Abstract
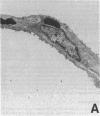
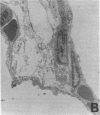
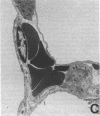
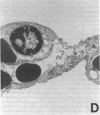
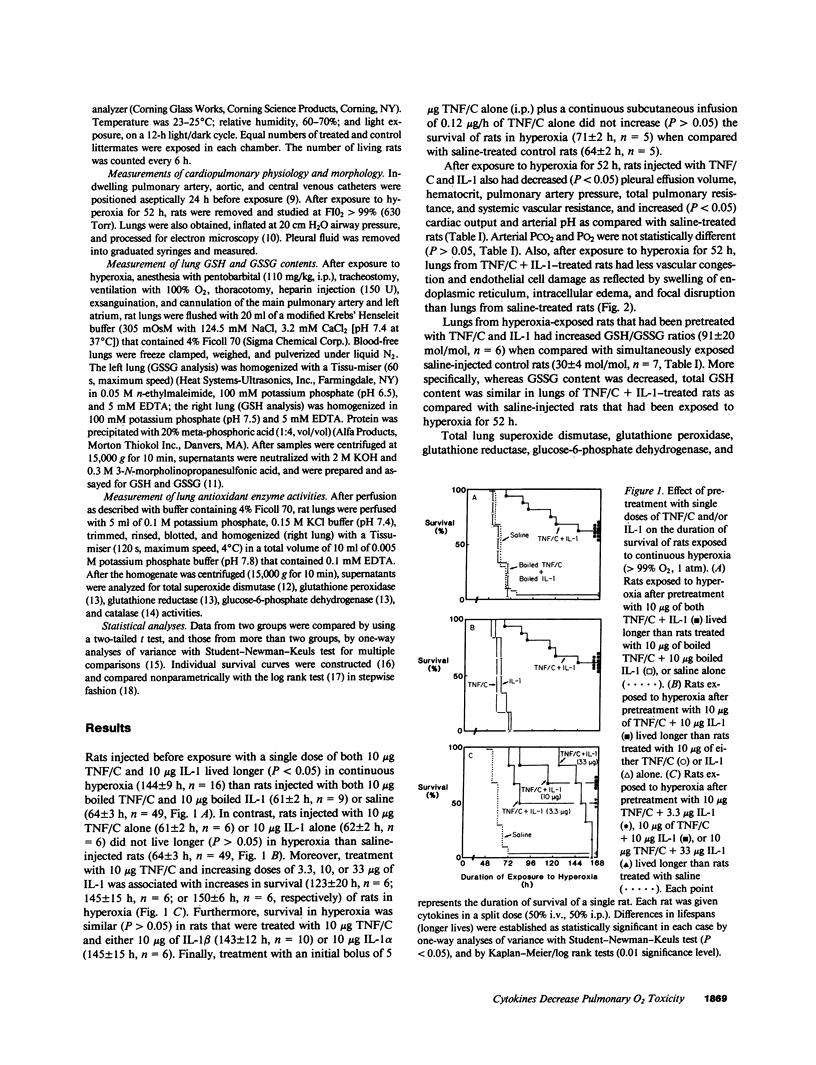
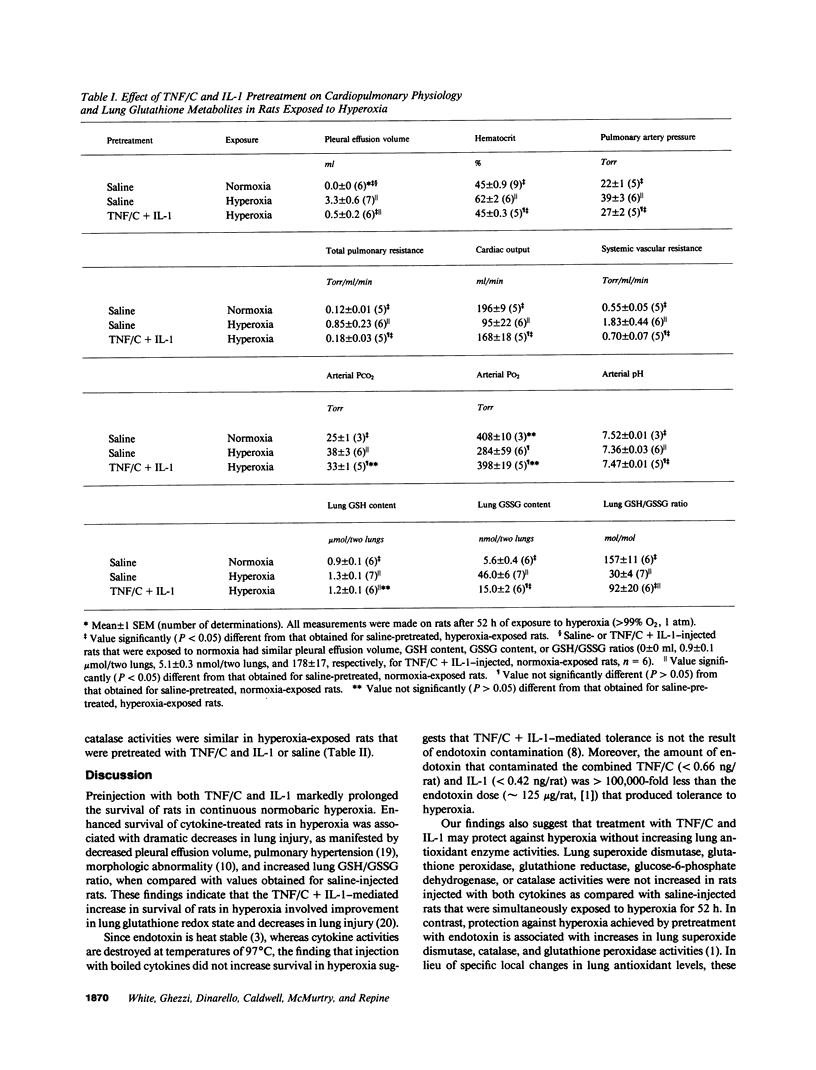
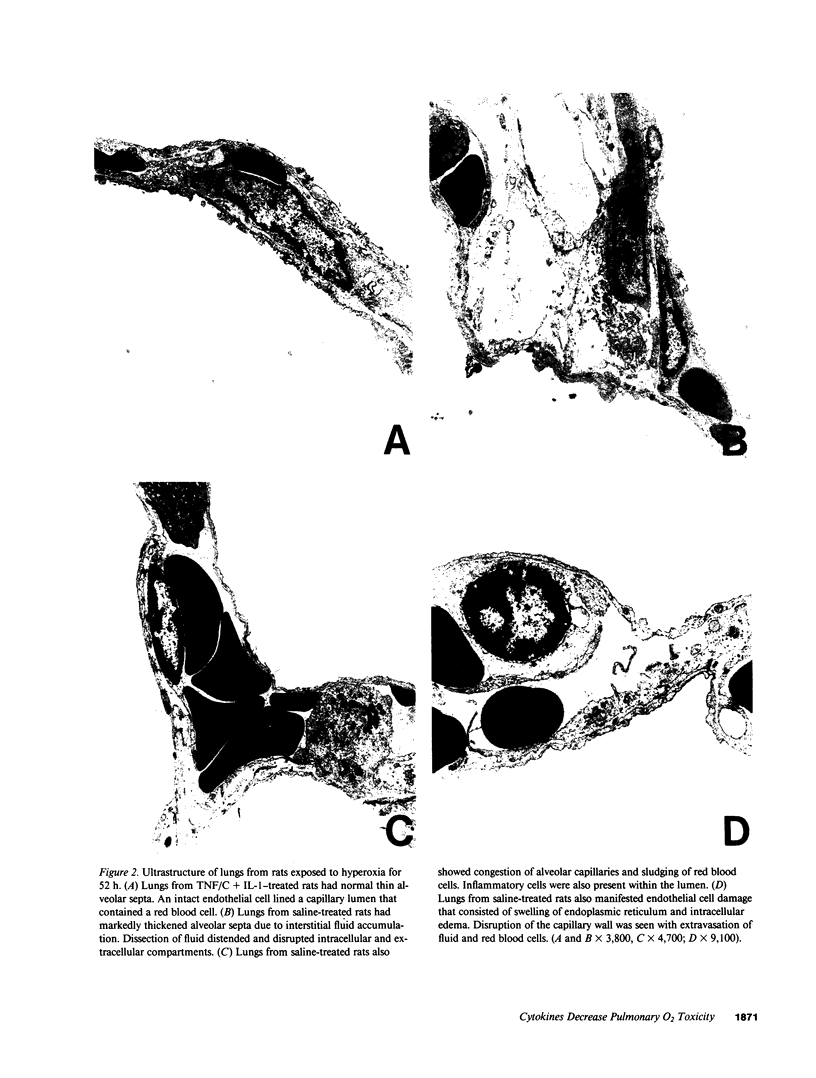
Single, preexposure, parenteral injection with both recombinant tumor necrosis factor/cachectin (TNF/C) and interleukin-1 (IL-1) prolonged the survival of rats (144 +/- 9 h) in continuous hyperoxia (greater than 99% O2 at 1 atm) when compared with rats injected with boiled TNF/C and boiled IL-1 (61 +/- 2 h), TNF/C alone (61 +/- 2 h), IL-1 alone (62 +/- 2 h), or saline (64 +/- 3 h). After exposure to hyperoxia for 52 h, pleural effusion volume, pulmonary artery pressure, total pulmonary resistance, and lung morphologic damage were decreased in those rats given TNF/C and IL-1 as compared with saline-injected rats. In parallel, ratios of reduced (GSH) to oxidized (GSSG) glutathione were greater (P less than 0.05) in lungs of TNF/C + IL-1-injected rats (91 +/- 20) than of saline-injected rats (30 +/- 4) that had been exposed to hyperoxia for 52 h. No differences were found in superoxide dismutase, glutathione peroxidase, glutathione reductase, glucose-6-phosphate dehydrogenase, or catalase activities in lungs of TNF/C + IL-1- or saline-treated, hyperoxia-exposed rats. Our results indicate that pretreatment with TNF/C and IL-1 favorably altered lung glutathione redox status, decreased lung injury, and enhanced survival of rats exposed to hyperoxia.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERGMEYER H. U. Zur Messung von Katalase-Aktivitäten. Biochem Z. 1955;327(4):255–258. [PubMed] [Google Scholar]
