Abstract
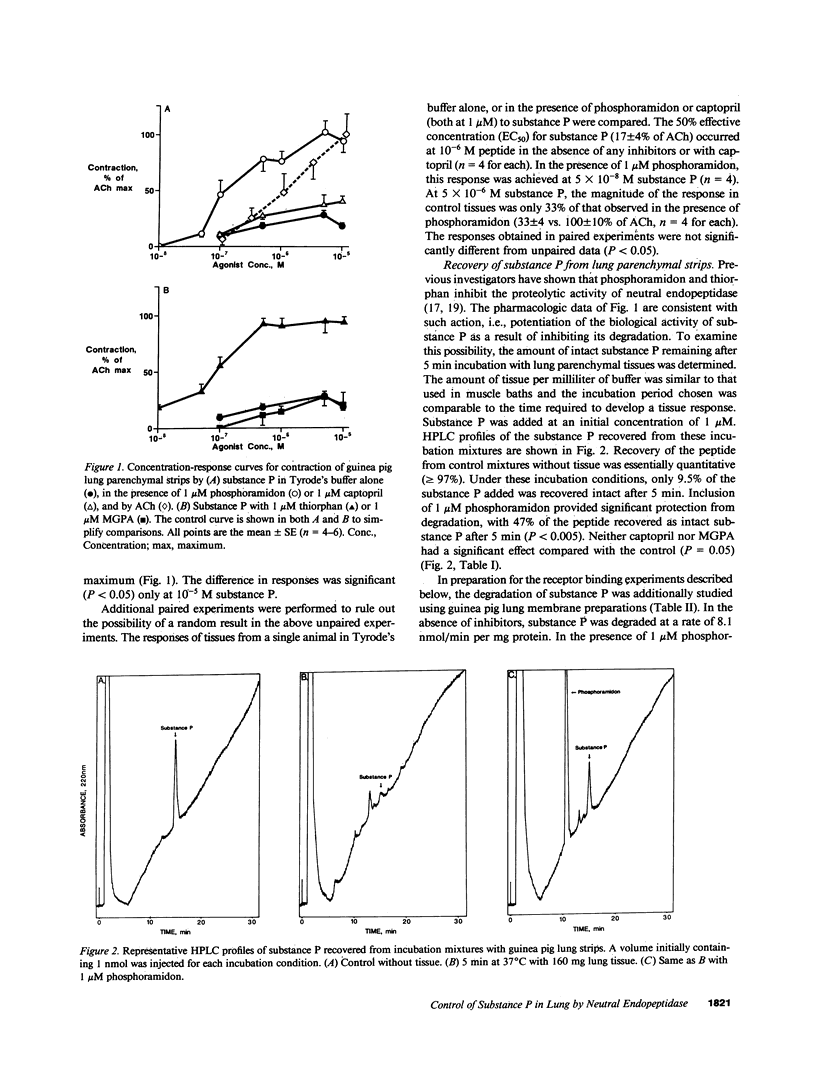
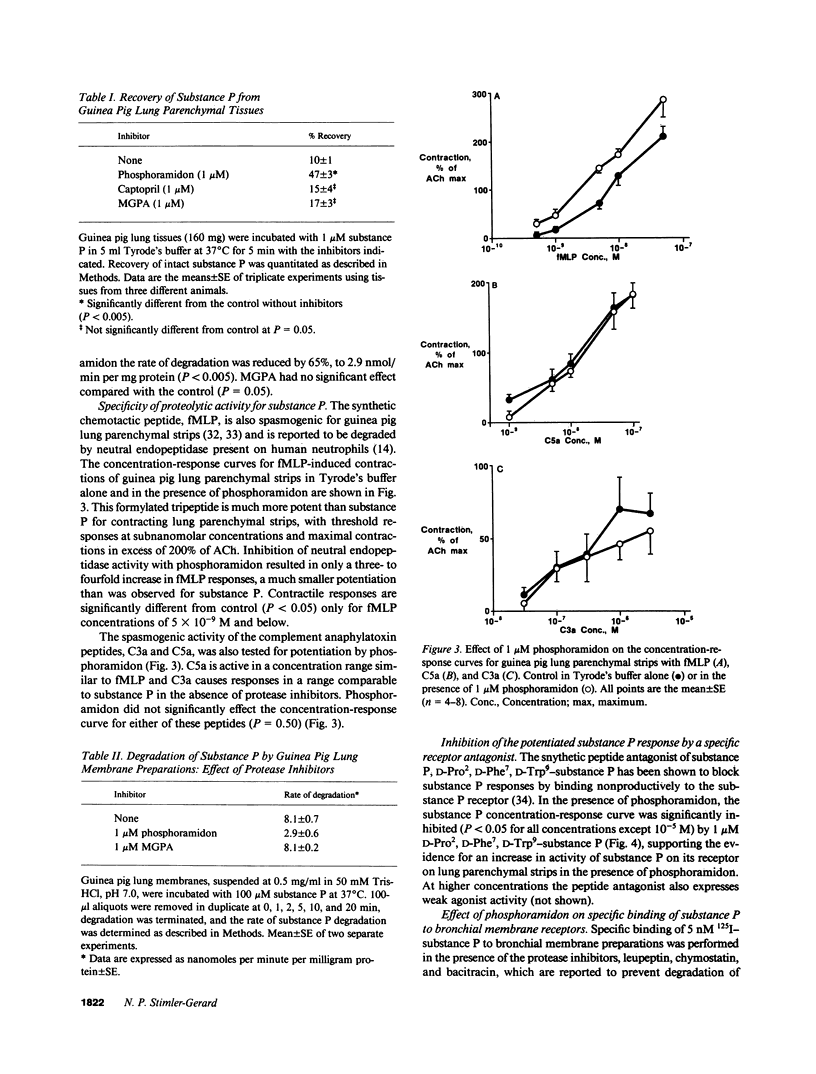
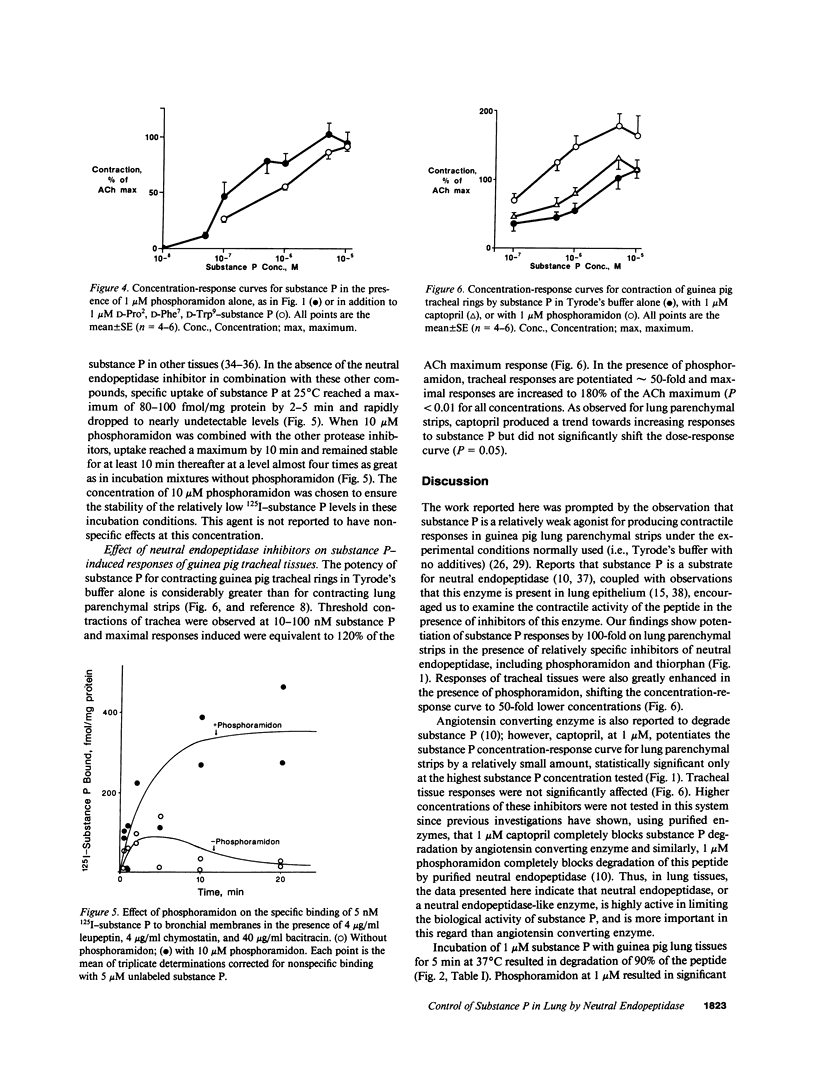
The responsiveness of isolated guinea pig lung parenchymal strips to substance P was enhanced by at least 100-fold in the presence of the endopeptidase inhibitors phosphoramidon (1 microM) or thiorphan (1 microM), but not with the converting enzyme inhibitor, captopril, or an inhibitor of serum carboxypeptidase N (both 1 microM). Responses of guinea pig tracheal rings to substance P were also markedly potentiated by phosphoramidon. The increase in tissue responsiveness by these inhibitors was relatively specific for substance P among several other spasmogenic peptides, including formyl-methionyl-leucyl-phenylalanine and the complement peptides C3a and C5a. The enhanced responses appear to result from a decrease in the rate of substance P degradation in the presence of neutral endopeptidase inhibitors. Specific binding of substance P to its receptor on bronchial membranes was increased by three- to fourfold in the presence of phosphoramidon. These data demonstrate an enhanced potential for substance P to contract lung tissues when degradation by a neutral endopeptidase-like enzyme is blocked.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bokisch V. A., Müller-Eberhard H. J. Anaphylatoxin inactivator of human plasma: its isolation and characterization as a carboxypeptidase. J Clin Invest. 1970 Dec;49(12):2427–2436. doi: 10.1172/JCI106462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burcher E., Buck S. H., Lovenberg W., O'Donohue T. L. Characterization and autoradiographic localization of multiple tachykinin binding sites in gastrointestinal tract and bladder. J Pharmacol Exp Ther. 1986 Mar;236(3):819–831. [PubMed] [Google Scholar]
- Burka J. F. Effects of compound 48/80 and formyl-methionyl-leucyl-phenylalanine on isolated guinea pig airways. Int Arch Allergy Appl Immunol. 1984;73(4):309–313. doi: 10.1159/000233490. [DOI] [PubMed] [Google Scholar]
