Abstract
Background
Liver metastases limit survival in colorectal cancer. Earlier detection of (occult) metastatic disease may benefit treatment and survival.
Objective
The objective of this article is to evaluate the potential of whole-liver CT texture analysis of apparently disease-free liver parenchyma for discriminating between colorectal cancer (CRC) patients with and without hepatic metastases.
Methods
The primary staging CT examinations of 29 CRC patients were retrospectively analysed. Patients were divided into three groups: patients without liver metastases (n = 15), with synchronous liver metastases (n = 10) and metachronous liver metastases within 18 months following primary staging (n = 4). Whole-liver texture analysis was performed by delineation of the apparently non-diseased liver parenchyma (excluding metastases or other focal liver lesions) on portal phase images. Mean grey-level intensity (M), entropy (E) and uniformity (U) were derived with no filtration and different filter widths (0.5 = fine, 1.5 = medium, 2.5 = coarse).
Results
Mean E1.5 and E2.5 for the whole liver in patients with synchronous metastases were significantly higher compared with the non-metastatic patients (p = 0.02 and p = 0.01). Mean U1.5 and U2.5 were significantly lower in the synchronous metastases group compared with the non-metastatic group (p = 0.04 and p = 0.02). Texture parameters for the metachronous metastases group were not significantly different from the non-metastatic group or synchronous metastases group (p > 0.05), although – similar to the synchronous metastases group – there was a subtle trend towards increased E1.5, E2.5 and decreased U1.5, U2.5 values. Areas under the ROC curve for the diagnosis of synchronous metastatic disease based on the texture parameters E1.5,2.5 and U1.5,2.5 ranged between 0.73 and 0.78.
Conclusion
Texture analysis of the apparently non-diseased liver holds promise to differentiate between CRC patients with and without metastatic liver disease. Further research is required to determine whether these findings may be used to benefit the prediction of metachronous liver disease.
Keywords: Colorectal cancer, liver metastases, CT texture, occult disease, metachronous metastases
Introduction
The liver is the most common and often unique site of metastasis in patients presenting with colorectal cancer (CRC).1–4 Studies have reported that >20% of CRC patients present with synchronous metastasis at primary staging.5 Furthermore, cumulative rates of metachronous liver metastases of 4.3% have been reported at one-year follow-up, increasing up to 14.5% at five-year follow-up.3 It has been hypothesised that these metachronous liver metastases may in fact develop from occult liver metastases that are already present at the time of primary staging.4,6 The presence of occult liver metastases (as established at histopathological examination after liver surgery) is associated with an unfavourable prognostic outcome regarding overall survival,7 and addition of chemotherapy may reduce the presence of micrometastases.8 With conventional staging tools it has so far not been feasible to establish the presence of micrometastases within the liver. This could, however, be of clinical importance as it may help establish a more risk-adapted (neo)adjuvant treatment strategy.
Previous studies have mainly focused on methods targeting liver perfusion to detect occult liver metastases, based on the hypothesis that changes in the hemodynamics of the liver occur before focal metastases become visible on morphological imaging. Some promising results have been reported for measuring the Doppler Perfusion Index (DPI) on ultrasound9,10 or hepatic perfusion indices (HPI) and hepatic transit time (HTT) on computed tomography (CT) or magnetic resonance imaging (MRI).11–18 Challenges with these techniques, however, are that they are operator dependent (DPI)19,20 and require a relatively complex image acquisition and post-processing algorithm.
An alternative approach would be to evaluate the spatial heterogeneity of the liver by means of texture analyses, which can be readily obtained from routinely acquired clinical CT data without the need for extra imaging procedures. Texture analysis focuses on the distribution and relationships of grey-level values within CT images of the liver. Similar to overall changes in perfusion, it can be expected that the texture of the liver parenchyma will change when the liver becomes invaded with tumoural cells. Apart from morphologically visible metastases, the presence of tumour may also affect the spatial heterogeneity of the remaining – apparently non-diseased – part of the liver.21,22 Previous studies in CRC patients without metastases have demonstrated that texture parameters (entropy and uniformity) of the normal liver parenchyma were significantly correlated with the hepatic hemodynamic status and glucose metabolism23,24 and may also be used as a predictor of survival.25 Furthermore, results from a study by Ganeshan et al. suggest that – in patients with known CRC liver metastases – texture parameters may be more sensitive to tumour-related changes in the apparently disease-free areas of the liver parenchyma than measurements of hepatic attenuation or perfusion.22
In order to determine whether texture analyses may be used to assess occult metastatic disease and predict the development of metachronous liver disease, a first step should be to investigate whether there are any changes in the overall liver CT texture in patients with known metastases. A next step would be to see whether such changes may already be observed in patients who will develop metastases at a later stage in the course of their disease. Hence, the aim of our study was to assess whether any differences can be detected in the CT texture of the apparently disease-free liver parenchyma of patients without and patients with hepatic metastasis from CRC.
