Abstract
Objectives
To develop and validate a Juvenile Spondyloarthritis (JSpA) Disease Activity (JSpADA) index for use in clinical practice and research.
Methods
Using modified Delphi consensus techniques, ten items were selected by participants in the international pediatric rheumatology list-serve, the Childhood Arthritis and Rheumatology Research Alliance, and the list-serve for the Pediatric Section of the American College of Rheumatology. Validation was performed in a retrospective multicenter cohort of 243 children.
Results
106 physicians representing 14 countries completed the initial questionnaire. Completion rates for the subsequent questionnaires were 84%, 75%, and 77% of the original respondents. Ten items reached 80% consensus: arthritis, enthesitis, patient pain assessment, inflammatory markers, morning stiffness, clinical sacroiliitis, uveitis, back mobility, and patient and physician assessments of disease activity. Two items were eliminated after item analysis (patient and physician assessments of disease activity). Factor analysis identified 3 primary domains that explain 58% of variance: peripheral disease, axial disease, and uveitis. Cronbach α coefficient was 0.66. The JSpADA had high or moderate correlations with the Juvenile Arthritis disease activity score (r=0.80), patient and physician assessments of disease activity (r=0.70 and 0.66), and the Childhood Health Assessment Questionnaire (r=0.56). The JSpADA discriminated well between subjects with active versus inactive disease (p<0.001) and was responsive to improvement or worsening in disease activity over time (p<0.001).
Conclusion
Using international input and consensus formation techniques, we developed and validated the first disease activity assessment for JSpA. Future studies should validate the JSpADA index in a prospective multi-center cohort.
Spondyloarthritis (SpA) is a chronic inflammatory arthritis that affects 0.9 to 1.4% of adults in the United States1 and is characterized by peripheral arthritis, enthesitis, dactylitis, acute anterior uveitis, HLA-B27 positivity, inflammatory back pain, and sacroiliitis. The term SpA encompasses ankylosing spondylitis (AS), undifferentiated SpA, inflammatory bowel disease associated arthritis, psoriatic arthritis, and reactive arthritis. The term juvenile SpA refers to SpA that starts during childhood. Under the newer International Leagues of Associations for Rheumatology (ILAR) juvenile idiopathic arthritis (JIA) classification scheme2, most children with JSpA fall into the categories of enthesitis related arthritis (ERA), psoriatic arthritis, and undifferentiated arthritis. Treatment regimens for JSpA are suboptimal and primarily based on those developed for adults with AS, adult RA and children with other JIA categories.3 In comparison to other categories of JIA, children with JSpA have more frequent and higher intensity pain and poorer health status.4-6 They are less likely to achieve and to sustain inactive disease than other JIA categories.7,8 Further, observational studies suggest that less than 20% of children with JSpA achieve remission within five years of diagnosis.9 Progress in finding more effective therapies for JSpA cannot be made without validated and highly discriminatory instruments to assess disease activity and response to treatments.
The frequent use of a highly responsive composite disease activity score (DAS) greatly contributes to improved outcomes in rheumatoid arthritis (RA).10 The Tight Control for Rheumatoid Arthritis (TICORA) study demonstrated that in comparison to routine care, intensive outpatient management that included close monitoring of a composite DAS, monthly follow-up and aggressive treatment decisions based on the DAS improved remission rates by 4-fold (16% versus 65%) at 18 months.10 Several disease activity indices have been developed for adults with AS, including the AS disease activity score (ASDAS)11, and the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI).12 Since JSpA has less axial disease than adults with ankylosing spondylitis and since the BASDAI is predominantly focused on axial symptoms it is unlikely to be as useful in this pediatric population. Neither of these indices has been validated in children. Outcome measures available for monitoring JIA disease outcomes and activity include the ACR pedi-30/50/70/9013, and the Juvenile Arthritis Disease Activity Score (JADAS).14 The pedi ACR 30/50/70/90 are dichotomous and must always have a baseline measurement for comparison; therefore, the measure does not provide a continuous scale by which to assess disease activity and response levels cannot be compared across patients because each has a unique baseline value. JSpA patients were included in the validation study of the JADAS but were a minority (<1%; ~26 of 4363 subjects).14 Neither of these measures has been validated in JSpA nor do they explicitly account for the unique disease activity features of the condition such as axial disease and enthesitis. Despite the lack of a composite validated disease activity measure, investigators doing trials in JSpA have traditionally used the pedi ACR 30/50/70/90, and single measures such as active joint count, tender entheses count, physician and parent global disease activity assessments, c-reactive protein (CRP) and the childhood health assessment questionnaire (CHAQ).15,16
To our knowledge, this is the first effort to develop and validate a disease activity assessment tool for use in JSpA. Creation of the JSpADA index will enable the design and analysis of much needed effectiveness and efficacy studies focused on JSpA, an understudied category of JIA.
