Abstract
Background
Hypospadias is a relatively common birth defect affecting the male urinary tract. We explored the etiology of hypospadias by examining its spatial distribution in North Carolina and the spatial clustering of residuals from individual and environmental risk factors.
Methods
We used data collected by the North Carolina Birth Defects Monitoring Program from 2003-2005 to estimate local Moran's I statistics to identify geographic clustering of overall and severe hypospadias, using 995 overall cases and 16,013 controls. We conducted logistic regression and local Moran's I statistics on standardized residuals to consider the contribution of individual variables (maternal age, maternal race/ethnicity, maternal education, smoking, parity, and diabetes) and environmental variables (block group land cover) to this clustering.
Results
Local Moran's I statistics indicated significant clustering of overall and severe hypospadias in eastern central North Carolina. Spatial clustering of hypospadias persisted when controlling for individual factors, but diminished somewhat when controlling for environmental factors. In adjusted models, maternal residence in a block group with more than 5% crop cover was associated with overall hypospadias (OR = 1.22; 95% CI = 1.04 – 1.43); that is living in a block group with greater than 5% crop cover was associated with a 22% increase in the odds of having a baby with hypospadias. Land cover was not associated with severe hypospadias.
Conclusions
This study illustrates the potential contribution of mapping in generating hypotheses about disease etiology. Results suggest that environmental factors including proximity to agriculture may play some role in the spatial distribution of hypospadias.
Keywords: Hypospadias, agriculture, North Carolina, Moran's I, spatial clustering
Introduction
Hypospadias is a relatively common urinary tract defect affecting between 1 of 200 to 1 of 300 live male births (Baskin and Ebbers, 2005). It is characterized by a urethral opening on the underside of the penis, and can vary in degree according to the location and size of the urethral opening, with first degree cases being the least severe and third degree cases being the most severe (Manson and Carr, 2003). Without surgery to repair the defect, it can result in urinary or sexual problems, particularly in more severe cases (Mieusset and Soulie, 2013). It is believed to have a multifactorial etiology where individual level and environmental level risk factors, as well as genetic influences, may play a role (Manson and Carr, 2003; Carmichael et al, 2012).
Previous studies have suggested a number of individual risk factors for hypospadias, including maternal health and other characteristics, parental genetics, and infant characteristics. Among maternal health factors, untreated hypertension (OR: 2.0-4.8) (Akre et al, 2008; Caton et al, 2007; Sun et al, 2009); thyroid disease (OR: 1.6) (Browne et al, 2009); and pre-existing diabetes (OR: 2.2) (Porter et al, 2005) all seem to increase risk. There is conflicting evidence regarding maternal BMI, with some researchers finding greater risk among mothers with a BMI above 26 (OR: 1.3) (Carmichael et al, 2007), but with others finding no evidence of an increased risk (Adams et al, 2011). Risk increases with maternal age (OR 1.5 for women over age 35, 1.7 for women over age 40) (Porter et al, 2005; Fisch et al, 2001), and is also highest among non-Hispanic whites (ORs for Black mothers 0.59 - 0.67, ORs for Hispanic mothers 0.46 - 0.66 and ORs for Asian mothers 0.55 – 0.73) (Porter et al, 2005; Carmichael et al, 2003). There is also conflicting evidence of an association between maternal education and hypospadias risk, with odds ratios ranging from 0.4 (Pierik et al, 2004) to 1.5 (Carmichael et al, 2003) for more highly educated mothers.
Behavioral risk factors include maternal use of progestins, which is associated with increased risk (OR 3.7) (Carmichael et al, 2005). There is some evidence that maternal diet may play a role, with certain dietary factors perhaps reducing risk (OR for Vitamin B12 = 0.7; OR for methionine = 0.6) (Carmichael et al 2008). One study suggests that maternal smoking may be associated with decreased risk (OR: 0.83) (Kallen, 2002), although confounding is suspected in that study because the association was only observed among mothers of parity 1 or 4+; and other studies find no association between smoking and hypospadias (Carmichael et al, 2005) Parental genetics also likely play a role, as boys whose fathers had hypospadias are also more likely to be born with the defect (OR = 9.7) (Brouwers et al, 2006). Among infant characteristics, first-borns are at a higher risk than higher-parity children (OR = 1.3 - 2.7) (Kallen, 2002; Carmichael et al, 2007)
The potential role played by environmental factors is less well understood. It is plausible that endocrine-disrupting chemicals, including some pesticides, might interrupt normal urethral closure and lead to hypospadias (Gray et al, 2004; Baskin and Ebbers, 2006). The evidence to support this hypothesis is mixed, however. A meta-analysis of studies conducted between 1966 and 2008 found a modest association between hypospadias and maternal occupational pesticide exposure (PRR = 1.4) and paternal occupational pesticide exposure (PRR = 1.2) (Rocheleau et al, 2009), but another review of environmental and genetic contributors to hypospadias concluded that a clear association cannot be made between endocrine-disrupting exposures and hypospadias, and called for further study (Carmichael et al, 2012).
