Abstract
Background
Where cancer patients receive surgical care has implications on policy and planning and on patients’ satisfaction and outcomes. We conducted a population-based analysis of where rectal cancer patients undergo surgery and a qualitative analysis of rectal cancer patients’ perspectives on location of surgical care.
Methods
We reviewed Manitoba Cancer Registry data on patients with colorectal cancer (CRC) diagnosed between 2004 and 2006. We interviewed rural patients with rectal cancer regarding their preferences and the factors they considered when deciding on treatment location. Interview data were analyzed using a grounded theory approach.
Results
From 2004 to 2006, 2086 patients received diagnoses of CRC in Manitoba (colon: 1578, rectal: 508). Among rural patients (n = 907), those with rectal cancer were more likely to undergo surgery at an urban centre than those with colon cancer (46.5% v. 28.8%, p < 0.001). Twenty rural patients with rectal cancer participated in interviews. We identified 3 major themes from the interview data: the decision-maker, treatment factors and personal factors. Participants described varying input into referral decisions, and often they did not perceive a choice regarding treatment location. Treatment factors, including surgeon factors and hospital factors, were important when considering treatment location. Personal factors, including travel, support, accommodation, finances and employment, also affected participants’ treatment experiences.
Conclusion
A substantial proportion of rural patients with rectal cancer undergo surgery at urban centres. The reasons are complex and only partly related to patient choice. Further studies are required to better understand cancer system access in geographically dispersed populations and to support cancer patients through the decision-making and treatment processes.
Abstract
Contexte
Le lieu où les patients atteints du cancer subissent une intervention chirurgicale a des répercussions sur les politiques et la planification, et sur la satisfaction du patient et ses résultats. Nous avons étudié dans une population le lieu où des patients atteints de cancer du rectum subissent leur chirurgie et effectué une analyse qualitative des points de vue exprimés par les patients au sujet du lieu où les soins chirurgicaux sont dispensés.
Méthodes
Nous avons consulté le Registre du cancer du Manitoba pour trouver des données sur des patients atteints de cancer colorectal diagnostiqué entre 2004 et 2006. Nous avons interviewé des patients de régions rurales atteints de cancer du rectum pour connaître leurs préférences et les facteurs dont ils avaient tenu compte en choisissant le lieu où ils allaient être traités. Nous avons analysé les données recueillies à l’aide d’une méthode théorique fondées sur les faits.
Résultats
Entre 2004 et 2006, au Manitoba, 2086 patients ont reçu un diagnostic de cancer colorectal (cancer du côlon : 1578; cancer du rectum : 508). Parmi les patients qui vivaient en milieu rural (n = 907), ceux atteints d’un cancer du rectum avaient plus tendance à subir leur chirurgie dans un établissement urbain que ceux atteints de cancer du côlon (46,5 % c. 28,8 %, p < 0,001). Vingt patients de milieu rural atteitns de cancer du rectum ont participé aux entrevues. Trois principaux éléments se dégagent des données recueillies : le décideur, des facteurs reliés au traitement et des facteurs d’ordre personnel. Les participants ont décrit diverses contributions qu’ils ont apportées à la décision relative à la référence de leur cas et dit que souvent, ils n’ont pas senti qu’un choix de lieux de traitement leur était offert. Les facteurs liés au traitement lui-même, y compris ceux liés au chirurgien et à l’hôpital, ont été importants dans le choix du lieu de traitement. Les facteurs d’ordre personnel, dont le déplacement, le soutien, l’hébergement, la situation financière et l’emploi ont aussi influé sur l’expérience thérapeutique des participants.
Conclusion
Une proportion considérable de patients atteints du cancer du rectum et vivant en milieu rural subissent leur chirurgie dans des établissements urbains. Les raisons sont complexes et ne sont qu’en partie reliées au choix du patient. Il faudrait mener d’autres études pour mieux comprendre l’accès aux services offerts aux personnes atteintes de cancer dans les populations géographiquement dispersées et pour les appuyer dans le processus de prise de décision et de traitement.