Materials and methods
Patients
This study retrospectively analysed 29 patients who were diagnosed with colon or rectal cancer at our institution between March 2007 and August 2011. All patients routinely underwent contrast-enhanced abdominal CT as a part of their primary staging work-up. Three groups of patients were considered: Group A (n = 15) included patients who had no evidence of liver metastasis at primary staging or during at least two years of follow-up after primary staging. Group B (n = 10) included patients who presented with ≥1 synchronous liver metastases at the time of primary staging. Group C (n = 4) included patients who had no evidence of metastatic disease at primary staging but developed metachronous liver metastases within 18 months after the primary staging CT. In the 14 patients with liver metastases (groups B and C), the presence of metastases was histopathologically proven (after liver resection) in 11/14 patients and by concordant 2-fluoro-2-deoxy-D-glucose positron-emission tomography (FDG-PET) findings in one of 14 patients. In the remaining two patients the liver lesions increased in size during follow-up and/or decreased in size after chemotherapy, confirming the presence of malignant disease. Inclusion criteria consisted of (a) histopathologically confirmed colorectal adenocarcinoma; (b) no evidence of other extrahepatic metastastic sites on contrast-enhanced CT of chest, abdomen and pelvis and/or 18F-FDG PET. Exclusion criteria consisted of (a) previous surgery of the liver, and (b) previous systemic treatment (chemotherapy), (c) diffuse hepatic metastases, owing to which too little apparently normal liver parenchyma remained for texture analysis, and (d) evidence of other diffuse liver disease such as steatosis or cirrhosis.
CT acquisition
Contrast-enhanced CT including a portal venous phase scan of the liver was performed as part of the routine liver/abdomen CT imaging protocol for CRC at our institution using multi-slice CT equipment (Siemens Somatom Sensation 16 or Somatom Definition Flash, Siemens Healthcare, Erlangen, Germany; Toshiba Aquilion 16, Toshiba Medical Systems, Tokyo, Japan; Philips Brilliance 64 or Philips Gemini TF 64, Philips Medical Systems, Best, The Netherlands). The portal venous phase CT images used for texture analysis were obtained with a tube voltage of 120 kVp. The in-plane pixel resolution for the liver images varied from 0.55 to 0.85 mm2, with a slice thickness of 3 mm.
Image assessment
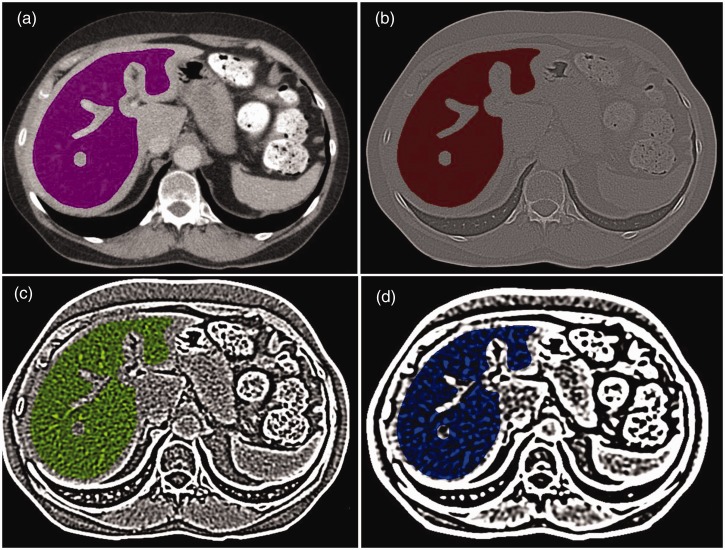
Images were transferred from the hospital’s picture archiving and communication system (PACS) to an offline workstation for texture analysis. Regions of interest (ROIs) covering the whole-liver volume were manually drawn on the portal phase CT images by a single reader (WvO). ROIs were drawn to cover the whole surface of the liver on each consecutive slice, excluding the border of the liver (to avoid partial volume effects), any visible lesions (metastases or benign focal liver lesions such as haemangiomas, or cysts), major portal and hepatic veins and the inferior vena cava. Because the border of the vena cava is often not clearly defined, we chose not to include the caudate lobe of the liver for the ROI delineation and texture analyses. A representative example of the manual ROI delineation is shown in Figure 1.
