Abstract
Background
Inflammatory bowel disease (IBD) patients are sometimes first diagnosed with irritable bowel syndrome (IBS), which may be construed as a misdiagnosis.
Objective
The objective of this article is to determine if this occurs more than expected by chance.
Methods
We conducted a case-control study nested in the General Practice Research Database. We selected incident cases of IBD and up to 10 matched controls for each. We assessed the proportions with IBS recorded prior to the IBD diagnosis and variation by age, sex, and calendar time. We compared proportions affected in fixed time periods and conducted conditional logistic regression to derive odds ratios.
Results
The 20, 193 cases were three times as likely as controls to have a prior record of IBS. Fifteen per cent of IBD cases and 5% of controls had IBS coded before diagnosis with 11% having a code for IBS over one year before IBD (cf. 5% of controls) and 6% over five years earlier (cf. 3%). These figures roughly doubled if typical antispasmodic therapies were assumed to represent IBS diagnoses.
Conclusion
If excess IBS diagnoses represent misdiagnoses of IBD, our results suggest that about 10% of IBD patients are misdiagnosed and in 3% of cases this may persist for five or more years.
Keywords: Inflammatory bowel diseases, irritable bowel syndrome, epidemiology, misdiagnosis, case-control study
Introduction
Though it is easy to find case reports of the delayed diagnosis of inflammatory bowel disease (IBD) and in particular of Crohn’s disease, and even to find statements that this is common, evidence as to how common is limited. The evidence available is primarily from small or dated studies and often individual case series within a single institution. There is, however, one relatively recent, and high-quality United Kingdom (UK)-wide study of childhood IBD indicating that this is a widespread problem, with symptoms present for in excess of a year in one-fifth of cases.1 For evidence of the rate of diagnostic delay in adults based on similarly robust population based data over a large UK population, we must go back to Northern Ireland in the 1960s and 1970s to find 33% of cases taking over a year to diagnose and 7.5% over five years.2
It is well recognised that an important differential diagnosis in IBD is that from irritable bowel syndrome (IBS) (or rather IBD is an important differential to exclude in the workup of IBS). A literature reporting tests to aid this differentiation has grown over recent years, clearly demonstrating that it remains an ongoing challenge without the use of invasive tests.3 There is, however, growing evidence of the value of faecal calprotectin in this regard.4 At the same time there has been interest in the presence of IBS-like symptoms in patients with an established diagnosis of IBD. Of particular interest to the subject of this report is a study from Canada showing that IBD patients with IBS-like symptoms experience symptoms for longer before diagnosis.5
One important mechanism of the delay in IBD diagnosis therefore is likely to be its misdiagnosis as IBS. We have therefore set out to examine how prevalent such misdiagnosis is in contemporary UK practise.
Methods
Design
A matched case-control study was conducted to determine the differences in prior diagnoses of IBS between patients newly diagnosed with IBD and people without IBD.
Setting
Data were extracted from the General Practice Research Database (GPRD) downloaded in January 2011. These data contain electronic information on consultations, diagnoses and prescriptions delivered in primary care in the UK, and have been validated for a wide variety of diagnoses including IBD.6,7 We used GPRD data from 1987 to October 2010 accessed under the University of Nottingham’s GPRD license. This dataset contains approximately 66 million person-years of available data for analysis among 11.26 million contributing patients within 613 general practices. Within the dataset, patients are labelled as ‘acceptable’ for use in research if follow-up is contiguous and data recorded do not raise worries about validity; data are also labelled as up to standard during the period for which an individual practice provided continuous data to a high standard defined by GPRD.
Study population
Incident cases of IBD were identified as acceptable patients with a first recording of IBD after the up-to-standard date of their practice or one year after their current registration date, whichever date was latest. The date of this first code for IBD has been previously validated as representing the date of diagnosis of IBD8 and is so construed here. Cases were assigned as Crohn’s disease or ulcerative colitis (UC) in a hierarchical fashion accepting that where both UC and Crohn’s disease were diagnosed, Crohn’s disease was the correct diagnosis. Cases with only codes for ‘inflammatory bowel disease’ or ‘indeterminate colitis’ throughout their record were not classified further into Crohn’s or UC but remained as a separate category of indeterminate IBD.
Controls were selected from all acceptable patients with no record of IBD recording in all their data. For each case up to 10 controls were selected matched on diagnosis date, practice, gender and age (±5 years). In order to provide a time point from which to assess controls, they were assigned the date of diagnosis of their matched case which we henceforth refer to as their pseudo-diagnosis date.
Data extracted from GPRD records
For each individual we assessed age at date of diagnosis/pseudo-diagnosis and categorised this into 10-year age bands. Records for all cases and controls were then examined for the presence of a code representing the diagnosis of IBS, and/or codes recording prescription of antispasmodic drugs typically used in the treatment of IBS (Mebeverine, Colpermin or alverine citrate) prior to the date of diagnosis/pseudo-diagnosis. The first date for each of these two coding options was retained as the first evidence of a consultation for IBS-like symptoms in an individual.