Colorectal cancer (CRC) is the third most common cancer diagnosis and the second most common cause of cancer-related death in Canada.1 In the province of Manitoba, nearly 900 people received diagnoses of CRC in 2012.1 Given the population distribution in which nearly half of the province’s population resides in nonurban regions, the number of people from rural areas requiring treatment for CRC is substantial.2
Numerous authors and health care policy-makers have advocated for the regionalization of cancer services (particularly of high-risk cancer procedures) to high-volume centres.3,4 These authors argue that regionalization may improve patient outcomes as well as lower systemic costs by concentrating and making more efficient use of resources and personnel. Much of their argument is based on studies that report an association between hospital or surgeon case volumes and outcomes for major cancer operations. For rectal cancer, several studies suggest that long-term survival may be improved when surgical resection is performed at high-volume hospitals5,6 and by surgeons with high case volumes.7,8 Numerous studies have also reported an association between case volume and sphincter preservation rates, suggesting the chances of being stoma-free may be higher when surgery is performed at high-volume hospitals5,6,9–11 and by surgeons with subspecialized colorectal or oncology training.8
There are, however, inconsistencies in the literature on the association between volume and outcomes pertaining specifically to rectal cancer.7 Furthermore, policies based solely on volume–outcome associations do not consider the preferences of patients residing in rural areas who may be most directly affected by regionalization. In fact, several studies suggest that patients are willing to risk significantly higher surgical mortality in order to be treated closer to home.12,13 Given a hypothetical scenario of resectable pancreatic cancer, Finlayson and colleagues12 reported that even if the operative mortality risk was doubled (6% at a local hospital v. 3% at a regional hospital), 45% of study participants would still prefer surgery at their local hospital to treatment at a regional centre.
While studies like those by Finlayson and colleagues attempt to determine where patients prefer to have surgery, they do not examine how patients make this choice. The present study aimed to quantify where patients from rural Manitoba underwent surgery for rectal cancer (local v. urban hospital) and to explore their perspectives on the factors that influenced their decision on where to undergo surgery.
Methods
Setting
The University of Manitoba Health Research Ethics Board approved this study. The province of Manitoba covers a vast geographic area of almost 650 000 km2. Its area is twice that of the entire United Kingdom. It has a population of more than 1.2 million people, 57% of whom live in Winnipeg, the capital city and only major urban centre.14 Manitoba has 2 urban tertiary care and 4 urban nontertiary care hospitals in Winnipeg and 8 rural hospitals outside of Winnipeg where major colorectal surgery can be performed. Manitoba’s health care system, like all of Canada’s, is publicly funded, and seeing a specialist usually requires a referral from another medical practitioner.
Population-based analysis of surgical care location
All patients who received a diagnosis of adenocarcinoma of the colon or rectum between Jan. 1, 2004, and Dec. 31, 2006, were identified from the population-based Manitoba Cancer Registry (MCR), which collects information on all patients with a cancer diagnosis in Manitoba.15 The MCR contains high-quality cancer reporting data16,17 and is maintained by CancerCare Manitoba, the province’s central cancer agency. We obtained patient demographic, tumour and treatment data from the MCR. We performed χ2 analyses to test associations between tumour site and place of residence and surgery (α = 0.05).
Patient interviews
A convenience sample of surgeons in Manitoba (from both rural and urban settings) who performed rectal cancer surgery were contacted and asked to enroll patients. English-speaking patients residing outside of Winnipeg who had stage I–III rectal cancer diagnoses and were scheduled to undergo curative-intent surgery were eligible to participate in our study. We offered participating patients a small honorarium (CAD $20 gift card).
We conducted telephone interviews with individual participants before surgery in order to more closely approximate the preoperative decision-making context (as opposed to the postoperative context when outcomes may affect perceptions).18 Interviews were conducted from June 2010 to July 2011. We used a semi-structured interview script (see the Appendix, available at canjsurg.ca) that included open-ended questions on the factors patients considered when deciding on treatment location, how this decision affected them and their personal supports (i.e., family, friends and caregivers), and their satisfaction with their decision. Interviews were audio-recorded and transcribed verbatim.
Data analysis
The data were analyzed for emergent themes using a grounded theory approach. Grounded theory describes a systematic approach to interpreting qualitative data that aims to generate theory in an area of social inquiry.19,20 Two researchers (M.N. and J.P.) read and analyzed the interview transcripts as they were collected. Themes were identified and further refined with each iteration of analysis. Sampling continued until no further themes were forthcoming from the data, consistent with a theoretical sampling strategy. A single researcher (M.N.) applied the final coding structure to the entire data set. We used NVivo qualitative data analysis software (QSR International PTY Ltd.) to organize the data.
