NATIONAL VACCINE ADVISORY COMMITTEE
Chair
Walter A. Orenstein, MD, Emory University, Atlanta, GA
Designated Federal Official
Bruce G. Gellin, MD, MPH, National Vaccine Program Office, U.S. Department of Health and Human Services, Washington, DC
Public Members
Richard H. Beigi, MD, MSc, Magee-Womens Hospital, Pittsburgh, PA
Sarah Despres, JD, Pew Charitable Trusts, Washington, DC
Philip S. LaRussa, MD, Columbia University, Department of Pediatrics, New York, NY
Ruth Lynfield, MD, Minnesota Department of Health, St. Paul, MN
Yvonne Maldonado, MD, Stanford University, Palo Alto, CA
Charles Mouton, MD, MS, Meharry Medical College, Nashville, TN
Amy Pisani, MS, Every Child by Two, Mystic, CT
Wayne Rawlins, MD, MBA, Aetna, Hartford, CT
Mitchel C. Rothholz, RPh, MBA, American Pharmacists Association, Washington, DC
Nathaniel Smith, MD, MPH, Arkansas Department of Health, Little Rock, AR
Thomas E. Stenvig, RN, PhD, MS, South Dakota State University College of Nursing, Brookings, SD
Catherine Torres, MD, Former New Mexico Secretary of Health, Las Cruces, NM
Kasisomayajula Viswanath, PhD, Harvard School of Public Health, Boston, MA
Representative Members
Seth Hetherington, MD, Genocea Biosciences, Cambridge, MA
Philip Hosbach, Sanofi Pasteur, Swiftwater, PA
EXECUTIVE SUMMARY
Maternal immunization provides important health benefits for pregnant women and their infants, and obstetrical care providers are now recommended to vaccinate all pregnant women against influenza and pertussis during each pregnancy. However, immunization coverage among pregnant women for influenza and pertussis-containing vaccines is suboptimal, leaving numerous pregnant women and their infants at risk for complications from vaccine-preventable diseases. Therefore, it is critical to understand the social, programmatic, and logistical barriers that both prevent pregnant women from receiving recommended vaccinations and prevent obstetrical care providers from recommending and administering vaccines within their practices.
To facilitate the successful development of a national maternal immunization program, in alignment with broader immunization goals such as those outlined in the National Vaccine Plan, the Assistant Secretary for Health (ASH) charged the National Vaccine Advisory Committee (NVAC) with reviewing the current state of maternal immunizations and existing best practices to identify programmatic gaps and/or barriers to the implementation of current recommendations regarding maternal immunization.
Through extensive analysis and input from subject matter experts, the NVAC identified five major areas of opportunity to strengthen maternal immunization programs and increase uptake of recommended vaccines among pregnant women. These areas for action include:
Enhance communication to address the safety and effectiveness of all currently recommended immunizations during pregnancy
Maximize obstetrical care provider recommendation and administration of recommended maternal immunizations
Focus efforts to improve financing for immunization services during pregnancy and postpartum
Support efforts to increase the use of electronic health records (EHRs) and Immunization Information Systems (IISs) among obstetrical care providers
Recognize and addresse current vaccine liability law barriers to optimize investigations and uptake of recommended and future vaccines during pregnancy
Within each area, the NVAC report details key recommendations to overcome challenges in these areas. The NVAC recommendations follow.
Recommendation 1: enhance communication to address the safety and effectiveness of all currently recommended immunizations during pregnancy
1.1. The ASH should provide regular updates to relevant stakeholders regarding vaccines that are recommended by the Advisory Committee for Immunization Practices (ACIP)/Centers for Disease Control and Prevention (CDC) for use in pregnant women. Doing so will maximize the potential for disease prevention through vaccine use, thereby benefiting the mother and her infant.
1.2. The ASH should work with federal partners and professional organizations to develop and distribute communication strategies and educational materials to health-care providers, especially those delivering obstetrical care. These educational materials should clearly state the benefits of maternal immunization, such as reducing the morbidity and mortality for mothers and young infants. In addition, they should enable providers to educate women who are pregnant or may become pregnant on the available clinical data regarding the safety and effectiveness of all ACIP/CDC-recommended maternal immunizations for themselves and their infants.
1.3. The ASH should encourage the use of current and newly emerging communication technologies to maximize the effectiveness and reach of communication efforts addressing the clinical benefits of maternal immunization.
1.4. The ASH should work with the appropriate federal agencies to assess data collected through post-marketing surveillance systems on the safety, efficacy, and effectiveness of currently recommended vaccines for pregnant women and their infants. The ASH also should work with federal agencies to determine the data needs for vaccine safety in pregnant women, confirm the ability of these systems to capture these data, and modify/develop new systems if data needs are not being met.
1.5. The ASH should encourage appropriate professional and health-care organizations to educate obstetrical care providers on the available post-marketing surveillance systems used to track vaccine safety data to improve provider knowledge and reporting of potential vaccine adverse events. Educational materials and trainings should include how to report possible events to the relevant post-marketing surveillance systems, the strengths and limitations of these systems, the importance of reporting possible serious vaccine adverse events, and information regarding federal reporting requirements.
Recommendation 2: maximize obstetrical care provider recommendation and administration of recommended maternal immunizations
2.1. The ASH should recommend that obstetrical care providers follow the published guidelines of professional organizations and government agencies to improve vaccination rates in their practices.
2.2. The ASH should collaborate with federal partners, professional educational organizations, professional societies, and other relevant maternal immunization stakeholders to develop curricula for trainees and health-care providers that should include information about the recognized benefits and risks of immunizations during pregnancy and postpartum. Curricula should also include information about both the scientific basis for immunizations as well as the basics of establishing and administering immunization services in outpatient obstetrical care settings.
2.3. The ASH should work with all relevant federal and non-federal partners to assure that focused efforts are undertaken to routinize obstetrical care provider vaccine recommendations and administration of all recommended vaccines during pregnancy.
2.4. The ASH should work with obstetrical care stakeholders to incorporate the widespread use of programs such as Assessment, Feedback, Incentives, and eXchange to support and evaluate the incorporation of immunization services into obstetrical care practices.
2.5. The ASH should work with the stakeholder community to evaluate the applicability of existing measures and/or the development of new measures for vaccines recommended to pregnant women. Standardized metrics will help to reliably measure rates of immunizations given by obstetrical care providers to improve vaccine delivery in this population and to better measure progress toward institutional and national goals.
Recommendation 3: focus efforts to improve financing for immunization services during pregnancy and postpartum
3.1. The ASH should work with the Centers for Medicare & Medicaid Services (CMS) and CDC to determine the costs to provide immunizations in various types of obstetrical practices to help evaluate the factors influencing the provision of adult maternal immunizations.
3.2. The ASH should work with CMS, the Health Resources and Services Administration (HRSA), and private payers to identify and improve upon current process issues related to billing, coding, and subsequent payment for the provision of maternal and other adult immunizations by obstetrical care providers, such as adult vaccine counseling and vaccine administration.
3.3. The ASH should continue to monitor the effectiveness of the evolving payment and delivery models, outside of fee-for-service, within the new framework of federal and state Exchanges, patient-centered medical homes, and accountable care organizations. These new models should be encouraged to use cost studies of efficient practices and evidence-based economic principles as they pertain to maternal immunization programs.
3.4. The ASH and the Department of Health and Human Services should work with professional organizations and other relevant maternal immunization stakeholders to develop a comprehensive toolkit that provides guidance on office and practice logistics (e.g., storage and inventory) to optimize the ability for providers to efficiently and effectively implement vaccination services within their practices. Such a toolkit should also provide technical assistance regarding efficient business practices including payer contracting for immunization services, appropriate vaccine billing practices, and participation in vaccine purchasing groups.
Recommendation 4: support efforts to increase the use of EHRs and IISs among obstetrical care providers
4.1. The ASH should continue to support efforts to promote increased adoption by all obstetrical care providers of EHRs that can exchange data with IISs of the appropriate public health jurisdictions. This support should include bidirectional data exchange standards where supported, according to current and future national standards and regulations set by CDC and the Office of the National Coordinator for Health Information Technology (ONC).
4.2. The ASH should promote collaborations among ONC, CDC, and the U.S. Food and Drug Administration (FDA) to establish automated, electronic interactions between EHRs and vaccine safety surveillance systems to strengthen vaccine safety monitoring systems in pregnant women.
Recommendation 5: recognize and address current vaccine liability law barriers to optimize investigations and uptake of recommended and future vaccines during pregnancy
5.1. The ASH should support efforts by HRSA to address the issue of including in utero injuries allegedly incurred following maternal immunization within the Vaccine Injury Compensation Program (VICP). The ASH should support resolution of the issue regarding infants born with alleged in utero injuries in favor of allowing such claims to be pursued under the VICP and in favor of providing settled liability protections to vaccine manufacturers and administrators.
INTRODUCTION
Maternal immunization provides important health benefits for pregnant women and their infants. Universal recommendations to vaccinate all pregnant women against influenza and pertussis during each pregnancy signify that immunizations should now be considered a routine component of obstetrical care. However, in a recent Internet panel survey of 1,702 pregnant women, 28.7% indicated that their obstetrical care provider had not recommended that they receive an influenza vaccination during the 2012–2013 season. Influenza vaccination coverage was significantly higher in women who both received a provider recommendation and were offered the vaccine compared with women who received no provider recommendation (70.5% vs. 16.1%).1 Likewise, numerous other studies have reported that a provider recommendation is the greatest predictor of pregnant women actually receiving either the influenza or pertussis vaccine.1–3
The majority of pregnant women report visiting their obstetrical care provider more than six times during pregnancy, creating numerous opportunities to offer and administer immunizations.1,3 A dedicated immunization program will increase influenza and pertussis vaccination coverage in pregnant women and help build a better system for the routine delivery of recommended vaccines to pregnant women (i.e., influenza and pertussis-containing vaccines), as well as vaccines for those women considered to be at high risk for certain vaccine-preventable diseases (VPDs) (e.g., hepatitis A, hepatitis B, meningococcal, or pneumococcal vaccines).4 In addition, incorporating immunizations into the standard of obstetrical care makes the development of new vaccines targeting pregnant women commercially viable, creating opportunities to protect against a greater number of infectious diseases in pregnant women, their infants, or both.
Strategies to improve maternal immunization that arise from a comprehensive understanding of these barriers will not only improve the quality of maternal and neonatal health, but they are also likely to provide additional insights into improving immunization efforts in general. It also is important to note that maternal immunization can help foster positive attitudes toward vaccines in pregnant women, which may result in greater vaccine awareness, acceptance, and demand for both themselves and their children during future health-care interactions.5,6
Charge to the National Vaccine Advisory Committee
In June 2012, the Assistant Secretary for Health (ASH) charged the National Vaccine Advisory Committee (NVAC) with reviewing the current state of maternal immunizations and existing best practices to identify programmatic gaps and/or barriers to the implementation of current recommendations regarding maternal immunization. The NVAC established the Maternal Immunization Working Group (MIWG) in August 2012 to conduct these assessments to provide recommendations for overcoming any identified barriers. The NVAC voted to adopt the recommendations put forth by the MIWG on June 11, 2014.
Both short- and long-term strategies are necessary to optimize the use of maternal immunizations for preventing disease in pregnant women and infants too young to be immunized. This report focuses on strategies for improving the uptake and delivery of currently recommended vaccines in pregnant women. Forthcoming efforts by the NVAC will explore longer-term strategies and policies that can facilitate research and development of new vaccines for use in pregnant women. These findings will be described in a subsequent report.
Definitions
Obstetrical care providers.
For the purpose of the NVAC considerations (and for the purpose of this report), obstetrical care providers include, but are not limited to, obstetrician-gynecologists (ob-gyns), family physicians, certified nurse-midwives, certified midwives, nurse practitioners, and physician assistants. Obstetrical care may also be provided by nurses, pharmacists, and other physicians and non-physician providers who administer health-care services to pregnant women.
Obstetrical care (i.e., perinatal care, maternal care, and maternity care).
Obstetrical care is defined here as providing prenatal/obstetrical care to pregnant and/or immediately postpartum women with the goal of optimizing maternal and infant outcomes (e.g., administration of recommended immunizations).
BACKGROUND
Pregnant women and young infants are at a higher risk for morbidity and mortality from various VPDs
Pregnant women are at a higher risk of severe complications from some infections, such as influenza. Although influenza infection rates in pregnant women are similar to those in the general population, several studies have demonstrated that hospitalizations and death due to influenza-attributable risks are higher in pregnant women than in non-pregnant and postpartum women.7–10 During the 2009 influenza pandemic, pregnant women accounted for 5% of all reported 2009 H1N1 influenza-associated deaths11 and were 7.2 times more likely to be hospitalized and 4.3 times more likely to require intensive care than non-pregnant women.12 The increased risk of disease complications appears to be at least partially due to the immunological and physiological changes that occur during pregnancy.13,14 However, vaccinated pregnant and non-pregnant women achieve similar concentrations of protective antibodies against influenza.15,16 As pregnant women are at a higher risk from severe complications from influenza than non-pregnant women, yet are expected to benefit similarly from immunization, it is desirable to optimize immunization strategies to protect pregnant women against influenza-related disease.
Infants too young to be vaccinated are also at considerable risk of morbidity and mortality due to VPDs. Studies have shown that complications due to influenza infection cause more hospitalizations in infants younger than six months of age than in any other age group, including the elderly.17,18 Pediatric deaths in the United States due to influenza from 2004 to 2012 are shown in the Table. Of note, neither influenza vaccine nor antiviral medications are licensed for use in infants ≤5 months of age.
Table.
CDC reported total number of infant deaths (aged 0–5 months) vs. total number of pediatric deaths (aged 0–17 years) in the U.S. from nine influenza seasons, 2004–2012a
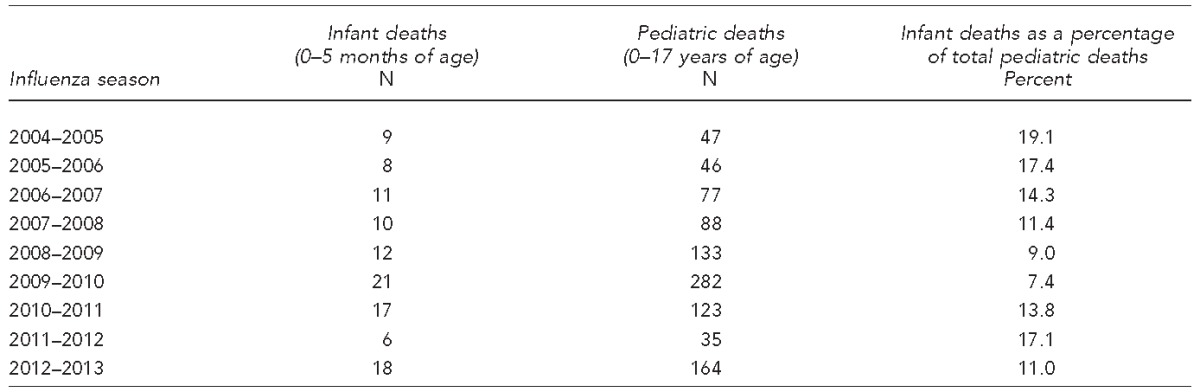
Source: Centers for Disease Control and Prevention (US). Influenza-associated pediatric mortality surveillance system [cited 2014 Aug 15]. Available from: URL: http://gis.cdc.gov/GRASP/Fluview/PedFluDeath.html
CDC = Centers for Disease Control and Prevention
Similarly, infants experience the highest rates of pertussis disease compared with any other age group, with incidence ranging from 27 to 127 cases per 100,000 population from 1990–2011 (Unpublished data. U.S. Centers for Disease Control and Prevention [CDC], 2012). From 2000 to 2012, 76% of all pertussis-related deaths occurred in infants younger than two months of age,19 before they were eligible to receive the first dose of the diphtheria-tetanus toxoid-acellular pertussis (DTaP) vaccine. Therefore, strategies such as maternal immunization should be actively pursued to protect young infants against VPDs.
Because young infants are immunologically naïve, they rely on maternal antibodies acquired in utero and through breast milk for protection against infectious diseases during the first months of life.20–22 Transplacental transfer of maternal antibodies to the fetus is a passive process that begins during the 17th week of gestation, with maximal transfer of antibodies occurring after the 30th week of gestation, when active transfer is initiated.21 Therefore, maternal antibody concentrations in the infant at the time of birth are dependent on maternal antibody concentrations during pregnancy and on the gestational age at birth.22 Although serum concentrations of maternally derived antibodies wane over time, studies looking at antibodies to pertussis and influenza in infants of mothers vaccinated against these pathogens during pregnancy suggest that protective antibodies are likely to persist until the infant is old enough to begin to receive his/her own immunizations.23–26
Maternal immunization is an effective strategy to protect young infants from disease
Maternal immunization has been described as a mechanism to protect infants against infectious diseases for more than a century.23,27 Since the 1970s, this strategy has been most successfully implemented globally to prevent maternal and neonatal tetanus.28 The United Nations Children's Fund estimates that more than 119 million pregnant women worldwide have been immunized with two or more doses of tetanus toxoid since 1999.29 As a result, maternal immunization, in combination with better surveillance and hygienic delivery practices, has reduced neonatal tetanus mortality by more than 90%.29 This achievement has led to the argument that maternal immunization efforts should be broadly expanded to include immunization against other VPDs (e.g., meningitis and pneumococcal disease), especially in resource-poor countries where there are still high infant mortality rates.30–32
Convincing data support the effectiveness of maternal immunization strategies in protecting infants younger than six months of age against influenza illness and influenza-related hospitalizations. The Mother's Gift project, a randomized, controlled trial in Bangladesh, found that infants younger than six months of age whose mothers had been immunized with inactivated influenza vaccine had a 63% reduction in laboratory-confirmed influenza and a 29% reduction in respiratory illness with fever compared with infants whose mothers had received pneumococcal vaccine. Moreover, mothers vaccinated against influenza were significantly less likely to develop febrile respiratory illness and had fewer clinical visits than mothers who received pneumococcal vaccine.26 In a prospective, observational study spanning three consecutive influenza seasons (November 2002 to September 2005), Eick et al. demonstrated a 41% reduction in laboratory-confirmed influenza and a 39% reduction in hospitalizations due to influenza-like illness in infants born to influenza vaccinated mothers compared with infants of unvaccinated mothers.33 Similarly, other studies have shown that maternal immunization can significantly reduce hospitalizations due to laboratory-confirmed influenza in infants younger than six months of age.34
Modeling studies suggest that immunizing pregnant women could reduce hospitalizations due to pertussis disease in infants younger than four months of age.35 A number of studies have demonstrated that maternal antibodies specific to pertussis antigens are efficiently transported across the placenta and can be detected in higher concentrations in infant umbilical cord sera than in maternal serum.24,36 Gall et al. demonstrated that cord serum concentrations of antibodies to pertussis antigens were higher in infants born to mothers vaccinated during pregnancy than in infants from unvaccinated mothers.37 Moreover, Munoz et al. demonstrated that infants born to mothers immunized with tetanus-diphtheria-acellular pertussis (Tdap) during pregnancy (30–32 weeks gestation) had significantly higher serum concentrations of antibodies against vaccine antigens at birth and two months of age than infants born to mothers immunized postpartum. Effectiveness could not be assessed in this study due to no reported cases of pertussis in any of the infants or mothers (either immunized during pregnancy or postpartum).38 Therefore, while it is biologically plausible that maternally derived pertussis-specific antibodies are likely to confer protection and could decrease the severity of disease in infants, the effectiveness of maternal antibodies in preventing infant pertussis is not yet known.
Maternal immunization can also have positive, indirect effects on fetal growth and birth outcomes, although studies on the benefits of maternal immunization for the infant are mostly limited to influenza vaccination. For instance, several studies indicate that infants of mothers vaccinated against influenza are less likely to be born preterm (<37 completed weeks of gestation) and were less likely to be born small for gestational age (birthweight <10th percentile for gestational age) than infants born to unvaccinated mothers during the same time period.39–41
Household contacts often serve as the primary source of infection to infants.42,43 The practice called “cocooning” protects young infants from VPDs by vaccinating all individuals who will come in frequent contact with the infant. Although this strategy is still strongly encouraged, recent studies indicate that cocooning is logistically difficult to implement, and the effectiveness of this practice in preventing neonatal disease is uncertain.44–46 Therefore, cocooning should be used whenever possible to optimize neonatal disease prevention but should be an adjunct to, not a substitute for, maternal immunization.