Figure 1.
Representative example of regions of interest drawn on a portal venous phase computed tomography (CT) image of the liver (a). Liver borders, any visible focal liver lesions, major vessels and the caudate lobe were manually excluded. The images represent the different types of filtrations: no filtration (a), fine filtration (b), medium filtration (c) and coarse filtration (d).
Texture analysis
Texture analyses were performed using a dedicated script written in MATLAB (MathWorks Inc, Natick, MA, USA) by one of the authors (RSS). The mathematical analysis technique used for this study is described in detail in the appendix. It comprised two main stages: (a) image filtration, followed by (b) quantification of texture. For image filtration, a Laplacian of Gaussian (LoG) band-pass filter was applied to the ROI using sigma (σ) values of 0.5 (fine-scale filtration), 1.5 (medium-scale filtration) and 2.5 (coarse-scale filtration). Subsequently, the texture of the liver parenchyma was characterized by mean grey-level intensity (M), entropy (E) and uniformity (U).
Statistical analyses
Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS Inc, version 16.0, Chicago, IL, USA). Texture parameters were compared between group A (no metastases) and group B (synchronous metastases), and between group A and group C (metachronous metastases) using a Student’s T test when normally distributed or Mann-Whitney U test when not normally distributed. Differences with a p value less than 0.05 were considered statistically significant. Receiver operating characteristics (ROC) analyses were constructed to determine the potential diagnostic performance of the respective texture parameters for diagnosing the presence of metastatic disease. Corresponding areas under the ROC curve (AUCs), sensitivities, specificities, positive predictive values (PPV) and negative predictive values (NPV) including 95% confidence intervals were calculated. For these analyses, cutoff values were determined according to the point nearest to the upper left corner in the ROC curve.
Results
Patient characteristics
Of the 29 study patients, 20 were male and nine female (median age 64 years, range 19–79 years). The primary tumours were located in the colon in 21 patients and in the rectum in eight patients. Baseline characteristics of the patients for the three subgroups are displayed in Table 1.
Table 1.
Baseline characteristics of patients with colorectal liver metastasis
| Group |
|||
|---|---|---|---|
| A | B | C | |
| Number | 15 | 10 | 4 |
| Median age (range) | 61 (19–72) | 65 (51–74) | 76 (69–79) |
| Male/female | 10/5 | 7/3 | 3/1 |
| Median years of follow-up (range)a | 6.0 (5.1–7.2) | 4.4 (3.5–6.7) | 4.3 (3.5–6.3) |
| Primary carcinomas: | 14/1 | 5/5 | 2/2 |
| Rectum/colon | |||
| Metastases: | |||
| Interval (months) staging-metastases (range)b | N/A | N/A | 5.3 (3.5–13) |
| Median number per patient (range) | N/A | 1 (1–6) | 3 (1–4) |
| Median lesion Dc in mm (range) | N/A | 29.1 (13.8–60.1) | 12.8 (11.6–69.2) |
Group A: patients without liver metastasis; Group B: patients with synchronous liver metastasis at the time of primary staging; Group C: patients with metachronous liver metastasis within 18 months after primary staging. N/A: not applicable.
Median follow-up from the date of the primary staging computed tomography (CT).
Median interval between the primary staging CT and the detection of metachronous metastatic disease.
D: the maximal axial diameter of the largest metastasis.
Texture analyses
The mean values for the texture parameters (mean grey-level intensity, entropy, uniformity) using different filtrations are shown in Table 2. The mean values of E1.5 and E2.5 in Group B (synchronous metastases) were significantly higher compared with Group A (no metastases; p = 0.02 and p = 0.01, respectively). Mean values of U1.5 and U2.5 in Group B were significantly lower compared with Group A (p = 0.04 and p = 0.02, respectively). Texture parameters were not significantly different between Group A and Group C (p > 0.05).
Table 2.