METHODS
Human subjects protections
The survey exercise was reviewed and determined exempt from Institutional Review Board limitations. The protocol for the retrospective validation was reviewed and approved by the Children's Hospital of Philadelphia Committee for the Protection of Human Subjects.
Development of the JSpADA index
Selection of items depicting disease activity
Volunteers were solicited from physicians who participate in the international pediatric electronic bulletin board, the Childhood Arthritis and Rheumatology Research Alliance (CARRA), and the list-serve for Pediatric Section of the American College of Rheumatology (ACR). Volunteers were sent four sequential electronic questionnaires that used modified Delphi techniques. Modified Delphi technique has been used successfully to develop the adult Ankylosing Spondylitis disease activity score (ASDAS) and inactive disease criteria for select categories of JIA.11,17 The initial questionnaire was open-ended and asked for signs and symptoms that should be considered when determining the disease activity of a child with JSpA. Respondents excluded from the full survey were those who were not pediatric rheumatologists (n=3), those who do not care for patients with JSpA (n=1), and those still in training (n=12). Seven respondents answered the screening questions but did not complete the actual survey. A team of 3 investigators (PFW, RAC, CAW) reviewed all items and grouped conceptually redundant items. For example, “swollen joint count” “inflamed joint count” “joint count” “active joint count” and “limited range of motion with warmth or pain” were all considered indicative of active arthritis. Items listed by >80% of respondents were considered to have consensus for inclusion in the JSpADA index. Items listed by greater than 10% but less than 80% of respondents formed the basis of the subsequent surveys.
The 2nd, 3rd, and 4th surveys were sent electronically to volunteers who answered the initial survey. Summary results of the antecedent exercise were presented to participants before administration of each new survey. The second survey contained 26 items (dichotomous, multiple choice, and open-ended items) and the third survey contained 9 dichotomous questions. Items receiving greater than 75% of votes were considered to have consensus. The fourth questionnaire consisted of a single dichotomous question aimed to clarify the definition of clinically relevant uveitis.
Items selected from the Delphi exercise were further tested in a retrospective database of 244 subjects from 5 pediatric rheumatology centers (Children's Hospital of Philadelphia, Philadelphia, PA; Cincinnati Children's Hospital, Cincinnati, OH; Texas Scottish Rite Hospital for Children, Dallas, TX; University of Alabama, Birmingham, AL; Meyer Children's Hospital, Florence, Italy) who had a new diagnosis of JSpA according to the treating physician from January 1989 to December 2012 (N=1,226 visits). Briefly, this database includes demographic, clinical, and laboratory data for each subject. Subjects in the database were followed for a median of 371 days (IQR: 311, 530 days).
The distribution and correlations of all items selected by the Delphi exercise were assessed. Continuous items were compared using spearman correlation. If two items were highly correlated with each other (r>0.75) and appeared to measure the same construct, one was removed from further analysis.18 Continuous items were transformed to values of 0, 0.5, or 1. The assignment of 0.5 or 1 for continuous items was based upon the distribution of the non-zero values for each item. Each item was considered to be of equal importance (value=1).
Validation
We assessed the validity of the JSpADA using the Outcome Measures in Rheumatoid Arthritis Clinical Trials (OMERACT) filter.19 This filter was created in 1992 to help standardize outcome measures utilized in rheumatology patient assessments and clinical trials. An outcome measure is accepted once it has passed 3 criteria: 1) Truth, which includes face, content, criterion, and construct validity; 2) Discrimination, which includes classification and sensitivity to change; and 3) Feasibility, which determines if the outcome measure can be easily applied given limitations of time and money. The OMERACT filter has been successfully applied to validate scales for JIA20,21.
Truth
Face and content validity were established through the modified Delphi questionnaire exercise. Face validity is demonstrated if the criteria are sensible on face value to physicians who utilize them in their field. Principal component analysis (PCA) with varimax rotation was used to verify that the candidate items measured a single construct. Internal consistency was assessed using Cronbach's α coefficient. Estimates greater than or equal to 0.6 were considered acceptable. Construct validity was tested using other measures of disease activity for JIA including the JADAS-10 (version of the JADAS that uses a 10-joint reduced count), patient and physician global assessments of disease activity, and the Childhood Health Assessment Questionnaire as reference standards. Spearman's correlation was used to evaluate the relationship between the JSpADA total score and other measures of JIA disease activity. A priori we hypothesized that the correlation with the JADAS-10, patient and physician global assessments of disease would be moderate to high secondary to closely related constructs. We also predicted that correlation with the Childhood Health Assessment Questionnaire (CHAQ) would be moderate.
