Abstract
Objective
Higher serum urate concentrations predict more favorable prognosis in individuals with Parkinson disease (PD). The purpose of this study was to test the causality of this association using a mendelian randomization approach.
Methods
The study was conducted among participants in DATATOP and PRECEPT, two randomized trials among patients with early PD. The 808 patients with available DNA were genotyped for three SLC2A9 single nucleotide polymorphisms that identify an allele associated with lower urate concentrations, and for selected SNPs in other genes encoding urate transporters that have modest or no effect on serum urate levels. A SLC2A9 score was created based on the total number of minor alleles at the three SLC2A9 loci . Primary outcome was disability requiring dopaminergic treatment.
Results
Serum urate concentrations were 0.69mg/dL lower among individuals with 4 or more SLC2A9 minor alleles as compared to those with two or less (p = 0.0002). The hazard ratio (HR) for progression to disability requiring dopaminergic treatment increased with increasing SLC2A9 score (HR=1.16; 95% confidence interval 1.00 to 1.35; p=.056). In a comparative analysis, the HR was 1.27 (1.00 to 1.61; p =0.0497) for a 0.5 mg/dL genetically conferred decrease in serum urate, and 1.05 (1.01 to 1.10; p=0.0133) for a 0.5 mg/dL decrease in measured serum urate. No associations were found between polymorphisms in other genes associated with urate that do not affect serum urate and PD progression.
Interpretation
This Mendelian randomization analysis adds to the evidence of a causal protective effect of high urate levels.
Introduction
Previous longitudinal investigations have shown that individuals with higher serum urate levels 1-3 or a diet that increases serum urate 4 have a lower risk of developing Parkinson disease (PD). Further, in individuals with early PD, higher urate predicts milder clinical and radiographic progression. 5, 6 Urate is a potent antioxidant 7 and several lines of evidence support a role for oxidative stress in the neurodegenerative process of PD 8, but whether the inverse association between serum urate and PD progression reflects a neuroprotective effect remains uncertain due to the possibility of unmeasured confounders. Because urate levels are in part heritable -- the estimate of between person variation due to inherited genetic factors ranges from 25% to 70%, 9 -- we sought to use a mendelian randomization design 10 to investigate whether genetic polymorphisms that predict serum urate levels predict the rate of clinical progression among individuals with early PD. Although several genes are associated with serum urate, and a multiple genes score has been used in a previous study of PD risk 11, we selected as an instrumental variable for this investigation only the gene for solute carrier family 2 [facilitated glucose transporter], member 9 (SLC2A9 , also known as GLUT9) 12, which explains most of the genetically specified variability in serum urate. 13-19 By using a single gene with a strong effect on serum urate, but no known direct effects in the central nervous system, we minimized the possibility of violating the assumption that there are no genetic effects on PD progression other than those mediated by urate levels. 10 Other genes encoding urate transporters that are known to have modest or no effects on serum urate, but could nevertheless modulate its biological effects, were included in exploratory analyses.
Methods
Study population
The source population for this study includes participants in two randomized clinical trials of PD: the Parkinson Research Examination of CEP-1347 (PRECEPT) and the Deprenyl and Tocopherol Antioxidative Therapy of Parkinsonism (DATATOP) trials. The details of these studies and their participants are described elsewhere. 20, 21 We have previously reported an inverse association between serum urate and rate of disease progression in 804 individuals enrolled in PRECEPT and 774 enrolled in DATATOP. 5, 6 The study population for this study comprises the subset of these individuals from whom DNA was also available. In DATATOP (two year study with enrollment from September 1987-November 1988), DNA was collected at the end of the extended follow-up in 1995. DNA was not collected during PRECEPT (a two year trial with enrollment from April 2002-April 2004), but DNA collection began during a follow-up investigation, known as POSTCEPT, in which all the surviving individuals previously enrolled in the original trial at participating sites were invited to participate. Overall, DNA was available for 808 individuals, of which 63 were excluded from the mendelian randomization analyses due to lack of serum urate levels or failure in genotyping of SLC2A9; further, we excluded 10 patients who reported use of allopurinol at baseline, leaving 735 patients (390 in DATATOP and 345 in PRECEPT). Exploratory analyses of other genes included between 759 and 783 patients, because we excluded only those patients missing the specific SNP of interest.