Vaccines recommended for use in pregnancy are generally considered safe
No currently U.S.-licensed vaccine has been studied in pregnant women in pre-licensure safety and efficacy trials to support an indication of the product in pregnant women. However, numerous post-licensure studies have been conducted by academic investigators to evaluate the safety and effectiveness of inactivated vaccines in pregnant women.47–51 To date, no evidence suggests that inactivated influenza vaccine (either seasonal or pandemic) causes any serious adverse events for the mother or infant. Numerous studies have also demonstrated no increased risk of outcomes such as preterm birth, stillbirth, low birthweight (i.e., birthweight <2,500 grams), or spontaneous abortion.39,41,47,52–55 However, because of a theoretical risk of viral transmission to the fetus, live-attenuated influenza vaccines are not currently recommended for use in women who are pregnant or are planning to become pregnant.
Similarly, the data available on the safety of the Tdap booster vaccine administered to pregnant women (albeit limited) does not suggest any elevated frequency or unusual patterns of adverse events.38,56–58 As mentioned previously, tetanus and diphtheria toxoid and tetanus toxoid vaccines have been used worldwide for more than 25 years in pregnant women to prevent neonatal tetanus and have not been shown to be teratogenic.59,60 Also, after week 14 of gestation, the fetal structures are fully formed so that the risk for fetal malformation due to immunization of the pregnant woman after this interval is biologically implausible. To evaluate the safety of administering Tdap in each pregnancy, the Advisory Committee on Immunization Practices (ACIP) reviewed the available safety data, including published data on receipt of two doses of Tdap in non-pregnant women and multiple doses of tetanus toxoid-containing vaccines in pregnant women. The ACIP/CDC concluded that experience with tetanus-toxoid-containing vaccines suggests no excess risk for severe adverse events for women receiving Tdap with every pregnancy regardless of interval since the last dose. Because U.S. birth statistics indicate that an average of two children are born per woman in a lifetime, most women would receive only two doses of Tdap vaccine, although a small proportion of women could receive ≥4 doses.61
Understanding the limitations of the available safety data for maternal immunizations
Concerns or uncertainties regarding the safety of vaccine administration during pregnancy remain important barriers to maternal immunization for both pregnant women and their health-care providers. Although available data have not demonstrated any vaccine-related adverse effects specific to pregnancy or pregnancy-related outcomes, additional studies are needed to reinforce these findings. Providers may remain concerned about theoretical risks or they may not know how to discuss the limitations of the current data with their patients. Moreover, as to date there are no U.S.-licensed vaccines specifically approved by the FDA for use in pregnancy, potential questions regarding vaccine safety are likely to lead to further hesitation among some obstetrical care providers in prescribing their use during pregnancy.
For the prescribing information of a vaccine to include an indication and usage statement that specifically addresses use in pregnancy, pre-licensure studies to evaluate the effectiveness and safety of the particular vaccine in pregnant women are required. For biological products, including vaccines, all indications must be supported by substantial evidence of effectiveness, which is based on adequate and well-controlled studies. Current regulations provide, with some exceptions, the inclusion of a pregnancy subsection in the prescribing information wherein each product is classified under one of five pregnancy categories. Vaccines currently recommended by the ACIP for use in pregnancy are labeled category B or C, allowing use of the vaccine during pregnancy if (1) the benefits of the vaccine for the pregnant mother may be acceptable despite its potential risk and (2) it is determined that the vaccine is clearly needed. Thus, even though these vaccines do not include a specific indication statement for use in pregnancy, they are not contraindicated and are not considered off-label use.57
Pregnancy and lactation labeling information
Manufacturers provide information on the use of vaccines in pregnant women in the product label in accordance with the Code of Federal Regulations Title 21, Section 201.57, established in 1979.62 This regulation specified five categories of use in pregnancy: A, B, C, D, and X. Products are categorized into these groups based on the risk of adverse events, or the risk of potential adverse events weighed against the potential benefits. However, the current pregnancy subsection of product labeling for U.S.-licensed vaccines provides limited data that could be misinterpreted, contributing to a provider's further uncertainty regarding whether or not to administer a specific vaccine to pregnant women.63
To this end, the FDA launched a major initiative to revise the current pregnancy labeling regulations to provide a framework to clearly communicate available scientific data on the potential risks of drugs and biologics used during pregnancy and lactation. The most significant change encompassed by the FDA Proposed Pregnancy and Lactation and Labeling Rule, when finalized, is the removal of the letter risk categories (A, B, C, D, or X), which will be replaced by a narrative summary of the risks of using a drug or biologic (e.g., vaccine) during pregnancy based on the available human and/or animal data.64 Also, the new label will include relevant clinical information intended to support health-care providers when making decisions about prescribing vaccines during pregnancy. However, it is very important to emphasize that label changes will improve clarity but do not translate into approval for a pregnancy indication. It is currently unknown whether or not the proposed rule will be finalized and, if so, when.
Coverage of influenza and pertussis vaccines during pregnancy
Recommendations by ACIP/CDC for influenza vaccination in pregnant women with high-risk medical conditions have been in place since 1960.65 Recommendations were broadened in 2004 to include influenza vaccination of all women who are pregnant (regardless of gestational age) or who will become pregnant during the influenza season.66 Despite this long-standing clinical guidance, from 2001 to 2009, influenza vaccination coverage among pregnant women aged 18–44 years with no high-risk conditions ranged from 11.2% during the 2001–2002 influenza season to 34.9% during the 2008–2009 influenza season.67–69
Public health efforts to increase vaccination of pregnant women were prioritized during the 2009 influenza A(H1N1) pandemic, in which pregnant women were significantly and disproportionately affected by severe influenza-associated outcomes.11,70,71 Increased awareness among patients and providers on the risks of influenza infection during pregnancy and the benefits of vaccination to pregnant women and their infants led to a median coverage rate of 47% of pregnant women vaccinated against seasonal influenza during the 2009–2010 season (40% vaccinated against the 2009 influenza A[H1N1] pandemic virus).72 Since then, CDC has reported promising increases in seasonal influenza vaccination coverage, with approximately 49% of pregnant women reporting having received influenza vaccination during the 2010-–2011 influenza seasons,2 47% in the 2011–2012 influenza season,3 and slightly more than 50% in the 2012–2013 influenza season.1
Regarding pertussis, maternal immunization is being used as the primary strategy for protecting infants too young to be fully vaccinated. In 2011, following recent nationwide increases in pertussis-related morbidity and mortality in infants younger than two months of age, ACIP/CDC recommended that women who had not previously been vaccinated with Tdap receive a single dose during pregnancy for the infants' protection via transplacental transfer of maternal antibodies.56 The following year, ACIP/CDC recommended that all pregnant women, regardless of previous Tdap vaccination status, receive Tdap vaccination during each pregnancy. This recommendation was based on data indicating that maternal antibodies against pertussis are short-lived and, therefore, not sustained at high enough levels to protect infants born from subsequent pregnancies.24,61 Because the optimal concentration of maternal antibodies for infant protection against pertussis are not well defined, vaccinating women between the 27th and 36th week of each gestation is thought to provide the highest concentration of maternal antibodies from Tdap vaccination to be transferred to the infant for maximal protection following birth.24
As recommendations for maternal immunization with Tdap are relatively recent, Tdap vaccination coverage in pregnant women remains low. CDC estimates that prior to the 2012 ACIP/CDC recommendation, Tdap coverage in pregnant women was only 2.6%.61 The most recent CDC estimates presented at the September 2013 NVAC meeting indicated that Tdap coverage in pregnant women may be as high as 29% (August 2012–April 2013). However, only 6.2% of these women were vaccinated during pregnancy (15.3% were vaccinated before pregnancy and 7.9% were vaccinated postpartum).73 Timing of vaccination should be considered when measuring coverage, as administering Tdap before the 27th week of gestation is unlikely to provide infant protection given the rapid waning of maternal pertussis antibodies.13 Modeling studies predict that, compared with postpartum vaccination, immunizing women during pregnancy will have the greatest effect on reducing pertussis-induced morbidity and mortality in infants younger than two months of age.74 Therefore, understanding the factors behind increasing Tdap uptake during pregnancy is considered a programmatic priority.
BARRIERS TO MATERNAL IMMUNIZATION
Strengthening a framework for the delivery of immunizations during pregnancy requires a full understanding of the patient and provider barriers that lead to missed opportunities for improving maternal immunization coverage. Overcoming these obstacles will improve the quality of obstetrical care and will facilitate efforts to enable patients to demand and access immunizations as a routine part of their preventive care.
Although efforts to improve maternal immunizations are not vaccine specific, most of the data on the investigation of patient and provider barriers to maternal immunizations relate to influenza vaccine, which is to be expected given the long-standing recommendation from the ACIP/CDC. However, many of the barriers cited for influenza immunization of pregnant women are expected to be similar to those for immunization with Tdap (and potential future vaccines), and strategies to overcome these barriers should be applicable for all maternal immunizations.
Patient barriers
Acceptance and uptake of recommended interventions, including maternal immunizations, are often affected by a patient's attitude, beliefs, demographic background, previous experiences, motivations, health literacy, expectations, and access to health care.75 In some instances, barriers to women's acceptance of immunization during pregnancy may reflect misperceptions of immunizations in general. For example, Henniger et al. showed that pregnant women who declined influenza vaccination were more likely than vaccinated pregnant women to believe that the vaccine can cause influenza (46% of unvaccinated women vs. 24% of vaccinated women).76 A recent study by Eppes etal. found that pregnant women from an urban tertiary medical center were significantly more likely to receive influenza vaccine during 2009 influenza A(H1N1) pandemic (both seasonal and pandemic vaccines) if they correctly answered more than 75% of the questions on an 88-question survey assessing their factual knowledge of influenza and influenza vaccination (n=80).77 A CDC Internet panel survey of 1,702 respondents showed that influenza vaccination coverage during the 2012–2013 influenza season was substantially higher in pregnant women who had positive attitudes toward the safety and effectiveness of the influenza vaccine than in pregnant women with negative attitudes (65.6% vs. 13.0% and 64.2% vs. 9.8%, respectively).1 Likewise, vaccine coverage among pregnant women is higher among women who reported receiving influenza vaccine during previous influenza seasons.3,78
Patient knowledge of the risks and benefits of maternal immunizations
For most pregnant women, concerns regarding the safety of vaccines during pregnancy are the greatest barriers to acceptance of maternal immunizations.1–3,72,79 In some instances, women who would not normally cite concerns about vaccine safety express concern that immunization is not safe for use during pregnancy.77 Women are often encouraged to avoid unnecessary medicine during pregnancy and may not understand the benefits of recommended immunizations against influenza and pertussis. In fact, one study of postpartum women revealed that 44% (106/242) of the women surveyed mistakenly believed that all vaccines should be avoided during pregnancy.78 Still other surveys indicate that pregnant women fear immunizations can harm the developing fetus,77,80–82 despite evidence to the contrary.
Vaccination uptake is lower in pregnant women who do not perceive VPDs such as influenza to be important risks to themselves or their infants. In a survey of 307 postpartum women conducted in a Delaware hospital, 23% of women who declined the 2009 H1N1 influenza vaccination cited that they did not feel at risk for influenza, whereas 24% stated that if they did get infected, they were not worried that they would get very sick.79 Yudin et al. found that 88% (51/58) of women surveyed did not know that influenza illness is often more severe in pregnant women than in non-pregnant women.81 Likewise, Henninger et al. found that compared with vaccinated women, unvaccinated pregnant women were much less likely to perceive themselves as susceptible to influenza infection, to feel at risk for severe influenza outcomes, and to anticipate feeling regret for not getting vaccinated if they did become ill.76 Others describe findings that women who express concerns regarding both the safety of the vaccine and their risk of infection may find it easier to default to inaction rather than shoulder the responsibility for even a very small risk of an adverse outcome associated with actively choosing to be immunized.83,84
Access to immunizations
Vaccination coverage in pregnant women can be related to the woman's ability or intention to access obstetrical care. CDC surveys show that vaccination coverage is lowest among women with fewer than five pregnancy-related provider visits. A study of more than 56,000 women in Ontario, Canada, found that vaccine uptake was lowest in women who did not have an obstetrical care provider or did not initiate prenatal care within the first trimester of pregnancy. The authors concluded that because all women in this study had access to free vaccine, disparities in vaccination coverage might reflect differences in access to medical information and/or fewer opportunities to be counseled by a provider on vaccine benefits and risks.85
In the U.S., vaccination among pregnant women was found to vary by the woman's type of medical coverage, with women covered by private or military insurance, Medicaid, or other types of public health insurance most likely to be vaccinated, and women with no insurance least likely to be vaccinated.1,3,86,87 Pregnant women facing barriers to accessing obstetrical care might not pursue immunization if it requires additional time and health-care visits and/or incurs additional copays or other out-of-pocket expenses.88 The Patient Protection and Affordable Care Act now requires group plans and private health insurance coverage to cover all ACIP/CDC routinely recommended vaccines for children, adolescents, and adults, with no cost sharing when vaccines are provided by an in-network provider.89 These provisions apply to all private, non-grandfathered plans, including non-grandfathered high-deductible health plans.
However, for pregnant women enrolled under public programs such as Medicaid, coverage of immunizations is more variable. Coverage of immunizations for individuals older than 21 years of age is considered an optional benefit, and individual states have the flexibility to determine if they will cover these types of services. To encourage states to increase the role Medicaid plays in providing preventive services, section 4106 of the Affordable Care Act gives states the opportunity to receive a one percentage point increase in their federal matching rate if they cover certain prevention services without cost sharing for these services. These preventive services include all ACIP/CDC-recommended immunizations and preventive services rated A or B by the U.S. Preventive Services Task Force (USPSTF).90 However, in a 2012 survey of state Medicaid fee-for-service programs by Stewart et al., only 17 out of 51 state programs covered all ACIP/CDC-recommended vaccines and prohibited cost sharing. Moreover, the majority of state immunization programs indicated that they did not plan to change their coverage or cost-sharing policies despite federal incentives.91 Therefore, out-of-pocket expenses for ACIP/CDC routinely recommended vaccines could continue to be a barrier for individuals enrolled in a state Medicaid program.
The importance of a provider recommendation
Interestingly, it has been repeatedly demonstrated that a strong recommendation from a health-care provider is the greatest predictor of vaccine acceptance among pregnant women. In a study across seven public hospitals in Sydney, Australia, investigators found that although only 25% of women (116/462) reported receiving influenza vaccination during their pregnancy, 78% (360/462) reported that they would accept vaccination during pregnancy if their physician recommended it.92 Beel et al. evaluated knowledge and beliefs toward both influenza and Tdap vaccines in 511 postpartum women in a public hospital in Houson, Texas, and found that 93% of respondents indicated they would be willing to receive both vaccines during pregnancy if recommended to them by their health-care provider.93 Although data regarding women's willingness to accept Tdap vaccination during pregnancy are somewhat limited, a survey of 815 pregnant women from Australia yielded similar results; 80% of women stated that they would be willing to receive the Tdap vaccine during pregnancy if it were recommended to them.94
The positive effect of a provider recommendation is further amplified if the provider both recommends and offers immunizations. During the 2011–2012 influenza season, CDC found that influenza vaccination coverage among women who received both a recommendation and an offer of vaccination from their provider was 73.6% compared with 47.9% among women who received a recommendation but no offer, and only 11.1% for women who did not receive either.3 Similar results were reported for the 2012–2013 influenza season (70.5%, 46.3%, and 16.1%, respectively).1
A provider recommendation and offer of vaccine can overcome other patient barriers. For instance, pregnant women who expressed negative attitudes toward vaccination were more likely to accept vaccination following a provider's recommendation and offer than women with positive attitudes who did not receive a provider recommendation.1–3 Reinforcing this finding, Meharry et al. found that “[i]f the provider states the influenza vaccine is important and it is not available, this contradicts the original message of the vaccine's importance.”84
Provider barriers
The influence of a provider recommendation on vaccination coverage clearly demonstrates that obstetrical care providers have a critical responsibility to inform health behaviors and overcome barriers to vaccination. In many cases, visits with obstetrical care providers may represent the only interactions that women have with the health-care system, and women often turn to these types of providers to receive preventive health services.95 In fact, the majority of women vaccinated during pregnancy report receiving these immunizations in their obstetrical care provider's office.2,3,79 Therefore, obstetrical care providers may create additional barriers if they do not regularly discuss, recommend, and offer immunizations during office visits.84,96 Provider knowledge about the relative benefits and risks of maternal immunization, the perceived role of immunization as part of routine obstetrical care, financial challenges to providing vaccine access for patients, and concerns about medical liability all contribute to provider barriers to immunization for pregnant women.
Provider knowledge
Many of the barriers cited for patients often apply to providers as well, including a lack of knowledge about the benefits of maternal immunizations. Providers may not be aware that pregnant women are at higher risk for severe outcomes from VPDs such as influenza. For example, Tong et al. surveyed 227 physicians (204 family physicians and 23 obstetricians) and found that 40% did not know pregnant women were at a higher risk of influenza-related complications.97 Other studies have highlighted a lack of understanding of the role that maternal immunization plays in protecting infants through the passive transfer of maternal antibodies.78,98 Moreover, several studies show that providers who were aware of ACIP/CDC and American College of Obstetricians and Gynecologists (ACOG) recommendations for maternal immunizations and demonstrated factual knowledge about the benefits of immunization and the risks of VPDs were more likely to recommend and offer vaccines.78,97–99
Safety concerns regarding immunizations during pregnancy remain an important provider barrier as well. A 2009 study found that one-third of physicians surveyed agreed with the statement, “We still do not know enough about the effects of vaccines on the fetus to administer them safely in pregnancy.”98 Wu et al. found that 23 of 37 physicians felt a healthy pregnant woman should not receive influenza vaccine until the second trimester, indicating continued vaccine safety concerns regarding fetal development100 despite ample evidence that immunization with inactivated influenza vaccine has not been shown to cause harm in either pregnant women or the developing fetus.101 Physicians have indicated that additional data concerning vaccine safety and efficacy during pregnancy could help to increase coverage.98
Viewing immunizations as a routine part of care activities
An additional barrier to maternal immunizations is that many obstetrical care providers simply may not view vaccine administration as a routine part of their patient care activities. Several studies indicate that obstetrical care providers feel that vaccines should be administered by a family physician or internist, while others assume that patients prefer to receive immunizations elsewhere.95,97,102 In a 2000 survey of 365 ob-gyns in Michigan, 62% of physicians stated that screening for VPDs was within their scope of practice, yet 25% did not offer any vaccinations at their office, citing “not part of my usual patient care activities” as the primary reason.99
However, newer studies suggest that these attitudes may be changing as more providers are acknowledging that immunizations should be an integral component of obstetrical care. Kissin et al. found that of the 873 ACOG fellows surveyed, the vast majority offered influenza vaccine during the 2009–2010 influenza season (77.6% offered seasonal influenza vaccine and 85.6% offered the 2009 H1N1 influenza vaccine).102 Likewise, a different survey of ACOG fellows found that 310/394 reported that they stocked and administered at least one vaccine in their practice—the most commonly stocked vaccines were human papillomavirus (HPV) (91%), influenza (67%), and Tdap (30%) vaccines.98
Financial and practical barriers to providing vaccines to patients
For many health-care providers, both those who provide obstetrical care and others, the most significant barriers to offering vaccines are financial, related to start-up costs (e.g., purchasing a refrigerator that is suitable for vaccine storage and vaccines) and reimbursement for vaccine costs and administration.95,98,99,102 Inadequate reimbursement is widely perceived as an important financial barrier deterring providers from offering immunizations to their pregnant patients.95,98–100 Power et al. found that more than 25% of physicians reported they had submitted insurance claims for vaccine administration and had not received any payment.98 Another study noted that insurance plans have refused reimbursement to some obstetricians for immunization services because they were not the patient's primary care provider for this preventive service.103 Adequate reimbursement for these services would serve as an incentive for obstetrical care providers to recommend and offer immunizations in their offices.78,104
Administrative costs for vaccines include procurement, costs associated with proper vaccine storage and handling, insurance against loss, opportunity costs, and personnel costs such as managing inventory, vaccine counseling, administration, and entering data into medical records and immunization registries. The costs related to administering vaccines have risen over time, a trend that some physicians indicate is a result of the need to stock, manage, and counsel patients on an ever-increasing number of vaccines (products and doses). For these reasons, those in solo practices may be less likely to offer vaccines than those in -multispecialty groups, where experience and distribution of costs may help alleviate the financial burden on a single provider.95,102
Costs must be balanced with variables at the practice level. First, physicians must have an adequate patient population to incur the costs of vaccines and vaccine storage and the ability to cover associated costs (e.g., participation in purchasing groups and business acumen of managing a practice). Second, there is considerable variation in the prices that physician practices pay for the same vaccine and in the reimbursement physicians negotiate with health plans.105 Third, patients today have access to vaccines at complementary sites, such as the local pharmacy, the grocery store, or their place of employment. Finally, patient attitudes toward immunization directly affect vaccine utilization and, thus, provider vaccine inventories and associated carrying costs. For example, Wu et al. found that physicians cited patient refusal as the main barrier that prevented them from administering influenza vaccines to their pregnant patients.98
Medical liability issues related to vaccine injury
Vaccine safety concerns also inhibit obstetrical care providers from recommending and/or administering vaccines during pregnancy due to fears about medical liabilities.95,100 Questions regarding medical liability are further complicated by uncertainties as to whether or not infants who may have sustained injuries in utero as a result of maternal immunization are eligible for compensation under the National Vaccine Injury Compensation Program (VICP).106
The VICP was established in 1986 following enactment by Congress of the National Childhood Vaccine Injury Act (the Vaccine Act).107 The VICP is a federal, no-fault compensation system that serves as an alternative to the civil tort system for vaccine-related injuries and deaths.108 Under the Vaccine Act, injured people may not file suits against vaccine administrators or vaccine manufacturers in almost all instances until they have first filed a petition for compensation under the VICP in the U.S. Court of Federal Claims and have exhausted their remedies with the Court.109 All VICP petitions are filed directly against the Secretary of the Department of Health and Human Services (HHS). To this end, the VICP maintains stability of the vaccine market by diverting lawsuits away from vaccine administrators and vaccine manufacturers, and provides compensation to those vaccinees whose injuries meet criteria established by the VICP. Ensuring that valid liability concerns regarding maternal immunization are appropriately addressed under the VICP and educating obstetrical care providers about the protections afforded could encourage more of them to offer and administer immunizations in their practice, thereby promoting wider implementation of immunization services and hopefully leading to increased vaccine coverage.