Previous studies of the geographic distribution of hypospadias have identified spatial clustering and suggested possible explanatory variables. Aho et al (2003) calculated hypospadias prevalence for each of the municipalities in Finland, and observed an association between prevalence and remoteness from an urban area, while Li et al (2011) calculated prevalence by region in China and observed an association between urban areas and hypospadias. Abdullah et al (2006) used Potthoff-Whittinghill tests of homogeneity to identify statistically significant clustering of hypospadias in Northern England, and found an association with lower levels of deprivation. Nelson et al (2003) used quasi-likelihood estimation to examine spatial heterogeneity of hypospadias in the United Kingdom, and found a positive association with districts with surface water supply and a negative association with districts with a large non-white population. There is also some evidence that the etiology of hypospadias may vary by severity. Siblings are at a lower risk of hypospadias when an older brother has a milder degree of hypospadias compared with siblings of older brothers who have severe hypospadias (Bauer et al 1979, Fredell et al 2002).This suggests that genetic, or individual, factors may play a more dominant role in more severe cases and environmental factors may be more important in the etiology of less severe cases.
In this study, we seek to build on the current knowledge of the etiology of hypospadias by identifying spatial clustering of hypospadias in North Carolina from 2003 to 2005. Then, because variations in the spatial distribution of disease may be due to individual effects (resulting from differences among people who live in different places) or due to environmental effects (resulting from external influences that differ between places), we seek to disentangle risk factors contributing to spatial clustering of hypospadias (Macintyre and Ellaway, 2003). We consider a number of individual maternal risk factors, including age, race/ethnicity, marital status, smoking, diabetes, socioeconomic status, and parity. Amongst environmental risk factors, we focus on land use, especially agriculture, and we consider the possibility of different etiologies for different levels of severity.
Each of the prior studies have used methods that required aggregation of cases and controls, either via mapping and comparing prevalence at the regional level (Aho et al, 2003; Li et al, 2011), or through use of the Potthoff-Whittinghill tests of homogeneity (Abdullah et al, 2006) or quasi-likelihood estimation (Nelson et al, 2003). We use an alternative approach to investigating the geographic distribution of hypospadias in North Carolina by examining clustering of residuals, which allows us to consider spatial clustering, and the influence of covariates on that clustering, at the individual level. To our knowledge, we are the first to examine individual and environmental factors affecting the spatial distribution of birth defects using this novel approach. To our knowledge, we are also the first to examine the spatial distribution of hypospadias in the United States.
Methods
Data
Data were collected by the North Carolina Birth Defects Monitoring Program (NCBDMP), which is a population-based, active surveillance system. NCBDMP field staff review hospital medical records and discharge reports and regularly report malformations to the Registry. NCBDMP also links data about cases and controls to vital records to provide demographic information about both mother and infant and geocodes maternal address at birth.
This study population included all successfully geocoded North Carolina resident women who delivered a live-born singleton infant with hypospadias (n = 995), and a 10% random sample of successfully geocoded women who delivered a singleton male infant without a known birth defect in North Carolina (n = 16,013) in 2003-2005. We conducted supplementary analyses on a subset of the cases with second and third degree hypospadias (n = 332). A total of124 cases (10.6% of all cases) and 11,518 controls (6.5% of the full male control population) were not successfully geocoded. We used data from 2003 to 2005 because these were the most recent years for which geocoded data was available and which overlapped with the environmental data used for the study.
In this study, we refer to first, second, and third degree cases, as well as cases with no degree specified (NOS), as overall hypospadias, and second and third degree cases as severe hypospadias. First-degree and hypospadias NOS may be more prone to differing diagnoses by different doctors. However, hypospadias screening is a routine element of regular newborn assessments (Baskin, 2000), which should reduce the number of overlooked cases. Further, the mechanism by which environmental factors affect hypospadias risk may be subtle, so we included first-degree and hypospadias NOS to allow for the possibility of varying etiologies at differing levels of severity.
We used birth certificate data to evaluate the role played by individual characteristics identified by existing literature. These characteristics included maternal age at delivery, maternal race/ethnicity (classified for this study as non-Hispanic white, non-Hispanic black, Hispanic, and other), maternal smoking, maternal diabetes, previous births, and maternal education.
We used land use characteristics to consider the role played by environmental variables in hypospadias risk. We used the 2006 National Land Cover Database from the US Geological Survey to classify the percent of each block group used by various land classes (developed land, crops, pasture, and forest). Due to the large number of block groups with no crops, we classified percent crop cover into the following three categories: block groups with zero crop cover, block groups with 0-5% crop cover; and block groups with greater than 5% crop cover. These environmental variables were created using ArcGIS v. 10.