- Cascieri M. A., Chicchi G. G., Liang T. Demonstration of two distinct tachykinin receptors in rat brain cortex. J Biol Chem. 1985 Feb 10;260(3):1501–1507. [PubMed] [Google Scholar]
- Castairs J. R., Barnes P. J. Autoradiographic mapping of substance P receptors in lung. Eur J Pharmacol. 1986 Aug 15;127(3):295–296. doi: 10.1016/0014-2999(86)90380-8. [DOI] [PubMed] [Google Scholar]
- Chang M. M., Leeman S. E., Niall H. D. Amino-acid sequence of substance P. Nat New Biol. 1971 Jul 21;232(29):86–87. doi: 10.1038/newbio232086a0. [DOI] [PubMed] [Google Scholar]
- Connelly J. C., Skidgel R. A., Schulz W. W., Johnson A. R., Erdös E. G. Neutral endopeptidase 24.11 in human neutrophils: cleavage of chemotactic peptide. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8737–8741. doi: 10.1073/pnas.82.24.8737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damerau B., Zimmermann B., Czorniak K., Wüstefeld H., Vogt W. Role of the N-terminal regions of hog C3a, C5a and C5a-desArg in their biological activities. Mol Immunol. 1986 Apr;23(4):433–440. doi: 10.1016/0161-5890(86)90141-0. [DOI] [PubMed] [Google Scholar]
- Gafford J. T., Skidgel R. A., Erdös E. G., Hersh L. B. Human kidney "enkephalinase", a neutral metalloendopeptidase that cleaves active peptides. Biochemistry. 1983 Jun 21;22(13):3265–3271. doi: 10.1021/bi00282a035. [DOI] [PubMed] [Google Scholar]
- Gerard C., Showell H. J., Hoeprich P. D., Jr, Hugli T. E., Stimler N. P. Evidence for a role of the amino-terminal region in the biological activity of the classical anaphylatoxin, porcine C5a des-Arg-74. J Biol Chem. 1985 Mar 10;260(5):2613–2616. [PubMed] [Google Scholar]
- Hamel R., Ford-Hutchinson A. W., Lord A., Cirino M. Bronchoconstriction induced by N-formyl-methionyl-leucyl-phenylalanine in the guinea pig; involvement of arachidonic acid metabolites. Prostaglandins. 1984 Jul;28(1):43–56. doi: 10.1016/0090-6980(84)90112-6. [DOI] [PubMed] [Google Scholar]
- Hudgin R. L., Charleson S. E., Zimmerman M., Mumford R., Wood P. L. Enkephalinase: selective peptide inhibitors. Life Sci. 1981 Dec 21;29(25):2593–2601. doi: 10.1016/0024-3205(81)90632-9. [DOI] [PubMed] [Google Scholar]