- Beutler B., Krochin N., Milsark I. W., Luedke C., Cerami A. Control of cachectin (tumor necrosis factor) synthesis: mechanisms of endotoxin resistance. Science. 1986 May 23;232(4753):977–980. doi: 10.1126/science.3754653. [DOI] [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Wheeler M. E., Cotran R. S., Gimbrone M. A., Jr Interleukin 1 acts on cultured human vascular endothelium to increase the adhesion of polymorphonuclear leukocytes, monocytes, and related leukocyte cell lines. J Clin Invest. 1985 Nov;76(5):2003–2011. doi: 10.1172/JCI112200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carswell E. A., Old L. J., Kassel R. L., Green S., Fiore N., Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crapo J. D., Barry B. E., Foscue H. A., Shelburne J. Structural and biochemical changes in rat lungs occurring during exposures to lethal and adaptive doses of oxygen. Am Rev Respir Dis. 1980 Jul;122(1):123–143. doi: 10.1164/arrd.1980.122.1.123. [DOI] [PubMed] [Google Scholar]
- Crapo J. D., McCord J. M., Fridovich I. Preparation and assay of superoxide dismutases. Methods Enzymol. 1978;53:382–393. doi: 10.1016/s0076-6879(78)53044-9. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A., Cannon J. G., Mier J. W., Bernheim H. A., LoPreste G., Lynn D. L., Love R. N., Webb A. C., Auron P. E., Reuben R. C. Multiple biological activities of human recombinant interleukin 1. J Clin Invest. 1986 Jun;77(6):1734–1739. doi: 10.1172/JCI112495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1. Rev Infect Dis. 1984 Jan-Feb;6(1):51–95. doi: 10.1093/clinids/6.1.51. [DOI] [PubMed] [Google Scholar]
- Frank L., Summerville J., Massaro D. Potection from oxygen toxicity with endotoxin. Role of the endogenous antioxidant enzymes of the lung. J Clin Invest. 1980 May;65(5):1104–1110. doi: 10.1172/JCI109763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble J. R., Harlan J. M., Klebanoff S. J., Vadas M. A. Stimulation of the adherence of neutrophils to umbilical vein endothelium by human recombinant tumor necrosis factor. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8667–8671. doi: 10.1073/pnas.82.24.8667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghezzi P., Saccardo B., Bianchi M. Recombinant tumor necrosis factor depresses cytochrome P450-dependent microsomal drug metabolism in mice. Biochem Biophys Res Commun. 1986 Apr 14;136(1):316–321. doi: 10.1016/0006-291x(86)90912-5. [DOI] [PubMed] [Google Scholar]