Results
Population-based analysis of surgical care location
Over the 3-year study period, 2086 patients received diagnoses of CRC in Manitoba (Table 1). The proportions of patients with rectal compared with colon/rectosigmoid cancer did not differ between patients in Winnipeg and those outside of Winnipeg (odds ratio [OR] 1.1, 95% confidence interval [CI] 0.9–1.3, p = 0.39). These proportions closely mirrored Manitoba’s population distribution, in which about 57% of the population lives in Winnipeg. However, a significantly higher proportion of patients with rectal compared with colon/rectosigmoid cancer underwent surgery in Winnipeg (OR 1.5, 95% CI 1.2–2.0, p < 0.001).
Table 1.
Clinical characteristics and treatment information on patients with colorectal cancer diagnosed in Manitoba between 2004 and 2006
| Characteristic | Cancer site; no. (%) or mean ± SD | ||
|---|---|---|---|
| Colon, n = 1376 | Rectosigmoid, n = 202 | Rectum, n = 508 | |
| Age, yr | 71 ± 13 | 70 ± 12 | 68 ± 12 |
| Sex, female | 678 (49.3) | 92 (45.5) | 193 (38.0) |
| Surgery | |||
| Major resection | 1107 (80.4) | 156 (77.2) | 365 (71.9) |
| Local excision or polypectomy | 45 (3.3) | 16 (7.9) | 67 (13.2) |
| None | 224 (16.3) | 30 (14.9) | 76 (15.0) |
| AJCC stage | |||
| 1 | 241 (17.5) | 40 (19.8) | 122 (24.0) |
| 2 | 403 (29.3) | 56 (27.7) | 116 (22.8) |
| 3 | 391 (28.4) | 57 (28.2) | 162 (31.9) |
| 4 | 302 (22.0) | 47 (23.3) | 90 (17.7) |
| Unknown | 39 (2.8) | 2 (1.0) | 18 (3.5) |
| Residing in Winnipeg | 788 (57.3) | 116 (57.4) | 302 (59.4) |
| Surgery in Winnipeg* | 793 (68.8) | 117 (68.0) | 333 (77.1) |
AJCC = American Joint Commission on Cancer; SD = standard deviation.
Parentheses show patients who underwent surgery in Winnipeg as a percentage of those who underwent surgery.
A total of 427 rectal cancer patients underwent surgical treatment (Table 2). Of the 172 patients from rural areas who had surgery, 80 had procedures in Winnipeg and 92 had procedures at a rural hospital. This means that 80 of 172 patients (46.5%) had procedures in Winnipeg, while 92 of 172 (53.5%) rural patients underwent surgery at a rural hospital. In comparison, 563 rural patients with colon/rectosigmoid cancer underwent surgery; of these, 162 (28.8%) had surgery in Winnipeg. The proportion of rural patients who underwent surgery in Winnipeg was significantly higher for patients with rectal cancers than those with colon/rectosigmoid cancers (OR 2.1, 95% CI 1.5–3.1, p < 0.001).
Table 2.
Clinical characteristics and treatment data on patients (n = 427) who underwent surgical treatment for rectal cancer in Manitoba based on place of surgery, 2004–2006
| Characteristic | Group; no. (%) or mean ± SD | |
|---|---|---|
| Surgery outside of Winnipeg, n = 94* | Surgery in Winnipeg, n = 333 | |
| Age, yr | 68 ± 11 | 67 ± 12 |
| Sex, female | 34 (36.2) | 130 (39.0) |
| Residence | ||
| Outside of Winnipeg | 92 (97.9) | 80 (24.0) |
| Winnipeg | 2 (2.1) | 253 (76.0) |
| Surgery | ||
| Major surgery | 76 (80.9) | 286 (85.9) |
| Local excision | 10 (10.6) | 31 (9.3) |
| Polypectomy | 8 (8.5) | 16 (4.8) |
| AJCC stage | ||
| 1 | 29 (30.9) | 85 (25.5) |
| 2 | 25 (26.6) | 76 (22.8) |
| 3 | 29 (30.9) | 126 (37.8) |
| 4 | 11 (11.7) | 40 (12.0) |
| Unknown | — | 6 (1.8) |
AJCC = American Joint Commission on Cancer; SD = standard deviation.
Five patients with rectal cancer underwent surgery outside of Manitoba.
