Abstract
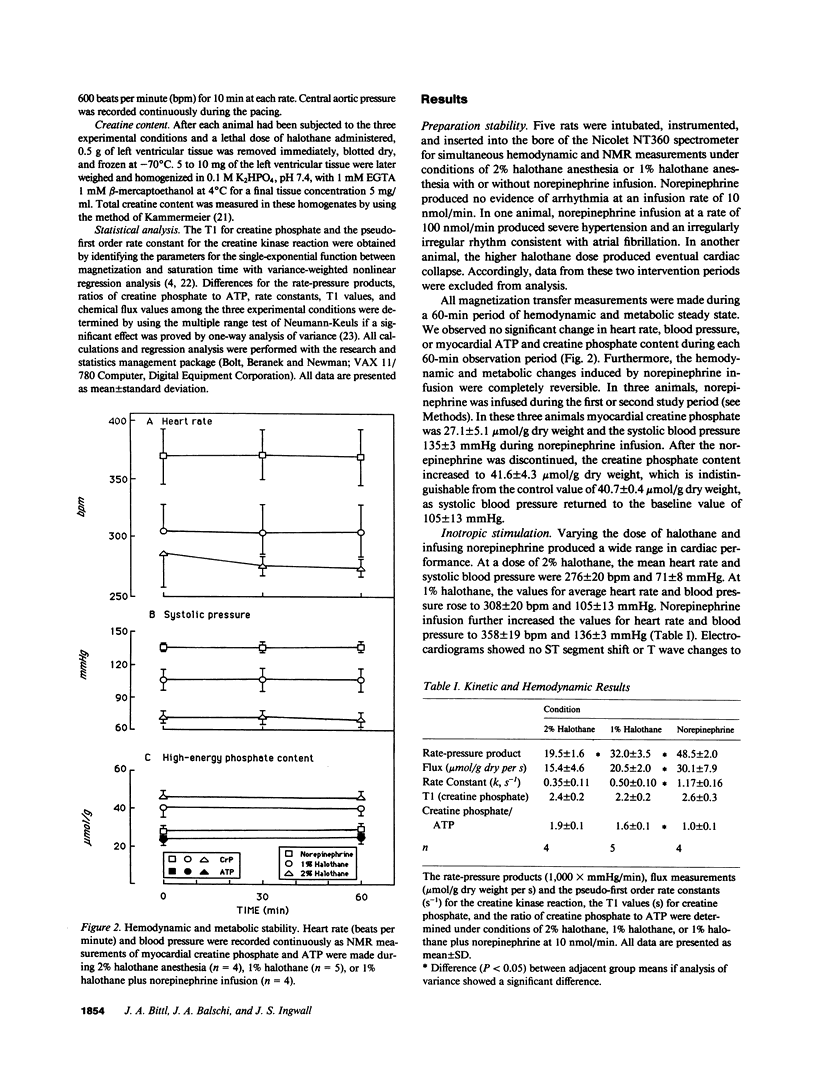
Using 31P-nuclear magnetic resonance, we studied the relationship between myocardial high-energy phosphate content and flux values for the creatine kinase reaction in the living rat under inotropic states achieved during norepinephrine infusion and halothane anesthesia. Under 2% halothane anesthesia (n = 4), 1% halothane anesthesia (n = 5) and norepinephrine infusion (n = 4), rats developed rate-pressure products of 19.5 +/- 1.6, 32.0 +/- 3.5, and 48.5 +/- 2.0 X 1,000 mmHg/min, respectively. Adenosine triphosphate content was not affected by inotropic state, ranging from 24.3 +/- 1.1 to 25.6 +/- 1.1 mumol/g dry weight, but creatine phosphate content varied inversely and reversibly with cardiac performance from 45.6 +/- 6.0 under 2% halothane to 26.0 +/- 6.5 mumol/g dry weight during norepinephrine infusion. The flux values for the creatine kinase reaction were 15.4 +/- 4.6, 20.5 +/- 2.0, and 30.1 +/- 7.9 mumol/g dry weight per s under 2% halothane, 1% halothane, and 1% halothane with norepinephrine, respectively. These results suggest that the turnover of myocardial high-energy phosphate compounds, not their tissue contents, matches cardiac performance during inotropic stimulation.