Analysis
To consider the geographic distribution of overall hypospadias and severe hypospadias, we estimated local Moran's I statistics to identify statistically significant clustering of high values (cases). Local Moran's I statistics provide “an indication of the extent of significant spatial clustering of similar values” around an observation (Anselin, 1995). In this case, we used local Moran's I statistics to identify individual “high-high” births throughout the state, which signify hypospadias cases clustered near other hypospadias cases. We applied a False Discovery Rate Correction to account for multiple testing. We then aggregated the total number of high-high births in each block group to allow visualization of the clusters without compromising patient confidentiality by displaying the locations of individual cases. The results of the local Moran's I analyses for overall hypospadias were validated with SaTScan's Bernoulli model, which uses a moving circular window of varying sizes up to 50% of the population at risk to identify the location and size of disease clusters (Kulldorff, 2010). SaTScan's Bernoulli model is most appropriate for the individual level, case/control data; however, it does not allow for adjustment for covariates.
To consider the association between individual level variables and hypospadias risk, we conducted logistic regression on all overall hypospadias cases and a 10% sample of controls, using individual level risk factors drawn from the existing literature. In order to consider whether these individual level variables helped explain the spatial clustering of hypospadias, we calculated the standardized residuals from the individual regression model by dividing individual raw residuals by their standard deviation. We repeated local Moran's I statistics on the standardized residuals from the individual-level analysis to identify high-high cases that remained after accounting for individual-level factors. We applied the False Discovery Rate Correction to account for multiple testing, and aggregated the total number of high-high cases to the block group level for visualization purposes.
To consider the combined effect of individual and environmental variables on the spatial distribution of hypospadias, we added environmental variables measuring land use into the individual-level regression models described above. Of the environmental variables considered, only crop use was statistically significant, so developed land, pasture, and forest were not included in the combined individual/environmental model. We compared goodness of fit between the individual level model and the combined individual/environmental model using Akaike information criterion. We also estimated a multilevel model to consider any potential nesting within block groups, but the interclass correlation was near 0 (0.04), so we returned to the single-level model.
To consider whether inclusion of these environmental variables helped to better explain the spatial distribution of hypospadias risk, we calculated standardized residuals from the combined individual/environmental model. We then repeated local Moran's I statistics on the standardized residuals, using the False Discovery Rate Correction, to identify remaining high-high values, and aggregated the total number of high-high values per block group for visualization.
We also conducted global Moran's I statistics to compare spatial clustering from the residuals of the individual model and the combined individual/environmental model. While local Moran's I statistics identify the location of clustered cases, global Moran's I statistics indicate whether there is clustering or dispersion throughout the study area, so we used global Moran's I statistics to compare overall unexplained clustering from the individual and combined models.
Given the possibility of ascertainment bias for first degree and hypospadias NOS, we repeated the Moran's I, as well as the individual and combined logistic regression analyses, using only severe hypospadias cases and the same population of controls to assess the robustness of the analysis of overall hypospadias.
Results
Descriptive statistics suggested that, on average, mothers of hypospadias cases tended to be slightly older, more likely to be non-Hispanic white, lower parity, and have more than high school education (Table 1).
Table 1.
Descriptive statistics for hypospadias cases and controls.
| Cases | Controls | ||||
|---|---|---|---|---|---|
| Characteristic | N | % | N | % | P-value |
| Maternal age | <0.01 | ||||
| <20 | 108 | 10.9 | 1,885 | 11.8 | |
| 20-24 | 219 | 22.0 | 4,295 | 26.8 | |
| 25-29 | 274 | 27.6 | 4,263 | 26.6 | |
| 30-34 | 250 | 25.1 | 3,623 | 22.6 | |
| 35+ | 144 | 14.5 | 1,946 | 12.2 | |
| Maternal race/ethnicity | <0.01 | ||||