Patient interviews
We conducted interviews with a convenience sample of 20 patients, all of whom went on to have surgery at 1 of 2 tertiary care centres in Winnipeg (Table 3). Participants lived a median of 186 (range 58–769) km from the centre where they were scheduled to have surgery. In comparison, they lived a median of 70 (range 0–187) km from the closest rural hospital that performed rectal cancer surgery. Our study protocol also included patients who planned to have surgery outside of Winnipeg; however, we were unable to accrue any of these patients.
Table 3.
Clinical characteristics and treatment data on patients who participated in the interviews, n = 20
| Characteristic | Median (range); mean ± SD* |
|---|---|
| Age, yr | 63 (35–86); 62 ± 11.2 |
| Sex, female:male | 6:14 |
| Distance of tumour from anal verge, cm | 8 (0–10); 6.6 ± 3.1 |
| Neoadjuvant treatment, no. | |
| Yes | 16 |
| No | 4 |
| Final pathological stage, no.† | |
| Stage 0 | 3 |
| Stage 1 | 6 |
| Stage 2 | 4 |
| Stage 3 | 7 |
SD = standard deviation; TNM = tumour–node–metastasis.
Unless otherwise indicated.
Final posttreatment pathological (TNM) stage.
Participants’ discussion on location of surgical care reflected 3 major themes: the decision-maker, treatment factors and personal factors. The decision-maker described who decided on where to send the referral. Treatment factors described how the hospital or surgeon might influence the patient’s choice based on treatments or perceived outcomes. Personal factors described how individual patients’ personal circumstances, including finances and support, were considered and/or affected by the location of care. Boxes 1 and 2 show quotations for the themes of treatment and personal factors.
Box 1. Example comments from rural patients with rectal cancer about treatment-related factors involved in decisions on location of surgery.
Surgeon-related
Skill and volume: “I would probably want to go to the city… because they would see more of that kind of surgery. They would have more experience. Obviously you want to have the surgery where someone’s doing it all the time, that particular surgery” (participant 011, 63-year-old woman).
Reputation: “I just wanted the best doctor that we referred to… I guess that’s pretty much it. I asked [the referring doctor] for the background on [the surgeon]. I guess who the best doctor was. The doctor here… had recommended Dr. A, and then I just asked a few doctors what he was like” (participant 010, 35-year-old man).
Interpersonal relationship: “Sometimes when you meet someone [like the surgeon], in a few minutes you trust them” (participant 017, 71-year-old man).
Hospital-related
Volume: “They do more surgery there [in Winnipeg], I think, than anywhere else. You feel more secure at a bigger hospital. The smaller towns, how many they do in a month? Where in Winnipeg, they be doing it regular, wouldn’t they?” (Participant 004, 57 year-old man).
Reputation: “If I did have a choice, I would have chosen Hospital X. It’s a top notch hospital.” (Participant 015, 63 year-old man).
Medical expertise and resources: “I never actually chose [where I wanted to have surgery]. I was going to go to [my local hospital], but they said that I had breathing problems.” (Participant 012, 75 year-old man).
Previous experiences: “[The tertiary care centre], I know, has some dandy doctors in there. Like, they’ve saved my wife’s life twice already, so...” (Participant 012, 75 year-old man).
Coordination of care: “That’s the reason that I like seeing my general practitioner… because he is in Hospital Y and I like that whole idea of my general practitioner is not far from there, and whatever he does, he does it at Hospital Y. When he made a referral, he referred me to somebody from there, and the tests were there. Then when I got referred to an oncologist, it’s somebody just across the street, and who also operates there. I like all of that. To me, it’s very more family-oriented” (participant 014, 60-year-old woman).
Box 2. Example comments from rural patients with rectal cancer about patient-related factors involved in decisions on location of surgery.
Travel: “It’s all the travelling. We’ll probably do a good 35–40 trips into Winnipeg. It involves a good part of the day each time. It’s 28 trips for the radiation alone... round trip, it’s 260 km, but I like to leave about 2¼ hours before my appointment. Now our world revolves around going to Winnipeg. It’s consumed our life. The distance doesn’t get any shorter. And we’re both not feeling well, so it’s easier if someone drives us in” (participant 020, 63-year-old man).
Personal supports: “It is a problem for family to get to wherever you are. So, the closer you can be to where family is, the better, basically” (participant 008, 53-year-old man).
Accommodation: “Once I got proper accommodation and I was able to secure an apartment, at least I had some kind of home base, instead of working out of a motel” (patient 011, 63-year-old woman).
Finances: “It’s just hard for someone like us that come from a rural area and the whole cost falls on us. It doesn’t matter what it is, the whole cost is just phenomenal. We’re in the thousands of dollars now” (participant 011, 63-year-old woman).