Discrimination
Discriminant validity was evaluated by comparing the mean JSpA scores of visits with physician determined inactive disease (physician global disease activity assessment = 0) versus active disease (physician global disease activity assessment >0) using a two-tailed t-test test. Responsiveness to change in clinical disease activity over time was tested by comparing the mean change in the JSpADA score between visits in subjects who had improvement or worsening of disease activity according to the treating physician using a two-tailed t-test and the standardized mean difference. A standardized mean difference of >0.8 was considered evidence of large responsiveness.22
Feasibility
The stated purpose of the modified Delphi exercise was to develop a list of items that physicians prioritize when considering disease activity for JSpA. The exercise was prefaced with a statement that the items were intended to be easily measured/collected during routine clinical assessment. Therefore, we relied on the modified Delphi exercise to create a set of items that were easily and quickly collected as part of routine care, inexpensive, and presented minimal or no risk to the patient.
All statistical analyses were performed with Stata version 12.1 (StataCorp, College Station, TX).
RESULTS
Delphi exercise and selection of assessment items
One hundred and six physicians representing 14 countries from the pediatric rheumatology international list-serve, ACR Pediatric section, and CARRA membership volunteered to participate in the exercise. Eighty-nine physicians met inclusion criteria and completed the first survey (Table 1). Three items achieved consensus for inclusion in the JSpADA. The items included active arthritis, active enthesitis, and abnormal inflammatory markers. Items that achieved between 10% and 80% consensus included measures of sacroiliitis, function, pain, quality of life and both physician and patient global assessments of disease activity.
Table 1.
Participants who met inclusion criteria from the first Delphi exercise
| Number of respondents (%) | |
|---|---|
| Pediatric Rheumatologists | 89 (100) |
| Years in practice | |
| < 5 | 18 (20) |
| 5 - 10 | 26 (29) |
| > 10 | 45 (51) |
| Country | |
| Brazil | 5 (6) |
| Canada | 6 (7) |
| Germany | 2 (2) |
| India | 1 (1) |
| Israel | 2 (2) |
| Italy | 1 (1) |
| Mexico | 2 (2) |
| Norway | 1 (1) |
| Spain | 3 (3) |
| The Netherlands | 1 (1) |
| Turkey | 2 (2) |
| United Kingdom | 1 (1) |
| United States | 62 (70) |
Legend. Volunteers are from the pediatric rheumatology international list-serve, ACR Pediatric section, and CARRA membership.
The response rate to the second survey was 84% (N=89/106). Items that achieved consensus for inclusion in the JSpADA included: clinical sacroiliitis, morning stiffness, physician assessment of disease activity, uveitis, and abnormal back mobility. There was also consensus to define active enthesitis as tenderness on direct palpation. Consensus was not achieved on the following issues: definition of clinically active sacroiliitis, definition of clinically relevant ocular inflammation, and inclusion of patient-reported pain, disease activity, and global well-being.
There was a 75% (N=79/106) response rate to the third questionnaire. Consensus on a number of issues was achieved: inclusion of patient-reported peripheral and axial pain; inclusion of patient-reported global disease activity; definition of probable clinical sacroiliitis (defined as the presence of 2 or more of the following: tenderness on examination, positive Patrick or Flexion Abduction External Rotation (FABER) test and inflammatory back pain23); definition of clinically relevant morning stiffness as greater than 15 minutes; definition of abnormal spinal mobility as a modified Schober's test < 20 cm. Consensus was not achieved regarding the definition of clinically relevant ocular inflammation.
Responses for the fourth questionnaire were received from 77% (N= 82/106) of physicians. There was consensus to define clinically relevant eye inflammation as any uveitis (acute/symptomatic or chronic/asymptomatic).
Selection of proposed measurement scales
Once items reached consensus for inclusion in the JSpADA index, we aimed to develop measurement scales to evaluate each item. In the second survey there was consensus that when considering arthritis as part of the JSpADA the number of active joints was important, but there was no consensus on how to quantify the number of active joints. Similarly, there was consensus that when considering enthesitis as part of the JSpADA the number of active entheses was significant but there was no consensus on how to quantify the number of active entheses or which entheses should be examined routinely.
In the third survey, consensus on how to measure several items was achieved. There was consensus to quantify the number of active joints by a reduced 10-joint count that includes any involved joint, except the sacroiliac joints which are considered as a separate item in the JSpADA, to a maximum of 10 without weighting of particular joints. This is analogous to how arthritis is quantified with the reduced 10-jont count in the JADAS.14,24 Second, there was consensus to quantify enthesitis by a 10-entheses count, analogous to the 10-joint count, which includes any tender entheses to a maximum of 10 without weighting of particular entheses. Third, there was consensus to measure patient-reported peripheral and axial pain and patient- reported global disease activity over the past week with a visual analogue numeric rating scale (0-10). Lastly, based on recently published normal values for the modified Schober's test in a multiracial cohort of children (median 22 cm, interquartile range 20, 23 cm)25, there was consensus to define abnormal spinal mobility as a modified Schober's test less than 20 cm.
Among the ten items, patient and physician reported global disease activity and patient pain assessments were all highly correlated (r>0.75) so only patient pain assessment was retained for subsequent evaluation. This resulted in 8 items to construct the JSpADA score and the range of possible scores was 0 to 8 by assuming equal importance of each item, with higher scores indicating more disease activity (Table 2).