SNPs and genotyping
Numerous SNPs in SLC2A9 -- a urate transporter 22 -- have been identified in several genome-wide association studies as the strongest genetic predictors of serum urate levels and gout. 13-19 Because these SNPs are in high linkage disequilibrium 15, and a single causal variant has not been identified, we selected three of the top SNPs for the present study. Specifically, the following SNPs in SLC2A9 were genotyped : rs6855911, an intronic SNP with minor allele frequency (MAF) of 0.31 (G allele); rs7442295 (intronic, MAF: 0.21 for G allele); and rs16890979 (missense mutation, MAF: 0.22 for T allele) (using HapMap data from Utah residents with ancestry from northern and western Europe, abbreviated CEU 23), for which each minor allele has been associated with a 0.30-0.43 mg/dL decrease in serum urate in individuals of European descent . 13, 18 Because these three SNPs are in strong linkage disequilibrium (LD; pairwise r2 range from 0.68-0.76 from Haploview 24 with HapMap CEU data), we used information from these three SNPs to create an SLC2A9 score with values equal to 0 (<= 2 minor alleles; i.e., preponderance of wild-type alleles); 1 (3 minor alleles and 3 wild-type alleles); and 2 (>=4 minor alleles; i.e., preponderance of minor alleles).
Other genes of interest because of their role in the transport of urate include solute carrier family 22, member 12 (URAT1/SLC22A12) - that encodes a urate-anion exchanger, 25 ATP-binding cassette sub-family G member 2 (ABCG2), and solute carrier family 19 (sodium phosphate), member 3 (SLC17A3). All genotyping was performed through the Harvard Partners Center for Genetics and Genomics (HPCGG) at the Harvard Partners Genotyping Facility using the OpenAssay SNP Genotyping System (BioTrove, Woburn, Massachusetts, USA). Concordance rates for blinded duplicates quality control samples were 100%. Test of Hardy-Weinberg equilibrium revealed no significant deviations (all p>0.05).
Serum urate and clinical outcomes
Serum urate was measured in PRECEPT and DATATOP participants at baseline prior to treatment assignment, as previously reported. 5, 6 The outcome evaluated in this study for both DATATOP and PRECEPT participants was the accumulation of disability sufficient to require dopaminergic therapy (this was also the primary outcome of the original studies). 20, 21 The mean duration of follow-up until endpoint or study termination was 13.6 months in DATATOP and 13.3 months in PRECEPT.
Statistical analysis
Initial analyses were conducted separately in DATATOP and PRECEPT. Because all tests of heterogeneity between studies were not significant (p>0.05), data from the two trials were pooled, and all models were adjusted for study group and treatment. Differences in serum urate according to genotype were assessed using generalized linear models. Primary analyses to assess the relation between genetic variants and PD progression assumed additive models (per unit increase in score for SLC2A9, per allele associations for other genes); secondary analyses used separate indicators for each genetic score category or genotype. Cox proportional hazards models were used to estimate hazard ratios (HRs) and 95% confidence intervals (95% CIs) for reaching the primary endpoint according to number of minor alleles or genotype. Analyses were adjusted for study, treatment, gender, age and use of thiazide diuretics at baseline. We assessed potential effect modification by gender and, in DATATOP, by randomization to α-tocopherol supplementation, which was included in some of the treatment arms in DATATOP. These interactions were assessed by including in the regression models an interaction term that was the cross product of the number of minor alleles of each individual SNP by gender (M/F) or α-tocopherol (yes/no). The association between genetically determined serum urate and PD progression was estimated by two stage regression: first, we fitted a generalized linear regression model with serum urate as the dependent variable and the SLC2A9 score and potential confounders (study, gender, age and use of thiazide diuretics) as independent variables, then the predicted urate level from the first stage regression was used as a continuous independent variable to determine its association with PD progression in a Cox proportional hazard model, adjusting for potential confounders. Sensitivity analyses were conducted estimating the genetically predicted urate level in a generalized linear model with separate indicators for each SLC2A9 SNP.