- Jiang Z., Dun N. J., Karczmar A. G. Substance P: a putative sensory transmitter in mammalian autonomic ganglia. Science. 1982 Aug 20;217(4561):739–741. doi: 10.1126/science.6179162. [DOI] [PubMed] [Google Scholar]
- Johnson A. R., Ashton J., Schulz W. W., Erdös E. G. Neutral metalloendopeptidase in human lung tissue and cultured cells. Am Rev Respir Dis. 1985 Sep;132(3):564–568. doi: 10.1164/arrd.1985.132.3.564. [DOI] [PubMed] [Google Scholar]
- Johnson A. R., Coalson J. J., Ashton J., Larumbide M., Erdös E. G. Neutral endopeptidase in serum samples from patients with adult respiratory distress syndrome. Comparison with angiotensin-converting enzyme. Am Rev Respir Dis. 1985 Dec;132(6):1262–1267. doi: 10.1164/arrd.1985.132.6.1262. [DOI] [PubMed] [Google Scholar]
- Llorens C., Gacel G., Swerts J. P., Perdrisot R., Fournie-Zaluski M. C., Schwartz J. C., Roques B. P. Rational design of enkephalinase inhibitors: substrate specificity of enkephalinase studied from inhibitory potency of various dipeptides. Biochem Biophys Res Commun. 1980 Oct 31;96(4):1710–1716. doi: 10.1016/0006-291x(80)91371-6. [DOI] [PubMed] [Google Scholar]
- Lundberg J. M., Brodin E., Saria A. Effects and distribution of vagal capsaicin-sensitive substance P neurons with special reference to the trachea and lungs. Acta Physiol Scand. 1983 Nov;119(3):243–252. doi: 10.1111/j.1748-1716.1983.tb07334.x. [DOI] [PubMed] [Google Scholar]
- Lundberg J. M., Martling C. R., Saria A. Substance P and capsaicin-induced contraction of human bronchi. Acta Physiol Scand. 1983 Sep;119(1):49–53. doi: 10.1111/j.1748-1716.1983.tb07304.x. [DOI] [PubMed] [Google Scholar]
- Lundberg J. M., Saria A. Bronchial smooth muscle contraction induced by stimulation of capsaicin-sensitive sensory neurons. Acta Physiol Scand. 1982 Dec;116(4):473–476. doi: 10.1111/j.1748-1716.1982.tb07170.x. [DOI] [PubMed] [Google Scholar]
- Malfroy B., Swerts J. P., Guyon A., Roques B. P., Schwartz J. C. High-affinity enkephalin-degrading peptidase in brain is increased after morphine. Nature. 1978 Nov 30;276(5687):523–526. doi: 10.1038/276523a0. [DOI] [PubMed] [Google Scholar]