Table 2.
JSpADA item definitions and distributions in the validation cohort (N=597 visits)
| Item | Score * | N= 597 visits |
|---|---|---|
| 1. Active joint count: includes any involved joint to a maximum of 10. There is no weighting of particular joints; mean ± SD | 0 joints= 0 | 1.3 ±3.0 |
| 1-2 joints= 0.5 | ||
| >2 joints= 1 | ||
| 2. Active enthesitis count: includes any involved enthesis to a maximum of 10. There is no weighting of particular entheses; mean ± SD | 0 entheses= 0 | 1.4 ± 2.1 |
| 1-2 entheses= 0.5 | ||
| >2 entheses= 1 | ||
| 3. Pain: patient reported pain over the past week, recorded on a visual analogue scale (0, 10); mean ± SD | 0= 0 | 2.4 ± 2.7 |
| 1-4= 0.5 | ||
| 5-10= 1 | ||
| 4. ESR or CRP related to JSpA activity; N (%) | Normal = 0 | 461 (77) |
| 1-2 times normal= 0.5 | 83 (14) | |
| >2 times normal= 1 | 53 (9) | |
| 5. Morning stiffness: Morning stiffness for greater than 15 minutes; N (%) | Absent= 0 | 302 (51) |
| Present= 1 | 295 (49) | |
| 6. Clinical sacroiliitis: defined as the presence of 2 or more of the following: tenderness on examination, positive Patrick's or FABER test and inflammatory back pain#; N (%) | Absent= 0 | 516 (86) |
| Present= 1 | 81 (14) | |
| 7. Uveitis: Presence of any uveitis (including acute/symptomatic and chronic/asymptomatic disease); N (%) | Absent= 0 | 581 (97) |
| Present= 1 | 16 (3) | |
| 8. Back mobility: Abnormal back mobility defined as modified Schober's < 20 cm; N (%) | Normal= 0 | 581 (97) |
Legend. JSpADA Items.
Score is obtained by summing the total for each item (maximum total per item=1).
There are no validated inflammatory back pain criteria for children. For this index the definition of inflammatory back pain was adapted from the ASAS criteria23 for adults and was defined as present when 3 of the following criteria are met: 1) insidious onset, 2) improvement with exercise, 3) no improvement with rest, 4) pain at night (with improvement upon getting up). Range of possible scores is 0 to 8, with higher scores indicating more disease activity. SD= Standard deviation
Validation
The source population for the validation exercise was a retrospective multicenter longitudinal data set of children with a new diagnosis of JSpA. Only those visits with complete data for all JSpADA items were retained for further analysis (N= 178 subjects, 597 visits). These 178 subjects did not significantly differ from those subjects with missing data with respect to age, sex, symptom duration, active joint count or active enthesitis count at presentation (data not shown). 175 children met ILAR criteria for ERA and 3 met criteria for ERA but also had a first-degree relative with psoriasis. One hundred and twenty-eight (72%) of subjects were male, mean age was 12 years (SD+ 3.49) and 89 (50%) were HLA-B27 positive. There were 16 children older than 16 years at diagnosis in whom symptoms started prior to age 16. There was active enthesitis, peripheral arthritis or hip arthritis present at 44%, 50%, and 8% of visits respectively. There was clinical sacroiliitis detected at 14% of visits and sacroiliitis documented by MRI at 8% of visits.
Eight items were included for validation (Table 2). PCA identified three primary factors with eigenvalues greater than 1 with a cumulative explained variance of 0.58 (Table 3). The first factor included items related to peripheral disease (arthritis, enthesitis, morning stiffness, patient assessment of pain, inflammatory markers). The second factor included items related to axial disease (clinical sacroiliitis and back mobility). The third factor only included one item, uveitis. Cronbach's α was 0.66 and 0.70 for the total score and peripheral disease, respectively demonstrating acceptable internal consistency.
Table 3.
Factor identification.
| Item | Factor 1 “Peripheral disease” | Factor 2 “Axial disease” | Factor 3 “Uveitis” |
|---|---|---|---|
| 1. Active joint count | 0.41 | −0.37 | 0.15 |
| 2. Active enthesitis count | 0.42 | 0.24 | 0.17 |
| 3. Patient assessment of pain (past week) | 0.49 | −0.08 | −0.14 |
| 4. ESR or CRP related to JSpA activity | 0.27 | −0.58 | −0.15 |
| 5. Morning stiffness | 0.48 | 0.07 | −0.01 |
| 6. Clinical sacroiliitis | 0.32 | 0.64 | 0.08 |
| 7. Uveitis | 0.02 | 0.02 | 0.63 |
| 8. Back mobility | 0.08 | 0.21 | −0.71 |
Legend. Items of the JSpADA index loaded on three primary factors after PCA. Items 1-5 load on factor 1 “Peripheral disease”, items 6 and 8 load on factor 2 “Axial disease” and item 7 loads significantly on factor 3 “Uveitis”.