The length of time before diagnosis/pseudo-diagnosis was categorised into three-month intervals for the first year, then yearly intervals up to 10 years prior to diagnosis/pseudo-diagnosis.
As potential covariates we extracted data on psychiatric co-morbidity (anxiety or depression) and on smoking as we hypothesised that those regarded as typical of patients with IBS (young, females, with psychiatric co-morbidity) might be more likely to be misdiagnosed, and those with risk factors for Crohn’s (smoking) might be more carefully investigated.
Statistical analysis
For all cases of IBD we present the proportion of cases and controls who had IBS recorded prior to the date of diagnosis of IBD/pseudo-diagnosis, overall and in the time periods specified above. These analyses are reported both based purely on diagnostic codes for IBS, and by using a combination of a diagnosis, and/or a prescription for antispasmodics typically used in IBS as the diagnostic codes allow more certainty of the GP’s opinion that the patient had IBS. Finally, we limited analysis to those with 10 years of follow-up and stratified by disease type.
Results are then stratified by gender, by age categorised into two groups (<50 years and 50+ years of age) and by the date of IBD diagnosis (before or after 1 January 2004 – chosen as approximately half of diagnoses are after this date). In all analyses we present absolute numbers of cases, and proportions with 95% confidence intervals (CIs). We consider significant differences in proportions where CIs are mutually exclusive. We have then calculated both univariate and multivariate odds ratios derived by conditional logistic regression, considering patients with at least five years of follow-up and excluding IBS diagnoses (combination of diagnosis and/or prescription) made less than one year before diagnosis with IBD.
All analyses were conducted in Stata version 11 StataCorp (College Station, TX, USA).
Ethical considerations
This study was approved by the Independent Scientific Advisory Committee of the GPRD (protocol 11_047).
Results
We identified 20,193 incident cases of IBD and matched these to 201,393 controls who had no evidence of IBD. Cases were predominantly of UC, were almost evenly distributed between the sexes (52% female) and were most commonly diagnosed in the fourth decade of life (Table 1).
Table 1.
Demographic characteristics of cases and controls
| Cases |
Controls |
|
|---|---|---|
|
n (%) |
n (%) |
|
| N = 20 193 | 201,393 | |
| Gender | ||
| Male | 9743 (48) | 97,159 (48) |
| Female | 10,450 (52) | 104,234 (52) |
| Age (years) | ||
| 0–9 | 156 (0.8) | 2225 (1.1) |
| 10–19 | 1405 (7.0) | 14,532 (7.2) |
| 20–29 | 2883 (14.3) | 26,935 (13.4) |
| 30–39 | 3478 (17.2) | 34,856 (17.3) |
| 40–49 | 3256 (16.1) | 33,268 (16.5) |
| 50–59 | 3082 (15.3) | 31,021 (15.4) |
| 60–69 | 2818 (14.0) | 27,880 (13.8) |
| 70–79 | 2114 (10.5) | 21,076 (10.5) |
| 80–89 | 911 (4.5) | 8834 (4.4) |
| 90+ | 87 (0.4) | 766 (0.4) |
| UC | 10,679 (52.9) | |
| Crohn’s | 7435 (36.8) | |
| Indeterminate IBD | 2079 (10.3) | |
| Psychiatric condition | 5085 (25.2) | 42,990 (21.4) |
| Ever smoker | 10,152 (50.3) | 87,538 (43.5) |
| Median follow-up (years) (interquartile range) | 5.23 (2.13, 9.28) | 5.22 (2.15, 9.27) |
UC: ulcerative colitis; IBD: inflammatory bowel disease.
When we examined for the presence of prior diagnoses of IBS, these were almost three times as common in the IBD patients as in their controls (15.2% versus 5% – p < 0.01). Many of these excess diagnoses occurred in the year before the diagnosis of IBD (4.4% of cases had a new diagnosis of IBS in this time versus 0.4% of controls – p < 0.01); however, there was a highly significant annual excess back as far as 10 years before diagnosis (Table 2).
Table 2.