Mean (±standard deviation) of CT texture parameters of the apparently non-diseased liver parenchyma measured at different scale values (σ) on portal venous phase CT
| Texture parameters | Group |
p value |
|||
|---|---|---|---|---|---|
| A (n = 15) | B (n = 10) | C (n = 4) | A vs. B | A vs. C | |
| Mean grey-level intensity | |||||
| No filtration | 104.8 ± 22.3 | 109.9 ± 16.66 | 103.8 ± 20.8 | 0.55 | 0.94 |
| σ = 0.5 | 0.09 ± 0.08 | 0.07 ± 0.06 | 0.13 ± 0.14 | 0.60 | 0.41 |
| σ = 1.5 | 0.60 ± 0.25 | 0.53 ± 0.18 | 0.58 ± 0.26 | 0.46 | 0.90 |
| σ = 2.5 | 1.67 ± 0.62 | 1.52 ± 0.39 | 1.5 ± 0.34 | 0.51 | 0.61 |
| Entropy | |||||
| No filtration | 6.44 ± 0.16 | 6.53 ± 0.20 | 6.39 ± 0.32 | 0.21 | 0.64 |
| σ = 0.5 | 8.08 ± 0.26 | 8.14 ± 0.31 | 7.96 ± 0.35 | 0.58 | 0.46 |
| σ = 1.5 | 7.59 ± 0.18 | 7.77 ± 0.20 | 7.63 ± 0.44 | 0.02 | 0.77 |
| σ = 2.5 | 6.96 ± 0.11 | 7.14 ± 0.21 | 7.02 ± 0.34 | 0.01 | 0.58 |
| Uniformity(×103) | |||||
| No filtration | 13.78 ± 1.71 | 13.00 ± 1.84 | 14.44 ± 2.90 | 0.29 | 0.56 |
| σ = 0.5 | 4.45 ± 0.82 | 4.28 ± 0.87 | 4.84 ± 1.04 | 0.62 | 0.43 |
| σ = 1.5 | 6.30 ± 0.94 | 5.53 ± 0.75 | 6.27 ± 1.90 | 0.04 | 0.97 |
| σ = 2.5 | 9.87 ± 0.92 | 8.82 ± 1.20 | 9.70 ± 2.42 | 0.02 | 0.82 |
Group A: patients without liver metastasis; Group B: patients with synchronous liver metastases at the time of primary staging; Group C: patients with metachronous liver metastases within 18 months following primary staging; Significant results are printed in bold. CT: computed tomography.
Diagnostic performance for assessment of synchronous liver metastases
Table 3 shows the AUCs, optimal threshold values and corresponding accuracy figures for the various texture measurements in diagnosing the presence of synchronous metastatic disease. For the texture measures showing significant differences between group A and B (see Table 2), the AUCs were as follows: for the entropy measurements, AUCs for detecting the presence of metastatic disease were 0.74 for E1.5 and 0.78 for E2.5, respectively. For the uniformity measurements, AUCs were 0.73 for U1.5 and 0.77 for U2.5, respectively.
Table 3.
Diagnostic performance for entropy and uniformity with or without filtrations in assessing synchronous colorectal liver metastasis by receiver operating characteristics (ROC) curve analyses
| AUC | Sensitivity | Specificity | PPV | NPV | Cutoff | |
|---|---|---|---|---|---|---|
| Mean grey-level intensity | ||||||
| No filtration | 0.59 | 0.40 (4/10) | 0.87 (13/15) | 0.67 (4/6) | 0.68 (13/19) | >118.98 |
| 95% CI | 0.37–0.78 | 0.14–0.73 | 0.58–0.98 | 0.24–0.94 | 0.43–0.86 | |
| σ = 0.5 | 0.54 | 1 (10/10) | 0.2 (3/15) | 0.45 (10/22) | 0.1 (3/3) | ≤0.16 |
| 95% CI | 0.33–0.74 | 0.66–1 | 0.05–0.48 | 0.25–0.67 | 0.30–1 | |
| σ = 1.5 | 0.56 | 0.3 (3/10) | 0.93 (14/15) | 0.75 (3/4) | 0.67 (14/21) | ≤0.34 |
| 95% CI | 0.35–0.75 | 0.08–0.65 | 0.66–1 | 0.22–0.99 | 0.43–0.85 | |
| σ = 2.5 | 0.54 | 0.5 (5/10) | 0.67 (10/15) | 0.5 (5/10) | 0.67 (10/15) | ≤1.45 |
| 95% CI | 0.33–0.74 | 0.2–0.8 | 0.39–0.87 | 0.2–0.8 | 0.39–0.87 | |
| Entropy | ||||||
| No filtration | 0.61 | 0.4 (4/10) | 1 (15/15) | 1 (4/4) | 0.71 (15/21) | >6.64 |
| 95% CI | 0.40–0.80 | 0.14–0.73 | 0.75–1 | 0.4–1 | 0.48–0.88 | |
| σ = 0.5 | 0.55 | 0.2 (2/10) | 1 (15/15) | 1 (2/2) | 0.65 (15/23) | >8.43 |
| 95% CI | 0.34–0.75 | 0.04–0.56 | 0.75–1 | 0.20–1 | 0.43–0.83 | |
| σ = 1.5 | 0.74 | 64 (9/14) | 91 (10/11) | 90 (9/10) | 67 (10/15) | ≥7.66 |
| 95% CI | 0.53–0.94 | 36–86 | 57–100 | 54–99 | 39–87 | |
| σ = 2.5 | 0.78 | 78 (7/9) | 81 (13/16) | 70 (7/10) | 87 (13/15) | ≥7.08 |
| 95% CI | 0.57–0.99 | 40–96 | 54–95 | 35–92 | 58–98 | |
| Uniformity | ||||||