As predicted, correlation with the JADAS-10 was high (r=0.81). Correlations with the patient and physician global disease activity assessments were moderate to high at 0.70 and 0.66, respectively, demonstrating construct validity. Also as predicted, correlation with the CHAQ was moderate (r=0.56).
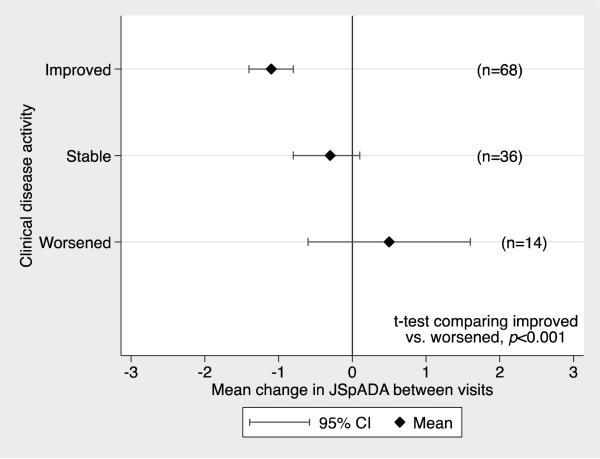
Discriminative validity was evaluated using physician global assessment as the external criterion. The mean JSpA scores for children with a physician VAS of 0 (N=211) and >0 (N=386) were 0.46 (SD=0.82) and 1.94 (SD=1.36), respectively (p-value <0.001). One hundred and eighteen subjects had a calculable JSpADA at 2 visits a median of 103 days apart (IQR: 55, 229 days). Thirty-six subjects had stable disease activity, 68 improved, and 14 were worse according to the treating physician. Using a two-tailed t-test, the mean change in JSpADA score was significantly different between those who improved and worsened (p<0.001), demonstrating excellent responsiveness (Figure 1). The standardized mean difference between those who improved and worsened was 1.07, demonstrating large responsiveness.
Figure 1. JSpADA index Responsiveness.
The difference in mean change in the JSpADA score between those who had improved and worsened disease was statistically significant (p<0.001).
DISCUSSION
This is the first disease activity assessment tool developed and validated for use in JSpA. We used modified Delphi consensus formation techniques with participation from an international group of pediatric rheumatologists. The eight items of the JSpADA include measures of active arthritis, active enthesitis, clinical sacroiliitis, morning stiffness, patient assessment of pain, uveitis, abnormal back mobility, and abnormal inflammatory markers. Each item was considered to be of equal importance (value=1) and the range of possible scores was 0 to 8, with higher scores indicating more disease activity. In a retrospective multicenter longitudinal database of newly diagnosed children with JSpA, the JSpADA had excellent face validity, construct validity, discrimination, and responsiveness to change. We aimed to limit the JSpADA items to those that are easily assessed during normal routine care so this tool would be feasible for both clinical care and research.
The JSpADA index surpasses other existing JIA disease activity assessment tools such as the pedi ACR 30/50/70/90 and the JADAS for the evaluation of JSpA disease activity as it will enable comparison of disease activity and medication response across patients and institutions, explicitly accounts for the unique disease activity features of JSpA and is based upon international input from a large group of pediatric rheumatologists. Strengths of the JSpADA index include the limited number of items, inclusion of disease features that are associated with poorer outcomes in JSpA (enthesitis, sacroiliac involvement)4-6, and all items are feasible to assess in the limited amount of time available during a routine clinic visit. Existing disease activity measures for adult SpA such as the BASDAI and ASDAS, which are unlikely to be as useful in the pediatric population given the differences in disease characteristics in adults and children, could not be evaluated in this retrospective cohort but should be assessed in future studies.
The results of this study should be interpreted in the context of several limitations. First, the validation was conducted on a retrospective cohort that was not developed specifically for this purpose. Therefore, we did not have complete data needed to calculate the JSpADA score on all subjects in the cohort. We only used those subjects with complete data for the validation (N= 178 subjects, 73% of the total cohort). These 178 subjects did not significantly differ from those subjects with missing data with respect to demographics or clinical presentation. This underscores the importance of making the next step in the JSpADA development assessment of the items in a prospective multicenter cohort, with an emphasis on completeness and uniformity of data collection. Second, since there is no gold standard or other pre-existing disease activity measure for JSpA concurrent validity could not be assessed. However, we demonstrated that the JSpA has construct validity by demonstrating a moderate to high correlation with other measures of disease activity and a moderate correlation with a global measure of function.
The ACR published recommendations for the development and validation of response criteria26 recommend that the development use appropriate consensus methodology to identify the list of criteria and that the criteria chose be reliable (reproducible), precise in measurement, easy and feasible to measure and clinically sensible while redundancy should be minimized. Validation should include the following: 1) Appropriate statistical factor analysis; 2) Identification of factors that differentiate patients across the full disease severity ; 3) Validation of the response criteria in a prospective clinical trial. Our methodology fulfills the recommendations for development of the criteria but only the first two validation recommendations. There are no large clinical trials in children with JSpA, precluding fulfillment of the third recommendation at this time. Future work is planned to evaluate the validity of the JSpADA index in a large multi-center prospective cohort, the next best option given the absence of a sufficiently large trial.