Results
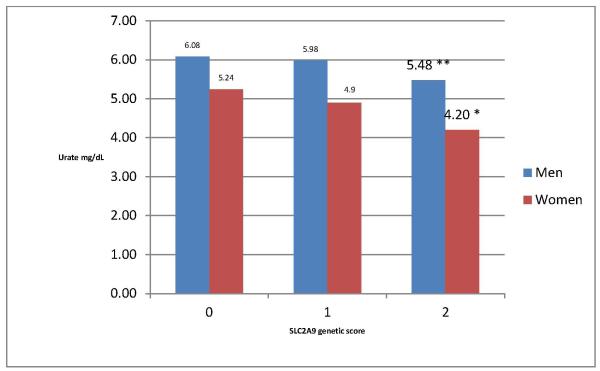
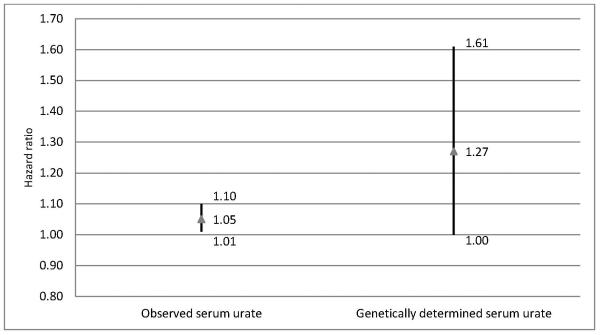
As expected, serum urate concentrations decrease with increasing number of minor SLC2A9 alleles, with a stronger association in women than in men (Figure 1). In an additive model, the rate of progression to a level of disability requiring dopaminergic treatment increased with the number of the minor alleles associated with lower serum UA (HR = 1.16 for each point increase in genetic score; 95% confidence interval: 1.00 to 1.35; p=0.056). As compared with individuals with <=2 minor SLC2A9 alleles, the HR was 1.12 (0.89 to 1.41) for individuals with 3 minor alleles, and 1.39 (0.98 to 1.96) for individuals with 4 or more minor alleles. The HR for a genetically determined lower serum urate was higher (HR for 0.5 mg/dL lower urate=1.27) than the corresponding HR for directly measured 0.5 mg/dL lower serum urate (HR=1.05) (figure 2). Results were not materially changed if each SLC2A9 SNP was used as an independent predictor of serum urate; in this analysis, the HR for 0.5 mg/dL genetically predicted lower urate was 1.24 (0.99 to 1.54). There was no significant effect modification by either gender or α-tocopherol supplementation (in DATATOP only) on the association between SLC2A9 score and initiation of dopaminergic therapy (all p for interaction > 0.05). Additionally, there was no evidence of interaction between SLC2A score and serum urate.
Figure 1.
Serum urate by SLC2A9 score. *p<.001, **p<.01 for comparison with score=0 (<=2 minor alleles).
Figure 2.
Hazard ratios and 95% confidence interval for initiation of dopaminergic therapy for a 0.5 mg/dL observed decrease in serum urate or for genetically conferred decrease in serum urate.
Overall, polymorphisms in genes other than SLC2A9 were not significantly associated with serum UA (Table 1) or subsequent initiation of dopaminergic therapy adjusting for age, gender and treatment (Table 2).
Table1.