NVAC CONCLUSIONS AND RECOMMENDATIONS
As awareness of the importance of maternal immunizations increases, obstetrical care providers will need guidance on how to fully incorporate immunizations into their routine practice. Currently, there are few data on the types of interventions that obstetrical care providers have used to improve vaccination coverage among their patient populations. Moreover, there are few data on how non-physician obstetrical care providers (e.g., certified midwives, certified nurse-midwives, and pharmacists) can be better utilized to deliver immunizations to pregnant women.
The NVAC has reviewed the aforementioned patient and provider barriers and identified five areas where efforts should be mobilized at the federal level to strengthen the foundation of a maternal immunization program. The NVAC recommends that the ASH encourage all obstetrical care providers and immunization stakeholders to consider the findings and recommendations of this report as strategies to improve immunization coverage as a measure of quality obstetrical care.
NVAC RECOMMENDATION 1: ENHANCE COMMUNICATION TO ADDRESS THE SAFETY AND EFFECTIVENESS OF ALL CURRENTLY RECOMMENDED IMMUNIZATIONS DURING PREGNANCY
Translating vaccine recommendations into provider practice
Prior to the 2009 influenza A(H1N1) pandemic, the ACIP/CDC and ACOG recommendations for influenza vaccination of all pregnant women were not widely adhered to, as evidenced by continually low coverage rates. In 2008, Johnson et al. found that 60% of physicians and 56% of physician assistants, nurse practitioners, and registered nurses surveyed stated that they did not use ACIP/CDC guidelines as a source of information about adult immunizations.110 Furthermore, studies have demonstrated that obstetrical care providers who are not familiar with current ACIP/CDC recommendations are less likely than those who are familiar with current ACIP/CDC recommendations to recommend vaccination to their patients.78,104,111 However, the disproportionate negative effect of the 2009 influenza pandemic on pregnant women and the growing number of infant pertussis cases have ignited a national conversation about maternal immunizations. Obstetrical care providers are becoming increasingly aware of the need to incorporate immunization recommendations into their standard practice.
ACIP/CDC recommendations for immunizations for adolescents and adults include information about the use of vaccines in pregnant and breastfeeding women, per ACIP standard guidelines. These recommendations state that specific information on disease burden in pregnant women and their infants should be included in a background section of the recommendations entitled “vaccination of women during pregnancy and breastfeeding.” The background section should include information regarding the rationale and the available scientific data to support vaccination in this population. ACIP documents also explicitly specify recommendations for the use of the targeted vaccines in pregnant and breastfeeding women, including identified contraindications and precautions.112 Currently, this information is found within the individual statements for recommendations. In the future, the ACIP could consider developing a separate statement that consolidates all of this information into a single document of ACIP/CDC recommendations specifically for pregnant women, similar to ACIP/CDC statements for health-care workers. This statement could be updated as new vaccines or information become available, thus streamlining information for obstetrical care providers.
The ACIP continually reviews immunization data as these become available and updates recommendations accordingly to ensure that all populations are receiving preventive care based on the best available evidence. Information from the ACIP is shared through ongoing public discussions at ACIP committee meetings and through publication in CDC's Morbidity and Mortality Weekly Report. For example, the updated ACIP/CDC recommendation for administration of Tdap vaccine during every pregnancy was deliberated and voted on by the ACIP at its October 2012 quarterly meeting and published the following February.61 Recommendations adopted by CDC are incorporated into the immunization schedules for children, adolescents, and adults and shared annually with professional organizations for review and endorsement. Many professional organizations may then distribute this information to their members through professional newsletters, updates to member websites, and formal position statements.
Conclusions and recommendations
The coordination of national efforts to enhance educational opportunities for all obstetrical care providers and health professionals who administer services to pregnant women concerning current ACIP/CDC recommendations is needed to sustain momentum and build additional support for maternal immunization efforts. Educational outreach should be inclusive of all obstetrical care professionals including, but not limited to, obstetricians and other physicians who may administer vaccines to pregnant women, certified midwives, certified nurse-midwives, nurse practitioners, physician assistants, nurses, and pharmacists. These efforts are particularly important as new recommendations for vaccine use in pregnant women are made, or as existing recommendations are updated.
Federal systems that monitor and report uptake of vaccines recommended for use in pregnant women can be used to keep maternal immunizations at the forefront of national public health discussions and promote further progress. For example, since 2011, HHS has included CDC's findings regarding seasonal influenza vaccination coverage among pregnant women in public discussions at an annual seasonal influenza press conference sponsored by the National Foundation for Infectious Diseases.113 These types of media coverage help socialize recommendations for maternal immunizations and simultaneously educate patients and providers of the risks of VPDs and the benefits of immunizations.
In addition, federal coordination can help unify maternal immunization messages among different professional organizations and maternal immunization stakeholders to reach a broader audience of obstetrical care providers. For example, during the 2009 influenza A(H1N1) pandemic, CDC, ACOG, the American Academy of Family Physicians, and the American Medical Association co-authored a letter to inform physicians of the risks of pandemic and seasonal influenza to pregnant women, and to strongly urge them to vaccinate their pregnant patients.114 CDC released a similar letter in 2011, this time including seven additional nonprofits and professional associations, underscoring the importance of all health-care providers who administer care to pregnant and postpartum women to recommend influenza vaccination.115 These types of communications reinforce to the community the responsibility that all obstetrical care providers have for immunizing pregnant patients.
NVAC recommendation 1.1: the ASH should provide regular updates to relevant stakeholders regarding vaccines that are recommended by ACIP/CDC for use in pregnant women to maximize the potential for disease prevention through vaccine use, thereby benefiting the mother and her infant
Helping pregnant women to better understand the risks and benefits of maternal immunizations
Health literacy plays a critical role in an individual's capacity to comprehend and use information to make informed decisions about their health, such as evaluating vaccine benefits and risks.116 As previously noted, inadequate knowledge about influenza infection and misperceptions about vaccines administered during pregnancy negatively affect vaccine uptake among pregnant women. Likewise, when pregnant women do not feel adequately informed to make health-care decisions, they may prefer inaction rather than actively pursuing vaccination.84
Surprisingly, in a survey of 200 health-care providers, more than 50% indicated they did not always inform patients about the consequences of being unvaccinated, indicating individuals may not be fully informed in making their health-care decisions.110 This lack of discussion may be due in part to the level of the provider's knowledge about an individual's risk of going unimmunized. As an example, implementing a provider education program focused on influenza vaccination in pregnant women in a hospital in Connecticut led to significantly more postpartum women recalling their provider discussing the vaccine during pregnancy, as well as greater vaccination coverage (31% vaccination coverage vs. 19% during the previous season).104
Pregnant women as a whole are highly motivated to make health-care decisions that will benefit their infants. Clearly communicating the benefits of maternal immunizations for both the infant and the pregnant woman can further enhance the mother's willingness to consider and accept vaccination.84,97,117 A study testing the effectiveness of an educational pamphlet on women's willingness to receive influenza vaccination during pregnancy found vaccination rates were highest among women who received an educational pamphlet on influenza and were verbally told “if you have the flu shot during pregnancy, you will also help protect your baby against influenza from birth to six months of age” compared with women who had only received the pamphlet and controls who received usual care (86.1%, 72.9%, and 46.9%, respectively).118
Information about VPDs and immunizations needs to be accessible to demographically and culturally diverse populations. Yet, all individuals do not access or use health information in the same way and a one-size-fits-all approach will not be effective for everyone. Many individuals, especially those who have difficulty understanding numerical health information, may be more influenced by narratives rather than statistical representation of information, which then has implications for their health-care decision making.119,120 Pregnant women may also benefit from tools that help them better visualize information about the risks of adverse outcomes from VPDs and the risks associated with immunization, as well as information to better put vaccination-associated risks in context by comparing them with everyday risks that individuals encounter.121
Verbal communication between providers and patients that includes culturally and linguistically appropriate material can also help increase the relatability of messages. For example, CDC has used culturally targeted messaging in the Spanish-language motion comic book Un Amor Perdido to tell the true story of a Hispanic couple expecting their second child, to help educate Hispanic mothers about the importance of influenza vaccination during pregnancy.122
Using diverse communication platforms to reach pregnant women
A number of studies show that while obstetrical care providers remain the primary, trusted source of information for pregnant women, women obtain pregnancy information from a number of different sources, including books, childbirth education classes, the Internet, media, and friends and relatives.123 Therefore, diverse communication platforms should be employed to better educate pregnant women and empower them to actively pursue maternal immunizations as part of their prenatal care.
The 2013 Listening to Mothers III (LTM III) report surveyed 2,400 mothers representative of the national birthing population for race/ethnicity, age, and education and found that 99% of first-time and experienced mothers used some form of electronic device with an Internet connection at least one time per week to access pregnancy information, even if this device was not their principal source for information.124 Women who accessed the Internet reported an average of 20 visits for information related to pregnancy and childbirth.123
Pregnancy-specific websites are becoming increasingly popular, and the LTM III report also found that 66% of first-time mothers and 60% of experienced mothers indicated they considered these sites to be very valuable sources of information.124 These types of websites create virtual communities for pregnant women where they can seek and share experiences and health-related information.125 Women can choose to have these websites send weekly e-mails with information tailored to their specific stage of pregnancy, thus providing opportunities to convey information about VPDs and the importance of immunizations. Immunization-specific websites (e.g., www.immunization-forwomen.org) and federally sponsored Web pages (e.g., www.cdc.gov/vaccines/adults/rec-vac/pregnant.html) are also available to pregnant women.
The widespread use of cell phones in the U.S. has made mobile health technologies an attractive platform for delivering information for use in health promotion and disease prevention, particularly among low-income and medically underserved populations. The majority of adults use cell phones, regardless of race or socioeconomic class,126 and an early release of estimates from CDC's National Health Interview Survey, January–June 2012, showed mobile use-only households are more common in adults with no health insurance and among those who report barriers to obtaining health care compared with adults in households with a landline telephone.127 Mobile health technologies are attractive because text messages and mobile applications provide cost-effective health interventions that are broadly accessible and can be easily tailored and scaled to meet the needs of individual target populations.128
To date, the most successful implementation of mobile technologies for maternal health promotion is the text-messaging Text4baby initiative. This program is a nationwide effort that is supported and promoted by more than 1,000 public and private partners, including HHS. Launched in 2010, Text4baby now includes 550,000 participants who receive free, 150-character text messages three times per week coordinated to their stage of pregnancy. Currently, more than 250 messages are available in both English and Spanish that include information on safe and healthy behaviors during pregnancy and up to one year after the baby's due date. Messages are continually reviewed and revised based on current data and user feedback.129
Preliminary evaluations of the Text4baby program indicate its success, especially among its target audience of low-income women who are pregnant or have recently given birth, as these women are often subject to disparities in their access to information regarding prenatal care.130 A 2011–2012 study evaluating outcomes and satisfaction among Text4baby users in San Diego County, California, found that two-thirds of the 626 women surveyed stated they had spoken with their obstetrical care provider about a topic they had learned about through a Text4baby message. In the same study, another 65% of women reported Text4baby was useful in reminding them of immunizations that they or their infants should be receiving.131
In contrast, Moniz et al. found no differences in influenza vaccination coverage among pregnant women receiving text messages containing only general information about pregnancy health vs. pregnant women who received similar text messages plus prompts for influenza vaccination.132 Reminder/recall messages to prompt immunizations are recommended by the Community Preventive Services Task Force as an evidence-based strategy for increasing immunization coverage in both adults and children.133 However, further research on the effectiveness of using text messaging and other digital communication strategies that encourage pregnant women to seek immunization services for their own health is still needed.
Other outreach strategies may include partnerships with national organizations, popular pregnancy magazines, patient advocate groups, social media, and highly accessed media sources such as television or radio to deliver public service announcements. These messages should all focus on informing pregnant women about the risks of VPDs and the benefits of immunizations to them and their infants and encourage them to discuss immunizations with their obstetrical care providers.
Conclusions and recommendations
Optimizing communication strategies about maternal immunizations requires a multifaceted approach aimed at addressing the underlying patient motivations for vaccination, reaching a broad and culturally diverse patient and provider population, and combating the effects of low health literacy on risk perception and the willingness of pregnant women to take action. Ideally, communication tools should include simple, culturally appropriate messages that help pregnant women contextualize the risks and benefits of vaccination compared with risks they may encounter in their everyday lives and risks they face if they continue to go unimmunized. Presenting this information using multiple formats and providing innovative communication tools to facilitate provider/patient counseling will help educate pregnant women and affect social norms around maternal immunization acceptance and uptake.
It is important to emphasize that implementation research is needed to determine the effectiveness of these strategies and their applicability to pregnant women of different socioeconomic backgrounds. Data to support the effectiveness of these strategies to increase vaccination coverage and raise awareness in a diverse group of pregnant women will help to better craft immunization messages, focus communication efforts, and help to increase health literacy around immunizations in general. Moreover, building stronger partnerships with organizations that develop and maintain resources for pregnant women will be critical for providing expertise in crafting appropriate messages and identifying the most effective tools to communicate important health information to pregnant women.
NVAC recommendation 1.2: the ASH should work with federal partners and professional organizations to develop and distribute communication strategies and educational materials to health-care providers, especially those delivering obstetrical care. These educational materials should clearly state the benefits of maternal immunization, such as reducing the morbidity and mortality for mothers and young infants. In addition, they should enable providers to educate women who are pregnant or may become pregnant on the available clinical data regarding the safety and effectiveness of all ACIP/CDC-recommended maternal immunizations for themselves and their infants.
NVAC recommendation 1.3: the ASH should encourage the use of current and newly emerging communication technologies to maximize the effectiveness and reach of communication efforts addressing the clinical benefits of maternal immunization.
Data collection for vaccine safety information in pregnant women
Vaccine manufacturers are reluctant to initiate clinical development programs to specifically study the safety and efficacy of a vaccine in pregnant women to support an indication for the product during pregnancy due to various reasons including financial and liability concerns. These barriers must be identified and addressed so that the clinical development of vaccines, particularly those that will specifically target diseases in pregnant women and young infants, can be pursued. Also, pregnant women are usually excluded from participation in clinical trials for products for which no specific indication for use during pregnancy is being pursued. Consideration should be given to include pregnant women in clinical studies for some vaccines conducted at advanced stages of product development to gather safety and effectiveness data in pregnant women even though the studies may not be powered to support an indication for use in pregnancy.
Post-marketing vaccine safety surveillance systems
As mentioned previously, for currently U.S.-licensed vaccines there have been no pre-licensure safety and efficacy trials conducted in pregnant women to -support an indication and usage statement for use in pregnancy in the vaccine's prescribing information. In general, data on vaccine safety in pregnant women are collected through post-marketing surveillance systems. Post-marketing vaccine safety surveillance includes the use of both passive and active surveillance systems to collect data on vaccine adverse events and to conduct epidemiologic investigations of any identified potential safety signals. These systems are necessary to detect new or rare but serious side effects that may not be detected during pre-licensure clinical trials due to study size and infrequency of the event. They have also been useful in tracking outcomes in specific populations not traditionally represented in clinical trials, such as pregnant women.
HHS agencies such as CDC and FDA play an important role in monitoring, analyzing, and communicating post-marketing vaccine safety information to manufacturers and the public. While post-marketing surveillance systems and their broader role in vaccine safety have been comprehensively reviewed in previous NVAC reports,134 a brief description of three of these systems and how they have been used to assess vaccine safety monitoring in pregnant women are provided hereafter.
Vaccine Adverse Event Reporting System (VAERS).
VAERS is a national surveillance system jointly sponsored by CDC and FDA for the early detection of vaccine safety signals. It is a passive surveillance system that depends on reports of possible vaccine adverse events submitted by health-care providers, manufacturers, and the public. Health-care providers and manufacturers are required to report the following to VAERS: (1) adverse events listed on the National Vaccine Injury Compensation Program's (VICP's) Vaccine Injury Table (hereinafter, Vaccine Injury Table) that occur within seven days of vaccination (or a longer period if specified on the Vaccine Injury Table), (2) adverse events identified as contraindicating reactions specified within the manufacturer's package insert, or (3) any other matters required by the HHS Secretary by regulation.135 VAERS can be used to rapidly identify new vaccine safety signals or increases in the frequency of known safety signals.136 However, VAERS reports in and of themselves are not evidence of causation.
VAERS data have been used to evaluate safety information in pregnant women for seasonal influenza vaccines (both inactivated and live-attenuated),137 pandemic H1N1 influenza vaccines,138 Tdap vaccines,58 and meningococcal polysaccharide-protein conjugate vaccines.139 None of these studies found any association between vaccination and adverse maternal or infant outcomes. It is important to note that VAERS data cannot be used to demonstrate any causal association between a reported signal and the vaccine.
Vaccine Safety Datalink (VSD).
The VSD is an active surveillance system led by CDC in collaboration with nine large managed-care organizations to collect health outcomes and vaccination registry data from linked health-care databases. Representing 3% of the U.S. population (approximately 9.5 million people), the VSD is used to investigate vaccine safety signals and conduct epidemiologic studies to verify the role of vaccination in reported adverse outcomes.48 The VSD has been shown to successfully link health outcomes and vaccine exposures in mother-infant pairs through electronic health records.140 Data from seven participating VSD sites (2002–2009) demonstrated no increased risk for adverse pregnancy-related outcomes for 75,906 women vaccinated with seasonal influenza vaccine (28.4% in the first trimester) compared with 147,992 unvaccinated women.50
Post-licensure Rapid Immunization Safety Monitoring (PRISM).
PRISM is one component of the FDA's Mini-Sentinel program, a pilot program to inform the broader implementation of FDA's Sentinel Initiative. The Sentinel Initiative, when launched, will be a comprehensive active surveillance system for monitoring all adverse events associated with the use of FDA-regulated products.141 PRISM is a collaboration among the FDA, the Harvard Pilgrim Healthcare Institute, and four major national health-care insurance providers to use information from claims data to identify possible vaccine adverse events.142 Importantly, PRISM includes the added advantage of linking to immunization information systems from seven states plus New York City to capture information about vaccination occurring outside the traditional provider practice that might be missing from claims data (e.g., immunizations occurring at local retail pharmacies or public health clinics). As of December 2012, PRISM includes the capacity to monitor more than 110 million individuals and is capable of capturing claims data from more than 44 million patient encounters per month.143 Its nationwide database and linkage to immunization registries can be used to provide substantial statistical power for capturing rare, vaccine adverse events in pregnant women, and PRISM is currently initiating studies to analyze potential adverse pregnancy outcomes associated with administration of seasonal influenza vaccines.144
Vaccines and Medications in Pregnancy Surveillance System (VAMPSS).