At the present there is no evidence to support the assignment of different measures of importance or weights for any item in the JSpADA. While the current scoring system (all items contribute equally for a total possible score of 8) has good construct validity, excellent discrimination, and responsiveness to change, future work should assess whether proportional weighting of the items improves the performance of the tool. Additionally, abnormal inflammatory markers and abnormal back mobility did not load strongly onto any of the 3 primary factors of the JSpADA (the strongest factor loadings for each were 0.27 and 0.21, respectively). Future prospective work with careful and complete data collection should assess whether these items should remain part of the JSpADA.
Studies have demonstrated that children with JIA spend the majority of time with continuous disease activity and that few children achieve remission as adults.27-29 Children with JSpA only have a 17% chance of remission within 5 years of diagnosis.30 Therefore, a validated tool to measure JSpA disease activity longitudinally is urgently needed so that we can perform crucial studies to improve these outcomes. In this exercise we developed the first disease activity assessment for children with JSpA and performed a preliminary validation using a retrospective multicenter cohort. The use of the JSpADA for this understudied and unique population will hopefully improve routine clinical care and enable effectiveness and efficacy studies focused on this category of JIA.
SIGNIFICANCE AND INNOVATION.
This is the first validated disease assessment tool for children with JSpA
The JSpADA index had evidence of construct validity, discrimination, and responsiveness to changes in clinical disease activity
Use of the JSpADA index will improve routine clinical care and enable the design and analysis of effectiveness and efficacy studies for this category of juvenile arthritis
Acknowledgements
The authors thank Colden Broderick, Andrew Klink, Keshia Maughn, Christy Reed, Davide Moretti, Roxanna Sabghi, Carla Upperman for their assistance in review of the medical charts and data entry. We would also like to thank all those who participated in the modified Delphi exercise who were not named authors on this publication: Leslie Abramson, Amita Aggarwal, Jonathan Akikusa, Shoghik Akoghlanian, Sheila Angeles-Han, Jordi Antón, Stacy Ardoin, Eileen Baildam, Marcia Bandeira, Fatima Barbar-Smiley, Mara Becker, Roberta Berard, Bryce Binstadt, Rosa Bou, Sharon Bout-Tabaku, Diane Brown, Amanda Brown, Ruben Burgos-Vargas, David Cabral, Scott Canna, Elizabeth Chalom, Randy Cron, Kaleo Ede, Barbara Edelheit, Melissa Elder, Natalya Fish, Mark Friswell, Harry Gewanter, Margarida Guedes, Kathleen Haines, Kristen Hayward, Claas Hinze, Mark Hoetzel, Sandy Hong, Daniel Horton, Kristin Houghton, Joyce Hsu, Adam Huber, Maria Ibarra, CJ Inman, John Ioannou, Rita Jerath, Karla Jones, Ankur Kamdar, Yuki Kimura, Daniel Kingsbury, Marisa Klein-Gitelman, Sharath Kumar, Tzielan Lee, Melissa Lerman, Deborah Levy, Suzanne Li, Clara Lin, Debby McCurdy, Jay Mehta, Diana Milojevic, Mary Moore, Terry Moore, Lakshmni Moorthy, Kabita Nanda, Marc Natter, Peter Nigrovic, Kathleen O’Neil, Sheila Oliveira, Judy Olson, Karen Onel, Barbara Ostrov, Jon Packham, Clare Pain, Christina Pelajo, Sampth Prahald, Egla Rabinovich, Homaira Rahimi, Angelo Ravelli, Andreas Reiff, Sarah Ringold, Angela Robinson, Tova Ronis, Deborah Rothman, Nicola Ruperto, Sandeep Saluga, Laura Schanberg, Ken Schikler, Kara Schmidt, Debajit Sen, Ethan Sen, Andrew Shulman, Judy Smith, Jorge Sotoca, Betul Sozeri, Hemalatha Srinivasalu, Matthew Stoll, Joost Swart, Grant Syverson, Ilona Szer, Flavio Sztajnbok, Melissa Tesher, Heather Tory, Mary Toth, Ralf Trauzeddel, Shirley Tse, Lori Tucker, Nikolay Tzaribachev, Yosef Uziel, Richard Vehe, Linda Wagner-Weiner, Andrew Zeft, and Lawrence Zemel.
Grant support: PF Weiss was supported by NIAMS NIH grant 1-K23-AR059749-01A1; RA Colbert was supported by the NIAMS Intramural Research Program Z01 AR041184.