| Non-Hispanic white | 701 | 70.5 | 9,426 | 58.9 | |
| Non-Hispanic black | 213 | 21.4 | 3,600 | 22.5 | |
| Hispanic | 56 | 5.6 | 2,316 | 14.5 | |
| Other race | 25 | 2.5 | 671 | 4.2 | |
| Smoking | 0.19 | ||||
| No | 886 | 89.1 | 14,026 | 87.7 | |
| Yes | 109 | 11.0 | 1,976 | 12.4 | |
| Previous live births | <0.01 | ||||
| No | 490 | 49.3 | 6,590 | 41.2 | |
| Yes | 505 | 50.7 | 9,415 | 58.8 | |
| Diabetes | 0.40 | ||||
| No | 965 | 97.0 | 15,600 | 97.4 | |
| Yes | 30 | 3.0 | 413 | 2.6 | |
| Maternal education | <0.01 | ||||
| Less than high school | 158 | 15.9 | 3,619 | 22.7 | |
| High school | 280 | 28.3 | 4,583 | 28.7 | |
| More than high school | 553 | 55.8 | 7,769 | 48.6 | |
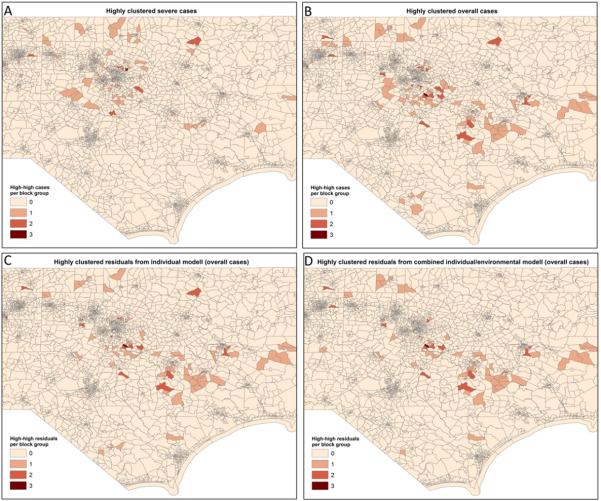
Local Moran's I statistics for hypospadias cases and controls showed significant high-high clustering in the eastern central portion of North Carolina for both overall hypospadias and second and third degree hypospadias. While there were fewer severe cases than overall cases, Census block groups with more than one highly clustered case tended be located in the same region (Figure 1, Panels A and B). SaTScan analyses (not shown) confirmed that the only Census block group in North Carolina with 3 high-high overall cases also contained the center of the primary cluster for overall hypospadias. Although it was not statistically significant, the primary cluster identified by SaTScan had 16 observed hypospadias cases falling within a 7.6 km radius where only 4.6 cases would have been expected. The block group with the 3 high-high cases and the primary SaTScan cluster is located in Johnston County, which has a very rapidly growing population, and which has historically been farmed.
Figure 1.
Local clustering of overall hypospadias, severe hypospadias, and model residuals for overall hypospadias in North Carolina, 2003-2005. High-high cases and residuals identified by Local Moran's I statistics are summed at the Census block group level to protect confidentiality. Map is focused on central North Carolina to show detail of the main cluster.
Of the eight individual variables considered based on existing literature, four were statistically significant at the p<0.05 level (Table 2). Consistent with descriptive statistics, increased maternal age, non-Hispanic white maternal race/ethnicity, and no previous births were associated with increased risk for overall and severe hypospadias. Smoking was significantly associated with decreased risk for more severe cases. Diabetes and maternal education were not associated with hypospadias. The local Moran's I of the standardized residuals from the individual model for overall hypospadias showed a very similar spatial pattern to that of the overall hypospadias cases. Census blocks with the greatest number of highly clustered cases or residuals remained in and around Johnston County (Figure 1, Panel C).
Table 2.
Results of logistic regression model including individual risk factors for hypospadias in North Carolina, 2003 – 2005.
| Hypospadias (all degree) | Hypospadias (2nd, 3rd degree) | |||
|---|---|---|---|---|
| Characteristic | OR | 95% CI | OR | 95% CI |
| Maternal age | ||||
| <20 | 1.00 | Referent | 1.00 | Referent |
| 20-24 | 1.01 | 0.78 – 1.31 | 1.09 | 0.69 – 1.75 |
| 25-29 | 1.30 | 0.99 – 1.70 | 1.57 | 0.97 – 2.55 |
| 30-34 | 1.38 | 1.04 – 1.84 | 1.98 | 1.19 – 3.27 |
| 35+ | 1.49 | 1.09 – 2.04 | 2.14 | 1.24 – 3.69 |
| Maternal race/ethnicity | ||||
| Non-Hispanic white | 1.00 | Referent | 1.00 | Referent |
| Non-Hispanic black | 0.85 | 0.72 – 1.00 | 0.84 | 0.63 – 1.12 |
| Hispanic | 0.33 | 0.24 – 0.45 | 0.31 | 0.18 – 0.52 |
| Other race | 0.50 | 0.34 – 0.76 | 0.37 | 0.16 – 0.84 |
| Smoking | ||||
| No | 1.00 | Referent | 1.00 | Referent |
| Yes | 0.83 | 0.67 – 1.04 | 0.40 | 0.24 – 0.65 |
| Previous live births | ||||
| No | 1.00 | Referent | 1.0 | Referent |
| Yes | 0.69 | 0.60 – 0.79 | 0.65 | 0.51 – 0.82 |
| Diabetes | ||||
| No | 1.00 | Referent | 1.00 | Referent |
| Yes | 1.15 | 0.79 – 1.68 | 0.81 | 0.38 – 1.73 |
| Maternal education | ||||
| Less than high school | 1.00 | Referent | 1.00 | Referent |
| High school | 1.02 | 0.82 – 1.27 | 0.97 | 0.66 – 1.44 |
| More than high school | 0.95 | 0-75 – 1.20 | 0.71 | 0.47 – 1.08 |
When environmental variables were incorporated into the combined individual/environmental model, living in a block group with greater than 5% crop cover as compared to living in a block group with zero crop cover was found to be significantly associated with overall hypospadias risk (OR = 1.22; 95% CI = 1.04 -1.43). Living in a block group with greater than 5% crop cover was not associated with severe hypospadias (Table 3). Other land use variables (developed land, pasture, and forest) were not significant and were excluded from the combined model.