Prior identification of IBS (through diagnostic codes only or diagnostic and therapeutic codes) for cases of inflammatory bowel disease and their matched controls
| Prior diagnosis of IBS | Cases N = 20,193 | % | CIs | Controls N = 201,393 | % | CIs | |||
|---|---|---|---|---|---|---|---|---|---|
| IBS from diagnostic codes only. | Ever | 3067 | 15.19 | 14.69 | 15.68 | 10,124 | 5.03 | 4.93 | 5.12 |
| Never | 17,126 | 84.81 | 84.32 | 85.31 | 191,269 | 94.97 | 94.88 | 95.07 | |
| 0–3 months | 329 | 1.63 | 1.45 | 1.80 | 220 | 0.11 | 0.09 | 0.12 | |
| 3–6 months | 240 | 1.19 | 1.04 | 1.34 | 212 | 0.11 | 0.09 | 0.12 | |
| 6–9 months | 172 | 0.85 | 0.73 | 0.98 | 227 | 0.11 | 0.10 | 0.13 | |
| 9–12 months | 142 | 0.70 | 0.59 | 0.82 | 203 | 0.10 | 0.09 | 0.11 | |
| 1–2 years | 337 | 1.67 | 1.49 | 1.85 | 852 | 0.42 | 0.39 | 0.45 | |
| 2–3 years | 253 | 1.25 | 1.10 | 1.41 | 790 | 0.39 | 0.36 | 0.42 | |
| 3–4 years | 193 | 0.96 | 0.82 | 1.09 | 743 | 0.34 | 0.40 | 0.40 | |
| 4–5 years | 196 | 0.97 | 0.84 | 1.11 | 718 | 0.36 | 0.33 | 0.38 | |
| 5–6 years | 135 | 0.67 | 0.56 | 0.78 | 697 | 0.37 | 0.33 | 0.38 | |
| 6–7 years | 148 | 0.73 | 0.62 | 0.85 | 606 | 0.30 | 0.28 | 0.32 | |
| 7–8 years | 102 | 0.51 | 0.41 | 0.60 | 553 | 0.27 | 0.25 | 0.30 | |
| 8–9 years | 93 | 0.46 | 0.37 | 0.55 | 542 | 0.27 | 0.25 | 0.29 | |
| 9–10 years | 101 | 0.50 | 0.40 | 0.60 | 485 | 0.24 | 0.22 | 0.26 | |
| 10+ years | 626 | 3.10 | 2.86 | 3.34 | 3276 | 1.63 | 1.57 | 1.68 | |
| IBS from diagnostic or prescription codes. | Ever | 5974 | 29.58 | 28.95 | 30.21 | 183,117 | 9.07 | 8.95 | 9.20 |
| Never | 14,219 | 70.42 | 69.79 | 71.05 | 18,276 | 90.93 | 90.80 | 91.05 | |
| 0–3 months | 1,051 | 5.20 | 4.90 | 5.51 | 530 | 0.26 | 0.24 | 0.29 | |
| 3–6 months | 596 | 2.95 | 2.72 | 3.18 | 532 | 0.26 | 0.24 | 0.29 | |
| 6–9 months | 394 | 1.95 | 1.76 | 2.14 | 522 | 0.26 | 0.24 | 0.28 | |
| 9–12 months | 324 | 1.60 | 1.43 | 1.78 | 481 | 0.24 | 0.22 | 0.26 | |
| 1–2 years | 727 | 3.60 | 3.34 | 3.86 | 1933 | 0.96 | 0.92 | 1.00 | |
| 2–3 years | 510 | 2.53 | 2.31 | 2.74 | 1733 | 0.86 | 0.82 | 0.90 | |
| 3–4 years | 370 | 1.83 | 1.65 | 2.02 | 1573 | 0.78 | 0.74 | 0.82 | |
| 4–5 years | 329 | 1.63 | 1.45 | 1.80 | 1524 | 0.76 | 0.72 | 0.79 | |
| 5–6 years | 261 | 1.29 | 1.14 | 1.45 | 1408 | 0.70 | 0.66 | 0.74 | |
| 6–7 years | 244 | 1.21 | 1.06 | 1.36 | 1214 | 0.60 | 0.57 | 0.64 | |
| 7–8 years | 209 | 1.04 | 0.90 | 1.17 | 1036 | 0.51 | 0.48 | 0.55 | |
| 8–9 years | 171 | 0.85 | 0.72 | 0.97 | 975 | 0.48 | 0.45 | 0.51 | |
| 9–10 years | 145 | 0.72 | 0.60 | 0.83 | 829 | 0.41 | 0.38 | 0.44 | |
| 10+ years | 643 | 3.18 | 2.94 | 3.43 | 3986 | 1.98 | 1.92 | 2.04 | |
IBS: irritable bowel syndrome; CI: confidence interval.