Contributor Information
Pamela F. Weiss, Division of Rheumatology, Department of Pediatrics, Center for Pediatric Clinical Effectiveness, University of Pennsylvania Perelman School of Medicine and Children's Hospital of Philadelphia, Philadelphia, PA.
Robert A. Colbert, NIAMS NIH, Bethesda, MD.
Rui Xiao, Department of Biostatistics and Epidemiology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA.
Chris Feudtner, Division of General Pediatrics, Department of Pediatrics, Center for Pediatric Clinical Effectiveness, University of Pennsylvania Perelman School of Medicine and Children's Hospital of Philadelphia, PA.
Timothy Beukelman, Division of Rheumatology, University of Alabama at Birmingham, Birmingham, AL.
Esi Morgan DeWitt, Division of Rheumatology, Cincinnati Children's Hospital, Cincinnati, OH.
Ilaria Pagnini, Department of Pediatrics, University of Florence, Anna Meyer Children's Hospital, Florence, Italy.
Tracey B. Wright, Division of Rheumatology, Texas Scottish Rite Hospital for Children, Dallas, TX.
Carol A. Wallace, University of Washington and Seattle Children's Hospital, Seattle, WA.
REFERENCES
- 1.Reveille JD. Epidemiology of spondyloarthritis in North America. Am J Med Sci. 2011 Apr;341(4):284–286. doi: 10.1097/MAJ.0b013e31820f8c99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petty RE, Southwood TR, Manners P, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004 Feb;31(2):390–392. [PubMed] [Google Scholar]
- 3.Saag KG, Teng GG, Patkar NM, et al. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 2008 Jun 15;59(6):762–784. doi: 10.1002/art.23721. [DOI] [PubMed] [Google Scholar]
- 4.Weiss P, Beukelman T, Schanberg LE, Kimura Y, Colbert R, investigators C. Enthesitis is a significant predictor of decreased quality of life, function, and arthritis-specific pain across juvenile arthritis (JIA) categories: Preliminary analyses from the CARRAnet registry. Arthritis & Rheumatism. 2011;62(10):S105. [Google Scholar]
- 5.Boiu S, Marniga E, Bader-Meunier B, et al. Functional status in severe juvenile idiopathic arthritis in the biologic treatment era: an assessment in a French paediatric rheumatology referral centre. Rheumatology (Oxford) 2012 Jul;51(7):1285–1292. doi: 10.1093/rheumatology/kes004. [DOI] [PubMed] [Google Scholar]
- 6.Selvaag AM, Lien G, Sorskaar D, Vinje O, Forre O, Flato B. Early disease course and predictors of disability in juvenile rheumatoid arthritis and juvenile spondyloarthropathy: a 3 year prospective study. J Rheumatol. 2005 Jun;32(6):1122–1130. [PubMed] [Google Scholar]
- 7.Donnithorne KJ, Cron RQ, Beukelman T. Attainment of inactive disease status following initiation of TNF-alpha inhibitor therapy for juvenile idiopathic arthritis: enthesitis-related arthritis predicts persistent active disease. The Journal of rheumatology. 2011 Dec;38(12):2675–2681. doi: 10.3899/jrheum.110427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Otten MH, Prince FH, Twilt M, et al. Tumor necrosis factor-blocking agents for children with enthesitis-related arthritis--data from the dutch arthritis and biologicals in children register, 1999-2010. The Journal of rheumatology. 2011 Oct;38(10):2258–2263. doi: 10.3899/jrheum.110145. [DOI] [PubMed] [Google Scholar]
- 9.Flato B, Aasland A, Vinje O, Forre O. Outcome and predictive factors in juvenile rheumatoid arthritis and juvenile spondyloarthropathy. J Rheumatol. 1998 Feb;25(2):366–375. [PubMed] [Google Scholar]
- 10.Grigor C, Capell H, Stirling A, et al. Effect of a treatment strategy of tight control for rheumatoid arthritis (the TICORA study): a single-blind randomised controlled trial. Lancet. 2004 Jul 17-23;364(9430):263–269. doi: 10.1016/S0140-6736(04)16676-2. [DOI] [PubMed] [Google Scholar]
- 11.Lukas C, Landewe R, Sieper J, et al. Development of an ASAS-endorsed disease activity score (ASDAS) in patients with ankylosing spondylitis. Ann Rheum Dis. 2009 Jan;68(1):18–24. doi: 10.1136/ard.2008.094870. [DOI] [PubMed] [Google Scholar]
- 12.Garrett S, Jenkinson T, Kennedy LG, Whitelock H, Gaisford P, Calin A. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol. 1994 Dec;21(12):2286–2291. [PubMed] [Google Scholar]