Serum urate according to urate transport-related genotype.
| SNP | Serum urate (Mean ± SD) |
P-Value * |
|---|---|---|
| URAT1-rs11231825 | N=764 | |
| TT | 5.5 ± 1.4 | 0.18 |
| TC | 5.3 ± 1.4 | |
| CC | 5.3 ± 1.4 | |
| URAT1-rs11602903 | N=780 | |
| AA | 5.5 ± 1.3 | 0.56 |
| AT | 5.3 ± 1.4 | |
| TT | 5.3 ± 1.4 | |
| URAT1-rs3825016 | N=780 | |
| CC | 5.6 ± 1.3 | 0.36 |
| CT | 5.3 ± 1.4 | |
| TT | 5.3 ± 1.4 | |
| URAT1-rs3825018 | N=759 | |
| AA | 5.3 ± 1.4 | 0.68 |
| AG | 5.4 ± 1.4 | |
| GG | 5.4 ± 1.4 | |
| URAT1-rs475688 | N=770 | |
| CC | 5.3 ± 1.5 | 0.95 |
| CT | 5.3 ± 1.3 | |
| TT | 5.4 ± 1.3 | |
| URAT1-rs476037 | N=763 | |
| AA | 6.1 ± 1.0 | 0.35 |
| AG | 5.4 ± 1.4 | |
| GG | 5.3 ± 1.4 | |
| URAT1-rs7932775 | N=783 | |
| CC | 5.7 ± 1.5 | 0.86 |
| CT | 5.2 ± 1.3 | |
| TT | 5.3 ± 1.4 | |
| URAT1-rs893006 | N=761 | |
| AA | 5.3 ± 1.4 | 0.46 |
| AC | 5.3 ± 1.4 | |
| CC | 5.6 ± 1.4 | |
| ABCG2-rs2231142 | N=779 | |
| GG | 5.3 ± 1.4 | 0.07 |
| GT | 5.6 ± 1.5 | |
| TT | 4.9 ± 1.1 | |
| SLC17A3-rs1165205 | N=773 | |
| AA | 5.3 ± 1.4 | 0.49 |
| AT | 5.3 ± 1.4 | |
| TT | 5.4 ± 1.4 |
P-value for trend test, adjusted for study.
Table 2.
Hazard ratio for initiating dopaminergic therapy according to urate transport related genotype.
| SNP | Genotypes | Risk Allele |
Genotype Frequencies |
HR (95% CI)* |
|---|---|---|---|---|
| URAT1-rs11231825 | TT/TC/CC | C | 89/313/375 | 1.10 (0.95, 1.27) |
| URAT1-rs11602903 | AA/AT/TT | T | 81/351/362 | 1.07 (0.92, 1.24) |
| URAT1-rs3825016 | CC/CT/TT | T | 78/365/351 | 1.10 (0.95, 1.28) |
| URAT1-rs3825018 | AA/AG/GG | G | 355/334/84 | 0.91 (0.78, 1.05) |
| URAT1-rs475688 | CC/CT/TT | T | 404/319/61 | 0.94 (0.81, 1.10) |
| URAT1-rs476037 | AA/AG/GG | G | 9/145/623 | 1.03 (0.83, 1.29) |
| URAT1-rs7932775 | CC/CT/TT | T | 34/258/505 | 1.05 (0.89, 1.25) |
| URAT1-rs893006 | AA/AC/CC | C | 358/332/85 | 0.92 (0.80, 1.07) |
| ABCG2-rs2231142 | GG/GT/TT | T | 614/169/10 | 1.04 (0.84, 1.29) |
| SLC17A3-rs1165205 | AA/AT/TT | T | 221/380/186 | 0.89 (0.78, 1.03) |
HR for increasing number of risk alleles, adjusted for gender and age, stratified by a 4-level treatment by study variable.
Discussion
In this investigation we found that among individuals with early PD, SNPs in SLC2A9 predicted differences in serum urate that are similar to those previously reported in the general population. 13-19 Further, the rate of progression to a level of disability requiring dopaminergic treatment was faster among those patients carrying the SLC2A9 genotypes associated with lower serum UA. Although the statistical significance was marginal according to conventional levels, these novel results suggest that among participants in DATATOP and PRECEPT the previously reported better prognosis of early PD patients with higher urate levels5,6 is due to a protective effect of urate itself rather than to confounding by unknown factors.