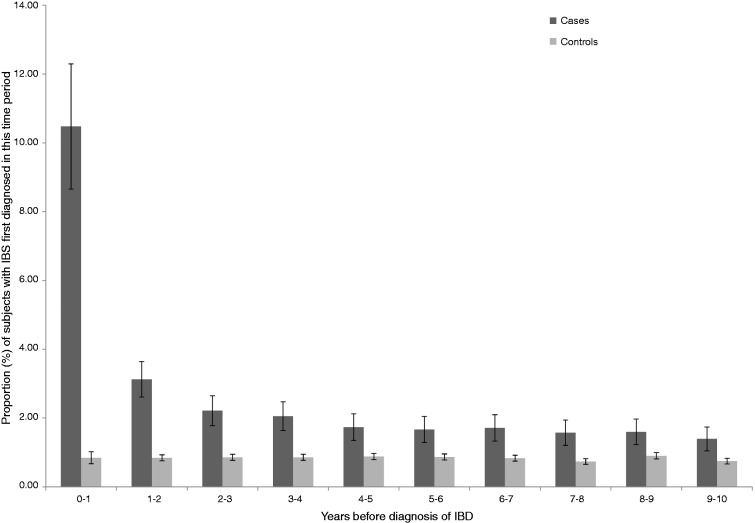
When the definition of IBS was broadened to include prescriptions for antispasmodic drugs as well as diagnostic codes, this accentuated the differences described above suggesting as many as 30% of IBD patients might have been diagnosed or treated as having IBS prior to diagnosis compared to 9% of controls. Again, though most marked in the year before diagnosis, this discrepancy persisted many years before the diagnosis of IBS (Table 2). Repeating this analysis in only those individuals with at least 10 years of follow-up yielded similar results (Table 3, Figure 1).
Table 3.
Prior identification of IBS (diagnostic or therapeutic codes) for cases of inflammatory bowel disease and their matched controls with at least 10 years of follow-up
| Prior diagnosis of IBS | Cases N = 4380 | % | 95% CIs | Controls N = 43,334 | % | 95% CIs | |
|---|---|---|---|---|---|---|---|
| IBS from diagnostic or prescription codes only in those with >10 years follow-up. | Ever | 1530 | 34.9 | 33.5, 36.3 | 5653 | 13.0 | 12.7, 13.4 |
| Never | 2850 | 65.1 | 63.7, 66.5 | 37681 | 87.0 | 86.6, 87.3 | |
| 0–3 months | 214 | 4.9 | 4.2, 5.5 | 79 | 0.2 | 0.1, 0.2 | |
| 3–6 months | 124 | 2.8 | 2.3, 3.3 | 103 | 0.2 | 0.2, 0.3 | |
| 6–9 months | 61 | 1.4 | 1.0, 1.7 | 93 | 0.2 | 0.2, 0.3 | |
| 9–12 months | 60 | 1.4 | 1.0, 1.7 | 93 | 0.2 | 0.2, 0.3 | |
| 1–2 years | 137 | 3.1 | 2.6, 3.6 | 368 | 0.8 | 0.8, 0.9 | |
| 2–3 years | 97 | 2.2 | 1.8, 2.6 | 374 | 0.9 | 0.8, 1.0 | |
| 3–4 years | 90 | 2.0 | 1.6, 2.5 | 374 | 0.9 | 0.8, 1.0 | |
| 4–5 years | 76 | 1.7 | 1.3, 2.1 | 384 | 0.9 | 0.8, 1.0 | |
| 5–6 years | 73 | 1.7 | 1.3, 2.1 | 378 | 0.9 | 0.8, 1.0 | |
| 6–7 years | 75 | 1.7 | 1.3, 2.1 | 363 | 0.8 | 0.8, 0.9 | |
| 7–8 years | 69 | 1.6 | 1.2, 1.9 | 321 | 0.7 | 0.7, 0.8 | |
| 8–9 years | 70 | 1.6 | 1.2, 2.0 | 392 | 0.9 | 0.8, 1.0 | |
| 9–10 years | 61 | 1.4 | 1.0, 1.7 | 326 | 0.7 | 0.7, 0.8 | |
| 10+ years | 323 | 7.4 | 6.6, 8.1 | 2005 | 4.6 | 4.4, 4.8 |
IBS: irritable bowel syndrome; CI: confidence interval.
Figure 1.
Proportion of cases and controls with prior diagnosis of IBS (diagnostic and therapeutic codes) in those with at least 10 years of follow-up.
IBS: irritable bowel syndrome; IBD: inflammatory bowel disease.
When similar analyses were conducted only in the subsets of cases with Crohn’s disease or with UC, the excess of IBS diagnoses between cases and controls was greater for Crohn’s disease (34.9% ever previously diagnosed or treated for IBS versus 8.7%) and smaller for UC (25.2% versus 9.2%). This also remained true in the analysis of only patients with 10 years of data (Table 4). Otherwise the pattern of results was similar to that for all IBD. Stratification by gender showed the absolute but not the relative excess of IBS diagnoses was higher in women, with a prior IBS diagnosis or therapy recorded in 35.9% of females and 22.8% of males compared to 12.6% of female and 5.3% of male controls. When stratified by age the excess of IBS diagnoses was greater in the young, with prior recording of IBS or its therapy in 31.3% of those under 50 years of age (compared to 7.8% of their controls) and 27.4% of those over 50 years of age (compared to 10.7% of their controls). Our final stratification of the analysis by the date of IBD diagnosis showed that the diagnosis and treatment of IBS was more common both in cases and controls in the later period. After 2004 the absolute increase in IBS before IBD diagnosis was greater but the relative excess was smaller (26.7% of cases and 7.4% of controls had such a record before 2004 compared to 32.5% of cases and 10.7% of controls after).