Work: “We ranch, and of course my husband won’t be here to help feed calves and stuff like that for the next couple weeks. His brother will be doing it” (participant 018, 55-year-old woman).
Decision-maker
Patients were referred to their surgeon by their family doctors, gastroenterologists, oncologists or another surgeon. At this referral decision point, 2 subthemes emerged: the patient had some input into the decision, or the patient was not offered a choice as to where they would have surgery.
Patients who had input in the referral decision cited many treatment-related factors (described below) as reasons for their decisions. Patients who were not offered the choice of surgical location fell into 2 categories: those who needed tertiary care for medical reasons and those who were referred without any perceived doctor–patient discussion. Some patients had extensive disease or significant medical comorbidities that required care at a tertiary centre. Other patients were simply referred without any discussion of options regarding surgeon or treatment location. Many of these patients described following through with the referring doctor’s decision because they trusted their doctor. One patient stated, “It wasn’t really a choice. I was just recommended by my family doctor, who’s been my family doctor for over 30 years. Whatever he decides for me, that’s what I’m going to do” (participant 015, 63-year-old man). However, some patients followed their referring doctors’ recommendations because they were otherwise too overwhelmed by the context of the cancer diagnosis and treatments, as demonstrated by the following: “So, all of a sudden I was told this and to think about, okay, like where should I have surgery, who should be the surgeon? I didn’t even think about those things. My doctor said, ‘there’s [a surgeon] in Winnipeg, she’s very good, yadda yadda...’ And I just said, ‘okay.’ There really wasn’t much discussion around other surgeons or other hospitals. I think providing a little more information might be helpful” (participant 019, 51-year-old woman).
Treatment factors
The treatment factors taken into account when participants made their decisions were divided into 2 subthemes: surgeon and hospital factors. Participants described how the surgeon’s skill and case volume, reputation and interpersonal relationship with them affected their decisions about where to have surgery. Some participants wanted to be referred to a specialist who was experienced in that particular field and who had performed the required operation many times. Others asked to be referred to the “best” doctor or had heard about a particular surgeon and asked to be referred to that surgeon. Participants often expressed establishing a good and trusting relationship with their surgeons after meeting them, which seemed to make them more comfortable about the location of surgery and the treatment process.
Hospital factors that influenced where participants chose to have surgery included the hospital’s reputation and case volume, other medical specialists and resources, participants’ previous experience with that hospital and the coordination of care. First, it was important to patients that the hospital they chose had a good reputation and substantial experience in the type of surgery they would be undergoing. Second, participants also mentioned the hospital’s medical expertise (including the concentration of other medical specialists) and resources as a factor in their decisions. This was especially important for those with other significant medical conditions who may have required tertiary care support in the peri- or postoperative periods.
Some participants quoted familiarity and past experiences with particular hospitals when considering a location of care. In addition, some patients were already seeing an oncologist or another doctor at a certain hospital and wanted to continue being cared for in the same place in order to better coordinate all of their care.
Personal factors
Personal factors, including travel, support, accommodation, finances and work, were considerations in participants’ decisions. However, for our study participants, all of whom travelled for treatment, these were less often described as primary reasons to have surgery in a given location. Exceptions to this included patients who actually had more personal supports in Winnipeg than in their local communities. Rather, most participants described these factors as something of a burden or trade-off that required their acquiescence in order to receive surgery at a tertiary care centre.
Many patients described travelling for long distances to appointments, some up to 9 hours of driving. This travel took up a lot of time and often negatively affected their work, personal supports and finances, but many accepted it as a necessary trade-off to receive treatment at their desired or prescribed location. However, many of the participants who had no choice but to have treatment at a tertiary care centre because of comorbidities expressed a preference to have surgery closer to home. Other participants stated that because they lived in rural locations, they were used to travelling long distances, and for some patients, travelling to their local hospitals was only marginally closer than travelling to Winnipeg.
Having personal supports nearby was important for patients. However, some patients had more family members in Winnipeg, which actually made travelling less of an ordeal. Another consideration for participants travelling from far away was accommodation. Some participants had family members with whom they stayed when coming for appointments or surgery, but many did not and instead bore the additional costs of accommodations themselves. Participants stated that most of the travel and accommodation costs were their responsibility, and some felt this responsibility was quite a burden.