| No filtration | 0.60 | 0.5 (5/10) | 0.87 (13/15) | 0.71 (5/7) | 0.72 (13/18) | ≤12.08 × 10−3 |
| 95% CI | 0.39–0.79 | 0.20–0.80 | 0.58–0.98 | 0.30–0.95 | 0.46–0.89 | |
| σ = 0.5 | 0.55 | 0.5 (5/10) | 0.73 (11/15) | 0.56 (5/9) | 0.69 (11/16) | ≤3.98 × 10−3 |
| 95% CI | 0.34–0.75 | 0.2–0.8 | 0.45–91 | 0.23–0.85 | 0.41–0.88 | |
| σ = 1.5 | 0.73 | 62 (8/13) | 83 (10/12) | 80 (8/10) | 67 (10/15) | ≤5.91 × 10−3 |
| 95% CI | 0.52–0.93 | 32–85 | 51–97 | 44–96 | 39–87 | |
| σ = 2.5 | 0.77 | 70 (7/10) | 80 (12/15) | 70 (7/10) | 80 (12/15) | ≤9.08 × 10−3 |
| 95% CI | 0.57–0.97 | 35–92 | 51–95 | 35–92 | 51–95 | |
Data are percentages; absolute numbers are given in parentheses. AUC: area under the ROC curve; PPV: positive predictive value; NPV: negative predictive value; CI: confidence interval.
Discussion
The goal of this study was to assess whether any differences can be detected in the CT texture of the apparently disease-free liver parenchyma of patients without and patients with hepatic metastasis from CRC. Our results indicate that entropy and uniformity with medium to coarse filtrations were useful for detecting differences in spatial heterogeneity between the normal-appearing liver parenchyma of patients with and without liver metastases at the time of primary staging. AUCs for assessment of the presence of synchronous liver metastasis using these texture parameters ranged between 0.73 and 0.78.
Apparently, CT texture analysis has the potential to detect changes in the overall structure of the liver at a level that goes beyond the resolution of morphological CT imaging. Higher entropy and corresponding lower uniformity were found in the non-diseased part of the liver (i.e. excluding the metastatic lesions) of patients with synchronous metastatic disease as compared to those without. In theory, entropy is a measure of texture irregularity. It is almost the opposite of uniformity, which is a measure of the uniformity of grey-level distribution indicating tissue homogeneity. The parenchymal structure of the non-diseased part of the liver in patients with known liver metastases appears to be more heterogeneous than that of a patient without any known liver metastases. There are several hypotheses that may explain this phenomenon. First, in patients with proven focal metastatic lesions, additional micrometastases may be present in the apparently disease-free remaining liver, resulting in a more heterogeneous overall liver structure. Previous studies reported that additional intrahepatic micrometastases (i.e. vascular, biliary, lymphatic infiltration and satellite nodules) were present within the surgically resected specimens in 58%–81% of CRC patients who had not undergone neoadjuvant chemotherapy.8,26,27 Second, the texture measures of the liver parenchyma may be influenced by tumour-induced changes in liver perfusion since different pre-clinical and clinical studies have suggested that measurements of liver texture on CT may be associated with liver vascularity.23,24,28 Interestingly, our results also showed a subtle trend – similar to the synchronous metastases group – towards increased entropy and decreased uniformity values in the patients who had no metastases, but developed metachronous liver disease within two years after the primary staging CT on which the texture analyses were performed. Although results were not significant and our metachronous group is obviously too small to draw any meaningful conclusions, this is a potentially interesting finding that warrants further research as it may indicate that tumoural changes – whether due to the presence of micrometastases or perfusional changes – are already occurring in the liver preceding any visible morphological changes.