VAMPSS is a collaborative effort among the American Academy of Allergy, Asthma and Immunology (AAAAI), the Organization of Teratology -Information Specialists (OTIS) of the University of California, San Diego, and the Slone Epidemiology Center (SEC) at Boston University to monitor the safety of vaccines and medications used by pregnant women. VAMPSS uses two complementary strategies for collecting exposure and health outcomes data in pregnant women exposed to targeted vaccines and medications: (1) prospective studies that enroll women reporting exposures (vaccinations) and that track these women for pregnancy and birth-related outcomes through medical records and interviews for comparison with unexposed pregnancies and (2) case-control studies of infants with and without congenital anomalies to compare the frequency of events among infants of exposed (vaccinated) women vs. unexposed women. Infants with congenital anomalies are also compared with infants without congenital anomalies whose mothers were exposed during pregnancy. All data are reviewed by investigative teams. Any potential vaccine safety signals that are identified are then reviewed by an independent advisory committee consisting of a biostatistician, a consumer representative, and representatives from CDC, the National Institutes of Health (NIH), ACOG, and the American Academy of Pediatrics (AAP).51 These systems were used to track the safety of the 2009 pandemic H1N1-containing vaccines in pregnant women and their infants. Similar to other studies, VAMPSS also found no meaningful evidence of adverse maternal or infant outcomes and no increased risk of congenital anomalies among infants born to vaccinated mothers compared with unvaccinated mothers.47,55
Conclusions and recommendations
The success of maternal immunization programs depends on the confidence of the provider and the pregnant woman that appropriate vaccine safety surveillance systems are being used to ensure that vaccines will not increase the risk of adverse maternal or infant outcomes. Clinical development programs to evaluate the safety and efficacy of a vaccine in pregnant women to support an indication for use in pregnancy should be encouraged. Future vaccines licensed for use in pregnant women may help obstetrical care providers view vaccines as part of their routine obstetrical care activities, as these vaccines will target their specific patient populations.
Post-marketing systems will always remain a critical component of vaccine safety surveillance, especially in pregnant women, because of lack of data from pre-licensure studies. These systems have provided reassuring data on the safety of vaccines used in pregnant women, but continued data collection is needed to ensure timely identification of vaccine safety signals. Federal support of these systems, particularly to determine where these systems could be adapted to better fill gaps in vaccine safety information regarding maternal and infant outcomes, should remain a priority.
Although providers are required to report certain possible vaccine adverse events to VAERS,135 not all providers are aware of these requirements. For example, Eckert et al. found that of 327 ob-gyns surveyed, fewer than 10% had ever used VAERS.145 Similarly, Kissen et al. found that fewer than half of ob-gyns surveyed reported suspected adverse events following administration of the influenza vaccine.102 In addition, providers may not know to report possible adverse events not listed in the Vaccine Injury Table or otherwise listed in the package insert. Failure to report weakens the robustness of safety systems to detect possible rare or unexpected adverse events. Therefore, additional outreach and education of providers on reporting requirements and the available vaccine safety surveillance systems could increase data collection and improve provider confidence in vaccine safety for use in pregnant women.
NVAC recommendation 1.4: the ASH should work with the appropriate federal agencies to assess data collected through post-marketing surveillance systems on the safety, efficacy, and effectiveness of currently recommended vaccines for pregnant women and their infants. The ASH also should work with federal agencies to determine the data needs for vaccine safety in pregnant women, confirm the ability of these systems to capture these data, and modify/develop new systems if data needs are not being met.
NVAC recommendation 1.5: the ASH should encourage appropriate professional and health-care organizations to educate obstetrical care providers on the available post-marketing surveillance systems used to track vaccine safety data to improve provider knowledge and reporting of potential vaccine adverse events. Educational materials and trainings should include how to report possible events to the relevant post-marketing surveillance systems, the strengths and limitations of these systems, the importance of reporting possible serious vaccine adverse events, and information regarding federal reporting requirements.
NVAC RECOMMENDATION 2: MAXIMIZE OBSTETRICAL CARE PROVIDER RECOMMENDATION AND ADMINISTRATION OF RECOMMENDED MATERNAL IMMUNIZATIONS
Supporting vaccine administrations as a routine standard of practice
ACOG issued an opinion statement in April 2013 indicating that all ob-gyns should consider immunizations as “an integral part of their women's health-care practice” and encouraged members to broaden their delivery of immunization services for both pregnant and non-pregnant women.146 In September 2013, the NVAC strengthened this guidance by introducing a set of updated standards for adult immunization practice by establishing expectations across the immunization stakeholder community emphasizing that it is a shared responsibility for every provider of health care to adults in all health-care settings to assess and recommend needed vaccines as a routine component of clinical care. The NVAC Standards for Adult Immunization Practices include a number of concise action steps and descriptions of model immunization practices to improve the uptake and delivery of adult immunizations.147 Several of these recommended best practices, and how they can be applied by obstetrical care providers to promote vaccination coverage among pregnant women, are described hereinafter.
Improving provider adherence to immunization recommendations for pregnant women through education and training opportunities.
Obstetricians are required to complete six months of primary care training, which includes immunizations, as part of their residency programs. However, a survey of ACOG fellows who had recently completed residency training reported that immunizations were among the topics least discussed with patients during wellness visits,148 indicating that their primary care training in vaccines and immunization services continues to be inadequate in terms of driving office behavior. Powers et al. found that more than one-third of ob-gyns reported that their immunization training was “barely adequate” in their medical school and residency training programs (39.8% and 34.9%, respectively).98 Similarly, obstetrical care providers have indicated a desire for post-graduate training materials focused on immunizations, such as Continuing Medical Education (CME) credits and educational tools for clinicians and their staff.98,149 While data on the behaviors and beliefs of certified midwives in the U.S. are not available, a study of midwives in England found that although 203/266 (76%) indicated that midwives should play a role in discussing and recommending immunizations to pregnant women, only 68/266 (26%) felt prepared to carry out this role. Even fewer felt that they were adequately trained to administer vaccines.150
Overcoming provider knowledge barriers is an important component of increasing immunization rates among pregnant women given the strong positive effect of a direct obstetrical care provider recommendation on pregnant women's vaccine acceptance.1,3 Thus, a number of efforts have focused on developing educational tools that can be widely disseminated to providers. For example, ACOG has developed an immunization-specific website (www.immunizationforwomen.org) that includes access to webinars, training, and committee opinion statements to educate its members on vaccines they may use in their practice.
Professional organizations also can direct their members to resources available through CDC or the Immunization Action Coalition. CDC has contributed to provider education efforts by developing a number of online resources for all types of providers seeking resources and training materials on immunizations.151 The Immunization Action Coalition, a nonprofit organization that provides immunization-specific educational materials for health-care providers and the public, provides numerous online resources including links to continuing education opportunities for increasing immunization competencies of various types of health-care providers.152
Other educational initiatives to improve immunization training have targeted medical education and professional training programs. For example, in 1989, CDC collaborated with the Association of Teachers of Preventive Medicine on a project called Teaching Immunization for Medical Education (TIME) to test different teaching strategies for improving immunization knowledge. Project objectives included evaluating the existing curriculum taught about immunizations and VPDs and developing improved case-based teaching materials that used interactive, problem-based learning and multistation clinical teaching scenarios based on actual cases for small group learning. Field testing of the project included 767 students and residents across 20 sites, and learning modules were successful in increasing knowledge of immunizations and VPDs, as assessed by comparing pre- and post-intervention test scores.153 The TIME project continues to be used, with its materials evaluated by an expert advisory committee that includes representatives from a number of professional and educational organizations, including ACOG.
Obstetrical care providers must not only be knowledgeable of vaccine recommendations, but they must also be familiar with VPD risk factors, the signs and symptoms of VPDs, and the potential for vaccine -failure in a small number of pregnant women. Innovative approaches to improving provider knowledge of VPDs and better using immunizations for disease prevention include Georgia's Educating Physicians In their Communities (EPIC) program. EPIC uses community-based training to educate Georgia providers and their staff about current immunization practices and recommendations.154 EPIC is a collaborative effort among the Georgia Immunization Program, the Georgia chapter of the American Academy of Pediatrics (GAAAP), the Georgia Academy of Family Physicians (GAFP), the Georgia chapter of the American College of Physicians, and the Georgia Obstetrical and Gynecological Society. Educational materials in the program are developed under the guidance of an expert advisory committee to reflect the most current standards of practice and ACIP guidelines. Educational programs are offered on site at no cost to all Georgia providers and their staff, and participants receive continuing education credits. The program offers scientific and practical information and resources for provider practices including, but not limited to, the risks of VPDs, understanding the most recent CDC recommendations for storage and handling of vaccines, defining herd immunity, and explaining the differences among a vaccine indication, vaccine recommendation, and vaccine requirement. EPIC has specifically worked with the Georgia Obstetrical and Gynecological Society to develop materials and curricula for immunizing pregnant women.
Implementing office-based practices to support the routine delivery of vaccines.
Successful immunization programs require logistical and organizational resources and support to achieve the recommended standards outlined for adult immunization practices.147 Professional and medical organizations and public health programs can assist members by developing toolkits and guidance documents that offer practical knowledge regarding the technical aspects of setting up and managing an office-based immunization program. These resources may include, but should not be limited to, talking points for providers, vaccine schedules, coding information for billing, frequently asked questions for consumers, vaccine information sheets, and information for reporting vaccine adverse events. Providers could also benefit from the development and sharing of best business practices concerning vaccine purchasing, payer contracting for immunization services, and appropriate billing.
For providers who already administer immunizations, there are several office-based strategies that can help increase vaccination coverage among their patients.155 Such practices have proven successful, especially for pediatricians and family physicians, and are predicted to have similar results for obstetrical care providers.146 For instance, standing orders allowing other eligible, non-provider staff (e.g., nurses and pharmacists) to administer immunizations can increase workflow efficiencies in busy practices, leading to increased vaccination coverage in both adult and pediatric settings. A meta-analysis of 11 studies reported a median 28% increase in vaccination coverage among targeted populations following the adoption of standing-order policies.155 In a large, multispecialty medical organization in Texas serving approximately 2,000 pregnant women per year, standing orders, provider education, and other interventions increased influenza vaccination from 2.5% in 2003–2004 to 46.5% in 2007–2008.87 Another study comparing interventions in one hospital vs. a control hospital found that standing orders alone increased Tdap vaccination rates in postpartum women from 18% to 69%.156
Even seemingly small organizational changes can have an effect on vaccine administration and improve office management of immunization services. In a multisite study in California, simple chart reminders reading “Think Flu Vaccine” were placed in the charts of all pregnant patients during the 2002–2003 influenza season to remind health-care providers to discuss and recommend influenza vaccination. As a result of this intervention, influenza vaccination rates increased from 1.5% pre-intervention to 21.9% post-intervention.103 An ob-gyn clinic in Milwaukee, Wisconsin, used a best practice alert for influenza vaccination that appeared as a prompt in a patient's medical record during each prenatal visit. Prompts ceased once the medical record contained documentation that the patient had been vaccinated, had received vaccination elsewhere, or had declined vaccination following counseling by a provider. Vaccination coverage among pregnant women rose from 41.8% in 2007–2008 to 60.9% in 2008–2009 following implementation of the alert.157 Likewise, a separate study showed that automated electronic prompts in patient medical records successfully increased pertussis vaccination among postpartum women.158 Other organizational changes to improve vaccine delivery in provider practices may include designating an office vaccine manager or champion, employing EHRs, setting office vaccination targets, and promoting vaccination among office staff.159
Using provider assessment and feedback to improve immunization services and delivery.
Increasing vaccination rates depends on having an accurate estimation of pre-intervention vaccination coverage to design, measure, and evaluate the effect of interventions as they are implemented. For obstetrical care providers who administer immunization services in their practices, both the ACIP and the Community Preventive Services Task Force recommend routine assessment and feedback of provider-based vaccination coverage data and immunization practices to improve rates across diverse patient populations and settings.155,160,161 Provider assessments, or audits, convey the ground truth highlighting missed opportunities and potential overestimations of vaccination coverage commonly occurring among providers when describing their own patient populations.159 These strategies motivate providers to develop processes to more accurately measure vaccination coverage among their patients and use these data for continual quality improvements.
The most successful demonstration of this strategy has occurred in pediatric practices under the Assessment, Feedback, Incentives, and eXchange (AFIX) program. AFIX is a nationwide initiative modeled on a pilot program by the Georgia Department of Public Health to increase childhood vaccination coverage in public clinics using quality improvement strategies.162 AFIX includes four components: (1) assessment of the provider's vaccination rates and immunization practices, (2) feedback of these results including recommendations to improve services, (3) incentives to reward improvements, and (4) exchange of best practices and follow-up with providers to monitor and support progress.163
AFIX has been applied widely across state immunization programs with great success. For example, an early evaluation of the AFIX program found that the program increased vaccination coverage among children in Missouri clinics by 49 percentage points in five years (from 44% in 1992 to 93% in 1997).164 In Maine, a statewide effort to improve AFIX outcomes through the development of quality improvement work plans based on AFIX feedback resulted in a 20 percentage-point increase in the number of children aged 24–35 months considered up-to-date for immunizations, improving from 49% in 2010 to 69% in 2011. Notably, 99% (92/93) of the participating providers found the AFIX feedback to be constructive, and 87% (81/93) found the assessments to be informative.165
The achievements gained by the AFIX program have led many to suggest that this program, or a similar program based on AFIX principles, should be applied to all providers who administer immunizations. In alignment with this thinking, initiatives such as the Quality Blue Physician Program led by Highmark, an independent licensee of Blue Cross/Blue Shield that offers health-care coverage to consumers in Pennsylvania and West Virginia,166 include assessments of all providers who deliver immunizations (i.e., childhood, adolescent, or adult immunizations). Clinical quality consultants work with practices to assess patient populations to identify immunization gaps, as well as to identify areas for improved immunization services through better office practices and workflow efficiencies. Resources and technical assistance are then made available to in-network providers to improve delivery of immunization services. The program also provides quarterly evaluations that compare vaccination coverage rates between provider practices. This program has succeeded in improving immunization rates across the Highmark network, including increasing adult coverage of influenza vaccination by 16%.167 Similar models serve as examples of how to achieve increases in multiple locales across the U.S.
Incorporating adult immunization standards into performance measures.
The use of performance measures that document how well providers adhere to the recommended standards for adult immunization practices have been proposed as a potential mechanism to increase awareness and improve immunization coverage rates.168,169 For example, the National Committee for Quality Assurance (NCQA) develops the Healthcare Effectiveness Data and Information Set (HEDIS) that is used to measure and compare performance and quality indicators between health plans.170 HEDIS measures are the most common metrics used by health plans, along with NCQA Consumer Assessment of Healthcare Providers and Systems (CAHPS) measures. Health plans are required to track HEDIS and CAHPS measures to obtain and maintain their NCQA accreditation and for public program contracting (i.e., with Medicare, Medicaid, and Medicare Advantage Star ratings). HEDIS measures are reported by 90% of health insurance plans in the U.S. In addition, health plans may incorporate and/or modify additional measures from other nationally recognized sources (e.g., NCQA chronic disease measures and some Centers for Medicare & Medicaid Services [CMS] measures). This information is then used by employers and consumers to compare products for purchase, and by health insurance plans to implement quality improvement interventions. There are six HEDIS measures documenting immunization status, including a recently revised 2014 HEDIS measure for evaluating patient influenza vaccination status that now applies to all individuals aged 18–64 years. Health-care plans should be encouraged to consider applying these existing quality measures to obstetrical care providers to promote provider recommendations for maternal immunizations and to improve immunization data collection among pregnant women. Data validation capabilities should ideally be in place at the time of initiating such programs to assure accurate measurement is attainable.
The National Quality Forum (NQF) initiated efforts in 2014 to identify performance measures to increase vaccination coverage and improve outcomes among adult populations as part of the National Quality Strategy.171 These efforts are intended to develop and prioritize measures that will have the greatest effect on health-care delivery and performance. However, it is not known if immunization measures specific to pregnant populations or obstetrical care providers will be included.
As quality metrics are discussed and developed for maternal immunizations, stakeholders will need to consider the unique challenges obstetrical care providers may face tracking vaccination coverage among pregnant patients. That is, pregnancy is time-limited and linking vaccination histories to pregnant women vs. non-pregnant women for reporting purposes may create additional administrative burdens. Moreover, methodologies used to measure adherence to maternal immunization recommendations are not standardized. How these measurements are performed and interpreted may affect provider assessments and the development of quality improvement strategies. Finally, many obstetrical care providers are relatively new to immunization services, or may not administer immunizations at all, thereby potentially creating technical barriers to tracking patient vaccination status. Some experts have suggested a potential role of partnerships/agreements between obstetrical care providers and other practices or organizations, such as pediatric practices or local pharmacies, to facilitate increasing vaccination coverage among pregnant women. However, all of these efforts should ensure that quality measures can be universally applied, result in improved quality of patient care, and do not create additional barriers for obstetrical care providers.
Conclusions and recommendations
Current standards of practice urge obstetrical care providers to routinely assess, recommend, and, when feasible, administer needed vaccines to their patients. Obstetrical care providers who identify as primary care providers are found to be more likely to assess their patients' vaccination status and administer vaccines within their own practice.98,99 Therefore, greater efforts are needed to educate and encourage obstetrical care providers to consider immunizations as a routine component of all women's health care, especially during obstetrical care, as pregnant women may be at higher risk from VPDs.
Providers may need additional technical assistance to establish or optimize immunization delivery services in their practices. Medical, professional, and public health organizations can offer providers support through the development of guidelines, best practices, and toolkits. Several evidence-based strategies have been successful in improving vaccination coverage rates in pediatric practices, and obstetrical care providers are strongly encouraged to consider implementing these interventions in their own practices. Periodic assessment of and feedback regarding a provider's performance and organizational practices should ensure that patients benefit from the highest quality of health care and that offices are effectively and efficiently managing their costs and workflow.
NVAC recommendation 2.1: the ASH should recommend that obstetrical care providers follow the published guidelines of professional organizations and government agencies to improve vaccination rates in their practices.
NVAC recommendation 2.2: the ASH should collaborate with federal partners, professional educational organizations, professional societies, and other relevant maternal immunization stakeholders to develop curricula for trainees and health-care providers that should include information about the recognized benefits and risks of immunizations during pregnancy and postpartum. Curricula should also include information about both the scientific basis for immunizations as well as the basics of establishing and administering immunization services in outpatient obstetrical care settings.
NVAC recommendation 2.3: the ASH should work with all relevant federal and nonfederal partners to assure that focused efforts are undertaken to routinize obstetrical care provider vaccine recommendations and administration of all recommended vaccines during pregnancy.
NVAC recommendation 2.4: the ASH should work with obstetrical care stakeholders to incorporate the widespread use of programs such as AFIX to support and evaluate the incorporation of immunization services into obstetrical care practices.
NVAC recommendation 2.5: the ASH should work with the stakeholder community to evaluate the applicability of existing measures and/or the development of new measures for vaccines recommended to pregnant women. Standardized metrics will help to reliably measure rates of immunizations given by obstetrical care providers to improve vaccine delivery in this population and to better measure progress toward institutional and national goals.
NVAC RECOMMENDATION 3: FOCUS EFFORTS TO IMPROVE FINANCING FOR IMMUNIZATION SERVICES DURING PREGNANCY AND POSTPARTUM
Meeting the needs for expanded access to immunization services
The paradigm for health care is now shifting toward an emphasis on the delivery of accountable care, with the goal of optimizing evidence-based approaches to maintain patient wellness while minimizing costs to the health-care system. In alignment with these goals, efforts to redefine the standards of maternal health and wellness should include maternal immunizations as a priority. Immunizations are one of the most cost-effective preventive services, and studies indicate that maternal immunization can provide direct savings to the health-care system.172–174
Full implementation of the Affordable Care Act will undoubtedly increase access to the benefits of maternal immunizations to a significant number of women. However, increasing the demand for immunization services does not automatically translate into providers having the resources necessary to establish or expand their immunization services to meet these growing needs (e.g., personnel, equipment, and technical and/or administrative assistance).175 In a 2011 study by Freed et al., only 27% of 849 physicians surveyed administered all ACIP-recommended vaccines for adults. Notably, only 12% of respondents indicated that they planned to increase the types of adult vaccines they offered in their practice and 79% did not expect to make any changes.176 While similar analyses have not been conducted to forecast the changing behaviors of obstetrical care providers, in a 2009 study of 310 obstetrical care providers who indicated that they stocked and administered at least one vaccine in their office, only 66.8% administered influenza vaccines and only 29.9% administered Tdap vaccines.98 Studies are needed to better characterize the types of immunizations administered by obstetrical care providers in light of raised awareness following the 2009 influenza A(H1N1) pandemic, the recent updates to CDC/ACIP and ACOG recommended use of Tdap in pregnant women, and changing models for obstetrical care.