Table 4.
Prior identification of IBS (diagnostic or therapeutic codes) for cases of Crohn’s disease and UC and their matched controls with at least 10 years of follow-up
| Prior diagnosis of IBS | Crohn’s disease |
Controls |
UC |
Controls |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N = 1551 | % | 95% CIs | N = 15,072 | % | 95% CIs | N = 2327 | % | 95% CIs | N = 22,997 | % | 95% CIs | ||
| IBS from diagnostic or prescription codes only in those with >10 years follow-up. | Ever | 622 | 40.1 | 37.7, 42.5 | 1914 | 12.7 | 12.2, 13.2 | 704 | 30.3 | 28.4, 32.1 | 3018 | 13.1 | 12.7,13.6 |
| Never | 929 | 59.9 | 57.5, 62.3 | 13,158 | 87.3 | 86.8, 87.8 | 1623 | 69.7 | 67.9, 71.6 | 19,979 | 86.9 | 86.4,87.3 | |
| 0–3 months | 93 | 6.0 | 4.8, 7.2 | 31 | 0.2 | 0.1, 0.3 | 93 | 4.0 | 3.2, 4.8 | 35 | 0.2 | 0.1, 0.2 | |
| 3–6 months | 65 | 4.2 | 3.2, 5.2 | 31 | 0.2 | 0.1, 0.3 | 52 | 2.2 | 1.6, 2.8 | 60 | 0.3 | 0.2, 0.3 | |
| 6–9 months | 27 | 1.7 | 1.1, 2.4 | 40 | 0.3 | 0.2, 0.3 | 27 | 1.2 | 0.7, 1.6 | 43 | 0.2 | 0.1, 0.2 | |
| 9–12 months | 27 | 1.7 | 1.1, 2.4 | 37 | 0.2 | 0.2, 0.3 | 27 | 1.2 | 0.7, 1.6 | 46 | 0.2 | 0.1, 0.2 | |
| 1–2 years | 66 | 4.3 | 3.3, 5.3 | 133 | 0.9 | 0.7, 1.0 | 53 | 2.3 | 1.7, 2.9 | 185 | 0.8 | 0.7, 0.9 | |
| 2–3 years | 36 | 2.3 | 1.6, 3.1 | 121 | 0.8 | 0.7, 0.9 | 49 | 2.1 | 1.5, 2.7 | 205 | 0.9 | 0.8, 1.0 | |
| 3–4 years | 28 | 1.8 | 1.1, 2.5 | 139 | 0.9 | 0.7, 1.0 | 49 | 2.1 | 1.5, 2.7 | 188 | 0.8 | 0.7, 0.9 | |
| 4–5 years | 31 | 2.0 | 1.3, 2.7 | 130 | 0.9 | 0.7, 1.0 | 31 | 1.3 | 0.9, 1.8 | 203 | 0.9 | 0.8, 1.0 | |
| 5–6 years | 31 | 2.0 | 1.3, 2.7 | 132 | 0.9 | 0.7, 1.0 | 31 | 1.3 | 0.9, 1.8 | 212 | 0.9 | 0.8, 1.0 | |
| 6–7 years | 35 | 2.3 | 1.5, 3.0 | 125 | 0.8 | 0.7, 1.0 | 32 | 1.4 | 0.9, 1.8 | 190 | 0.8 | 0.7, 0.9 | |
| 7–8 years | 26 | 1.7 | 1.0, 2.3 | 105 | 0.7 | 0.6, 0.8 | 32 | 1.4 | 0.9, 1.8 | 179 | 0.8 | 0.7, 0.9 | |
| 8–9 years | 17 | 1.1 | 0.6, 1.6 | 125 | 0.8 | 0.7, 1.0 | 43 | 1.9 | 1.3, 2.4 | 217 | 0.9 | 0.8, 1.1 | |
| 9–10 years | 25 | 1.6 | 1.0, 2.2 | 109 | 0.7 | 0.6, 0.9 | 28 | 1.2 | 0.8, 1.6 | 175 | 0.8 | 0.6, 0.9 | |
| 10+ years | 115 | 7.4 | 6.1, 8.7 | 656 | 4.4 | 4.0, 4.7 | 157 | 6.8 | 5.8, 7.8 | 1080 | 4.7 | 4.4, 5.0 | |
IBS: irritable bowel syndrome; UC: ulcerative colitis; CI: confidence interval.
When estimating the odds ratio for prior IBS diagnosis among newly diagnosed IBD patients and controls, we found the odds ratio for this to be 3.0 (2.8, 3.2) overall. This was higher among Crohn’s patients (3.6 (3.3, 4.0)), and lower in UC patients (2.5 (2.3, 2.8)). In our adjusted model there was significant statistical interaction between IBD diagnosis and past psychiatric history and so we have presented our analysis stratified by this factor, but adjusted for smoking (Table 5). Overall the increased odds of prior IBS was greater at 3.5 (95% CI 3.1–3.9) among those without a psychiatric history and slightly lower at 2.4 (2.0–2.9) in those with one.