Table 3.
Results of logistic regression model including individual and environmental risk factors for hypospadias in North Carolina, 2003 – 2005.
| Hypospadias (all degree) | Hypospadias (2nd, 3rd degree) | |||
|---|---|---|---|---|
| Characteristic | OR | 95% CI | OR | 95% CI |
| Maternal age | ||||
| <20 | 1.00 | Referent | 1.00 | Referent |
| 20-24 | 1.04 | 0.81 – 1.34 | 1.18 | 0.75 – 1.87 |
| 25-29 | 1.33 | 1.02 – 1.73 | 1.59 | 0.99 – 2.56 |
| 30-34 | 1.39 | 1.05 – 1.84 | 1.94 | 1.18 – 3.19 |
| 35+ | 1.50 | 1.10 – 2.03 | 2.14 | 1.26 – 3.66 |
| Maternal race/ethnicity | ||||
| Non-Hispanic white | 1.00 | Referent | 1.00 | Referent |
| Non-Hispanic black | 0.87 | 0.74 – 1.02 | 0.91 | 0.68 – 1.20 |
| Hispanic | 0.36 | 0.27 – 0.48 | 0.36 | 0.22 – 0.60 |
| Other race | 0.49 | 0.32 – 0.73 | 0.37 | 0.17 – 0.85 |
| Smoking | ||||
| No | 1.00 | Referent | 1.00 | Referent |
| Yes | 0.86 | 0.69 – 1.06 | 0.42 | 0.26 – 0.67 |
| Previous live births | ||||
| No | 1.00 | Referent | 1.00 | Referent |
| Yes | 0.71 | 0.62 – 0.81 | 0.69 | 0.54 – 0.87 |
| Diabetes | ||||
| No | 1.00 | Referent | 1.00 | Referent |
| Yes | 1.17 | 0.81 – 1.68 | 0.77 | 0.36 – 1.65 |
| Maternal education | ||||
| Less than high school | 1.00 | Referent | 1.00 | Referent |
| High school | 1.03 | 0.83 – 1.27 | 1.00 | 0.68 – 1.46 |
| More than high school | 0.96 | 0.77 – 1.21 | 0.74 | 0.50 – 1.11 |
| Block group crop cover | ||||
| 0% in crops | 1.00 | Referent | 1.00 | Referent |
| 0-5% in crops | 1.05 | 0.90 – 1.22 | 1.22 | 0.95 – 1.57 |
| >5% in crops | 1.22 | 1.04 – 1.43 | 0.99 | 0.74 – 1.34 |
Overall model fit remained similar after introducing environmental variables to the combined model. The Akaike information criterion (AIC) reduced slightly from 7787.4 for the individual model to 7785.5 for the combined model for overall hypospadias, indicating a small improvement in model fit when environmental variables were incorporated into models for overall hypospadias. On the other hand, AIC increased slightly from 3235.1 for the individual model to 3236.4 for the combined model for severe hypospadias, indicating that model fit worsened slightly when environmental variables were included into models for severe hypospadias.
The global Moran's I for the unexplained clustering from the two models is also similar (Index = 0.0004 for the individual model and Index = 0.0007 for the combined individual/environmental model). Moran's I values range from -1, which indicates perfect dispersion throughout the study area, to +1, which indicates perfect clustering throughout the study area. Global Moran's I values near zero for both the individual model and the combined model indicate that clustering across the study area is essentially random.
The local Moran's I of the standardized residuals from the combined model for overall hypospadias shows somewhat less spatial clustering of unexplained risk in the eastern central portion of the state. When compared to the map of highly clustered cases and the map of highly clustered residuals from the individual model, the number of highly clustered residuals from the combined model declines in some of the block groups, although the primary cluster remains in Johnston County (Figure 1, Panel D).