Unlike with pediatric immunizations, data characterizing the direct and indirect costs of immunization services for obstetrical care providers or other providers of adult vaccines are not available. Many variables impact vaccine financing at the practice level, and a 2011 study by Freed et al. found that among the providers surveyed, there was no single dominant or group of factors that led to providers choosing to stock a particular vaccine for adults.176 Regardless, inadequate reimbursement for vaccine purchase and administration is cited by obstetrical care providers as an important barrier to offering immunization services in their practice.95,98,100 Whether these barriers are actual or perceived, provider concerns regarding reimbursement can affect access to vaccines and immunization services.176,177 For example, a 2007 study of 385 family physicians and ob-gyns found physicians that cited reimbursement as a barrier to vaccination were 55% less likely to recommend HPV vaccines to their patients than those who did not see reimbursement as a notable barrier.178 Therefore, it is necessary to explore all of these issues more fully to determine the extent that financial factors, including vaccine purchasing, stocking, and reimbursement for immunization services, create barriers to vaccine administration for obstetrical care providers. Of equal importance is the need to increase provider awareness about processes that can improve practice management and facilitate the provision of recommended vaccines for adult patients.
Financial considerations affecting immunization services
Immunization services require significant upfront investments, including the initial purchase of vaccine products, equipment for proper storage and handling, and the costs to manage vaccine inventories and data entry into immunization registries.179 These costs are not insignificant and are factored into the provider's decision to offer immunization services within their practice. Moreover, practices must purchase and stock these vaccines in advance of patient demand. As they are only reimbursed for the vaccines they administer, providers may only be willing to offer vaccines with a predictable, high demand.176,177
In 2007, Freed et al. surveyed 76 pediatric practices and found significant variations in the prices paid by providers for vaccine purchases and the reimbursement providers received for vaccines and immunization services.180 Practices may pay more than the price of the vaccine if purchased for immediate delivery or in smaller quantities, typically the most costly way to -purchase vaccines. Practices that choose not to participate in vaccine purchasing cooperatives or lack guidance or knowledge of best practices to improve vaccine administration efficiencies and administrative costs may be less inclined to stock more expensive vaccines or add new vaccines to their menu of routine services.
In cases where costs exceed reimbursement, providers must absorb their financial losses, and some have worried that inadequate reimbursement rates may eventually cause providers to discontinue immunization services.175,181 This concern is especially true for small or rural practices not affiliated with integrated health-care organizations.182 Ensuring that providers receive reimbursement to cover immunization services not only helps to secure a more robust network of vaccinators, but studies have also shown a positive association between increased reimbursement rates and higher vaccination rates among both publicly and privately insured individuals.183,184
In addition, the time needed to evaluate an individual's vaccination status and counsel him/her on the risks of VPDs and the benefits of immunization is often not reimbursed under the current payment systems. These activities take time away from other reimbursable interventions, and there may be a lack of incentive for providers to discuss and make referrals for immunizations.177 On the other hand, pregnant women who receive their provider's recommendation to receive vaccination but are not offered vaccines by their provider are still more likely to be vaccinated than women who are neither recommended nor offered vaccine.1 Because obstetrical care providers who cannot incorporate immunizations into their practice still have the responsibility to refer their patients to places where vaccine is more readily available, some within the stakeholder community have suggested creating a billing code specific to vaccine counseling to compensate for these services.177
Determination of reimbursement rates.
Reimbursement rates for vaccines administered to eligible adults aged 21 years and older enrolled in public health insurance programs such as Medicaid (and Children's Health Insurance Program [CHIP] in the case of pregnant women) are set by each state's Medicaid program. These rates can vary widely by state and generally cover minimal administration costs. For example, in 2007, Medicaid reimbursement for vaccine administration varied from $2.00 in Hawaii to $17.86 in New York, with a mean of $9.17 among the 50 states.184 Additionally, Medicaid reimbursement rates may differ from Medicare reimbursement rates, depending on the state's coverage of benefits. These differences result because Medicare's rates are based on the Resource-Based Relative Value Scale (RBRVS), which takes into account the costs associated with vaccine purchasing, vaccine labor, administration, overhead, and malpractice costs.179
These deficiencies in Medicaid reimbursement rates were acknowledged in November 2012, when the CMS issued a rule that would allow specified physicians (designated specialties to include family medicine, general internal medicine, or pediatric medicine, or a related sub-specialty) to receive Medicaid reimbursement rates for eligible primary care services (including immunizations) at the level of Medicare Part B rates for calendar years 2013–2014.185 The payment increase is for services provided through both fee-for-service and managed care delivery systems. While the ruling is intended to increase the number of providers administering adult immunizations,186 it should be emphasized that ob-gyn care providers are not designated specialties and are not eligible for these increases.
Reimbursement rates set by private plans depend on a number of factors including the retail price of the vaccines, the estimated value of the services provided, market forces, and geographic location.187 These rates can vary considerably among providers and are outlined in the reimbursement agreements negotiated between individual health plan and provider.180,188 In 2009, a survey of medical or quality improvement directors from 15 major private health insurance plans cited that one of the biggest factors they considered when determining changes to vaccine administration reimbursement was physician feedback.187 These findings suggest the importance for providers to have a more detailed understanding of the direct and indirect costs their practices incur from immunization-related activities, which would enhance their ability to negotiate reimbursement with health insurance payers. To address this gap, a 2012 RAND report proposed the development of decision-making tools to assist providers in documenting and evaluating the economic considerations associated with providing immunization services specific to their practices.177 Finally, many private health insurance plans also use Medicare's RBRVS as a basis for setting physician reimbursement.
Disparities that exist between public and private payer reimbursement rates have important consequences for providers. A 2009 economic analysis provided by Coleman et al. found that the net financial loss or gain to pediatric providers for vaccine services was directly linked to the proportion of publicly to privately insured individuals in a practice, with greater losses associated with greater percentages of Medicaid-enrolled patients.189 While on average there was a positive net return from vaccinating private pay patients, public programs do not keep pace with these increasing costs. As a result, practices in the study experienced a net loss when vaccinating large numbers of publicly insured individuals. These findings also suggest that private payers may bear an unfair proportion of the costs to fund immunizations.190 As the Medicaid- and CHIP-eligible population is expected to grow with implementation of the Affordable Care Act, the consequences that may be imposed on obstetrical care providers and the health-care system must be carefully assessed.
Conclusions and recommendations
The value of maternal immunizations as a preventive measure should be reflected not only through expanded access within the new models of patient care, but also in evaluating the reimbursement processes for providers who recommend and offer these vital services. Maternal immunizations play a fundamental role in obstetrical care, with health benefits extending to both the pregnant woman and her newborn. However, immunizations are still considered an optional benefit under many state Medicaid programs, and efforts to improve reimbursement rates for providers that deliver immunizations to Medicaid-enrolled individuals have not included all obstetrical care providers. Future initiatives to evaluate the effects that increased Medicaid reimbursement rates have on improving immunization coverage should include obstetrical care providers as a designated specialty.
Also, as new payment models such as pay-for-performance, accountable care organizations, and patient-centered medical homes continue to roll out, much attention will be paid to whether or not these models are resulting in improved quality and better outcomes, such as higher vaccination coverage among pregnant women. Similar to the current fee-for-service models, if reimbursement for immunizations under these new payment and delivery models is considered to be inadequate, it is likely that financial barriers will limit the number of obstetrical care providers willing to offer these services. This analysis is crucial for supporting provider decisions in establishing or expanding immunization services, and can aid providers when advocating for increases in reimbursement rates both with public and private payers.
Information about the effects of different payment models on the delivery of immunization services in specialty practices such as obstetrical care providers is also needed to evaluate the effect of health reform, both on access to health care and indicators of quality improvement. In particular, publicly funded programs need to identify stress points in the health-care system and provide appropriate incentives to absorb the growing demand for preventive services. Examples of the types of information to inform publicly funded programs include using data to educate state immunization programs and policy makers about the importance of maternal immunizations, to view all obstetrical care providers as primary care providers, and to ensure that states include maternal immunizations as a covered benefit under public programs, such as Medicaid.
Other strategies improving the return on investment for providers who offer immunizations include approaches to reduce the costs of immunization services within practices by developing more efficient office management practices. These strategies include promoting the adoption of EHRs, improving coding and billing processes, helping smaller practices develop negotiating and procurement skills, and better integrating community vaccinators as in-network providers to promote the concept of the medical home.179 Efforts to facilitate provider implementation of best practices could include developing toolkits and resources for obstetrical care providers, similar to those already available to pediatricians (e.g., American Academy of Pediatrics—Vaccine Finance Resources for Physicians).191 Similarly, ACOG has produced resources for providers with information on billing for immunization services.192
NVAC recommendation 3.1: the ASH should work with CMS and CDC to determine the costs to provide immunizations in various types of obstetrical practices to help evaluate the factors influencing the provision of adult maternal immunizations.
NVAC recommendation 3.2: the ASH should work with CMS, HRSA, and private payers to identify and improve upon current process issues related to billing, coding, and subsequent payment for the provision of maternal and other adult immunizations by obstetrical care providers, such as adult vaccine counseling and vaccine administration.
NVAC recommendation 3.3: the ASH should continue to monitor the effectiveness of the evolving payment and delivery models, outside of fee-for-service, within the new framework of federal and state Exchanges, patient-centered medical homes, and accountable care organizations. These new models should be encouraged to use cost studies of efficient practices and evidence-based economic principles as they pertain to maternal immunization programs.
NVAC recommendation 3.4: the ASH and HHS should work with professional organizations and other relevant maternal immunization stakeholders to develop a comprehensive toolkit that provides guidance on office and practice logistics (e.g., storage and inventory) to optimize providers' ability to efficiently and effectively implement vaccination services within their practices. Such a toolkit should also provide technical assistance regarding efficient business practices including payer contracting for immunization services, appropriate vaccine billing practices, and participation in vaccine purchasing groups.
NVAC RECOMMENDATION 4: SUPPORT EFFORTS TO INCREASE THE USE OF ELECTRONIC HEALTH RECORDS AND IMMUNIZATION INFORMATION SYSTEMS AMONG OBSTETRICAL CARE PROVIDERS
Using EHRs to support maternal immunization programs
Since 2009 and the passage of the Health Information Technology for Economic and Clinical Health Act,193 HHS has aggressively promoted the use of health information technology, and specifically the adoption of EHRs, as a mechanism to improve the quality and efficiency of health-care delivery. EHRs accomplish such improvements by increasing communication among a patient's multiple providers within a health-care organization and ideally across a patient's continuum of care.194 For example, pregnant women may receive health care from a number of providers in both the office and hospital settings. EHRs can facilitate obstetrical care delivery by making all relevant health-care information accessible at the point of care, including information regarding a patient's immunization status or possible vaccine contraindications.
Clinical decision-support modules within EHRs can improve provider adherence to standards of care, may be tailored to prompt obstetrical care providers and health-care staff to inquire about a woman's immunization status, and, in some cases, may automatically order immunizations during a patient visit. For example, to combat low Tdap vaccination among postpartum women in their facility, investigators at a teaching hospital in Chicago developed an algorithm linking prompts for Tdap vaccination in the EHRs of postpartum women directly to the ordering of treatments typical in postpartum care (e.g., iron supplements). Using this algorithm, Tdap vaccine order and administration in postpartum women increased from 0% pre-intervention to 59% post-intervention.158
EHRs, meaningful use, and promoting information exchange with Immunization Information Systems (IISs)
EHRs can also serve as powerful sources of health data to inform public health and broader immunization efforts. For instance, aggregated data from an organization's EHR system have been used to estimate vaccine effectiveness in different patient populations.195 In future studies, EHRs could be a critical data source for confirming the overall effectiveness of vaccines used for the specific purpose of preventing illness in infants by linking maternal and infant health records (e.g., future group B Streptococcus or respiratory syncytial virus vaccines). Immunization data within a practice's EHR system can also provide verified coverage rates within a practice/organization for overall quality assessments.196 With increasing interoperability of EHRs across care settings, providers will be able to access more accurate and complete immunization histories regardless of the location where care is received, promoting the idea of accountable care across a population, not just within an organization's walls.
HHS recognizes the potential effect that EHRs could have on improving patient care and coordination within the health-care system. However, the utility of EHR data for supplemental purposes such as public health reporting, research, patient-safety event reporting, and coverage determination has been limited due to lack of uniformity in the terminology and definitions of data elements across EHRs. In addition, clinicians often report information in unstructured free text. Linking EHR data with other data in a uniform and structured way could accelerate population health, safety, and quality improvement, and provide opportunities for large-scale research into coverage, safety, and other important endpoints. To this end, a newly formed HHS Structured Data Capture Public Health Tiger Team has begun to identify public health use cases, develop and consolidate common data elements, and build metadata that can be used to pre-populate forms on EHRs.
To assist in creating greater interoperability of EHRs across products, health-care providers, and institutions, CMS is collaborating with the HHS Office of the National Coordinator for Health Information Technology (ONC) to create standards for demonstrating meaningful use of EHR products. Providers and health-care facilities are eligible for financial incentives when they achieve a number of core objectives for data capture and exchange in each of the three stages of meaningful use when using ONC-certified EHRs (Figure).197 Because immunizations may be offered at multiple locations where patients receive health care (e.g., retail pharmacies or public health clinics), patient immunization histories in EHRs are often fragmented, resulting in missed opportunities to vaccinate. As part of meeting meaningful use stage 2 core objectives, all eligible participants must achieve ongoing submission of patient immunization data from their EHR to a centralized public health IIS. The long-term goal of these efforts is to achieve an ongoing, bidirectional flow of information between these systems to capture a patient's full immunization history, independent of where vaccines were administered, to better direct patient care activities and improve preventive care.128
Figure.
Stages of meaningful usea for electronic health records
aHITECH Answers. Meaningful use: the stages of meaningful use [cited 2014 Sep 1]. Available from: URL: http://www.hitechanswers.net/ehr-adoption-2/meaningful-use
HIE = health information exchange
However, it is important to note that the current inclusion of immunization data for adults in IISs remains regrettably low. In 2012, the number of state IISs that included an adult (19 years of age or older) with at least one immunization recorded in the system ranged from 0.7% in Texas to 85.4% in Minnesota, with the average percentage of adults in the population participating in a state's system being 25%.198 Additional efforts are ongoing to optimize the use of IISs for adults and improve the added value of providing vaccination information not already captured in patient EHRs. For example, CDC has awarded 20 grantees with funds to support enhanced interoperability of EHRs with IISs. As a result of this funding, more than 380 grantee sites, including >1,800 providers, have enhanced their systems to achieve bidirectional data exchange between IISs and EHRs. Stage 2 of the meaningful use program also includes objectives for providing clinical summaries for each office visit, providing a summary care record for each transition of care or referral, and using clinical decision support tools for high-priority health conditions.
Leveraging the use of EHRs to enhance vaccine safety surveillance systems
Importantly, EHRs linked to large databases of patient outcomes are playing a growing role in vaccine safety monitoring and causality assessments. Although the current use of EHRs is still somewhat limited, data encoded within EHRs would facilitate both prospective and retrospective cohort studies to actively monitor pregnancy and infant outcomes associated with maternal immunizations and to compare these results with outcomes observed in non-pregnant women and pregnant women who did not receive immunizations.48,57
For example, QueryHealth is an ONC-led initiative focused on using distributed networks to analyze data from multiple organizations in aggregate form for secondary uses (e.g., disease surveillance, comparative effectiveness, and medical product safety). The QueryHealth model takes individual-level data, de-identifies the information in compliance with the Health Insurance Portability and Accountability Act and aggregates information for population health use. These activities can serve to strengthen broader vaccine safety surveillance systems.199
As described previously, active surveillance systems such as CDC's Vaccine Safety Datalink (VSD) and the FDA's Post-licensure Rapid Immunization Safety Monitoring (PRISM) are now being adapted to specifically answer vaccine safety questions in pregnant patient populations through International Classification of Diseases, Ninth Revision (ICD-9) diagnostic codes recorded in EHRs and claims data (see Post-Marketing Vaccine Safety Surveillance Systems, page 24). Although not specific to obstetrical care, others have explored natural language algorithms to identify additional vaccine safety signals in the clinical notes section of a patient's EHR that may not be recognized using ICD-9 diagnostic codes and claims data alone.200
Conclusions and recommendations
The use of EHRs can help obstetrical care providers more accurately capture data on a pregnant woman's immunization status for better management of the woman's overall care through clinical support tools and monitoring of health outcomes. Promoting the use of EHRs capable of bidirectional exchange of health data between different EHRs and with state/local IISs will further improve tracking of a pregnant woman's immunization history, prompting providers to offer vaccines when needed to avoid potential missed opportunities. More complete immunization information can also help obstetrical care providers avoid potentially vaccinating a pregnant woman who is already fully immunized, resulting in unnecessary costs to the health-care system. However, efforts to enhance the uptake, use, and interoperability of EHRs must be matched by efforts to support the development, use, and interoperability of state and local IISs.
Finally, EHRs can provide a source of data regarding the safety and effectiveness of vaccines recommended by ACIP/CDC for use in pregnancy. The use of EHRs for active surveillance is typically needed to test hypotheses on large populations of patients to evaluate causality regarding uncommon adverse events. However, more work is needed to create opportunities to use EHR data to generate vaccine safety signals and report these signals to providers in real time. In addition, innovations in health technologies have demonstrated the ability to incorporate passive vaccine safety monitoring into the clinical support modules of EHRs at the point of patient care. For example, investigators at a large, multispecialty provider group in Boston used the clinical support function of their organization's EHRs to incorporate prompts for recognizing vaccine adverse events during patient encounters (both visits and telephone consultations) and reporting to VAERS.201 Future developments should continue to strive to include bidirectional exchange of these types of data among providers, IISs, public health authorities, vaccine safety surveillance systems, and pregnancy exposure registries to automatically generate alerts when possible vaccine adverse events are identified.
NVAC recommendation 4.1: the ASH should continue to support efforts to promote increased adoption by all obstetrical care providers of EHRs that can exchange data with IISs of the appropriate public health jurisdictions. This support should include bidirectional data exchange standards where supported, according to current and future national standards and regulations set by CDC and ONC.
NVAC recommendation 4.2: the ASH should promote collaborations among ONC, CDC, and FDA to establish automated, electronic interactions between EHRs and vaccine safety surveillance systems to strengthen vaccine safety monitoring systems in pregnant women.
NVAC RECOMMENDATION 5: RECOGNIZE AND ADDRESS CURRENT VACCINE LIABILITY LAW BARRIERS TO OPTIMIZE INVESTIGATIONS AND UPTAKE OF RECOMMENDED AND FUTURE VACCINES DURING PREGNANCY
Vaccine liability under the Vaccine Injury Compensation Program
Uncertainties surrounding maternal immunizations and vaccine liability under the VICP create barriers that limit obstetrical care providers' willingness to administer immunizations during pregnancy. To be considered for compensation, a petitioner must demonstrate by a preponderance of the evidence that the injury (or death) was caused or significantly aggravated by the vaccine and that the vaccine received was listed in the Vaccine Injury Table.202 A presumption of causation is provided if an injury meets all of the requirements for an injury listed in the Vaccine Injury Table. Vaccines included in the Vaccine Injury Table are all those that have been recommended by CDC for routine use in children, for which an excise tax has been imposed, and that the Secretary of HHS has added to the VICP. The VICP trust fund provides funds to the VICP through the excise tax that is imposed on these vaccines. Both influenza and Tdap vaccines are included in the Vaccine Injury Table. Categories of vaccines that are not recommended for routine use in children are not covered under the VICP.203
It is clear that vaccine administrators and manufacturers are afforded generous medical malpractice and product liability protections for injuries sustained by pregnant women as a result of their own, direct immunization (e.g., anaphylaxis). However, the Courts have not definitively resolved whether injuries sustained by a live-born child while in utero as a result of immunization of the mother are eligible for compensation under the VICP. To receive compensation, the Vaccine Act requires VICP petitioners to prove that the injured person “received” a vaccine.204 In the case of in utero injuries, the question is whether this statutory requirement includes in utero receipt, or whether it only extends to direct receipt by the mother.