A limitation of this study is that DNA was collected only several years after the trial completion and was only available for a subset of participants in DATATOP and PRECEPT, so that patients with more rapidly progressive disease may be underrepresented. It is unlikely, however, that this selection would result in a spurious inverse association between the SLC2A9 SNPs and the rate of PD progression during the trials. Further, as in the previous studies including all trial participants, baseline serum urate was inversely related to time to initiation of dopaminergic therapy.
A mendelian randomization approach has been used to investigate the causality of the association between serum urate and PD risk in three previous studies. In the first, conducted among individuals with PD in Italy, Croatia, and Germany, a SLC2A9 SNP predicting lower serum urate was associated with a younger age at onset of PD. 26 In the second, a case-control study in Spain, individuals in the highest tertile of a genetic score predicting lower serum urate were found to have a 50% higher risk of PD. 11 In the third study, only one of 12 genotyped SNPs in SLC2A9 was associated with a significantly increased PD risk in women, and none in men. 27 In this last study, however, serum urate levels were not available, and it is thus possible that the association between the genotyped SNPs and serum urate in the study population was weaker than expected. An important limitation of these studies is that even for those SNPs with the most robust associations with serum urate, the expected effects on PD risk are small, and power to detect an association is thus modest. Considering these limitations, overall the results of these studies support the hypothesis that higher urate levels reduce PD risk.
The association between genetically decreased serum UA levels and PD prognosis was somewhat stronger than the comparable association for measured circulating UA, suggesting that the latter may have been attenuated by unmeasured confounding. The lower measurement error and long term stability of genetically determined changes in serum urate may have contributed to this difference, but it is also possible that the association between serum urate and PD progression is attenuated by unmeasured confounders and thus underestimate the true effect of urate on PD progression. Serum urate is associated with obesity and insulin resistance, which in some investigations has been associated with an increased risk of PD 28, 29 , and could be a marker of dysfunctional energy metabolism. 30 In vitro, urate production is stimulated by compounds that lower ATP, including inhibitors of mitochondrial respiration 30, which have been implicated in the pathogenesis of PD. 31 The observed association between serum urate and PD progression could thus reflect in part the protective effect of urate on neurodegeneration, and in part the adverse effects of the upstream metabolic dysregulation that results in elevated serum urate. Although we did not find a significant interaction between SLC2A9 genotype and α-tocopherol supplementation or gender, the power for these analyses was modest, and effect modification by these factors therefore cannot be excluded. Whereas among DATATOP and PRECEPT participants serum urate was found to be a stronger prognostic predictor in men than in women5,6 it is noteworthy that the results of a recent phase 2 randomized trial in patients with early PD suggested that urate elevation may be more effective in women than in men. 32
We a priori considered SLC2A9 the primary gene of interest in relation to serum urate levels, so we did not consider potential joint or synergistic effects of a combination of SNPs recently considered by other authors. 19, 33 Although a composite genetic score incorporating several loci could be used,11, 19, 33 the contribution of the additional genes to serum urate is small relative to SLC2A9. The inclusion of numerous genes with modest effects on serum UA could increase the possibility that at least one of these genes affects PD progression via mechanisms other than serum urate, thus violating a key assumption of the mendelian randomization method. 10 In particular, the second strongest genetic predictor of serum urate levels is the ATP-binding cassette, subfamily G, isoform 2 protein (ABCG2), which has been related to the clearance of neurotoxic polypeptides from the brain 34 and neuroregeneration 35, and whose expression in brain capillaries is altered in an animal model of PD. 36 We cannot therefore exclude the possibility that variations in ABCG2 could affect PD progression through mechanisms independent from its effects on urate. The validity of the mendelian randomization approach in our study is supported by the fact that the genotype used as an instrumental variable (SLC2A9) is strongly associated with the exposure of interest (serum urate), and is most likely independent of the factors that confound the association between serum urate and PD progression. The fact that other genes involved in urate transport but without sizable effects on serum urate were not related to PD progression indirectly supports this conclusion. Because urate is also inversely associated with PD risk, one might expect that SNPs in SLC2A9 that predict lower urate levels should have been found to be associated with PD risk in large genome-wide association studies (GWAS). So its absence 37 may appear to contradict the hypothesis of a genuine protective effect of urate. However, because of the stringent significance criteria imposed by the large number of tests performed, even large GWAS are underpowered to detect the small effects attributable to single SLC2A9 SNPs.