Twelve of 20 interview participants were working, whereas 8 were retired. Among those who were still working, their work was affected to varying degrees. Some patients’ employers had no problem allowing them time off work. Other patients had to personally make work arrangements; for example, 1 participant owned livestock and had to ensure his animals were cared for while he was away.
Discussion
In the first part of this study, we conducted a population-based analysis to examine patterns of treatment among patients with rectal cancer in Manitoba. There are few comparable population-based studies in the medical literature examining rural patients with CRC, and there is little information specific to Canadian populations. A recent Australian study similarly reported that rural patients in New South Wales who had CRC were less likely to undergo surgery in a specialist cancer centre than patients from metropolitan or urban areas.21 In their study, only 11% of rural patients underwent surgery at specialist cancer centres. In contrast, almost half of all patients with rectal cancer in rural Manitoba underwent surgery in Winnipeg. Many Canadian provinces, like Manitoba, cover extremely wide geographic areas, but each may have unique characteristics with respect to population distribution and treatment patterns. Undertaking reviews of regional treatment patterns are thus important from policy and regional planning standpoints.
In the second part of this study, we performed a qualitative analysis to gain insight on how these treatment patterns develop and their effects on patients. We found that the underlying reasons for the patterns reported in the first part of this study were not always straightforward and that the effects could be quite burdensome for some patients. Previous qualitative studies involving rural obstetrical patients22 and patients undergoing more minor procedures23 reported decision factors similar to those in our study, including the desire for safety, availability of family support, familiarity with hospital staff and their environment, and financial costs. A major theme that was not described in these studies, however, was the lack of choice pertaining to treatment provider and location perceived by many rural patients.
While a lack of choice was understandable in patients with particularly extensive cancers or other significant medical problems, in other cases the underlying reasons were less clear. Previous qualitative studies have reported that health care professionals often do not involve patients in important treatment decisions.24 Some patients may not want an active role and are more than willing to let their doctors select their care providers.25,26 Other patients may be too overwhelmed, especially when given the cancer diagnosis, to process all the information and ask questions about the referral process.27,28 It may also be, however, that some doctors practise in a more paternalistic manner without making any efforts to engage patients.25 The consequences of these decisions are substantial and potentially burdensome, and multiple studies have shown that most patients desire involvement in the decision-making process.29
We do not necessarily support a strict policy of centralization for rectal cancer surgery; instead, we have adopted an approach with provincial guidelines and a system of education with selective referrals to improve province-wide outcomes. In this context, the implications of our study include the need to understand the perspectives of referring doctors and how they make referral decisions and the need to understand when and how much patients want to be involved in decision-making and to develop resources to facilitate the process accordingly.
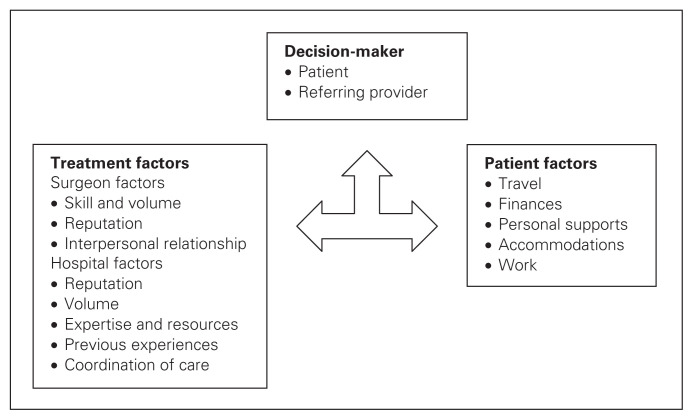
We propose a conceptual model of the decision process with the decision-maker, either the patient or referring physician, deciding where to make the referral based on treatment or patient-related factors (Fig. 1). Depending on individual patient circumstances and desires, these factors may work in concert to support a decision or they may work in opposition; in the latter case, some factors have to be prioritized at the expense of others. For example, rural patients who seek treatment at high-volume centres may have to yield on personal factors to receive this treatment. Alternatively, for an urban patient seeking treatment at a high-volume centre, treatment and personal factors both support treatment at a tertiary centre. Further study is, however, required to test this model.
Fig. 1.
Conceptual model of the decision process showing the decision-maker considering treatment and patient-related factors.