Full text
PDF

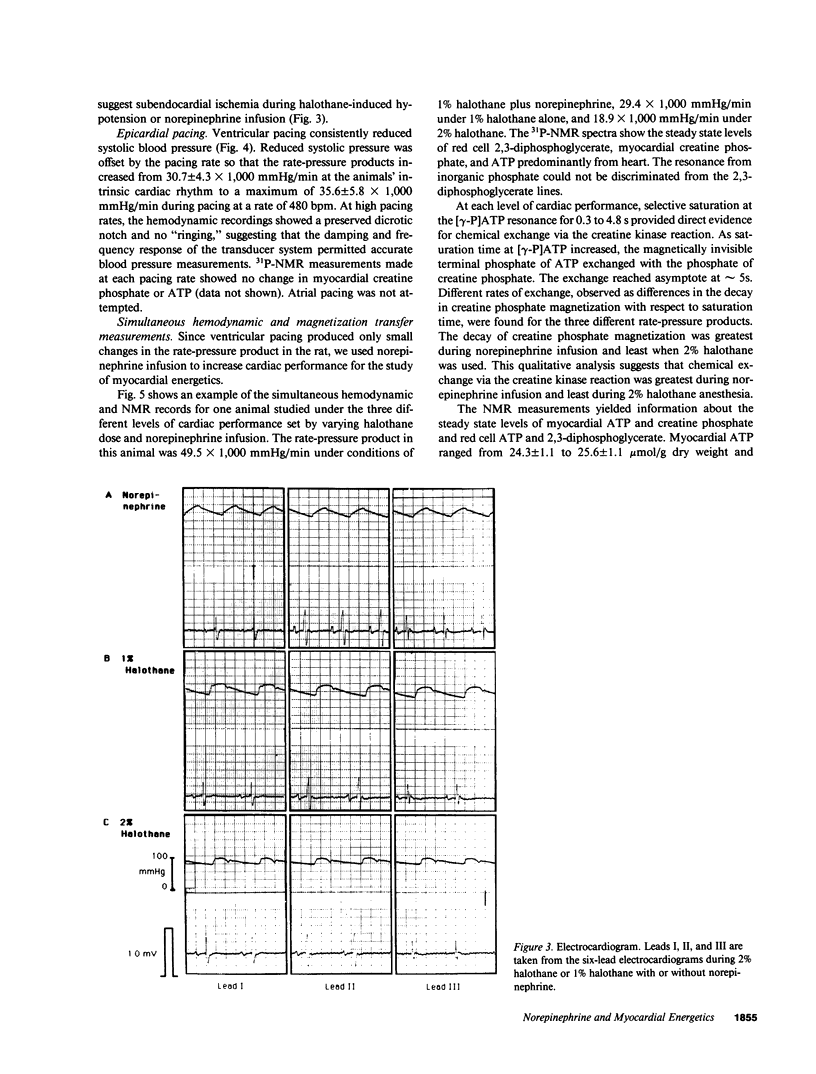



Images in this article
Selected References
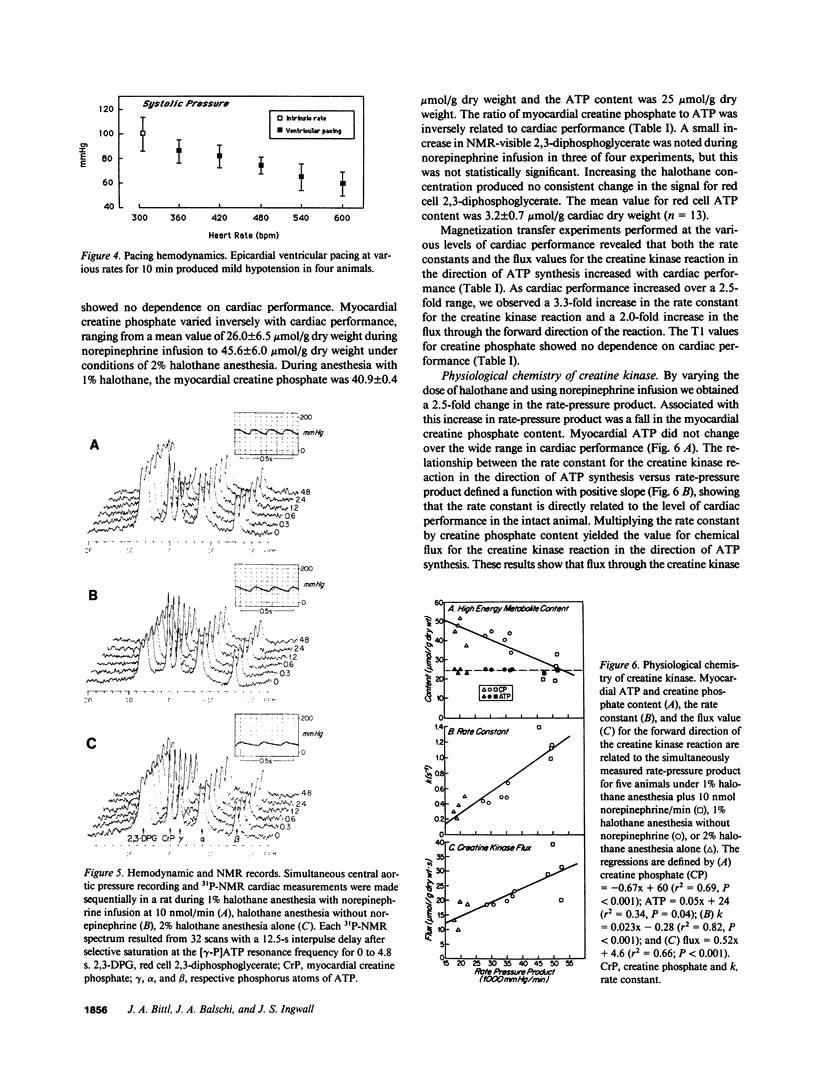
These references are in PubMed. This may not be the complete list of references from this article.