Discussion
Moran's I analysis identified significant local spatial clustering of overall hypospadias risk in eastern central North Carolina between 2003 and 2005. Spatial clustering of severe cases was reduced somewhat due to the smaller number of cases, although statistically significant clustering remained in Johnston County even after excluding first degree and unspecified hypospadias. Logistic regression identified several important individual factors contributing to overall and more severe hypospadias risk. However, even when controlling for these effects, local spatial clustering remained, which suggested that environmental factors or unmeasured individual factors that vary spatially might be playing a role in the distribution of hypospadias in this area.
Spatial clustering of residuals was concentrated in eastern central North Carolina, which is known for its agricultural production, including corn, tobacco, soybeans, sweet potatoes, and winter wheat. In fact, logistic regression indicated that living in a block group with greater than 5% crop cover was associated with a 22% increase with the odds of having a baby with hypospadias. This suggests that exposure to agriculture might be correlated with overall hypospadias. This may also be consistent with previous studies conducted by Meyer et al (2006) who found an association between hypospadias and diclofop-methyl, which is used on wheat, and previous studies by Agopian et al (2013) who found an association between hypospadias and atrazine, which is used on broadleaf crops including corn.
Contrary to overall hypospadias, crop cover was not associated with severe hypospadias. One possible explanation of the different result is that there may be different etiologies for different levels of severity. Another possible explanation is that clustering of overall hypospadias might be influenced by greater diagnosis of first degree hypospadias or assignment of hypospadias NOS at a particular facility or in a particular area. Spatial clustering of hypospadias remains in Johnston County even when cluster analyses are conducted on severe cases, which are less likely to be subject to differential diagnosis than first degree cases. Spatial variation in diagnosis does remain a possibility, however, both in Johnston County, and elsewhere in the state. Further, while we have controlled to the extent possible for both individual and environmental risk factors that might explain the spatial distribution of hypospadias, it is possible that other unmeasured hypospadias risk factors such as maternal/paternal behavior or genetics might also play a role in this distribution.
In addition, this study only has access to information about maternal address at birth, which means that if mothers moved during their pregnancy, we may not be accurately capturing environmental effects during the relevant time of gestation. Yet a study of exposure to air pollution using New York data found that only 16.5% of mothers moved during pregnancy, and most moved within such short distances that exposure assignments did not change substantially (Chen et al, 2010). Other studies have found much higher mobility during pregnancy – 33% of case and 31% of control mothers – but suggested that while maternal mobility may lead to exposure misclassification, any such misclassification is likely to be non-differential, which would tend to bias the results toward the null (Canfield et al, 2006).
One of the main limitations of the local Moran's I statistic is the possibility of multiple testing and spatial dependency, which might lead to false identification of clustering. To address this concern, we applied a False Discovery Rate Correction to reduce the critical p-value thresholds for each local Moran's I in this study.
Previous studies of the spatial distribution of hypospadias have focused on Europe and Asia, and have aggregated data in their cluster analyses. This study builds upon that work by using individual level data from a population-based birth defects surveillance system, and by considering the spatial distribution of hypospadias in North Carolina. There is also some ambiguity in the existing literature about a possible association between hypospadias and exposure to pesticides. This study lends some support to the possibility of such association, although further research is needed.
This study illustrates the potential contribution of mapping the spatial distribution of birth defects and other diseases to generating hypotheses about disease etiology, and to investigating the relative contribution of individual and environmental effects. The associations found with hypospadias in this analysis should not be interpreted as implying causality, and further research is needed to evaluate these findings. Future work will investigate the mechanism by which exposure to agriculture may be contributing to hypospadias risk in North Carolina. This ultimately might help inform policy interventions to help reduce hypospadias risk.
Acknowledgements
This research received support from the Population Research Training grant (T32 HD007168) and the Population Research Infrastructure Program (R24 HD050924) awarded to the Carolina Population Center at the University of North Carolina at Chapel Hill by the Eunice Kennedy Shriver National Institute of Child Health and Human Development. This research also received support from the National Science Foundation (BCS-0924479).
Footnotes
The co-authors have no conflict of interest to declare.