Limitations
Several limitations require discussion. First, all interview participants planned to undergo surgery in Winnipeg. Despite our attempts, we were unable to accrue any rural patients in the presurgery setting who were planning to undergo surgery at their local hospital. One reason for this may have been that only a small number of rural patients (we estimate about 30) actually underwent surgery outside of Winnipeg during the interview period. Another reason may have been a lack of incentive for rural surgeons to enroll patients, or even possible concern over how a study asking about decisions on location of care for cancer surgery might be perceived by their patients. As a result, we were unable to gain the perspectives of rural patients undergoing surgery at their local hospitals, which may be different than those who planned to travel for their surgeries. Given other studies reporting that patients greatly prefer to have surgery closer to home,12 the uninterviewed sample may represent patients who actually had more input into the referral decision. Second, we limited our questions to more generic aspects of rectal cancer surgery. We did not specifically ask more technical questions, such as the likelihood of avoiding a stoma, as our interviews were conducted before surgery; we did not want to influence participants’ perceptions going forward, particularly when we were still trying to recruit patients planning to undergo surgery at a rural hospital. Third, our conceptual model is limited by context. Further study to include the perspectives of referring physicians and a broader range of patients is needed to test our model and assess its transferability.
Conclusion
Almost half of all rural patients with rectal cancer in Manitoba underwent surgery at urban hospitals. The reasons for this pattern are complex and only partly related to patient choice. We plan further studies to include referring physicians’ perspectives in order to better understand access and cancer treatment pathways in a publicly funded system with geographically dispersed populations. These studies can play important steps in informing health policy and planning, educating practitioners, promoting patient-centred care and supporting patients through the cancer-related decision-making and treatment processes.
Footnotes
Presented as a poster at the Canadian Surgery Forum, Calgary, Alta., Sept. 13–16, 2012
Competing interests: None declared.
Contributors: All authors designed the study. M. Nostedt, J. Park, A. McKay and B. Yip acquired the data, which M. Nostedt and J. Park analyzed. M. Nostedt and J. Park wrote the article, which all authors reviewed and approved for publication.
References
- 1.Canadian Cancer Society’s Steering Committee on Cancer Statistics. Canadian Cancer Statistics 2012. Toronto (ON): The Society; 2012. [Google Scholar]
- 2.City of Winnipeg. Population of Winnipeg: 2013. [accessed 2013 Nov. 8]. Available: www.winnipeg.ca/cao/pdfs/population.pdf.
- 3.Birkmeyer JD. Should we regionalize major surgery? Potential benefits and policy considerations. J Am Coll Surg. 2000;190:341–9. doi: 10.1016/s1072-7515(99)00270-7. [DOI] [PubMed] [Google Scholar]
- 4.Epstein AM. Volume and outcome–it is time to move ahead. N Engl J Med. 2002;346:1161–4. doi: 10.1056/NEJM200204113461512. [DOI] [PubMed] [Google Scholar]
- 5.Hodgson DC, Zhang W, Zaslavsky AM, et al. Relation of hospital volume to colostomy rates and survival for patients with rectal cancer. J Natl Cancer Inst. 2003;95:708–16. doi: 10.1093/jnci/95.10.708. [DOI] [PubMed] [Google Scholar]
- 6.Simons AJ, Ker R, Groshen S, et al. Variations in treatment of rectal cancer: the influence of hospital type and caseload. Dis Colon Rectum. 1997;40:641–6. doi: 10.1007/BF02140891. [DOI] [PubMed] [Google Scholar]
- 7.Schrag D, Panageas KS, Riedel E, et al. Hospital and surgeon procedure volume as predictors of outcome following rectal cancer resection. Ann Surg. 2002;236:583–92. doi: 10.1097/00000658-200211000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Porter GA, Soskolne CL, Yakimets WW, et al. Surgeon-related factors and outcome in rectal cancer. Ann Surg. 1998;227:157–67. doi: 10.1097/00000658-199802000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marusch F, Koch A, Schmidt U, et al. Hospital caseload and the results achieved in patients with rectal cancer. Br J Surg. 2001;88:1397–402. doi: 10.1046/j.0007-1323.2001.01873.x. [DOI] [PubMed] [Google Scholar]
- 10.Meyerhardt JA, Tepper JE, Niedzwiecki D, et al. Impact of hospital procedure volume on surgical operation and long-term outcomes in high-risk curatively resected rectal cancer: findings from the Intergroup 0114 Study. J Clin Oncol. 2004;22:166–74. doi: 10.1200/JCO.2004.04.172. [DOI] [PubMed] [Google Scholar]
- 11.Harling H, Bulow S, Moller LN, et al. Hospital volume and outcome of rectal cancer surgery in Denmark 1994–99. Colorectal Dis. 2005;7:90–5. doi: 10.1111/j.1463-1318.2004.00751.x. [DOI] [PubMed] [Google Scholar]
- 12.Finlayson SR, Birkmeyer JD, Tosteson AN, et al. Patient preferences for location of care: implications for regionalization. Med Care. 1999;37:204–9. doi: 10.1097/00005650-199902000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Chang RK, Joyce JJ, Castillo J, et al. Parental preference regarding hospitals for children undergoing surgery: a trade-off between travel distance and potential outcome improvement. Can J Cardiol. 2004;20:877–82. [PubMed] [Google Scholar]
- 14.Manitoba Health. Manitoba Health Population Report 2012. 2012. Jun 1, [accessed 2013 Nov. 8]. Available: www.gov.mb.ca/health/population/pr2012.pdf.