Table 5.
Odds ratios for prior IBS diagnosis of or treatment for ≥1 year before diagnosis with IBD for patients with at least five years of follow-up stratified by psychiatric history and mutually adjusted for smoking history
| With psychiatric diagnosis | Without psychiatric diagnosis | (Number of observations) | |
|---|---|---|---|
| All IBD | |||
| Diagnosis of IBS | 2.4 (2.0, 2.9) | 3.5 (3.1, 3.9) | (8480) |
| Smoking | 1.2 (1.1, 1.4) | 1.4 (1.3, 1.5) | (42, 856) |
| Crohn’s | |||
| Diagnosis of IBS | 2.3 (1.7, 3.3) | 4.3 (3.6, 5.3) | (3055) |
| Smoking | 1.4 (1.1, 1.8) | 1.6 (1.4, 1.8) | (15, 160) |
| UC | |||
| Diagnosis of IBS | 2.2 (1.7, 3.0) | 2.9 (2.5, 3.4) | (4270) |
| Smoking | 1.2 (1.0, 1.4) | 1.3 (1.2, 1.4) | (23, 588) |
UC: ulcerative colitis; IBS: irritable bowel syndrome.
Discussion
We have shown that in UK general practice, patients newly diagnosed with IBD are more likely to have a previous diagnosis of IBS than are matched controls. IBD patients are in fact three times as likely to have a diagnosis or typical therapy for IBS, with this occurring in 29.58% of IBD patients and only 9.07% of controls. This excess is greater in Crohn’s disease than in UC, is greater in the young and in those without a previous psychiatric diagnosis, and is greater in absolute but not in relative terms in women than in men. The numbers of such diagnoses have not diminished in recent years. It is not possible to prove that these diagnoses of IBS represent misdiagnoses as we cannot verify retrospectively that IBD was (or was not) present at a time before it was actually diagnosed (i.e. around the time of the IBS diagnosis). That IBD was present previously is, however, the most obvious interpretation of our findings.
Before we accept these findings and their interpretation, however, we must consider two questions. First, is it likely that the association we have found is genuine? And second, might it be explained by other mechanisms than misdiagnosis? The answer to the first question depends upon the opportunities for error and bias in our work. Though the GPRD is known to provide diagnoses of high validity,6 there is potential for some error in the diagnoses we have relied upon. We should consider therefore whether the specific diagnoses here considered (IBS and IBD) are valid. For IBD a validation study has been conducted and demonstrates that in excess of 90% of those identified with IBD have good evidence to support this.7 For IBS no such validation has occurred and in fact the possibility of errors has been demonstrated.9 In the current study however since our intent is to study whether some diagnoses of IBS in general practice may be invalid, we do not see the potential for over-diagnosis of IBS in IBD cases as a weakness, rather it is part of the study design. We do, however, need to be sure that IBS is not under-identified in controls. In this regard we are reassured by the fact that the 9.07% of controls we found to have records of diagnosis or treatment for IBS corresponds quite closely to the prevalence figures found in UK-based community surveys10,11 (which also found that a large proportion of IBS was not formally diagnosed but may still be treated). We must also consider the potential for error in the timing of the diagnoses we have used. If the ‘incident’ cases of IBD we have studied were in truth prevalent, then the diagnosis of IBS may not predate them. We believe that this is unlikely to be a major problem, however, based upon the previous demonstration by Lewis and colleagues12 of the validity of the algorithm for identifying incident cases which we have used.
We must also consider the possibilities of bias. Since the cases and controls were selected in an unbiased manner and their data collected prospectively for reasons unrelated to the study, neither selection nor recall bias should have occurred. It is, however, possible that an ascertainment bias might act if patients with IBS by dint of receiving more medical attention to gastrointestinal (GI) symptoms were rendered more likely to have IBD diagnosed. Since the greatest effect of such a mechanism would be likely to occur around the time of the initial diagnostic workup of a patient, this might well at least in part explain the excess of IBS diagnoses in the year before IBD diagnosis; however, it is harder to conceive of how such a bias would act at earlier time points. Another potential bias in our analysis is due to the fact that we have not limited all analyses to patients with follow-up throughout the time period studied. However, when we included only cases and controls who had at least 10 years of follow-up data and repeated our analyses in this restricted group (Table 3 and Figure 1(c)), the larger apparent excess of IBS diagnoses in this analysis suggests that if anything such a bias is causing us to underestimate the true size of the problem we are studying.