- Acad B. A., Guggenheimer E., Sonn J., Kedem J. Differential effects of various inotropic agents on the intracellular NADH redox level in the in vivo dog heart. J Cardiovasc Pharmacol. 1983 Mar-Apr;5(2):284–290. doi: 10.1097/00005344-198303000-00020. [DOI] [PubMed] [Google Scholar]
- Balaban R. S., Kantor H. L., Katz L. A., Briggs R. W. Relation between work and phosphate metabolite in the in vivo paced mammalian heart. Science. 1986 May 30;232(4754):1121–1123. doi: 10.1126/science.3704638. [DOI] [PubMed] [Google Scholar]
- Bittl J. A., Ingwall J. S. Reaction rates of creatine kinase and ATP synthesis in the isolated rat heart. A 31P NMR magnetization transfer study. J Biol Chem. 1985 Mar 25;260(6):3512–3517. [PubMed] [Google Scholar]
- Boerth R. C., Covell J. W., Seagren S. C., Pool P. E. High-energy phosphate concentrations in dog myocardium during stress. Am J Physiol. 1969 May;216(5):1103–1106. doi: 10.1152/ajplegacy.1969.216.5.1103. [DOI] [PubMed] [Google Scholar]
- Boerth R. C., Hammermeister K. E., Warbasse J. R. Comparative influence of ouabain, norepinephrine and heart rate on myocardial oxygen consumption and inotropic state in dogs. Am Heart J. 1978 Sep;96(3):355–362. doi: 10.1016/0002-8703(78)90047-9. [DOI] [PubMed] [Google Scholar]
- Brown B. R., Jr, Crout J. R. A comparative study of the effects of five general anesthetics on myocardial contractility. I. Isometric conditions. Anesthesiology. 1971 Mar;34(3):236–245. doi: 10.1097/00000542-197103000-00007. [DOI] [PubMed] [Google Scholar]
- Clancy R. L., Graham T. P., Jr, Powell W. J., Jr, Gilmore J. P. Inotropic augmentation of myocardial oxygen consumption. Am J Physiol. 1967 May;212(5):1055–1061. doi: 10.1152/ajplegacy.1967.212.5.1055. [DOI] [PubMed] [Google Scholar]
- Hoffman W. E., Seals C., Miletich D. J., Albrecht R. F. Plasma and myocardial catecholamine levels in young and aged rats during halothane anesthesia. Neurobiol Aging. 1985 Summer;6(2):117–120. doi: 10.1016/0197-4580(85)90028-4. [DOI] [PubMed] [Google Scholar]
- Ingwall J. S. Phosphorus nuclear magnetic resonance spectroscopy of cardiac and skeletal muscles. Am J Physiol. 1982 May;242(5):H729–H744. doi: 10.1152/ajpheart.1982.242.5.H729. [DOI] [PubMed] [Google Scholar]
- Jacobs H., Heldt H. W., Klingenberg M. High activity of creatine kinase in mitochondria from muscle and brain and evidence for a separate mitochondrial isoenzyme of creatine kinase. Biochem Biophys Res Commun. 1964 Aug 11;16(6):516–521. doi: 10.1016/0006-291x(64)90185-8. [DOI] [PubMed] [Google Scholar]
- Jacobus W. E., Lehninger A. L. Creatine kinase of rat heart mitochondria. Coupling of creatine phosphorylation to electron transport. J Biol Chem. 1973 Jul 10;248(13):4803–4810. [PubMed] [Google Scholar]
- Kammermeier H. Microassay of free and total creatine from tissue extracts by combination of chromatographic and fluorometric methods. Anal Biochem. 1973 Dec;56(2):341–345. doi: 10.1016/0003-2697(73)90199-1. [DOI] [PubMed] [Google Scholar]