- Malo P. E., Wasserman M. A., Torphy T. J., Parris D. J., Pfeiffer D. F. Characterization of substance P-induced contractions of guinea-pig trachea. J Pharmacol Exp Ther. 1986 Jun;237(3):782–786. [PubMed] [Google Scholar]
- Matsas R., Fulcher I. S., Kenny A. J., Turner A. J. Substance P and [Leu]enkephalin are hydrolyzed by an enzyme in pig caudate synaptic membranes that is identical with the endopeptidase of kidney microvilli. Proc Natl Acad Sci U S A. 1983 May;80(10):3111–3115. doi: 10.1073/pnas.80.10.3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizrahi J., Couture R., Caranikas S., Regoli D. Pharmacological effects of peptides on tracheal smooth muscle. Pharmacology. 1982;25(1):39–50. doi: 10.1159/000137722. [DOI] [PubMed] [Google Scholar]
- Mumford R. A., Pierzchala P. A., Strauss A. W., Zimmerman M. Purification of a membrane-bound metalloendopeptidase from porcine kidney that degrades peptide hormones. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6623–6627. doi: 10.1073/pnas.78.11.6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino N., Powers J. C. Design of potent reversible inhibitors for thermolysin. Peptides containing zinc coordinating ligands and their use in affinity chromatography. Biochemistry. 1979 Oct 2;18(20):4340–4347. doi: 10.1021/bi00587a012. [DOI] [PubMed] [Google Scholar]
- Pong S. S., DeHaven R. N. Characterization of a leukotriene D4 receptor in guinea pig lung. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7415–7419. doi: 10.1073/pnas.80.24.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regal J. F., Eastman A. Y., Pickering R. J. C5a induced tracheal contraction: a histamine independent mechanism. J Immunol. 1980 Jun;124(6):2876–2878. [PubMed] [Google Scholar]
- Regal J. F., Johnson D. E. Indomethacin alters the effects of substance-P and VIP on isolated airway smooth muscle. Peptides. 1983 Jul-Aug;4(4):581–584. doi: 10.1016/0196-9781(83)90065-7. [DOI] [PubMed] [Google Scholar]