Also relevant to the assessment of the validity of our work is the manner in which it fits with pre-existing knowledge of the relationship we have studied. Over recent years there has been much interest in IBS symptoms in those already diagnosed with IBD and some interest in these symptoms prior to diagnosis, but surprisingly little has been published directly relevant to prior IBS diagnoses. What we do know is that when previously formally studied, there was a marked delay in the diagnosis of IBD in the UK.2 This is reflected also in Canadian data which suggest that delays are greater in those with IBS-like symptoms, and which found that 33% of patients had a prior diagnosis of IBS at the time of IBD diagnosis (closely mirroring our own figures).5 In contrast to our results, however, this group found that delays were greater in the old (while we found that IBS diagnoses were more common in the young). Far more is published upon the presence of IBS symptoms within those already diagnosed with IBD. There can be no doubt from this literature that IBS symptoms are often present in those with IBD, being present in remission in about a third of patients in a number of studies.13,14 In one study the proportion of patients so affected is even higher than the proportion we found to have been previously diagnosed with IBS, with 46% of UC patients in remission having IBS-like symptoms in a study from Tehran.15
Considering the above, we believe that our methodology is sound and our results coherent with the existing literature. We must then consider what mechanisms other than misdiagnosis might produce this result. It has previously been questioned whether IBS symptoms prior to IBD diagnosis represent a prodrome16 with presumably the implication that the disease is not fully present at this time and might not be diagnosable. Since GI inflammation in various sites has been associated with a number of subgroups of IBS,17–19 one might hypothesise that a proportion of these cases are passing through a mild to a more severe inflammation in some cases as a stage in the evolution of IBD. Equally if the frequency of IBS in IBD patients in remission is increased due to shared risk factors (of which we are as yet unaware) rather than to a direct effect of the inflammatory damage from IBD, then one might expect increased IBS prior to IBD developing also (in effect this would suggest the association is explained by residual unknown confounders). The counterpoint to these arguments is of course the suggestion that many of the IBS symptoms in IBD may be due to clinically occult disease activity.20
If we accept that the excess IBS diagnoses we have described do represent incorrect diagnoses and diagnostic delays, our results suggest that about 10% of IBD patients receive an incorrect diagnosis of IBS prior to receiving their diagnosis of IBD. Roughly one-third of these incorrect diagnoses occur in the year before a final diagnosis is made (and probably therefore during the investigation leading to the diagnosis of IBD), but the rest occur earlier, and a small subgroup (3% of all eventual IBD diagnoses) attract an erroneous diagnosis of IBS for their symptoms five or more years before the eventual identification of IBD as the cause of their problems. If GPs using Mebeverine, Colpermin and alverine citrate do so because they believe that they are treating IBS, then the frequency of these delays to diagnosis may be even greater, with combined figures for IBS diagnosis or these therapies suggesting this may occur in as many as 22% of all eventual IBD diagnoses and persist for more than a year in 12% and for over five years in 6%. Recent guidance from the National Institute for Health and Care Excellence (NICE),21 and from the American College of Gastroenterology (ACG)22 on the diagnosis and management of IBS has recommended that in people who meet the IBS diagnostic criteria antibody testing should be undertaken to exclude coeliac disease. To exclude IBD in the young without alarm symptoms or family history, however, the ACG does not recommend tests, and NICE recommends only a full blood count, erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP). CRP is of course at best an imperfect test, and can be clearly outperformed by faecal calprotectin.4 Given that approaching 1% of UK IBS diagnoses will be followed by a diagnosis of IBD within four years, and that based on UK costs at least there is evidence that screening IBS patients for IBD using faecal calprotectin is more cost effective than current practice in the UK,23 we believe that this test should be routinely used.
Funding
This work was supported by Crohn’s and Colitis UK (grant number M10/1).
Conflict of interest
None declared.