In contrast with our results, a previous study by Ganeshan et al. reported lower entropy and higher uniformity values in the liver parenchyma of patients with liver metastasis.22 A contributing factor to these conflicting results could be that in this previous report a different imaging protocol was used, including both arterial and portal phase CT data which were derived from a dynamic scan protocol (consequently using lower contrast doses and higher injection rates). As a result, the portal venous phase will occur earlier than in a routine multi-phase CT imaging protocol as used in our study. In their study, significant results were mainly found in the arterial phase, while no significant results could be demonstrated in the portal venous phase (contradictory to our findings). Ganeshan reported that different contrast phases resulted in variations in entropy and uniformity, which may contribute to these inconsistent findings. There were also some differences in the study groups: Ganeshan et al. included patients during surveillance after primary CRC resection, while our study included CRC patients at the time of primary staging (prior to any treatment). This might result in different effects on hepatic haemodynamics caused by the presence vs. absence of the primary tumour.11,24 Moreover, in the study by Ganeshan et al. it was unknown from the archival study dataset whether the study patients had been exposed to any chemotherapeutic agents (which was known not to be the case in any of our study patients), which may have had a considerable effect on the texture measurements. Finally, in our study the whole volume of the liver was assessed rather than a single mid-liver axial section, as was the case in their previous study. Whole-volume measurements may be more representative of tissue heterogeneity and result in smaller measurement variations compared with single-slice analyses. On the other hand, the texture of specific hepatic segments in which liver metastases are located might be different from that of other segments. These subtle changes in more focal areas of the liver may be averaged out by choosing a whole-volume approach, an issue that requires further investigation. Altogether, larger study cohorts with uniform CT protocols and study populations are required to gain better insight into the complex relationship between texture measurements, treatment effects and underlying micro-architectural changes in the liver parenchyma.
Our study is limited by its retrospective nature and the single-reader design. Although we believe that interobserver variations in whole-volume delineation will likely be limited as has also previously been demonstrated in other cancer types, this is an issue that will need to be addressed by future studies.29 Also, the total number of patients – particularly for the metachronous liver metastasis – is small. Further research is required to validate the value of CT texture analyses and particularly to prospectively investigate its potential for the prediction of the development of metachronous disease.
Clinical impact and conclusions
In CRC patients, the presence of intrahepatic micrometastases has been demonstrated to be associated with poorer prognosis.7 Previous studies have shown that preoperative neoadjuvant chemotherapy may reduce the incidence of intrahepatic micrometastases in patients with colorectal liver metastasis.8 Prediction of the presence of micrometastases by non-invasive imaging methods may therefore potentially impact the therapeutic strategy. For the patients with colorectal liver metastases who are scheduled to undergo hepatectomy, preoperative identification of those patients who would likely have micrometastases in addition to the visible liver metastases might allow a better stratification for preoperative neoadjuvant chemotherapy. For CRC patients without overt liver metastasis, accurate screening of micrometastases at the time of primary staging may allow personalized treatment strategies, offering chemotherapy for the high-risk patients, while omitting it for the lower-risk patients. Conventional imaging methods are incapable of detecting occult liver metastasis. CT texture analysis is a promising noninvasive approach that allows quantification of the spatial heterogeneity of the liver parenchyma. Texture data can be readily obtained from routine CT scans without the need for additional imaging procedures. Our results suggest that texture analysis holds promise to detect subtle changes in the ‘normal’ liver parenchyma of patients with known colorectal liver metastases that might reflect the presence of tumour-induced structural and/or haemodynamic changes. Furthermore, a similar subtle trend might be observed in patients who develop metachronous liver lesions later during the course of their disease, although our current results on this matter are too preliminary to draw any meaningful conclusions. These results warrant further investigation to evaluate whether CT texture analysis will have potential value for the detection of occult liver metastases and predict which patients are at risk to develop metachronous liver disease.
Appendix
Texture analysis consists of two main stages: (a) image filtration and (b) quantification of texture.
I. Image filtration
For image filtration, a Laplacian of Gaussian (LoG) band-pass filter was chosen. Before applying the filter, regions outside the ROI were set to the average value of the pixels inside the ROI, to prevent non-target regions to influence the analysis. The LoG filter is mathematically defined as ΔG(x,y), with Δ the Laplace operator and G(x,y) a two-dimensional Gaussian. We have chosen to normalize the Gaussian as follows:
, which results in a LoG filter that is described by
We applied the filter in the Fourier space, as this is less computationally demanding. To calculate the convolution of the data and our filter, we multiplied their Fourier transforms in Fourier space. This has the additional benefit of reducing the discretization errors, as the filter has a larger width in Fourier space. The Fourier transform of the filter is given by
with kx and ky in the range (−0.5 to 0.5). The LoG-filter was applied for a range of σ values. The width of the filters in pixels (at the reference resolution of 0.84 mm) and mm is shown in Table 4. For each computed tomography (CT) scan with a resolution different than 0.84 mm, the value of σ was modified to keep the physical size (in mm) of the filter constant. We defined the width of the filter as the distance between the zero crossings (see Figure 2). These occur for x2 + y2 = 2σ2, so the total width of the filter is given by 2.