- Ghezzi P., Saccardo B., Villa P., Rossi V., Bianchi M., Dinarello C. A. Role of interleukin-1 in the depression of liver drug metabolism by endotoxin. Infect Immun. 1986 Dec;54(3):837–840. doi: 10.1128/iai.54.3.837-840.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonder J. C., Proctor R. A., Will J. A. Genetic differences in oxygen toxicity are correlated with cytochrome P-450 inducibility. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6315–6319. doi: 10.1073/pnas.82.18.6315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Chua A. O., Stern A. S., Hellmann C. P., Vitek M. P., DeChiara T. M., Benjamin W. R., Collier K. J., Dukovich M., Familletti P. C. Recombinant human interleukin 1 alpha: purification and biological characterization. J Immunol. 1986 Apr 1;136(7):2492–2497. [PubMed] [Google Scholar]
- Jones R., Zapol W. M., Reid L. Pulmonary artery remodeling and pulmonary hypertension after exposure to hyperoxia for 7 days. A morphometric and hemodynamic study. Am J Pathol. 1984 Nov;117(2):273–285. [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J., Vadas M. A., Harlan J. M., Sparks L. H., Gamble J. R., Agosti J. M., Waltersdorph A. M. Stimulation of neutrophils by tumor necrosis factor. J Immunol. 1986 Jun 1;136(11):4220–4225. [PubMed] [Google Scholar]
- Neta R., Douches S., Oppenheim J. J. Interleukin 1 is a radioprotector. J Immunol. 1986 Apr 1;136(7):2483–2485. [PubMed] [Google Scholar]