- 15.Public Health Act: Reporting of Diseases and Conditions Regulation. 2009. [accessed 2014 Oct. 22]. Available: http://web2.gov.mb.ca/laws/regs/current/_pdf-regs.php?reg=37/2009.
- 16.Singh H, De Coster C, Shu E, et al. Wait times from presentation to treatment for colorectal cancer: a population-based study. Can J Gastroenterol. 2010;24:33–9. doi: 10.1155/2010/692151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Copeland G, Lake A, Firth R, et al., editors. Cancer in North America: 2003–2007. Springfield (IL): North American Association of Central Cancer Registries; 2010. [Google Scholar]
- 18.Chapple A. The use of telephone interviewing for qualitative research. Nurs Res. 1999;6:85–93. [Google Scholar]
- 19.Kennedy TJ, Lingard LA. Making sense of grounded theory in medical education. Med Educ. 2006;40:101–8. doi: 10.1111/j.1365-2929.2005.02378.x. [DOI] [PubMed] [Google Scholar]
- 20.Park J, Woodrow SI, Reznick RK, et al. Patient care is a collective responsibility: perceptions of professional responsibility in surgery. Surgery. 2007;142:111–8. doi: 10.1016/j.surg.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 21.Goldsbury D, Harris MF, Pascoe S, et al. Socio-demographic and other patient characteristics associated with time between colonoscopy and surgery, and choice of treatment centre for colorectal cancer: a retrospective cohort study. BMJ Open. 2012;2 doi: 10.1136/bmjopen-2012-001070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith M, Askew DA. Choosing childbirth provider location — rural women’s perspective. Rural Remote Health. 2006;6:510. [PubMed] [Google Scholar]
- 23.Humber N, Dickinson P. Rural patients’ experiences accessing surgery in British Columbia. Can J Surg. 2010;53:373–8. [PMC free article] [PubMed] [Google Scholar]
- 24.McCall K, Rice AM. What influences decisions around the place of care for terminally ill cancer patients? Int J Palliat Nurs. 2005;11:541–7. doi: 10.12968/ijpn.2005.11.10.19983. [DOI] [PubMed] [Google Scholar]
- 25.Wilson CT, Woloshin S, Schwartz LM. Choosing where to have major surgery: Who makes the decision? Arch Surg. 2007;142:242–6. doi: 10.1001/archsurg.142.3.242. [DOI] [PubMed] [Google Scholar]
- 26.Suarez-Almazor ME, Soskolne CL, Fung K, et al. Empirical assessment of the effect of different summary worklife exposure measures on the estimation of risk in case-referent studies of occupational cancer. Scand J Work Environ Health. 1992;18:233–41. doi: 10.5271/sjweh.1584. [DOI] [PubMed] [Google Scholar]
- 27.Park J, Neuman HB, Bennet AV, et al. Patients’ expectations of functional outcomes following rectal cancer surgery: a qualitative study. Dis Colon Rectum. 2014;57:151–7. doi: 10.1097/DCR.0000000000000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wright FC, Crooks D, Fitch M, et al. Qualitative assessment of patient experiences related to extended pelvic resection for rectal cancer. J Surg Oncol. 2006;93:92–9. doi: 10.1002/jso.20382. [DOI] [PubMed] [Google Scholar]
- 29.Guadagnoli E, Ward P. Patient participation in decision-making. Soc Sci Med. 1998;47:329–39. doi: 10.1016/s0277-9536(98)00059-8. [DOI] [PubMed] [Google Scholar]