Figure 2.
Example of the Laplacian of Gaussian (LoG) filter with σ = 2.5.
Table 4.
Filter widths for different values of σ
| σ (pixels) | Full width (pixels) | Full width (mm) |
|---|---|---|
| 0.5 | 1.4 | 1.2 |
| 1.5 | 4.2 | 3.6 |
| 2.5 | 7.0 | 5.9 |
II. Quantification of texture
For image quantification of texture, mean grey-level intensity, entropy and uniformity were used. These parameters were calculated from a histogram of the points within the ROI with a bin size of 1 HU. From the histogram, the parameters were calculated using:
where N is the total number of pixels in the ROI, M the number of bins in the histogram and n(i) the number of pixels in bin number i if the histogram. The entropy gives an indication of the width of the histogram and the uniformity of the narrowness.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
None declared.
References
- 1. Pulitanò C, Castillo F, Aldrighetti L, et al. What defines ‘cure’ after liver resection for colorectal metastases? Results after 10 years of follow-up. HPB (Oxford) 2010; 12: 244–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Parks R, Gonen M, Kemeny N, et al. Adjuvant chemotherapy improves survival after resection of hepatic colorectal metastases: Analysis of data from two continents. J Am Coll Surg 2007; 204: 753–761. [DOI] [PubMed] [Google Scholar]
- 3. Manfredi S, Lepage C, Hatem C, et al. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg 2006; 244: 254–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Leen E. The detection of occult liver metastases of colorectal carcinoma. J Hepatobiliary Pancreat Surg 1999; 6: 7–15. [DOI] [PubMed] [Google Scholar]
- 5. van der Pool AE, Damhuis RA, Ijzermans JN, et al. Trends in incidence, treatment and survival of patients with stage IV colorectal cancer: A population-based series. Colorectal Dis 2012; 14: 56–61. [DOI] [PubMed] [Google Scholar]
- 6. Conzelmann M, Linnemann U, Berger MR. Detection of disseminated tumour cells in the liver of cancer patients. Eur J Surg Oncol 2005; 31: 977–985. [DOI] [PubMed] [Google Scholar]
- 7. Viganò L, Capussotti L, De Rosa G, et al. Liver resection for colorectal metastases after chemotherapy: Impact of chemotherapy-related liver injuries, pathological tumour response, and micrometastases on long-term survival. Ann Surg 2013; 258: 731–740. [DOI] [PubMed] [Google Scholar]
- 8. Wakai T, Shirai Y, Sakata J, et al. Histologic evaluation of intrahepatic micrometastases in patients treated with or without neoadjuvant chemotherapy for colorectal carcinoma liver metastasis. Int J Clin Exp Pathol 2012; 5: 308–314. [PMC free article] [PubMed] [Google Scholar]
- 9. Kopljar M, Brkljacic B, Doko M, et al. Nature of Doppler perfusion index changes in patients with colorectal cancer liver metastases. J Ultrasound Med 2004; 23: 1295–1300. [DOI] [PubMed] [Google Scholar]
- 10. Leen E, Goldberg JA, Angerson WJ, et al. Potential role of doppler perfusion index in selection of patients with colorectal cancer for adjuvant chemotherapy. Lancet 2000; 355: 34–37. [DOI] [PubMed] [Google Scholar]
- 11. Tsushima Y, Blomley MJ, Yokoyama H, et al. Does the presence of distant and local malignancy alter parenchymal perfusion in apparently disease-free areas of the liver? Dig Dis Sci 2001; 46: 2113–2119. [DOI] [PubMed] [Google Scholar]
- 12. Totman JJ, O’Gorman RL, Kane PA, et al. Comparison of the hepatic perfusion index measured with gadolinium-enhanced volumetric MRI in controls and in patients with colorectal cancer. Br J Radiol 2005; 78: 105–109. [DOI] [PubMed] [Google Scholar]
- 13. White MJ, O’Gorman RL, Charles-Edwards EM, et al. Parametric mapping of the hepatic perfusion index with gadolinium-enhanced volumetric MRI. Br J Radiol 2007; 80: 113–120. [DOI] [PubMed] [Google Scholar]