Some special masters and judges of the U.S. Court of Federal Claims have rendered decisions concluding that Congress intended “receipt” to mean only direct injection, thereby precluding compensation for in utero injuries, whereas others have concluded that Congress intended “receipt” to have a broader meaning that includes in utero receipt.202 However, none of these decisions are binding, because only the U.S. Court of Appeals for the Federal Circuit (or the U.S. Supreme Court) sets binding precedent over the VICP. To date, the U.S. Court of Appeals for the Federal Circuit has not addressed the issue of compensability of in utero injuries, so the question has not been resolved. As such, short of a decision by the U.S. Court of Appeals for the Federal Circuit or a statutory amendment by Congress specifically addressing whether in utero injuries are compensable, the uncertainty remains regarding whether or not liability protections extend to in utero injuries.
The Advisory Commission on Childhood Vaccines
The Advisory Commission on Childhood Vaccines (ACCV) serves as the federal advisory committee to the HHS Secretary to advise the Secretary on VICP responsibilities, to recommend changes to the Vaccine Injury Table, and to provide guidance for VICP implementation. In June 2012, the ACCV was asked to recommend how the VICP could accommodate evolving recommendations for immunizations administered during pregnancy.205 As part of this charge, ACCV contemplated eligibility for compensation for injuries sustained by a live-born infant from covered vaccines received by the mother while the infant was in utero. Their consideration of eligibility includes vaccines recommended for use in pregnant women as well as those not routinely recommended but sometimes inadvertently administered during pregnancy.
In its proceedings, the ACCV agreed that live-born infants are the primary beneficiaries of maternal immunization and recommended that the HHS Secretary support eligibility of live-born infants to seek compensation under the VICP for injuries sustained in utero due to maternal immunization.205 The ACCV also provided possible avenues for the HHS Secretary to adopt and implement this recommendation, including (1) supporting a statutory amendment to the legislation (to be made by Congress) to include language that specifies eligibility for live-born infants of mothers vaccinated during pregnancy to pursue injury claims, (2) pursuing administrative rule-making to adopt a broader interpretation of the current statute, or (3) supporting a litigation strategy to seek a binding decision on this issue through the U.S. Court of Appeals. A full report detailing the ACCV's findings and recommendations was formally adopted by the ACCV on September 5, 2013, and transmitted to the HHS Secretary for consideration.
The ACCV noted that broadening eligibility is necessary to meet the changing needs of the national immunization program and would “contribute to the … continued development of new vaccines by addressing unsettled liability concerns for vaccine manufacturers and immunization program administrators.” However, the ACCV also recognized that each of its proposed options has both pros and cons, and the ACCV encouraged the HHS Secretary to seek further guidance from vaccine and immunization stakeholders, including the general public.205
Conclusions and recommendations
It is important to assure that infants who are injured from vaccines administered in utero are eligible for compensation. Overcoming important barriers to such compensation for injuries as a result of maternal immunization requires collaboration between both the National Vaccine Program and the VICP. Consensus among multiple advisory groups that this issue is important sends a powerful message to HHS and others and helps build solidarity around the proposed recommendations. The NVAC has worked closely with the ACCV and agrees with its findings and recommendations to HHS to support eligibility for live-born infants of mothers vaccinated during pregnancy to seek compensation under the VICP for injuries sustained in utero.
NVAC recommendation 5.1: the ASH should support efforts by HRSA to address the issue of including in utero injuries allegedly incurred following maternal immunization within the VICP. The ASH should support resolution of the issue regarding infants born with alleged in utero injuries in favor of allowing such claims to be pursued under the VICP and in favor of providing settled liability protections to vaccine manufacturers and administrators.
CONCLUSION
Ensuring pregnant women receive vaccinations specifically recommended for use during pregnancy (e.g., those against influenza and pertussis disease) should be incorporated as a standard of obstetrical care as well as a standard of practice among any and all providers who administer health-care services to pregnant women. Currently, many pregnant women do not receive recommended vaccinations due to ongoing patient and provider barriers. Overcoming these challenges is necessary for the benefits of maternal immunizations to be fully realized. Moreover, many of these described barriers are also relevant to broader adult immunization efforts, and evidenced-based solutions are likely applicable to strengthening adult immunization efforts overall. The NVAC report describes these barriers in depth and the resulting recommendations are intended to offer evidence-based solutions for strengthening maternal immunization efforts. The NVAC submits these recommendations to the ASH for consideration.
Footnotes
The views represented in this report are those of the National Vaccine Advisory Committee. The positions expressed and recommendations made in this report do not necessarily represent those of the U.S. Department of Health and Human Services, the U.S. government, or the individual working group members who served as authors of, or otherwise contributed to, this report.
REFERENCES
- 1.Ball S, Donahue S, Izrael D, Walker DK, Martonik R, DiSogra C, et al. Influenza vaccination coverage among pregnant women—United States, 2012–13 influenza season. MMWR Morb Mortal Wkly Rep. 2013;62(38):787–92. [PMC free article] [PubMed] [Google Scholar]
- 2.Walker DK, Ball S, Black R, Izrael D, Ding H, Euler GL, et al. Influenza vaccination coverage among pregnant women—United States, 2010–11 influenza season. MMWR Morb Mortal Wkly Rep. 2011;60(32):1078–82. [PubMed] [Google Scholar]
- 3.Walker DK, Ball S, Donahue S, Izrael D, Srinath KP, Ding H, et al. Influenza vaccination coverage among pregnant women: 2011–12 influenza season, United States. MMWR Morb Mortal Wkly Rep. 2012;61(38):758–63. [PubMed] [Google Scholar]
- 4.Gall SA, Poland GA. A maternal immunization program (MIP): developing a schedule and platform for routine immunization during pregnancy. Vaccine. 2011;29:9411–3. doi: 10.1016/j.vaccine.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 5.Link-Gelles R, Chamberlain AT, Schulkin J, Ault K, Whitney E, Seib K, et al. Missed opportunities: a national survey of obstetricians about attitudes on maternal and infant immunization. Matern Child Health J. 2012;16:1743–7. doi: 10.1007/s10995-011-0936-0. [DOI] [PubMed] [Google Scholar]
- 6.Návar AM, Halsey NA, Carter TC, Montgomery MP, Salmon DA. Prenatal immunization education. Am J Prev Med. 2007;33:211–3. doi: 10.1016/j.amepre.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 7.Neuzil KM, Reed GW, Mitchel EF, Simonsen L, Griffin MR. Impact of influenza on acute cardiopulmonary hospitalizations in pregnant women. Am J Epidemiol. 1998;148:1094–102. doi: 10.1093/oxfordjournals.aje.a009587. [DOI] [PubMed] [Google Scholar]
- 8.Rogers VL, Sheffield JS, Roberts SW, McIntire DD, Luby JP, Trevino S, et al. Presentation of seasonal influenza A in pregnancy: 2003–2004 influenza season. Obstet Gynecol. 2010;115:924–9. doi: 10.1097/AOG.0b013e3181da0c5e. [DOI] [PubMed] [Google Scholar]
- 9.Cox S, Posner SF, McPheeters M, Jamieson DJ, Kourtis AP, Meikle S. Hospitalizations with respiratory illness among pregnant women during influenza season. Obstet Gynecol. 2006;107:1315–22. doi: 10.1097/01.AOG.0000218702.92005.bb. [DOI] [PubMed] [Google Scholar]
- 10.Rasmussen SA, Jamieson DJ, Uyeki TM. Effects of influenza on pregnant women and infants. Am J Obstet Gynecol. 2012;207(3 Suppl):S3–8. doi: 10.1016/j.ajog.2012.06.068. [DOI] [PubMed] [Google Scholar]
- 11.Siston AM, Rasmussen SA, Honein MA, Fry AM, Seib K, Callaghan WM, et al. Pandemic 2009 influenza A(H1N1) virus illness among pregnant women in the United States. JAMA. 2010;303:1517–25. doi: 10.1001/jama.2010.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Creanga AA, Johnson TF, Graitcer SB, Hartman LK, Al-Samarrai T, Schwarz AG, et al. Severity of 2009 pandemic influenza A (H1N1) virus infection in pregnant women. Obstet Gynecol. 2010;115:717–26. doi: 10.1097/AOG.0b013e3181d57947. [DOI] [PubMed] [Google Scholar]
- 13.Blanchard-Rohner G, Siegrist CA. Vaccination during pregnancy to protect infants against influenza: why and why not? Vaccine. 2011;29:7542–50. doi: 10.1016/j.vaccine.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 14.Mor G, Cardenas I. The immune system in pregnancy: a unique complexity. Am J Reprod Immunol. 2010;63:425–33. doi: 10.1111/j.1600-0897.2010.00836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jamieson DJ, Kissin DM, Bridges CB, Rasmussen SA. Benefits of influenza vaccination during pregnancy for pregnant women. Am J Obstet Gynecol. 2012;207(3 Suppl):S17–20. doi: 10.1016/j.ajog.2012.06.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jackson LA, Patel SM, Swamy GK, Frey SE, Creech CB, Munoz FM, et al. Immunogenicity of an inactivated monovalent 2009 H1N1 influenza vaccine in pregnant women. J Infect Dis. 2011;204:854–63. doi: 10.1093/infdis/jir440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poehling KA, Edwards KM, Weinberg GA, Szilagyi P, Staat MA, Iwane MK, et al. The underrecognized burden of influenza in young children. N Engl J Med. 2006;355:31–40. doi: 10.1056/NEJMoa054869. [DOI] [PubMed] [Google Scholar]
- 18.Poehling KA, Edwards KM, Griffin MR, Szilagyi PG, Staat MA, Iwane MK, et al. The burden of influenza in young children, 2004–2009. Pediatrics. 2013;131:207–16. doi: 10.1542/peds.2012-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clark TA. Pertussis epidemiology and vaccination in the United States. National Vaccine Advisory Committee Meeting; June 2012; Washington. Also available from: URL: http://www.hhs.gov/nvpo/nvac/meetings/pastmeetings/2012/clark_and_messonnier_062512.pdf [cited 2013 Oct 7] [Google Scholar]
- 20.Van de Perre P. Transfer of antibody via mother's milk. Vaccine. 2003;21:3374–6. doi: 10.1016/s0264-410x(03)00336-0. [DOI] [PubMed] [Google Scholar]
- 21.Van den Berg JP, Westerbeek EA, van der Klis FR, Berbers GA, van Elburg RM. Transplacental transport of IgG antibodies to preterm infants: a review of the literature. Early Hum Dev. 2011;87:67–72. doi: 10.1016/j.earlhumdev.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 22.Van den Berg JP, Westerbeek EA, Berbers GA, van Gageldonk PG, van der Klis FR, van Elburg RM. Transplacental transport of IgG antibodies specific for pertussis, diphtheria, tetanus, haemophilus influenzae type b, and Neisseria meningitidis serogroup C is lower in preterm compared with term infants. Pediatr Infect Dis J. 2010;29:801–5. doi: 10.1097/inf.0b013e3181dc4f77. [DOI] [PubMed] [Google Scholar]
- 23.Mooi FR, de Greeff SC. The case for maternal vaccination against pertussis. Lancet Infect Dis. 2007;7:614–24. doi: 10.1016/S1473-3099(07)70113-5. [DOI] [PubMed] [Google Scholar]
- 24.Healy CM, Rench MA, Baker CJ. Importance of timing of maternal combined tetanus, diphtheria, and acellular pertussis (Tdap) immunization and protection of young infants. Clin Infect Dis. 2013;56:539–44. doi: 10.1093/cid/cis923. [DOI] [PubMed] [Google Scholar]
- 25.Zuccotti G, Pogliani L, Pariani E, Amendola A, Zanetti A. Transplacental antibody transfer following maternal immunization with a pandemic 2009 influenza A(H1N1) MF59-adjuvanted vaccine. JAMA. 2010;304:2360–1. doi: 10.1001/jama.2010.1729. [DOI] [PubMed] [Google Scholar]
- 26.Zaman K, Roy E, Arifeen SE, Rahman M, Raqib R, Wilson E, et al. Effectiveness of maternal influenza immunization in mothers and infants [published erratum appears in N Engl J Med 2009;360:648] N Engl J Med. 2008;359:1555–64. doi: 10.1056/NEJMoa0708630. [DOI] [PubMed] [Google Scholar]
- 27.Englund JA. The influence of maternal immunization on infant immune responses. J Comp Pathol. 2007;137(Suppl):S16–9. doi: 10.1016/j.jcpa.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 28.Fischer GW, Ottolini MG, Mond JJ. Prospects for vaccines during pregnancy and in the newborn period. Clin Perinatol. 1997;24:231–49. [PubMed] [Google Scholar]
- 29.UNICEF. Elimination of maternal and neonatal tetanus [cited 2014 May 3] Available from: URL: http://www.unicef.org/health/index_43509.html.
- 30.Greenwood B. Maternal immunisation in developing countries. Vaccine. 2003;21:3436–41. doi: 10.1016/s0264-410x(03)00346-3. [DOI] [PubMed] [Google Scholar]
- 31.Munoz FM, Ferrieri P. Group B streptococcus vaccination in pregnancy: moving toward a global maternal immunization program. Vaccine. 2013;31(Suppl 4):D46–51. doi: 10.1016/j.vaccine.2012.11.026. [DOI] [PubMed] [Google Scholar]
- 32.Ortiz JR, Neuzil KM, Ahonkhai VI, Gellin BG, Salisbury DM, Read JS, et al. Translating vaccine policy into action: a report from the Bill & Melinda Gates Foundation Consultation on the prevention of maternal and early infant influenza in resource-limited settings. Vaccine. 2012;30:7134–40. doi: 10.1016/j.vaccine.2012.09.034. [DOI] [PubMed] [Google Scholar]
- 33.Eick AA, Uyeki TM, Klimov A, Hall H, Reid R, Santosham M, et al. Maternal influenza vaccination and effect on influenza virus infection in young infants. Arch Pediatr Adolesc Med. 2011;165:104–11. doi: 10.1001/archpediatrics.2010.192. [DOI] [PubMed] [Google Scholar]
- 34.Benowitz I, Esposito DB, Gracey KD, Shapiro ED, Vázquez M. Influenza vaccine given to pregnant women reduces hospitalization due to influenza in their infants. Clin Infect Dis. 2010;51:1355–61. doi: 10.1086/657309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peters TR, Banks GC, Snively BM, Poehling KA. Potential impact of parental Tdap immunization on infant pertussis hospitalizations. Vaccine. 2012;30:5527–32. doi: 10.1016/j.vaccine.2012.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Healy CM, Munoz FM, Rench MA, Halasa NB, Edwards KM, Baker CJ. Prevalence of pertussis antibodies in maternal delivery, cord, and infant serum. J Infect Dis. 2004;190:335–40. doi: 10.1086/421033. [DOI] [PubMed] [Google Scholar]
- 37.Gall SA, Myers J, Pichichero M. Maternal immunization with tetanus-diphtheria-pertussis vaccine: effect on maternal and neonatal serum antibody levels. Am J Obstet Gynecol. 2011;204:334.e1–5. doi: 10.1016/j.ajog.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 38.Munoz FM, Bond NH, Maccato M, Pinell P, Hammill HA, Swamy GK, et al. Safety and immunogenicity of tetanus diphtheria and acellular pertussis (Tdap) immunization during pregnancy in mothers and infants: a randomized clinical trial. JAMA. 2014;311:1760–9. doi: 10.1001/jama.2014.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fell DB, Sprague AE, Liu N, Yasseen AS, III, Wen SW, Smith G, et al. H1N1 influenza vaccination during pregnancy and fetal and neonatal outcomes. Am J Public Health. 2012;102:e33–40. doi: 10.2105/AJPH.2011.300606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Richards JL, Hansen C, Bredfeldt C, Bednarczyk RA, Steinhoff MC, Adjaye-Gbewonyo D, et al. Neonatal outcomes after antenatal influenza immunization during the 2009 H1N1 influenza pandemic: impact on preterm birth, birth weight, and small for gestational age birth. Clin Infect Dis. 2013;56:1216–22. doi: 10.1093/cid/cit045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Omer SB, Goodman D, Steinhoff MC, Rochat R, Klugman KP, Stoll BJ, et al. Maternal influenza immunization and reduced likelihood of prematurity and small for gestational age births: a retrospective cohort study. PLoS Med. 2011;8:e1000441. doi: 10.1371/journal.pmed.1000441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bisgard KM, Pascual FB, Ehresmann KR, Miller CA, Cianfrini C, Jennings CE, et al. Infant pertussis: who was the source? Pediatr Infect Dis J. 2004;23:985–9. doi: 10.1097/01.inf.0000145263.37198.2b. [DOI] [PubMed] [Google Scholar]
- 43.Wendelboe AM, Njamkepo E, Bourillon A, Floret DD, Gaudelus J, Gerber M, et al. Transmission of Bordetella pertussis to young infants. Pediatr Infect Dis J. 2007;26:293–9. doi: 10.1097/01.inf.0000258699.64164.6d. [DOI] [PubMed] [Google Scholar]
- 44.Healy CM, Rench MA, Baker CJ. Implementation of cocooning against pertussis in a high-risk population. Clin Infect Dis. 2011;52:157–62. doi: 10.1093/cid/ciq001. [DOI] [PubMed] [Google Scholar]
- 45.Grizas AP, Camenga D, Vázquez M. Cocooning: a concept to protect young children from infectious diseases. Curr Opin Pediatr. 2012;24:92–7. doi: 10.1097/MOP.0b013e32834e8fe9. [DOI] [PubMed] [Google Scholar]
- 46.Munoz F, Englund J. Infant pertussis: is cocooning the answer? Clin Infect Dis. 2011;53:893–6. doi: 10.1093/cid/cir542. [DOI] [PubMed] [Google Scholar]
- 47.Chambers CD, Johnson D, Xu R, Luo Y, Louik C, Mitchell AA, et al. Risks and safety of pandemic H1N1 influenza vaccine in pregnancy: birth defects, spontaneous abortion, preterm delivery, and small for gestational age infants. Vaccine. 2013;31:5026–32. doi: 10.1016/j.vaccine.2013.08.097. [DOI] [PubMed] [Google Scholar]
- 48.Kharbanda EO, Vazquez-Benitez G, Shi WX, Lipkind H, Naleway A, Molitor B, et al. Assessing the safety of influenza immunization during pregnancy: the Vaccine Safety Datalink. Am J Obstet Gynecol. 2012;207(3 Suppl):S47–51. doi: 10.1016/j.ajog.2012.06.073. [DOI] [PubMed] [Google Scholar]
- 49.Naleway AL, Irving SA, Henninger ML, Li DK, Shifflett P, Ball S, et al. Safety of influenza vaccination during pregnancy: a review of subsequent maternal obstetric events and findings from two recent cohort studies. Vaccine. 2014;32:3122–7. doi: 10.1016/j.vaccine.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nordin JD, Kharbanda EO, Benitez GV, Nichol K, Lipkind H, Naleway A, et al. Maternal safety of trivalent inactivated influenza vaccine in pregnant women. Obstet Gynecol. 2013;121:519–25. doi: 10.1097/AOG.0b013e3182831b83. [DOI] [PubMed] [Google Scholar]
- 51.Schatz M, Chambers CD, Jones KL, Louik C, Mitchell AA. Safety of influenza immunizations and treatment during pregnancy: the Vaccines and Medications in Pregnancy Surveillance System. Am J Obstet Gynecol. 2011;204(6 Suppl 1):S64–8. doi: 10.1016/j.ajog.2011.01.047. [DOI] [PubMed] [Google Scholar]
- 52.Steinhoff MC, Omer SB, Roy E, El Arifeen S, Raqib R, Dodd C, et al. Neonatal outcomes after influenza immunization during pregnancy: a randomized controlled trial. CMAJ. 2012;184:645–53. doi: 10.1503/cmaj.110754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pasternak B, Svanström H, Mølgaard-Nielsen D, Krause TG, Emborg HD, Melbye M, et al. Risk of adverse fetal outcomes following administration of a pandemic influenza A(H1N1) vaccine during pregnancy. JAMA. 2012;308:165–74. doi: 10.1001/jama.2012.6131. [DOI] [PubMed] [Google Scholar]
- 54.Pasternak B, Svanström H, Mølgaard-Nielsen D, Krause TG, Emborg HD, Melbye M, et al. Vaccination against pandemic A/H1N1 2009 influenza in pregnancy and risk of fetal death: cohort study in Denmark. BMJ. 2012;344:e2794. doi: 10.1136/bmj.e2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Louik C, Ahrens K, Kerr S, Pyo J, Chambers C, Jones KL, et al. Risks and safety of pandemic H1N1 influenza vaccine in pregnancy: exposure prevalence, preterm delivery, and specific birth defects. Vaccine. 2013;31:5033–40. doi: 10.1016/j.vaccine.2013.08.096. [DOI] [PubMed] [Google Scholar]
- 56.Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine (Tdap) in pregnant women and persons who have or anticipate having close contact with an infant aged <12 months—Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011;60(41):1424–6. [PubMed] [Google Scholar]
- 57.Shakib JH, Korgenski K, Sheng X, Varner MW, Pavia AT, Byington CL. Tetanus, diphtheria, acellular pertussis vaccine during pregnancy: pregnancy and infant health outcomes. J Pediatr. 2013;163:1422–6.e1-4. doi: 10.1016/j.jpeds.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zheteyeva YA, Moro PL, Tepper NK, Rasmussen SA, Barash FE, Revzina NV, et al. Adverse event reports after tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccines in pregnant women. Am J Obstet Gynecol. 2012;207:59.e1–7. doi: 10.1016/j.ajog.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 59.Silveira CM, Cáceres VM, Dutra MG, Lopes-Camelo J, Castilla EE. Safety of tetanus toxoid in pregnant women: a hospital-based case-control study of congenital anomalies. Bull World Health Organ. 1995;73:605–8. [PMC free article] [PubMed] [Google Scholar]
- 60.Czeizel AE, Rockenbauer M. Tetanus toxoid and congenital abnormalities. Int J Gynaecol Obstet. 1999;64:253–8. doi: 10.1016/s0020-7292(99)00008-9. [DOI] [PubMed] [Google Scholar]
- 61.Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (Tdap) in pregnant women—Advisory Committee on Immunization Practices (ACIP), 2012. MMWR Morb Mortal Wkly Rep. 2013;62(7):131–5. [PMC free article] [PubMed] [Google Scholar]
- 62. 21 C.F.R., 201.57 (2014)
- 63.Marshall V, Gruber M. Influenza immunization during pregnancy: US regulatory perspective. Am J Obstet Gynecol. 2012;207(3 Suppl):S57–62. doi: 10.1016/j.ajog.2012.06.075. [DOI] [PubMed] [Google Scholar]
- 64.Food and Drug Administration (US) Summary of proposed rule on pregnancy and lactation labeling. 2009. [cited 2014 Jan 12]. Available from: URL: http://www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/Labeling/ucm093310.htm.