In summary, we found that patients in the early stages of PD who carry the variant SLC2A9 alleles associated with lower urate levels have a faster rate of disease progression than those homozygous for the wild-type alleles. This finding suggests that the previously reported inverse association between higher urate levels and rate of PD progression is not explained by unmeasured confounders and is thus likely to reflect a genuine neuroprotective effect of urate. Genotypic characterization may be useful in identifying those most likely to respond to urate-elevating interventions. These data raise the possibility that modulation of SLC2A9 might be an equally or even more effective approach to urate elevation, compared to urate precursor administration38, as a candidate strategy for slowing PD progression.
Acknowledgments
This work was supported by the National Institutes of Health / National Institute of Neurological Diseases and Stroke [grant numbers K24NS060991 to MAS and R01NS061858 to AA] and the Department of Defense [DoD W81XWH-11-1-0150]. We also acknowledge support from the Parkinson Disease Foundation and we thank Cephalon (Teva Pharmaceutical Inc.) and Lundbeck for providing access to the data from PRECEPT and technical support. The authors thank Leslie Unger for aid in the preparation of this manuscript and Majken Jensen for expert advice.
LITERATURE CITED
- 1.Davis JW, Grandinetti A, Waslien CI, Ross GW, White LR, Morens DM. Observations on serum uric acid and the risk of idiopathic Parkinson's disease. Am J Epidemiol. 1996;144:480–4. doi: 10.1093/oxfordjournals.aje.a008954. [DOI] [PubMed] [Google Scholar]
- 2.de Lau LM, Koudstaal PJ, Hofman A, Breteler MM. Serum uric acid levels and the risk of Parkinson disease. Ann Neurol. 2005 Oct 20;58(5):797–800. doi: 10.1002/ana.20663. [DOI] [PubMed] [Google Scholar]
- 3.Weisskopf MG, O'Reilly E, Chen H, Schwarzschild MA, Ascherio A. Plasma urate and risk of Parkinson's disease. Am J Epidemiol. 2007 Sep 1;166(5):561–7. doi: 10.1093/aje/kwm127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao X, Chen H, Choi HK, Curhan G, Schwarzschild MA, Ascherio A. Diet, urate, and Parkinson's disease risk in men. Am J Epidemiol. 2008 Apr 1;167(7):831–8. doi: 10.1093/aje/kwm385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwarzschild MA, Schwid SR, Marek K, et al. Serum urate as a predictor of clinical and radiographic progression in Parkinson's disease. Arch Neurol. 2008;65(6):716–23. doi: 10.1001/archneur.2008.65.6.nct70003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ascherio A, Lewitt PA, Xu K, et al. Urate as a predictor of the rate of clinical decline in Parkinson disease. Arch Neurol. 2009;66(12):1460–8. doi: 10.1001/archneurol.2009.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci U S A. 1981;78(11):6858–62. doi: 10.1073/pnas.78.11.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cipriani S, Chen X, Schwarzschild MA. Urate: a novel biomarker of Parkinson's disease risk, diagnosis and prognosis. Biomark Med. 2010 Oct;4(5):701–12. doi: 10.2217/bmm.10.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang Q, Guo CY, Cupples LA, Levy D, Wilson PW, Fox CS. Genome-wide search for genes affecting serum uric acid levels: the Framingham Heart Study. Metabolism. 2005 Nov;54(11):1435–41. doi: 10.1016/j.metabol.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 10.Lawlor DA, Harbord RM, Sterne JA, Timpson N, Davey Smith G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. 2008 Apr 15;27(8):1133–63. doi: 10.1002/sim.3034. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez-Aramburu I, Sanchez-Juan P, Jesus S, et al. Genetic variability related to serum uric acid concentration and risk of Parkinson's disease. Mov Disord. 2013 Oct;28(12):1737–40. doi: 10.1002/mds.25507. [DOI] [PubMed] [Google Scholar]