Literature Cited
- Abdulllah NA, Pearce MS, Parker L, Wilkinson JR, McNally RJQ. Evidence of an environmental contribution to the aetiology of cryptorchidism and hypospadias? European Journal of Epidemiology. 22(9):615–620. doi: 10.1007/s10654-007-9160-z. [DOI] [PubMed] [Google Scholar]
- Adams SV, Hastert TA, Huang Y, Starr JR. No association between maternal pre-pregnancy obesity and risk of hypospadias or cryptorchidism in male newborns. Birth Defects Research Part A: Clinical and Molecular Teratology. 2011;91(4):241–248. doi: 10.1002/bdra.20805. doi:10.1002/bdra.20805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agopian AJ, Lupo PJ, Canfield MA, Langlois PH. Case-Control Study of Maternal Residential Atrazine Exposure and Male Genital Malformations. American Journal of Medical Genetics Part A. 2013;161(5):977–982. doi: 10.1002/ajmg.a.35815. [DOI] [PubMed] [Google Scholar]
- Aho MO, Koivito AM, Tammela TLJ, Auvinen AP. Geographical differences in the prevalence of hypospadias in Finland. Environmental Research. 92(2):118–123. doi: 10.1016/s0013-9351(02)00089-0. [DOI] [PubMed] [Google Scholar]
- Akre O, Boyd HA, Ahlgren M, Wilbrand K, Westergaard T, Hjalgrim H, Nordenskjold A, Ekbom A, Melbye M. Maternal and gestational risk factors for hypospadias. Environmental Health Perspectives. 2008;116:8, 1071–1078. doi: 10.1289/ehp.10791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anselin L. Local Indicators of Spatial Association – LISA. Geographical Analysis. 1995;27(2):93–115. [Google Scholar]
- Baskin LS. Hypospadias and urethral development. The Journal of Urology. 2000;163(3):951–956. [PubMed] [Google Scholar]
- Baskin LS, Ebbers MB. Hypospadias: anatomy, etiology, and technique. Journal of Pediatric Surgery. 2006;41(3):463–472. doi: 10.1016/j.jpedsurg.2005.11.059. [DOI] [PubMed] [Google Scholar]
- Bauer SB, Bull MJ, Retik AB. Hypospadias: A familial study. The Journal of Urology. 1979;121(4):474–477. doi: 10.1016/s0022-5347(17)56832-9. [DOI] [PubMed] [Google Scholar]
- Brouwers MM, Feitz WFJ, Roelofs LAJ, Kiemeney LALM, de Gier RPE, Roeleveld N. Risk factors for hypospadias. European Journal of Pediatrics. 2007;166(7):671–678. doi: 10.1007/s00431-006-0304-z. [DOI] [PubMed] [Google Scholar]
- Browne ML, Rasmussen SA, Hoyt AT, Waller DK, Druschel CM, Caton AR, Canfield MA, Lin AE, Carmichael SL, Romitti PA. Maternal thyroid disease, thyroid medication use, and selected birth defects in the National Birth Defects Prevention Study. Birth Defects Research Part A: Clinical and Molecular Teratology. 2009;85(7):621–628. doi: 10.1002/bdra.20573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canfield MA, Ramadhani TA, Langlois PA, Waller K. Residential mobility patterns and exposure misclassification in epidemiologic studies of birth defects. Journal of Exposure Science and Environmental Epidemiology. 2006;16:538–543. doi: 10.1038/sj.jes.7500501. [DOI] [PubMed] [Google Scholar]
- Carmichael SL, Shaw GM, Lammer EJ. Environmental and genetic contributors to hypospadias: A review of the epidemiologic evidence. Birth Defects Research Part A: Clinical and Molecular Teratology. 2012;94:499–510. doi: 10.1002/bdra.23021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael SL, Shaw GM, Laurent C, Croughan MS, Olney RS, Lammer EJ. Maternal progestin intake and risk of hypospadias. Archives of Pediatrics and Adolescent Medicine. 2005;159(10):957–-962. doi: 10.1001/archpedi.159.10.957. [DOI] [PubMed] [Google Scholar]
- Carmichael SL, Shaw GM, Laurent C, Lammer EJ, Olney RS. Hypospadias and maternal exposures to cigarette smoke. Paediatric and Perinatal Epidemiology. 2005;19(6):406–412. doi: 10.1111/j.1365-3016.2005.00680.x. [DOI] [PubMed] [Google Scholar]
- Carmichael SL, Shaw GM, Laurent C, Olney RS, Lammer EJ. Maternal reproductive and demographic characteristics as risk factors for hypospadias. Paediatric and Perinatal Epidemiology. 2007;21(3):210–218. doi: 10.1111/j.1365-3016.2007.00809.x. [DOI] [PubMed] [Google Scholar]
- Carmichael SL, Shaw GM, Nelson V, Selvin S, Torfs CP, Curry CJ. Hypospadias in California: trends and descriptive epidemiology. Epidemiology. 2003;14(6):701–706. doi: 10.1097/01.ede.0000091603.43531.d0. [DOI] [PubMed] [Google Scholar]