References
- 1. Sawczenko A, Sandhu BK, Logan RF, et al. Prospective survey of childhood inflammatory bowel disease in the British Isles. Lancet 2001; 357: 1093–1094. [DOI] [PubMed] [Google Scholar]
- 2. Brown JS, Humphreys WG, Parks TG. Some clinical aspects of Crohn’s disease in Northern Ireland: An aid to earlier diagnosis? J R Coll Gen Pr 1988; 38: 549–551. [PMC free article] [PubMed] [Google Scholar]
- 3. van Rheenen PF, Van de Vijver E, Fidler V. Faecal calprotectin for screening of patients with suspected inflammatory bowel disease: Diagnostic meta-analysis. BMJ 2010; 341: c3369–c3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schoepfer AM, Trummler M, Seeholzer P, et al. Discriminating IBD from IBS: Comparison of the test performance of fecal markers, blood leukocytes, CRP, and IBD antibodies. Inflamm Bowel Dis 2008; 14: 32–39. [DOI] [PubMed] [Google Scholar]
- 5. Burgmann T, Clara I, Graff L, et al. The Manitoba Inflammatory Bowel Disease Cohort Study: Prolonged symptoms before diagnosis – how much is irritable bowel syndrome? Clin Gastroenterol Hepatol 2006; 4: 614–620. [DOI] [PubMed] [Google Scholar]
- 6. Herrett E, Thomas SL, Schoonen WM, et al. Validation and validity of diagnoses in the General Practice Research Database: A systematic review. Br J Clin Pharmacol 2010; 69: 4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lewis JD, Brensinger C, Bilker WB, et al. Validity and completeness of the General Practice Research Database for studies of inflammatory bowel disease. Pharmacoepidemiol Drug Saf 2002; 11: 211–218. [DOI] [PubMed] [Google Scholar]
- 8. Lewis JD, Bilker WB, Weinstein RB, et al. The relationship between time since registration and measured incidence rates in the General Practice Research Database. Pharmacoepidemiol Drug Saf 2005; 14: 443–451. [DOI] [PubMed] [Google Scholar]
- 9. García Rodríguez LA, Ruigómez A, Wallander MA, et al. Detection of colorectal tumor and inflammatory bowel disease during follow-up of patients with initial diagnosis of irritable bowel syndrome. Scand J Gastroenterol 2000; 35: 306–311. [DOI] [PubMed] [Google Scholar]
- 10. Hungin APS, Whorwell PJ, Tack J, et al. The prevalence, patterns and impact of irritable bowel syndrome: An international survey of 40,000 subjects. Aliment Pharmacol Ther 2003; 17: 643–650. [DOI] [PubMed] [Google Scholar]
- 11. Wilson S, Roberts L, Roalfe A, et al. Prevalence of irritable bowel syndrome: A community survey. Br J Gen Pract 2004; 54: 495–502. [PMC free article] [PubMed] [Google Scholar]
- 12. Lewis JD, Aberra FN, Lichtenstein GR, et al. Seasonal variation in flares of inflammatory bowel disease. Gastroenterology 2004; 126: 665–673. [DOI] [PubMed] [Google Scholar]
- 13. Minderhoud IM, Oldenburg B, Wismeijer JA, et al. IBS-like symptoms in patients with inflammatory bowel disease in remission; relationships with quality of life and coping behavior. Dig Dis Sci 2004; 49: 469–474. [DOI] [PubMed] [Google Scholar]
- 14. Isgar B, Harman M, Kaye MD, et al. Symptoms of irritable bowel syndrome in ulcerative colitis in remission. Gut 1983; 24: 190–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ansari R, Attari F, Razjouyan H, et al. Ulcerative colitis and irritable bowel syndrome: Relationships with quality of life. Eur J Gastroenterol Hepatol 2008; 20: 46–50. [DOI] [PubMed] [Google Scholar]
- 16. Pimentel M, Chang M, Chow EJ, et al. Identification of a prodromal period in Crohn’s disease but not ulcerative colitis. Am J Gastroenterol 2000; 95: 3458–3462. [DOI] [PubMed] [Google Scholar]
- 17. Gwee KA, Collins SM, Read NW, et al. Increased rectal mucosal expression of interleukin 1beta in recently acquired post-infectious irritable bowel syndrome. Gut 2003; 52: 523–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chadwick VS, Chen W, Shu D, et al. Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology 2002; 122: 1778–1783. [DOI] [PubMed] [Google Scholar]
- 19. Törnblom H, Lindberg G, Nyberg B, et al. Full-thickness biopsy of the jejunum reveals inflammation and enteric neuropathy in irritable bowel syndrome. Gastroenterology 2002; 123: 1972–1979. [DOI] [PubMed] [Google Scholar]
- 20. Keohane J, O’Mahony C, O’Mahony L, et al. Irritable bowel syndrome-type symptoms in patients with inflammatory bowel disease: A real association or reflection of occult inflammation? Am J Gastroenterol 2010; 105: 1788, 1789–1794; quiz 1795. [DOI] [PubMed]
- 21. National Institute for Health and Care Excellence. NICE clinical guideline 61 – Irritable bowel syndrome in adults: Diagnosis and management of irritable bowel syndrome in primary care, http://www.nice.org.uk/CG61 (2008, accessed 30 January 2012).
- 22. Brandt LJ, Chey WD, Foxx-Orenstein AE, et al. An evidence-based position statement on the management of irritable bowel syndrome. Am J Gastroenterol 2009; 104(Suppl 1): S1–S35. [DOI] [PubMed] [Google Scholar]
- 23. Whitehead S, Hutton J. Economic report: Value of calprotectin in screening out irritable bowel syndrome. CEP09041. Centre for Evidence-based Purchasing, NHS Purchasing and Supplies Agency, National Health Service, http://webarchive.nationalarchives.gov.uk/20100110130808/http://www.pasa.nhs.uk/pasa/Doc.aspx?Path=[MN][SP]/NHSprocurement/CEP/CEP09041.pdf (2010, accessed 13 July 2012).