- 65.Burney LE. Influenza immunization: statement. Public Health Rep. 1960;75:944. [PMC free article] [PubMed] [Google Scholar]
- 66.Harper SA, Fukuda K, Uyeki TM, Cox NJ, Bridges CB. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Morb Mortal Wkly Rep. 2004;53(RR-6):1–40. [PubMed] [Google Scholar]
- 67.Kennedy ED, Ahluwalia IB, Ding H, Lu PJ, Singleton JA, Bridges CB. Monitoring seasonal influenza vaccination coverage among pregnant women in the United States. Am J Obstet Gynecol. 2012;207(3 Suppl):S9–16. doi: 10.1016/j.ajog.2012.06.069. [DOI] [PubMed] [Google Scholar]
- 68.Zhao G, Ford ES, Tsai J, Li C, Ahluwalia IB, Pearson WS, et al. Trends in health-related behavioral risk factors among pregnant women in the United States: 2001–2009. J Womens Health (Larchmt) 2012;21:255–63. doi: 10.1089/jwh.2011.2931. [DOI] [PubMed] [Google Scholar]
- 69.Lu P, Bridges CB, Euler GL, Singleton JA. Influenza vaccination of recommended adult populations, U.S., 1989–2005. Vaccine. 2008;26:1786–93. doi: 10.1016/j.vaccine.2008.01.040. [DOI] [PubMed] [Google Scholar]
- 70.Jamieson DJ, Honein MA, Rasmussen SA, Williams JL, Swerdlow DL, Biggerstaff MS, et al. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet. 2009;374:451–8. doi: 10.1016/S0140-6736(09)61304-0. [DOI] [PubMed] [Google Scholar]
- 71.Louie JK, Acosta M, Jamieson DJ, Honein MA. Severe 2009 H1N1 influenza in pregnant and postpartum women in California. N Engl J Med. 2010;362:27–35. doi: 10.1056/NEJMoa0910444. [DOI] [PubMed] [Google Scholar]
- 72.Ahluwalia IB, Harrison L, Ding H, Austin T, D'Angelo D, Hastings P, et al. Influenza vaccination coverage among pregnant women—29 states and New York City, 2009–10 season. MMWR Morb Mortal Wkly Rep. 2012;61(7):113–8. [PubMed] [Google Scholar]
- 73.Bridges CB. Preliminary results Tdap vaccination coverage among pregnant women. National Vaccine Advisory Committee Meeting; September 2013; Washington. Also available from: URL: http://www.hhs.gov/nvpo/nvac/meetings/pastmeetings/2013/tdap_vaccination_women_coverage_sept2013.pdf [cited 2014 Jan 12] [Google Scholar]
- 74.Terranella A, Asay GR, Messonnier ML, Clark TA, Liang JL. Pregnancy dose Tdap and postpartum cocooning to prevent infant pertussis: a decision analysis. Pediatrics. 2013;131:e1748–56. doi: 10.1542/peds.2012-3144. [DOI] [PubMed] [Google Scholar]
- 75.Walsh JM, McPhee SJ. A systems model of clinical preventive care: an analysis of factors influencing patient and physician. Health Educ Q. 1992;19:157–75. doi: 10.1177/109019819201900202. [DOI] [PubMed] [Google Scholar]
- 76.Henninger M, Naleway A, Crane B, Donahue J, Irving S. Predictors of seasonal influenza vaccination during pregnancy. Obstet Gynecol. 2013;121:741–9. doi: 10.1097/AOG.0b013e3182878a5a. [DOI] [PubMed] [Google Scholar]
- 77.Eppes C, Wu A, You W, Cameron KA, Garcia P, Grobman W. Barriers to influenza vaccination among pregnant women. Vaccine. 2013;31:2874–8. doi: 10.1016/j.vaccine.2013.04.031. [DOI] [PubMed] [Google Scholar]
- 78.Silverman NS, Greif A. Influenza vaccination during pregnancy. Patients' and physicians' attitudes. J Reprod Med. 2001;46:989–94. [PubMed] [Google Scholar]
- 79.Drees M, Johnson O, Wong E, Stewart A, Ferisin S, Silverman PR, et al. Acceptance of 2009 H1N1 influenza vaccine among pregnant women in Delaware. Am J Perinatol. 2012;29:289–94. doi: 10.1055/s-0031-1295660. [DOI] [PubMed] [Google Scholar]
- 80.Ahluwalia IB, Jamieson DJ, Rasmussen SA, D'Angelo D, Goodman D, Kim H. Correlates of seasonal influenza vaccine coverage among pregnant women in Georgia and Rhode Island. Obstet Gynecol. 2010;116:949–55. doi: 10.1097/AOG.0b013e3181f1039f. [DOI] [PubMed] [Google Scholar]
- 81.Yudin MH, Salaripour M, Sgro MD. Pregnant women's knowledge of influenza and the use and safety of the influenza vaccine during pregnancy. J Obstet Gynaecol Can. 2009;31:120–5. doi: 10.1016/s1701-2163(16)34095-6. [DOI] [PubMed] [Google Scholar]
- 82.Moniz MH, Vitek WS, Akers A, Meyn LA, Beigi RH. Perceptions and acceptance of immunization during pregnancy. J Reprod Med. 2013;58:383–8. [PubMed] [Google Scholar]
- 83.Lyerly AD, Mitchell LM, Armstrong EM, Harris LH, Kukla R, Kuppermann M, et al. Risks, values, and decision making surrounding pregnancy. Obstet Gynecol. 2007;109:979–84. doi: 10.1097/01.AOG.0000258285.43499.4b. [DOI] [PubMed] [Google Scholar]
- 84.Meharry PM, Colson ER, Grizas AP, Stiller R, Vázquez M. Reasons why women accept or reject the trivalent inactivated influenza vaccine (TIV) during pregnancy. Matern Child Health J. 2013;17:156–64. doi: 10.1007/s10995-012-0957-3. [DOI] [PubMed] [Google Scholar]
- 85.Liu N, Sprague AE, Yasseen AS, III, Fell DB, Wen SW, Smith GN, et al. Vaccination patterns in pregnant women during the 2009 H1N1 influenza pandemic: a population-based study in Ontario, Canada. Can J Public Health. 2012;103:e353–8. doi: 10.1007/BF03404440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Munoz FM, Greisinger AJ, Wehmanen OA, Mouzoon ME, Hoyle JC, Smith FA, et al. Safety of influenza vaccination during pregnancy. Am J Obstet Gynecol. 2005;192:1098–106. doi: 10.1016/j.ajog.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 87.Vitek WS, Akers A, Meyn LA, Switzer GE, Lee BY, Beigi RH. Vaccine eligibility and acceptance among ambulatory obstetric and gynecologic patients. Vaccine. 2011;29:2024–8. doi: 10.1016/j.vaccine.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 88.Mouzoon ME, Munoz FM, Greisinger AJ, Brehm BJ, Wehmanen OA, Smith FA, et al. Improving influenza immunization in pregnant women and healthcare workers. Am J Manag Care. 2010;16:209–16. [PubMed] [Google Scholar]
- 89.Department of Health and Human Services (US) The Affordable Care Act and immunization [cited 2014 Jan 12] Available from: URL: http://www.hhs.gov/healthcare/facts/factsheets/2010/09/The-Affordable-Care-Act-and-Immunization.html.
- 90. Pub. L. No. 111-148, Stat. 119 (2009)
- 91.Stewart AM, Lindley MC, Chang KHM, Cox MA. Vaccination benefits and cost-sharing policy for non-institutionalized adult Medicaid enrollees in the United States. Vaccine. 2014;32:618–23. doi: 10.1016/j.vaccine.2013.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Maher L, Hope K, Torvaldsen S, Lawrence G, Dawson A, Wiley K, et al. Influenza vaccination during pregnancy: coverage rates and influencing factors in two urban districts in Sydney. Vaccine. 2014;31:5557–64. doi: 10.1016/j.vaccine.2013.08.081. [DOI] [PubMed] [Google Scholar]
- 93.Beel ER, Rench MA, Montesinos DP, Mayes B, Healy CM. Knowledge and attitudes of postpartum women toward immunization during pregnancy and the peripartum period. Hum Vaccin Immunother. 2013;9:1926–31. doi: 10.4161/hv.25096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wiley KE, Massey PD, Cooper SC, Wood N, Quinn HE, Leask J. Pregnant women's intention to take up a post-partum pertussis vaccine, and their willingness to take up the vaccine while pregnant: a cross sectional survey. Vaccine. 2013;31:3972–8. doi: 10.1016/j.vaccine.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 95.Schrag SJ, Fiore AE, Gonik B, Malik T, Reef S, Singleton JA, et al. Vaccination and perinatal infection prevention practices among obstetrician-gynecologists. Obstet Gynecol. 2003;101:704–10. doi: 10.1016/s0029-7844(03)00010-3. [DOI] [PubMed] [Google Scholar]
- 96.Beigi RH, Switzer GE, Meyn LA. Acceptance of a pandemic avian influenza vaccine in pregnancy. J Reprod Med. 2009;54:341–6. [PubMed] [Google Scholar]
- 97.Tong A, Biringer A, Ofner-Agostini M, Upshur R, McGeer A. A cross-sectional study of maternity care providers' and women's knowledge, attitudes, and behaviours towards influenza vaccination during pregnancy. J Obstet Gynaecol Can. 2008;30:404–10. doi: 10.1016/s1701-2163(16)32825-0. [DOI] [PubMed] [Google Scholar]
- 98.Power ML, Leddy MA, Anderson BL, Gall SA, Gonik B, Schulkin J. Obstetrician-gynecologists' practices and perceived knowledge regarding immunization. Am J Prev Med. 2009;37:231–4. doi: 10.1016/j.amepre.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 99.Gonik B, Jones T, Contreras D, Fasano N, Roberts C. The obstetrician-gynecologist's role in vaccine-preventable diseases and immunization. Obstet Gynecol. 2000;96:81–4. doi: 10.1016/s0029-7844(00)00860-7. [DOI] [PubMed] [Google Scholar]
- 100.Wu P, Griffin MR, Richardson A, Gabbe SG, Gambrell MA, Hartert TV. Influenza vaccination during pregnancy: opinions and practices of obstetricians in an urban community. South Med J. 2006;99:823–8. doi: 10.1097/01.smj.0000231262.88558.8e. [DOI] [PubMed] [Google Scholar]
- 101.Bednarczyk RA, Adjaye-Gbewonyo D, Omer SB. Safety of influenza immunization during pregnancy for the fetus and the neonate. Am J Obstet Gynecol. 2012;207(3 Suppl):S38–46. doi: 10.1016/j.ajog.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 102.Kissin DM, Power ML, Kahn EB, Williams JL, Jamieson DJ, MacFarlane K, et al. Attitudes and practices of obstetrician-gynecologists regarding influenza vaccination in pregnancy. Obstet Gynecol. 2011;118:1074–80. doi: 10.1097/AOG.0b013e3182329681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wallis DH, Chin JL, Sur DK. Influenza vaccination in pregnancy: current practices in a suburban community. J Am Board Fam Pract. 2004;17:287–91. doi: 10.3122/jabfm.17.4.287. [DOI] [PubMed] [Google Scholar]
- 104.Panda B, Stiller R, Panda A. Influenza vaccination during pregnancy and factors for lacking compliance with current CDC guidelines. J Matern Neonatal Med. 2011;24:402–6. doi: 10.3109/14767058.2010.497882. [DOI] [PubMed] [Google Scholar]
- 105.Freed GL, Cowan AE, Clark SJ. Primary care physician perspectives on reimbursement for childhood immunizations. Pediatrics. 2008;122:1319–24. doi: 10.1542/peds.2008-2033. [DOI] [PubMed] [Google Scholar]
- 106.Al-Safi ZA, Shavell VI, Gonik B. Vaccination in pregnancy. Womens Health (Lond Engl) 2011;7:109–19. doi: 10.2217/whe.10.86. [DOI] [PubMed] [Google Scholar]
- 107. 42 U.S.C. Ch. 6A, Subchapter XIX (1994)
- 108. 42 U.S.C. §300aa-10 et seq.
- 109. 42 U.S.C. § 300aa-11(a)(2)(A)
- 110.Johnson DR, Nichol KL, Lipczynski K. Barriers to adult immunization. Am J Med. 2008;121(7 Suppl 2):S28–35. doi: 10.1016/j.amjmed.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 111.Leddy MA, Gonik B, Gall SA, Anderson BA, Schulkin J. Changes in immunization practices, knowledge and beliefs of Michigan obstetrician-gynecologists since 2000. Michigan J Public Health. 2009;3:20–32. [Google Scholar]
- 112.Centers for Disease Control and Prevention (US), Advisory Committee on Immunization Practices Workgroup on the Use of Vaccines During Pregnancy and Breastfeeding. Guiding principles for development of ACIP recommendations for vaccination during pregnancy and breastfeeding. 2008. [cited 2013 Nov 16]. Available from: URL: http://www.cdc.gov/vaccines/acip/committee/downloads/preg-principles-2008.pdf.
- 113.National Foundation for Infectious Diseases. 2013 NFID influenza/pneumococcal news conference. 2013. Sep 26, [cited 2013 Nov 16]. Available from: URL: http://www.adultvaccination.org/newsroom/events/2013-news-conference.
- 114.Frieden TF, Heim LJ, Joseph GF, Jr, Rohack JJ. Dear colleague. 2009. [cited 2013 Nov 15]. Available from: URL: http://www.cdc.gov/h1n1flu/clinicians/pdf/Dear_Colleague_final.pdf.
- 115.Centers for Disease Control and Prevention (US) Dear colleague letter. 2011. [cited 2013 Nov 15]. Available from: URL: http://www.cdc.gov/flu/pdf/nivw/influenza-pregnancy-letter.pdf.
- 116.Lipkus IM. Numeric, verbal, and visual formats of conveying health risks: suggested best practices and future recommendations. Med Decis Making. 2007;27:696–713. doi: 10.1177/0272989X07307271. [DOI] [PubMed] [Google Scholar]
- 117.Lynch MM, Mitchell EW, Williams JL, Brumbaugh K, Jones-Bell M, Pinkney DE, et al. Pregnant and recently pregnant women's perceptions about influenza A pandemic (H1N1) 2009: implications for public health and provider communication. Matern Child Health J. 2012;16:1657–64. doi: 10.1007/s10995-011-0865-y. [DOI] [PubMed] [Google Scholar]
- 118.Meharry PM, Cusson RM, Stiller R, Vázquez M. Maternal influenza vaccination: evaluation of a patient-centered pamphlet designed to increase uptake in pregnancy. Matern Child Health J. 2014;18:1205–14. doi: 10.1007/s10995-013-1352-4. [DOI] [PubMed] [Google Scholar]
- 119.Betsch C, Renkewitz F, Haase N. Effect of narrative reports about vaccine adverse events and bias-awareness disclaimers on vaccine decisions: a simulation of an online patient social network. Med Decis Making. 2013;33:14–25. doi: 10.1177/0272989X12452342. [DOI] [PubMed] [Google Scholar]
- 120.Winterbottom A, Bekker HL, Conner M, Mooney A. Does narrative information bias individual's decision making? A systematic review. Soc Sci Med. 2008;67:2079–88. doi: 10.1016/j.socscimed.2008.09.037. [DOI] [PubMed] [Google Scholar]
- 121.Fagerlin A, Zikmund-Fisher BJ, Ubel PA. Helping patients decide: ten steps to better risk communication. J Natl Cancer Inst. 2011;103:1436–43. doi: 10.1093/jnci/djr318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Centers for Disease Control and Prevention (US) Seasonal influenza (flu)—un amor perdido (a lost love) 2013. [cited 2013 Nov 15]. Available from: URL: http://www.cdc.gov/flu/freeresources/video/un-amor-perdido.htm.
- 123.Declercq ER, Sakala C, Corry MP, Applebaum S. New York: Childbirth Connection; 2006. Listening to Mothers II pregnancy and birth: report of the second national U.S. survey of women's childbearing experiences. Also available from: URL: https://www.childbirthconnection.org/pdfs/LTMII_report.pdf [cited 2013 Nov 17] [Google Scholar]
- 124.Declercq ER, Sakala C, Corry MP, Applebaum S, Herrlich A. Listening to Mothers III pregnancy and birth. New York: Childbirth Connection; 2013. Also available from: URL: http://transform.childbirthconnection.org/wp-content/uploads/2013/06/LTM-III_Pregnancy-and-Birth.pdf [cited 2013 Nov 17] [Google Scholar]
- 125.Romano AM. A changing landscape: implications of pregnant women's Internet use for childbirth educators. J Perinat Educ. 2007;16:18–24. doi: 10.1624/105812407X244903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Smith A. Americans and text messaging. Washington: PewResearch Internet Project; 2011. Also available from: URL: http://pewinternet.org/Reports/2011/Cell-Phone-Texting-2011.aspx [cited 2013 Nov 17] [Google Scholar]
- 127.Blumberg SJ, Luke JV. Rockville (MD): National Center for Health Statistics; 2012. Wireless substitution: early release of estimates from the National Health Interview Survey, January–June 2012. Also available from: URL: http://www.cdc.gov/nchs/data/nhis/earlyrelease/wireless201212.pdf [cited 2013 Nov 17] [Google Scholar]
- 128.Stockwell MS, Fiks AG. Utilizing health information technology to improve vaccine communication and coverage. Hum Vaccin Immunother. 2013;9:1802–11. doi: 10.4161/hv.25031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.National Healthy Mothers Healthy Babies Coalition. Text4baby [cited 2014 Jun 1] Available from: URL: https://www.text4baby.org.