- Schwartz J. C., Malfroy B., De La Baume S. Biological inactivation of enkephalins and the role of enkephalin-dipeptidyl-carboxypeptidase ("enkephalinase") as neuropeptidase. Life Sci. 1981 Oct 26;29(17):1715–1740. doi: 10.1016/0024-3205(81)90182-x. [DOI] [PubMed] [Google Scholar]
- Shults C. W., Quirion R., Chronwall B., Chase T. N., O'Donohue T. L. A comparison of the anatomical distribution of substance P and substance P receptors in the rat central nervous system. Peptides. 1984 Nov-Dec;5(6):1097–1128. doi: 10.1016/0196-9781(84)90177-3. [DOI] [PubMed] [Google Scholar]
- Skidgel R. A., Engelbrecht S., Johnson A. R., Erdös E. G. Hydrolysis of substance p and neurotensin by converting enzyme and neutral endopeptidase. Peptides. 1984 Jul-Aug;5(4):769–776. doi: 10.1016/0196-9781(84)90020-2. [DOI] [PubMed] [Google Scholar]
- Stimler N. P., Bach M. K., Bloor C. M., Hugli T. E. Release of leukotrienes from guinea pig lung stimulated by C5ades Arg anaphylatoxin. J Immunol. 1982 May;128(5):2247–2252. [PubMed] [Google Scholar]
- Stimler N. P., Hugli T. E., Bloor C. M. Pulmonary injury induced by C3a and C5a anaphylatoxins. Am J Pathol. 1980 Aug;100(2):327–348. [PMC free article] [PubMed] [Google Scholar]
- Sullivan S., Akil H., Barchas J. D. In vitro degradation of enkephalin: evidence for cleavage at the Gly-Phe bond. Commun Psychopharmacol. 1978;2(6):525–531. [PubMed] [Google Scholar]
- Turner A. J., Matsas R., Kenny A. J. Are there neuropeptide-specific peptidases? Biochem Pharmacol. 1985 May 1;34(9):1347–1356. doi: 10.1016/0006-2952(85)90669-0. [DOI] [PubMed] [Google Scholar]
- Waksman G., Hamel E., Fournié-Zaluski M. C., Roques B. P. Autoradiographic comparison of the distribution of the neutral endopeptidase "enkephalinase" and of mu and delta opioid receptors in rat brain. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1523–1527. doi: 10.1073/pnas.83.5.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H. Y., Erdös E. G. Second kininase in human blood plasma. Nature. 1967 Sep 23;215(5108):1402–1403. doi: 10.1038/2151402a0. [DOI] [PubMed] [Google Scholar]