- 12.Phay JE, Hussain HB, Moley JF. Cloning and expression analysis of a novel member of the facilitative glucose transporter family, SLC2A9 (GLUT9) Genomics. 2000 Jun 1;66(2):217–20. doi: 10.1006/geno.2000.6195. [DOI] [PubMed] [Google Scholar]
- 13.Li S, Sanna S, Maschio A, et al. The GLUT9 gene is associated with serum uric acid levels in Sardinia and Chianti cohorts. PLoS Genet. 2007 Nov 9;3(11):2194. doi: 10.1371/journal.pgen.0030194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wallace C, Newhouse SJ, Braund P, et al. Genome-wide Association Study Identifies Genes for Biomarkers of Cardiovascular Disease: Serum Urate and Dyslipidemia. Am J Hum Genet. 2008 Jan 10;82(1):139–49. doi: 10.1016/j.ajhg.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doring A, Gieger C, Mehta D, et al. SLC2A9 influences uric acid concentrations with pronounced sex-specific effects. Nat Genet. 2008 Apr;40(4):430–6. doi: 10.1038/ng.107. [DOI] [PubMed] [Google Scholar]
- 16.Vitart V, Rudan I, Hayward C, et al. SLC2A9 is a newly identified urate transporter influencing serum urate concentration, urate excretion and gout. Nat Genet. 2008 Mar 9; doi: 10.1038/ng.106. [DOI] [PubMed] [Google Scholar]
- 17.Stark K, Reinhard W, Neureuther K, et al. Association of common polymorphisms in GLUT9 gene with gout but not with coronary artery disease in a large case-control study. PLoS ONE. 2008;3(4):e1948. doi: 10.1371/journal.pone.0001948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dehghan A, Kottgen A, Yang Q, et al. Association of three genetic loci with uric acid concentration and risk of gout: a genome-wide association study. The Lancet. 2008 doi: 10.1016/S0140-6736(08)61343-4. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stefan N, Thamer C, Staiger H, et al. Genetic variations in PPARD and PPARGC1A determine mitochondrial function and change in aerobic physical fitness and insulin sensitivity during lifestyle intervention. J Clin Endocrinol Metab. 2007;92(5):1827–33. doi: 10.1210/jc.2006-1785. [DOI] [PubMed] [Google Scholar]
- 20.The Parkinson Study Group PRECEPT Investigators Mixed lineage kinase inhibitor CEP-1347 fails to delay disability in early Parkinson disease. Neurology. 2007 Oct 9;69(15):1480–90. doi: 10.1212/01.wnl.0000277648.63931.c0. [DOI] [PubMed] [Google Scholar]
- 21.Parkinson Study Group DATATOP: a multicenter controlled clinical trial in early Parkinson's disease. Arch Neurol. 1989 Oct;46(10):1052–60. doi: 10.1001/archneur.1989.00520460028009. [DOI] [PubMed] [Google Scholar]
- 22.Caulfield MJ, Munroe PB, O'Neill D, et al. SLC2A9 Is a high-hapacity urate transporter in humans. PLoS Med. 2008 Oct 7;5(10):e197. doi: 10.1371/journal.pmed.0050197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.The International HapMap Consortium The International HapMap Project. Nature. 2003 Dec 18;426(6968):789–96. doi: 10.1038/nature02168. [DOI] [PubMed] [Google Scholar]
- 24.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005 Jan 15;21(2):263–5. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 25.Woodward OM, Kottgen A, Coresh J, Boerwinkle E, Guggino WB, Kottgen M. Identification of a urate transporter, ABCG2, with a common functional polymorphism causing gout. Proc Natl Acad Sci U S A. 2009 Jun 23;106(25):10338–42. doi: 10.1073/pnas.0901249106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Facheris MF, Hicks AA, Minelli C, et al. Variation in the Uric Acid Transporter Gene SLC2A9 and Its Association with AAO of Parkinson's Disease. J Mol Neurosci. 2011 Mar;43(3):246–50. doi: 10.1007/s12031-010-9409-y. [DOI] [PubMed] [Google Scholar]