- 14. Hohmann J, Müller C, Oldenburg A, et al. Hepatic transit time analysis using contrast-enhanced ultrasound with BR1: A prospective study comparing patients with liver metastases from colorectal cancer with healthy volunteers. Ultrasound Med Biol 2009; 35: 1427–1435. [DOI] [PubMed] [Google Scholar]
- 15. Zhang H, He Y, Du L, et al. Shorter hepatic transit time can suggest coming metastases: Through-monitoring by contrast-enhanced ultrasonography? J Ultrasound Med 2010; 29: 719–726. [DOI] [PubMed] [Google Scholar]
- 16. Zhou JH, Li AH, Cao LH, et al. Haemodynamic parameters of the hepatic artery and vein can detect liver metastases: Assessment using contrast-enhanced ultrasound. Br J Radiol 2008; 81: 113–119. [DOI] [PubMed] [Google Scholar]
- 17. Cuenod C, Leconte I, Siauve N, et al. Early changes in liver perfusion caused by occult metastases in rats: Detection with quantitative CT. Radiology 2001; 218: 556–561. [DOI] [PubMed] [Google Scholar]
- 18. Hohmann J, Newerla C, Müller A, et al. Hepatic transit time analysis using contrast enhanced MRI with Gd-BOPTA: A prospective study comparing patients with liver metastases from colorectal cancer and healthy volunteers. J Magn Reson Imaging 2012; 36: 1389–1394. [DOI] [PubMed] [Google Scholar]
- 19. Fong Y. Doppler perfusion index in colorectal cancer. Lancet 2000; 355: 5–6. [DOI] [PubMed] [Google Scholar]
- 20. Roumen RM, Scheltinga MR, Slooter GD, et al. Doppler perfusion index fails to predict the presence of occult hepatic colorectal metastases. Eur J Surg Oncol 2005; 31: 521–527. [DOI] [PubMed] [Google Scholar]
- 21. Ganeshan B, Miles KA, Young RC, et al. Texture analysis in non-contrast enhanced CT: Impact of malignancy on texture in apparently disease-free areas of the liver. Eur J Radiol 2009; 70: 101–110. [DOI] [PubMed] [Google Scholar]
- 22. Ganeshan B, Miles KA, Young RC, et al. Hepatic entropy and uniformity: Additional parameters that can potentially increase the effectiveness of contrast enhancement during abdominal CT. Clin Radiol 2007; 62: 761–768. [DOI] [PubMed] [Google Scholar]
- 23. Ganeshan B, Miles KA, Young RC, et al. In search of biologic correlates for liver texture on portal-phase CT. Acad Radiol 2007; 14: 1058–1068. [DOI] [PubMed] [Google Scholar]
- 24. Ganeshan B, Miles KA, Young RC, et al. Hepatic enhancement in colorectal cancer: Texture analysis correlates with hepatic hemodynamics and patient survival. Acad Radiol 2007; 14: 1520–1530. [DOI] [PubMed] [Google Scholar]
- 25. Miles KA, Ganeshan B, Griffiths MR, et al. Colorectal cancer: Texture analysis of portal phase hepatic CT images as a potential marker of survival. Radiology 2009; 250: 444–452. [DOI] [PubMed] [Google Scholar]
- 26. Wakai T, Shirai Y, Sakata J, et al. Appraisal of 1 cm hepatectomy margins for intrahepatic micrometastases in patients with colorectal carcinoma liver metastasis. Ann Surg Oncol 2008; 15: 2472–2481. [DOI] [PubMed] [Google Scholar]
- 27. Yokoyama N, Shirai Y, Ajioka Y, et al. Immunohistochemically detected hepatic micrometastases predict a high risk of intrahepatic recurrence after resection of colorectal carcinoma liver metastases. Cancer 2002; 94: 1642–1647. [DOI] [PubMed] [Google Scholar]
- 28. Bézy-Wendling J, Kretowski M, Rolland Y, et al. Towards a better understanding of texture in vascular CT scan simulated images. IEEE Trans Biomed Eng 2001; 48: 120–124. [DOI] [PubMed] [Google Scholar]
- 29. Lambregts DM, Beets GL, Maas M, et al. Tumour ADC measurements in rectal cancer: Effect of ROI methods on ADC values and interobserver variability. Eur Rad 2011; 21: 2567–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]