- Peto R., Pike M. C., Armitage P., Breslow N. E., Cox D. R., Howard S. V., Mantel N., McPherson K., Peto J., Smith P. G. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br J Cancer. 1977 Jan;35(1):1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pober J. S., Bevilacqua M. P., Mendrick D. L., Lapierre L. A., Fiers W., Gimbrone M. A., Jr Two distinct monokines, interleukin 1 and tumor necrosis factor, each independently induce biosynthesis and transient expression of the same antigen on the surface of cultured human vascular endothelial cells. J Immunol. 1986 Mar 1;136(5):1680–1687. [PubMed] [Google Scholar]
- Repine J. E., Fox R. B., Berger E. M. Hydrogen peroxide kills Staphylococcus aureus by reacting with staphylococcal iron to form hydroxyl radical. J Biol Chem. 1981 Jul 25;256(14):7094–7096. [PubMed] [Google Scholar]
- Stanbrook H. S., Morris K. G., McMurtry I. F. Prevention and reversal of hypoxic pulmonary hypertension by calcium antagonists. Am Rev Respir Dis. 1984 Jul;130(1):81–85. doi: 10.1164/arrd.1984.130.1.81. [DOI] [PubMed] [Google Scholar]
- Sugarman B. J., Aggarwal B. B., Hass P. E., Figari I. S., Palladino M. A., Jr, Shepard H. M. Recombinant human tumor necrosis factor-alpha: effects on proliferation of normal and transformed cells in vitro. Science. 1985 Nov 22;230(4728):943–945. doi: 10.1126/science.3933111. [DOI] [PubMed] [Google Scholar]
- Tsujimoto M., Yokota S., Vilcek J., Weissmann G. Tumor necrosis factor provokes superoxide anion generation from neutrophils. Biochem Biophys Res Commun. 1986 Jun 30;137(3):1094–1100. doi: 10.1016/0006-291x(86)90337-2. [DOI] [PubMed] [Google Scholar]
- Turrens J. F., Freeman B. A., Crapo J. D. Hyperoxia increases H2O2 release by lung mitochondria and microsomes. Arch Biochem Biophys. 1982 Sep;217(2):411–421. doi: 10.1016/0003-9861(82)90519-7. [DOI] [PubMed] [Google Scholar]
- Wang A. M., Creasey A. A., Ladner M. B., Lin L. S., Strickler J., Van Arsdell J. N., Yamamoto R., Mark D. F. Molecular cloning of the complementary DNA for human tumor necrosis factor. Science. 1985 Apr 12;228(4696):149–154. doi: 10.1126/science.3856324. [DOI] [PubMed] [Google Scholar]