- 27.Gao J, Xu H, Huang X, Chen H. Short communication: genetic variations of SLC2A9 in relation to Parkinson's disease. Translational neurodegeneration. 2013;2(1):5. doi: 10.1186/2047-9158-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abbott RD, Ross GW, White LR, et al. Midlife adiposity and the future risk of Parkinson's disease. Neurology. 2002 Oct 8;59(7):1051–7. doi: 10.1212/wnl.59.7.1051. [DOI] [PubMed] [Google Scholar]
- 29.Chen H, Zhang SM, Schwarzschild MA, Hernan MA, Willett WC, Ascherio A. Obesity and the risk of Parkinson's disease. Am J Epidemiol. 2004 Mar 15;159(6):547–55. doi: 10.1093/aje/kwh059. [DOI] [PubMed] [Google Scholar]
- 30.Petrie JL, Patman GL, Sinha I, Alexander TD, Reeves HL, Agius L. The rate of production of uric acid by hepatocytes is a sensitive index of compromised cell ATP homeostasis. American journal of physiology Endocrinology and metabolism. 2013;305(10):E1255–65. doi: 10.1152/ajpendo.00214.2013. [DOI] [PubMed] [Google Scholar]
- 31.Greenamyre JT, Cannon JR, Drolet R, Mastroberardino PG. Lessons from the rotenone model of Parkinson's disease. Trends Pharmacol Sci. 2010;31(4):141–2. doi: 10.1016/j.tips.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwarzschild MA, Macklin EA, Ascherio A, Parkinson Study Group SURE-PD Investigators Urate and neuroprotection trials. Lancet Neurol. 2014 Aug;13(8):758. doi: 10.1016/S1474-4422(14)70138-3. [DOI] [PubMed] [Google Scholar]
- 33.Kolz M, Johnson T, Sanna S, et al. Meta-analysis of 28,141 individuals identifies common variants within five new loci that influence uric acid concentrations. PLoS Genet. 2009 Jun;5(6):e1000504. doi: 10.1371/journal.pgen.1000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Do TM, Noel-Hudson MS, Ribes S, et al. ABCG2- and ABCG4-mediated efflux of amyloid-beta peptide 1-40 at the mouse blood-brain barrier. J Alzheimers Dis. 2012;30(1):155–66. doi: 10.3233/JAD-2012-112189. [DOI] [PubMed] [Google Scholar]
- 35.Schumacher T, Krohn M, Hofrichter J, et al. ABC transporters B1, C1 and G2 differentially regulate neuroregeneration in mice. PLoS ONE. 2012;7(4):e35613. doi: 10.1371/journal.pone.0035613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vautier S, Milane A, Fernandez C, Chacun H, Lacomblez L, Farinotti R. Role of two efflux proteins, ABCB1 and ABCG2 in blood-brain barrier transport of bromocriptine in a murine model of MPTP-induced dopaminergic degeneration. Journal of pharmacy & pharmaceutical sciences : a publication of the Canadian Society for Pharmaceutical Sciences, Societe canadienne des sciences pharmaceutiques. 2009;12(2):199–208. doi: 10.18433/j3b596. [DOI] [PubMed] [Google Scholar]
- 37.International Parkinson Disease Genomics Consortium Imputation of sequence variants for identification of genetic risks for Parkinson's disease: a meta-analysis of genome-wide association studies. Lancet. 2011 Feb 19;377(9766):641–9. doi: 10.1016/S0140-6736(10)62345-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.The Parkinson Study Group SURE-PD Investigators. Schwarzschild MA, Ascherio A, et al. Inosine to increase serum and cerebrospinal fluid urate in Parkinson disease: a randomized clinical trial. JAMA Neurol. 2014;71(2):141–50. doi: 10.1001/jamaneurol.2013.5528. [DOI] [PMC free article] [PubMed] [Google Scholar]