- Caton AR, Bell EM, Druschel CM, Werler M, Mitchell AA, Browne ML, McNutt LA, Romitti PA, Olney RS, Correa A. Maternal hypertension, antihypertensive medication use, and the risk of severe hypospadias. Birth Defects Research Part A: Clinical and Molecular Teratology. 2007;82(1):34–40. doi: 10.1002/bdra.20415. [DOI] [PubMed] [Google Scholar]
- Chen L, Bell EM, Caton AR, Druschel CM, Lin S. Residential mobility during pregnancy and the potential for ambient air pollution exposure misclassification. Environmental Research. 2010;110(2):162–168. doi: 10.1016/j.envres.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Fisch H, Golden RJ, Libersen GL, Hyun GS, Madsen P, New MI, Hensle TW. Maternal age as a risk factor for hypospadias. Urology. 2001;165(3):934–936. [PubMed] [Google Scholar]
- Fredell L, Kockum I, Hansson E, Holmner S, Lundquist L, Lackgren G, Pedersen J, Stenberg A, Westbacke G, Nordenskjold A. Heredity of hypospadias and the significance of low birth weight. The Journal of Urology. 2002;167(3):1426–1427. [PubMed] [Google Scholar]
- Gray LE, Ostby J, Furr J, Wolf C, Lambright C, Wilson V, Noriega N. Toxicant-induced hypospadias in the male rat. Advances in Experimental Medicine and Biology. 2004;545:217–241. doi: 10.1007/978-1-4419-8995-6_14. [DOI] [PubMed] [Google Scholar]
- Hussain N, Chaghtai A, Herndon A, Herson VC, Rosenkrantz TS, McKenna PH. Hypospadias and early gestation growth restriction in infants. Pediatrics. 2002;109(3):473–478. doi: 10.1542/peds.109.3.473. [DOI] [PubMed] [Google Scholar]
- Kallen K. Role of maternal smoking and maternal reproductive history in the etiology of hypospadias in the offspring. Birth Defects Research Part A: Clinical and Molecular Teratology. 2002;66(4):185–191. doi: 10.1002/tera.10092. [DOI] [PubMed] [Google Scholar]
- Kulldorff M. SaTScan user guide for version 9.0. 2010.
- Li Y, Mao M, Dai L, Li K, Li X, Zhou G, Wang Y, Li Q, He C, Liang J, Zhu J. Time trends and geographic variations in the prevalence of hypospadias in China. Birth Defects Research Part A: Clinical and Molecular Teratology. 94(1):36–41. doi: 10.1002/bdra.22854. [DOI] [PubMed] [Google Scholar]
- Macintyre S, Ellaway A. Neighborhoods and health: An overview. In: Kawachi I, Berkman L, editors. Neighborhoods and health. Oxford University Press; New York: 2003. pp. 20–43. [Google Scholar]
- Manson JA, Carr MC. Molecular epidemiology of hypospadias: Review of genetic and environmental risk factors. Birth Defects Research Part A: Clinical and Molecular Teratology. 2003;67(10):825. doi: 10.1002/bdra.10084. [DOI] [PubMed] [Google Scholar]
- Meyer KJ, Reif JS, Veermacheneni DNR, Luben TJ, Mosley BS, Nuckols JR. Agricultural Pesticide Use and Hypospadias in Eastern Arkansas. Environmental Health Perspectives. 2006;114(10):1589–1595. doi: 10.1289/ehp.9146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mieusset R, Soulie R. Hypospadias: psychosocial, sexual, and reproductive consequences in adult life. Journal of Andrology. 2005;26(2):163–168. doi: 10.1002/j.1939-4640.2005.tb01078.x. [DOI] [PubMed] [Google Scholar]
- Nelson P, Elliot P, Nieuwenhuijsen M, Kold-Jensen T, Marshall C, Dudley ML. Geographical Epidemiology of Hypospadias (Abstract). Epidemiology. 14(5) [Google Scholar]
- Pierik FH, Burdorf A, Deddens JA, Juttmann RE, Weber RFA. Maternal and Paternal Risk Factors for Cryptorchidism and Hypospadias: A Case-Control Study in Newborn Boys. Environmental Health Perspectives. 2004;112:15, 1570–1576. doi: 10.1289/ehp.7243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter MP, Faizan MK, Grady RW, Mueller BA. Hypospadias in Washington State: Maternal risk factors and prevalence trends. Pediatrics. 2005;115(4):495–499. doi: 10.1542/peds.2004-1552. [DOI] [PubMed] [Google Scholar]
- Reefhuis J, Honein MA. Maternal age and non-chromosomal birth defects, Atlanta— 1968–2000: Teenager or thirty-something, who is at risk? Birth Defects Research Part A: Clinical and Molecular Teratology. 2004;70(9):572–579. doi: 10.1002/bdra.20065. [DOI] [PubMed] [Google Scholar]
- Rocheleau CM, Romitti PA, Dennis LK. Pesticides and hypospadias: A meta-analysis. Journal of Pediatric Urology. 2009;5(1):17–24. doi: 10.1016/j.jpurol.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun G, Tang D, Liang J, Wu M. Increasing prevalence of hypospadias associated with various perinatal risk factors in Chinese newborns. Urology. 2009;73(6):1241–1245. doi: 10.1016/j.urology.2008.12.081. [DOI] [PubMed] [Google Scholar]