- Kupriyanov V. V., Ya Steinschneider A., Ruuge E. K., Kapel'ko V. I., Yu Zueva M., Lakomkin V. L., Smirnov V. N., Saks V. A. Regulation of energy flux through the creatine kinase reaction in vitro and in perfused rat heart. 31P-NMR studies. Biochim Biophys Acta. 1984 Dec 11;805(4):319–331. doi: 10.1016/0167-4889(84)90014-4. [DOI] [PubMed] [Google Scholar]
- Langer G. A., Rich T. L. Augmentation of sarcolemmal Ca by anionic amphiphile: contractile response of three ventricular tissues. Am J Physiol. 1986 Feb;250(2 Pt 2):H247–H254. doi: 10.1152/ajpheart.1986.250.2.H247. [DOI] [PubMed] [Google Scholar]
- Matthews P. M., Bland J. L., Gadian D. G., Radda G. K. A 31P-NMR saturation transfer study of the regulation of creatine kinase in the rat heart. Biochim Biophys Acta. 1982 Nov 17;721(3):312–320. doi: 10.1016/0167-4889(82)90084-2. [DOI] [PubMed] [Google Scholar]
- Matthews P. M., Williams S. R., Seymour A. M., Schwartz A., Dube G., Gadian D. G., Radda G. K. A 31P-NMR study of some metabolic and functional effects of the inotropic agents epinephrine and ouabain, and the ionophore R02-2985 (X537A) in the isolated, perfused rat heart. Biochim Biophys Acta. 1982 Apr 29;720(2):163–171. doi: 10.1016/0167-4889(82)90008-8. [DOI] [PubMed] [Google Scholar]
- Ogawa S., Dreifus L. S., Shenoy P. N., Brockman S. K., Berkovits B. V. Hemodynamic consequences of atrioventricular and ventriculoatrial pacing. Pacing Clin Electrophysiol. 1978 Jan;1(1):8–15. doi: 10.1111/j.1540-8159.1978.tb03435.x. [DOI] [PubMed] [Google Scholar]
- Polimeni P. I., Page E. Chloride distribution and exchange in rat ventricle. Am J Physiol. 1980 May;238(5):C169–C176. doi: 10.1152/ajpcell.1980.238.5.C169. [DOI] [PubMed] [Google Scholar]
- SARNOFF S. J., BRAUNWALD E., WELCH G. H., Jr, CASE R. B., STAINSBY W. N., MACRUZ R. Hemodynamic determinants of oxygen consumption of the heart with special reference to the tension-time index. Am J Physiol. 1958 Jan;192(1):148–156. doi: 10.1152/ajplegacy.1957.192.1.148. [DOI] [PubMed] [Google Scholar]
- Saito D., Yasuhara K., Nishiyama O., Kusachi S., Haraoka S. Comparative effects of heart rate and aortic blood pressure on MVO in the anesthetized open-chest dog. Jpn Heart J. 1981 Sep;22(5):833–837. doi: 10.1536/ihj.22.833. [DOI] [PubMed] [Google Scholar]
- Saks V. A., Lipina N. V., Smirnov V. N., Chazov E. I. Studies of energy transport in heart cells. The functional coupling between mitochondrial creatine phosphokinase and ATP ADP translocase: kinetic evidence. Arch Biochem Biophys. 1976 Mar;173(1):34–41. doi: 10.1016/0003-9861(76)90231-9. [DOI] [PubMed] [Google Scholar]
- Sonnenblick E. H., Ross J., Jr, Covell J. W., Kaiser G. A., Braunwald E. Velocity of contraction as a determinant of myocardial oxygen consumption. Am J Physiol. 1965 Nov;209(5):919–927. doi: 10.1152/ajplegacy.1965.209.5.919. [DOI] [PubMed] [Google Scholar]
- Turner D. C., Wallimann T., Eppenberger H. M. A protein that binds specifically to the M-line of skeletal muscle is identified as the muscle form of creatine kinase. Proc Natl Acad Sci U S A. 1973 Mar;70(3):702–705. doi: 10.1073/pnas.70.3.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veech R. L., Lawson J. W., Cornell N. W., Krebs H. A. Cytosolic phosphorylation potential. J Biol Chem. 1979 Jul 25;254(14):6538–6547. [PubMed] [Google Scholar]