Risk factors for mortality and ischemic heart disease in patients with long-term type 1 diabetes. . Journal of Diabetes & its Complications 24:223–228. [DOI] [PubMed] [Google Scholar]
- 28. Harjutsalo V, Forsblom C, Groop P-H (2011) Time trends in mortality in patients with type 1 diabetes: nationwide population based cohort study. BMJ. 343:d5364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Joner G, Patrick S (1991) The mortality of children with type 1 (insulin-dependent) diabetes mellitus in Norway, 1973–1988. . Diabetologia 34:29–32. [DOI] [PubMed] [Google Scholar]
- 30. Laing SP, Swerdlow AJ, Slater SD, Burden AC, Morris A, et al. (2003) Mortality from heart disease in a cohort of 23,000 patients with insulin-treated diabetes. . Diabetologia 46:760–765. [DOI] [PubMed] [Google Scholar]
- 31. Muggeo M, Verlato G, Bonora E, Bressan F, Girotto S, et al. (1995) The Verona diabetes study: a population-based survey on known diabetes mellitus prevalence and 5-year all-cause mortality. . Diabetologia 38:318–325. [DOI] [PubMed] [Google Scholar]
- 32. Raymond NT, Langley JD, Goyder E, Botha JL, Burden AC, et al. (1995) Insulin treated diabetes mellitus: causes of death determined from record linkage of population based registers in Leicestershire, UK. Journal of Epidemiology & Community Health. 49:570–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Riley MD, McCarty DJ, Couper DJ, Humphrey AR, Dwyer T, et al. (1995) The 1984 Tasmanian insulin treated diabetes mellitus prevalence cohort: an eight and a half year mortality follow-up investigation. . Diabetes Research & Clinical Practice 29:27–35. [DOI] [PubMed] [Google Scholar]
- 34. Roberts SE, Goldacre MJ, Neil HAW (2004) Mortality in young people admitted to hospital for diabetes: database study. . BMJ 328:741–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sartor G, Dahlquist G (1995) Short-term mortality in childhood onset insulin-dependent diabetes mellitus: a high frequency of unexpected deaths in bed. . Diabetic Medicine 12:607–611. [DOI] [PubMed] [Google Scholar]
- 36. Skrivarhaug T, Bangstad HJ, Stene LC, Sandvik L, Hanssen KF, et al. (2006) Long-term mortality in a nationwide cohort of childhood-onset type 1 diabetic patients in Norway. . Diabetologia 49:298–305. [DOI] [PubMed] [Google Scholar]
- 37. Soedamah-Muthu SS, Fuller JH, Mulnier HE, Raleigh VS, Lawrenson RA, et al. (2006) All-cause mortality rates in patients with type 1 diabetes mellitus compared with a non-diabetic population from the UK general practice research database, 1992–1999. . Diabetologia 49:660–666. [DOI] [PubMed] [Google Scholar]
- 38. Swerdlow AJ, Jones ME (1996) Mortality during 25 years of follow-up of a cohort with diabetes. . International Journal of Epidemiology 25:1250–1261. [DOI] [PubMed] [Google Scholar]
- 39. Waernbaum I, Blohme G, Ostman J, Sundkvist G, Eriksson JW, et al. (2006) Excess mortality in incident cases of diabetes mellitus aged 15 to 34 years at diagnosis: a population-based study (DISS) in Sweden. [Erratum appears in Diabetologia. 2006 Jun;49(6):1457]. . Diabetologia 49:653–659. [DOI] [PubMed] [Google Scholar]
- 40. Wibell L, Nystrom L, Ostman J, Arnqvist H, Blohme G, et al. (2001) Increased mortality in diabetes during the first 10 years of the disease. A population-based study (DISS) in Swedish adults 15–34 years old at diagnosis. . Journal of Internal Medicine 249:263–270. [DOI] [PubMed] [Google Scholar]
- 41. Smolina K, Wright FL, Rayner M, Goldacre MJ (2012) Determinants of the decline in mortality from acute myocardial infarction in England between 2002 and 2010: linked national database study. . BMJ 344:d8059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Seshasai SR, Kaptoge S, Thompson A, Di Angelantonio E, Gao P, et al. (2011) Diabetes mellitus, fasting glucose, and risk of cause-specific death. . N Engl J Med 364:829–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jorgensen ME, Almdal TP, Carstensen B (2013) Time trends in mortality rates in type 1 diabetes from 2002 to 2011. . Diabetologia 56:2401–2404. [DOI] [PubMed] [Google Scholar]
- 44. Livingstone SJ, Looker HC, Hothersall EJ, Wild SH, Lindsay RS, et al. (2012) Risk of cardiovascular disease and total mortality in adults with type 1 diabetes: Scottish registry linkage study. . PLoS Med 9:e1001321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Murray P, Chune GW, Raghavan VA (2010) Legacy effects from DCCT and UKPDS: what they mean and implications for future diabetes trials. . Curr Atheroscler Rep 12:432–439. [DOI] [PubMed] [Google Scholar]
- 46. Ranjit Unnikrishnan I, Anjana RM, Mohan V (2011) Importance of controlling diabetes early-the concept of metabolic memory, legacy effect and the case for early insulinisation. J Assoc Physicians India 59 Suppl: 8–12. [PubMed] [Google Scholar]
- 47. Jess T, Gamborg M, Munkholm P, Sorensen TI (2007) Overall and cause-specific mortality in ulcerative colitis: meta-analysis of population-based inception cohort studies. . Am J Gastroenterol 102:609–617. [DOI] [PubMed] [Google Scholar]
- 48. Yurkovich M, Vostretsova K, Chen W, Avina-Zubieta JA (2014) Overall and cause-specific mortality in patients with systemic lupus erythematosus: a meta-analysis of observational studies. . Arthritis Care Res (Hoboken) 66:608–616. [DOI] [PubMed] [Google Scholar]
- 49.Soltesz GPC, Dahlquist G (2003) Diabetes in the young: a global perspective.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1 and S2 and Figures S1 and S2. Table S1. Summary of terms used in the Medline search strategy. Table S2. STROBE checklist for cohort studies. Figure S1. Cumulative meta-analysis by median study date (< = 1970, 1971–1980, 1981–1990,>1990). Horizontal bars and circles widths denote 95% CIs, and box sizes indicate relative weight in the analysis. Figure S2. Trim-and-fill analysis of the included estimates. No trimming of data was performed, suggesting no evidence of publication bias.
(DOCX)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.