- 13.Giannini EH, Ruperto N, Ravelli A, Lovell DJ, Felson DT, Martini A. Preliminary definition of improvement in juvenile arthritis. Arthritis Rheum. 1997 Jul;40(7):1202–1209. doi: 10.1002/1529-0131(199707)40:7<1202::AID-ART3>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 14.Consolaro A, Ruperto N, Bazso A, et al. Development and validation of a composite disease activity score for juvenile idiopathic arthritis. Arthritis and Rheumatism. 2009 May 15;61(5):658–666. doi: 10.1002/art.24516. [DOI] [PubMed] [Google Scholar]
- 15.Horneff G, Burgos-Vargas R, Constantin T, et al. Efficacy and safety of open-label etanercept on extended oligoarticular juvenile idiopathic arthritis, enthesitis-related arthritis and psoriatic arthritis: part 1 (week 12) of the CLIPPER study. Annals of the Rheumatic Diseases. 2013 May 21; doi: 10.1136/annrheumdis-2012-203046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burgos-Vargas R, Vazquez-Mellado J, Pacheco-Tena C, Hernandez-Garduno A, Goycochea-Robles MV. A 26 week randomised, double blind, placebo controlled exploratory study of sulfasalazine in juvenile onset spondyloarthropathies. Annals of the Rheumatic Diseases. 2002 Oct;61(10):941–942. doi: 10.1136/ard.61.10.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wallace CA, Ruperto N, Giannini E. Preliminary criteria for clinical remission for select categories of juvenile idiopathic arthritis. J Rheumatol. 2004 Nov;31(11):2290–2294. [PubMed] [Google Scholar]
- 18.Juniper EF, Guyatt GH, Streiner DL, King DR. Clinical impact versus factor analysis for quality of life questionnaire construction. J Clin Epidemiol. 1997 Mar;50(3):233–238. doi: 10.1016/s0895-4356(96)00377-0. [DOI] [PubMed] [Google Scholar]
- 19.Boers M, Brooks P, Strand CV, Tugwell P. The OMERACT filter for Outcome Measures in Rheumatology. The Journal of rheumatology. 1998 Feb;25(2):198–199. [PubMed] [Google Scholar]
- 20.Wallace CA, Ravelli A, Huang B, Giannini EH. Preliminary validation of clinical remission criteria using the OMERACT filter for select categories of juvenile idiopathic arthritis. J Rheumatol. 2006 Apr;33(4):789–795. [PubMed] [Google Scholar]
- 21.Iglesias MJ, Cuttica RJ, Herrera Calvo M, Micelotta M, Pringe A, Brusco MI. Design and validation of a new scale to assess the functional ability in children with juvenile idiopathic arthritis (JIA). Clin Exp Rheumatol. 2006 Nov-Dec;24(6):713–718. [PubMed] [Google Scholar]
- 22.Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas. 1960;20:37–46. [Google Scholar]
- 23.Sieper J, van der Heijde D, Landewe R, et al. New criteria for inflammatory back pain in patients with chronic back pain: a real patient exercise by experts from the Assessment of SpondyloArthritis international Society (ASAS). Annals of the Rheumatic Diseases. 2009 Jun;68(6):784–788. doi: 10.1136/ard.2008.101501. [DOI] [PubMed] [Google Scholar]
- 24.McErlane F, Beresford MW, Baildam EM, et al. Validity of a three-variable Juvenile Arthritis Disease Activity Score in children with new-onset juvenile idiopathic arthritis. Annals of the Rheumatic Diseases. 2012 Dec 20; doi: 10.1136/annrheumdis-2012-202031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woolston SL, Beukelman T, Sherry DD. Back mobility and interincisor distance ranges in racially diverse North American healthy children and relationship to generalized hypermobility. Pediatr Rheumatol Online J. 2012;10(1):17. doi: 10.1186/1546-0096-10-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh JA, Solomon DH, Dougados M, et al. Development of classification and response criteria for rheumatic diseases. Arthritis and Rheumatism. 2006 Jun 15;55(3):348–352. doi: 10.1002/art.22003. [DOI] [PubMed] [Google Scholar]
- 27.Oen K, Malleson PN, Cabral DA, Rosenberg AM, Petty RE, Cheang M. Disease course and outcome of juvenile rheumatoid arthritis in a multicenter cohort. J Rheumatol. 2002 Sep;29(9):1989–1999. [PubMed] [Google Scholar]
- 28.Oen K, Tucker L, Huber AM, et al. Predictors of early inactive disease in a juvenile idiopathic arthritis cohort: results of a Canadian multicenter, prospective inception cohort study. Arthritis Rheum. 2009 Aug 15;61(8):1077–1086. doi: 10.1002/art.24539. [DOI] [PubMed] [Google Scholar]
- 29.Zak M, Pedersen FK. Juvenile chronic arthritis into adulthood: a long-term follow-up study. Rheumatology (Oxford) 2000 Feb;39(2):198–204. doi: 10.1093/rheumatology/39.2.198. [DOI] [PubMed] [Google Scholar]
- 30.Minden K, Kiessling U, Listing J, et al. Prognosis of patients with juvenile chronic arthritis and juvenile spondyloarthropathy. J Rheumatol. 2000 Sep;27(9):2256–2263. [PubMed] [Google Scholar]