- 130.Evans WD, Wallace JL, Snider J. Pilot evaluation of the Text4baby mobile health program. BMC Public Health. 2012;12:1031. doi: 10.1186/1471-2458-12-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hoff A, Nunez-Alvarez A, Martinez KM, Lacoursiere DY. Maternal and newborn health: Text4baby San Diego. Evaluation overview: October 2011–October 2012. 2012 [cited 2013 Nov 17] Available from: URL: https://www.csusm.edu/nlrc/documents/report_archives/Text4Baby_SanDiego_Evaluation_Overview.pdf.
- 132.Moniz MH, Hasley S, Meyn LA, Beigi RH. Improving influenza vaccination rates in pregnancy through text messaging: a randomized controlled trial. Obstet Gynecol. 2013;121:734–40. doi: 10.1097/AOG.0b013e31828642b1. [DOI] [PubMed] [Google Scholar]
- 133.Community Preventive Services Task Force. Increasing appropriate vaccination: client reminder and recall systems. 2008. [cited 2013 Nov 16]. Available from: URL: http://www.thecommunityguide.org/vaccines/clientreminder.html.
- 134.Department of Health and Human Services (US), National Vaccine Advisory Committee. White paper on the United Stated vaccine safety system: final. Washington: Department of Health and Human Services (US); 2011. Also available from: URL: http://www.hhs.gov/nvpo/nvac/nvac_vswp.pdf [cited 2013 May 19] [Google Scholar]
- 135.Department of Health and Human Services (US) Vaccine Adverse Event Reporting System: frequently asked questions [cited 2014 May 12] Available from: URL: http://vaers.hhs.gov/about/faqs#who_reports.
- 136.Varricchio F, Iskander J, Destefano F, Ball R, Pless R, Braun MM, et al. Understanding vaccine safety information from the Vaccine Adverse Event Reporting System. Pediatr Infect Dis J. 2004;23:287–94. doi: 10.1097/00006454-200404000-00002. [DOI] [PubMed] [Google Scholar]
- 137.Moro PL, Broder K, Zheteyeva Y, Walton K, Rohan P, Sutherland A, et al. Adverse events in pregnant women following administration of trivalent inactivated influenza vaccine and live attenuated influenza vaccine in the Vaccine Adverse Event Reporting System, 1990–2009. Am J Obstet Gynecol. 2011;204:146.e1–7. doi: 10.1016/j.ajog.2010.08.050. [DOI] [PubMed] [Google Scholar]
- 138.Moro PL, Broder K, Zheteyeva Y, Revzina N, Tepper N, Kissin D, et al. Adverse events following administration to pregnant women of influenza A (H1N1) 2009 monovalent vaccine reported to the Vaccine Adverse Event Reporting System. Am J Obstet Gynecol. 2011;205:473.e1–9. doi: 10.1016/j.ajog.2011.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zheteyeva Y, Moro PL, Yue X, Broder K. Safety of meningococcal polysaccharide-protein conjugate vaccine in pregnancy: a review of the Vaccine Adverse Event Reporting System. Am J Obstet Gynecol. 2013;208:478.e1–6. doi: 10.1016/j.ajog.2013.02.027. [DOI] [PubMed] [Google Scholar]
- 140.Naleway AL, Gold R, Kurosky S, Riedlinger K, Henninger ML, Nordin JD, et al. Identifying pregnancy episodes, outcomes, and mother-infant pairs in the Vaccine Safety Datalink. Vaccine. 2013;31:2898–903. doi: 10.1016/j.vaccine.2013.03.069. [DOI] [PubMed] [Google Scholar]
- 141.Food and Drug Administration (US) Safety: FDA's sentinel initiative [cited 2013 Nov 24] Available from: URL: http://www.fda.gov/Safety/FDAsSentinelinitiative/ucm2007250.htm.
- 142.Nguyen M, Ball R, Midthun K, Lieu TA. The Food and Drug Administration's Post-Licensure Rapid Immunization Safety Monitoring program: strengthening the federal vaccine safety enterprise. Pharmacoepidemiol Drug Saf. 2012;21(Suppl 1):291–7. doi: 10.1002/pds.2323. [DOI] [PubMed] [Google Scholar]
- 143.Mini-Sentinel. Post-Licensure Rapid Immunization Safety Monitoring (PRISM). 2012 [cited 2013 Nov 24] Available from: URL: http://mini-sentinel.org/work_products/PRISM/PRISM_Summary.pdf.
- 144.Mini-Sentinel. Welcome to Mini-Sentinel [cited 2014 Jul 8] Available from: URL: www.mini-sentinel.org.
- 145.Eckert LO, Anderson BL, Gonik B, Schulkin J. Reporting vaccine complications: what do obstetricians and gynecologists know about the Vaccine Adverse Event Reporting System? Infect Dis Obstet Gynecol. 2013;2013:285257. doi: 10.1155/2013/285257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.American College of Obstetricians and Gynecologists. ACOG committee opinion no. 558: integrating immunizations into practice. Obstet Gynecol. 2013;121:897–903. doi: 10.1097/01.AOG.0000428788.74725.90. [DOI] [PubMed] [Google Scholar]
- 147.Department of Health and Human Services (US), National Vaccine Advisory Committee. Recommendations from the National Vaccine Advisory Committee: standards for adult immunization practice. Public Health Rep. 2014;129:115–23. doi: 10.1177/003335491412900203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Coleman VH, Laube DW, Hale RW, Williams SB, Power ML, Schulkin J. Obstetrician-gynecologists and primary care: training during obstetrics-gynecology residency and current practice patterns. Acad Med. 2007;82:602–7. doi: 10.1097/ACM.0b013e3180556885. [DOI] [PubMed] [Google Scholar]
- 149.Leddy MA, Anderson BL, Power ML, Gall S, Gonik B, Schulkin J. Changes in and current status of obstetrician-gynecologists' knowledge, attitudes, and practice regarding immunization. Obstet Gynecol Surv. 2009;64:823–9. doi: 10.1097/OGX.0b013e3181c4bbb7. [DOI] [PubMed] [Google Scholar]
- 150.Ishola DA, Jr, Permalloo N, Cordery RJ, Anderson SR. Midwives' influenza vaccine uptake and their views on vaccination of pregnant women. J Public Health (Oxf) 2013;35:570–7. doi: 10.1093/pubmed/fds109. [DOI] [PubMed] [Google Scholar]
- 151.Centers for Disease Control and Prevention (US) Vaccines and immunizations: education & training for health professionals [cited 2014 Jul 8] Available from: URL: http://www.cdc.gov/vaccines/ed/default.htm.
- 152.Immunization Action Coalition. Immunization Action Coalition [cited 2014 Jul 8] Available from: URL: www.immunize.org.
- 153.Zimmerman RK, Barker WH, Strikas RA, Ahwesh ER, Mieczkowski TA, Janosky JE, et al. Developing curricula to promote preventive medicine skills. JAMA. 1997;278:705–11. [PubMed] [Google Scholar]
- 154.EPIC Program. EPIC® Educating Physicians In their Communities [cited 2014 Jul 8] Available from: URL: www.gaepic.org.
- 155.Briss PA, Rodewald LE, Hinman AR, Shefer AM, Strikas RA, Bernier RR, et al. Reviews of evidence regarding interventions to improve vaccination coverage in children, adolescents, and adults. The Task Force on Community Preventive Services. Am J Prev Med. 2000;18(1 Suppl):97–140. doi: 10.1016/s0749-3797(99)00118-x. [DOI] [PubMed] [Google Scholar]
- 156.Yeh S, Mink C, Kim M, Naylor S, Zangwill KM, Allred NJ. Effectiveness of hospital-based postpartum procedures on pertussis vaccination among postpartum women. Am J Obstet Gynecol. 2014;210:237.e1–6. doi: 10.1016/j.ajog.2013.09.043. [DOI] [PubMed] [Google Scholar]
- 157.Klatt TE, Hopp E. Effect of a best-practice alert on the rate of influenza vaccination of pregnant women. Obstet Gynecol. 2012;119(2 Pt 1):301–5. doi: 10.1097/AOG.0b013e318242032a. [DOI] [PubMed] [Google Scholar]
- 158.Trick WE, Linn ES, Jones Z, Caquelin C, Kee R, Morita JY. Using computer decision support to increase maternal postpartum tetanus, diphtheria, and acellular pertussis vaccination. Obstet Gynecol. 2010;116:51–7. doi: 10.1097/AOG.0b013e3181e40a9f. [DOI] [PubMed] [Google Scholar]
- 159.Shavell VI, Moniz MH, Gonik B, Beigi RH. Influenza immunization in pregnancy: overcoming patient and health care provider barriers. Am J Obstet Gynecol. 2012;207(3 Suppl):S67–74. doi: 10.1016/j.ajog.2012.06.077. [DOI] [PubMed] [Google Scholar]
- 160.Community Preventive Services Task Force. Recommendations regarding interventions to improve vaccination coverage in children, adolescents, and adults. Task Force on Community Preventive Services. Am J Prev Med. 2000;18(1 Suppl):92–6. [PubMed] [Google Scholar]
- 161.Centers for Disease Control and Prevention (US) Recommendations of the Advisory Committee on Immunization Practices: programmatic strategies to increase vaccination rates—assessment and feedback of provider-based vaccination coverage information. MMWR Morb Mortal Wkly Rep. 1996;45(10):219–20. [PubMed] [Google Scholar]
- 162.Dini EF, Chaney M, Moolenaar RL, LeBaron CW. Information as intervention: how Georgia used vaccination coverage data to double public sector vaccination coverage in seven years. J Public Health Manag Pract. 1996;2:45–9. doi: 10.1097/00124784-199600210-00008. [DOI] [PubMed] [Google Scholar]
- 163.Centers for Disease Control and Prevention (US) AFIX (Assessment, Feedback, Incentives, and eXchange) [cited 2014 Jan 5] Available from: URL: http://www.cdc.gov/vaccines/programs/afix/index.html.
- 164.LeBaron CW, Mercer JT, Massoudi MS, Dini E, Stevenson J, Fischer WM, et al. Changes in clinic vaccination coverage after institution of measurement and feedback in 4 states and 2 cities. Arch Pediatr Adolesc Med. 1999;153:879–86. doi: 10.1001/archpedi.153.8.879. [DOI] [PubMed] [Google Scholar]
- 165.Maine Immunization Program. Augusta (ME): Maine Center for Disease Control and Prevention; 2011. Evaluation of AFIX process—using a quality improvement workplan to improve delivery service and immunization rates. Also available from: URL: http://www.maine.gov/dhhs/mecdc/infectious-disease/immunization/documents/2010-qi-evaluation-report.pdf [cited 2014 Jan 5] [Google Scholar]
- 166.Highmark. Highmark. [cited 2014 Jul 8] Available from: URL: www.highmark.com.
- 167.America's Health Insurance Plans. Highmark—quality blue physician program [cited 2014 Jan 5] Available from: URL: http://www.ahip.org/HighmarkQualityBlueProgram.
- 168.Department of Health and Human Services (US), National Vaccine Advisory Committee. A pathway to leadership for adult immunization: recommendations of the National Vaccine Advisory Committee. Public Health Rep. 2012;127(Suppl 1):1–42. doi: 10.1177/00333549121270s101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Pickering LK, Baker CJ, Freed GL, Gall SA, Grogg SE, Poland GA, et al. Immunization programs for infants, children, adolescents, and adults: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49:817–40. doi: 10.1086/605430. [DOI] [PubMed] [Google Scholar]
- 170.National Committee for Quality Assurance. National Committee for Quality Assurance [cited 2014 Jul 8] Available from: URL: www.ncqa.org.
- 171.National Quality Forum. National Quality Forum [cited 2014 Jul 8] Available from: URL: www.qualityforum.org.
- 172.Roberts S, Hollier LM, Sheffield J, Laibl V, Wendel GD., Jr Cost-effectiveness of universal influenza vaccination in a pregnant population. Obstet Gynecol. 2006;107:1323–9. doi: 10.1097/01.AOG.0000210225.45986.99. [DOI] [PubMed] [Google Scholar]
- 173.Beigi RH, Wiringa AE, Bailey RR, Assi TM, Lee BY. Economic value of seasonal and pandemic influenza vaccination during pregnancy. Clin Infect Dis. 2009;49:1784–92. doi: 10.1086/649013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Westra TA, de Vries R, Tamminga JJ, Sauboin CJ, Postma MJ. Cost-effectiveness analysis of various pertussis vaccination strategies primarily aimed at protecting infants in the Netherlands. Clin Ther. 2010;32:1479–95. doi: 10.1016/j.clinthera.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 175.Shen AK, Sobzcyk E, Buchanan A, Wu L, Duggan-Goldstein S. Second National Immunization Congress 2010: addressing vaccine financing for the future in the US. Hum Vaccin. 2011;7:12–8. doi: 10.4161/hv.7.1.13949. [DOI] [PubMed] [Google Scholar]
- 176.Freed GL, Clark SJ, Cowan AE, Coleman MS. Primary care physician perspectives on providing adult vaccines. Vaccine. 2011;29:1850–4. doi: 10.1016/j.vaccine.2010.12.097. [DOI] [PubMed] [Google Scholar]
- 177.Harris KM, Uscher-Pines L, Mattke S, Kellerman AL. A blueprint for improving the promotion and delivery of adult vaccination in the United States. Arlington (VA): RAND Corp.; 2012. [PMC free article] [PubMed] [Google Scholar]
- 178.Young JL, Bernheim RG, Korte JE, Stoler MH, Guterbock TM, Rice LW. Human papillomavirus vaccination recommendation may be linked to reimbursement: a survey of Virginia family practitioners and gynecologists. J Pediatr Adolesc Gynecol. 2011;24:380–5. doi: 10.1016/j.jpag.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 179.America's Health Insurance Plans. AHIP vaccines and immunizations roundtable report. Washington: America's Health Insurance Plans; 2009. Also available from: URL: http://www.ahip.org/vaccine-financing [cited 2013 Dec 4] [Google Scholar]
- 180.Freed GL, Cowan AE, Gregory S, Clark SJ. Variation in provider vaccine purchase prices and payer reimbursement. Pediatrics. 2009;124(Suppl 5):S459–65. doi: 10.1542/peds.2009-1542E. [DOI] [PubMed] [Google Scholar]
- 181.Berman S. Is our vaccine system at risk for a future financial “meltdown?”. Pediatrics. 2008;122:1372–3. doi: 10.1542/peds.2008-2881. [DOI] [PubMed] [Google Scholar]
- 182.Coleman MS, Fontanesi J, Meltzer MI, Shefer A, Fishbein DB, Bennett NM, et al. Estimating medical practice expenses from administering adult influenza vaccinations. Vaccine. 2005;23:915–23. doi: 10.1016/j.vaccine.2004.07.028. [DOI] [PubMed] [Google Scholar]
- 183.McInerny TK, Cull WL, Yudkowsky BK. Physician reimbursement levels and adherence to American Academy of Pediatrics well-visit and immunization recommendations. Pediatrics. 2005;115:833–8. doi: 10.1542/peds.2004-1510. [DOI] [PubMed] [Google Scholar]
- 184.Yoo B-K, Berry A, Kasajima M, Szilagyi PG. Association between Medicaid reimbursement and child influenza vaccination rates. Pediatrics. 2010;126:e998–1010. doi: 10.1542/peds.2009-3514. [DOI] [PubMed] [Google Scholar]
- 185.Centers for Medicare & Medicaid Services (US) Affordable Care Act, provisions: provider payments [cited 2013 Dec 15] Available from: URL: http://www.medicaid.gov/AffordableCareAct/Provisions/Provider-Payments.html.
- 186.Association of State and Territorial Health Officials. Increase in vaccine administration rates—a summary of state stakeholder meetings. Arlington (VA): ASTHO; 2012. Also available from: URL: http://www.astho.org/programs/immunization/increase-in-vaccine-administration-rates [cited 2013 Dec 15] [Google Scholar]
- 187.Shen AK, Hunsaker J, Gazmararian JA, Lindley MC, Birkhead GS. Role of health insurance in financing vaccinations for children and adolescents in the United States. Pediatrics. 2009;124(Suppl):S522–31. doi: 10.1542/peds.2009-1542L. [DOI] [PubMed] [Google Scholar]
- 188.Glazner JE, Beaty B, Berman S. Cost of vaccine administration among pediatric practices. Pediatrics. 2009;124(Suppl):S492–8. doi: 10.1542/peds.2009-1542H. [DOI] [PubMed] [Google Scholar]
- 189.Coleman MS, Lindley MC, Ekong J, Rodewald L. Net financial gain or loss from vaccination in pediatric medical practices. Pediatrics. 2009;124(Suppl 5):S472–91. doi: 10.1542/peds.2009-1542G. [DOI] [PubMed] [Google Scholar]
- 190.Hunsaker J, Veselovskiy G, Gazmararian JA. Health insurance plans and immunization: assessment of practices and policies, 2005–2008. Pediatrics. 2009;124(Suppl 5):S532–9. doi: 10.1542/peds.2009-1542M. [DOI] [PubMed] [Google Scholar]
- 191.Sobczyk E. Vaccine finance resources for physicians. Pediatrics. 2009;124(Suppl 5):S573–6. doi: 10.1542/peds.2009-1542V. [DOI] [PubMed] [Google Scholar]
- 192.American College of Obstetricians and Gynecologists. Immunization resources for obstetrician-gynecologists—a comprehensive tool kit. Washington: American College of Obstetricians and Gynecologists; 2013. Also available from: URL: https://www.acog.org/∼/media/Departments/Immunization/ImmunizationToolkit.pdf [cited 2014 May 11] [Google Scholar]
- 193.HealthIT.gov. Health IT legislation: HITECH Act [cited 2013 Nov 24] Available from: URL: http://www.healthit.gov/policy-researchers-implementers/hitech-act-0.
- 194.Buntin MB, Jain SH, Blumenthal D. Health information technology: laying the infrastructure for national health reform. Health Aff (Millwood) 2010;29:1214–9. doi: 10.1377/hlthaff.2010.0503. [DOI] [PubMed] [Google Scholar]
- 195.Hardelid P, Fleming DM, Andrews N, Barley M, Durnall H, Mangtani P, et al. Effectiveness of trivalent and pandemic influenza vaccines in England and Wales 2008–2010: results from a cohort study in general practice. Vaccine. 2012;30:1371–8. doi: 10.1016/j.vaccine.2011.12.038. [DOI] [PubMed] [Google Scholar]
- 196.Kharbanda EO, Parker ED, Nordin JD, Hedblom BD, Rolnick SJ. Influenza and pertussis vaccination coverage among privately insured women of reproductive age. Matern Child Health J. 2013;17:1631–7. doi: 10.1007/s10995-012-1176-7. [DOI] [PubMed] [Google Scholar]
- 197.HealthIT.gov. EHR incentives and certification: how to attain meaningful use [cited 2014 Jan 12] Available from: URL: http://www.healthit.gov/providers-professionals/how-attain-meaningful-use.
- 198.Centers for Disease Control and Prevention (US) Immunization Information Systems (IIS): 2012 IISAR data participation rates [cited 2013 Nov 26] Available from: URL: http://www.cdc.gov/vaccines/programs/iis/annual-report-IISAR/2012-data.html#adult.
- 199.S&I Framework. Query Health [cited 2014 Jul 8] Available from: URL: http://wiki.siframework.org/Query+Health.
- 200.Hazlehurst B, Naleway A, Mullooly J. Detecting possible vaccine adverse events in clinical notes of the electronic medical record. Vaccine. 2009;27:2077–83. doi: 10.1016/j.vaccine.2009.01.105. [DOI] [PubMed] [Google Scholar]
- 201.Hinrichsen VL, Kruskal B, O'Brien MA, Lieu TA, Platt R. Using electronic medical records to enhance detection and reporting of vaccine adverse events. J Am Med Informatics Assoc. 2007;14:731–5. doi: 10.1197/jamia.M2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Jacobs AL. Liability and maternal immunization: in utero injury claims in the VICP. Am J Obstet Gynecol. 2012;207(3 Suppl):S63–6. doi: 10.1016/j.ajog.2012.06.076. [DOI] [PubMed] [Google Scholar]
- 203.Health Resources and Services Administration (US) Vaccine injury table [cited 2014 Jul 8] Available from: URL: http://www.hrsa.gov/vaccinecompensation/vaccinetable.html.
- 204. 42 U.S.C. §300aa-11(c)(1)(A)
- 205.Health Resources and Services Administration (US) Advisory Commission on Childhood Vaccines (ACCV) [cited 2014 Mar 1] Available from: URL: http://www.hrsa.gov/vaccinecompensation/commissionchildvaccines.html.


