Abstract
Background and Purpose
Milk fat globule–EGF factor-8 (MFGE8) has been reported to be neuroprotective in ischemic stroke. However the effects of MFGE8 in early brain injury after subarachnoid hemorrhage (SAH) have not been investigated. We investigated the role of MFGE8 in early brain injury and the potential mechanisms in anti-oxidation after SAH.
Methods
Two dosages (1 µg and 3.3 µg) of recombinant human MFGE8 (rhMFGE8) were injected intracerebroventricularly at 1.5 hours after SAH. SAH grades, neurological scores and brain water content were measured at 24 hours and 72 hours. For mechanistic study, MFGE8 siRNA, integrin β3 siRNA and heme oxygenase (HO) inhibitor SnPP IX were used for intervention. The oxidative stress and expression of MFGE8, integrin β3, HO-1, ERK and Nrf2 were measured by western blots 24 hours after SAH.
Results
The expression of MFGE8 and HO-1 increased and peaked 24 hours after SAH. Administration of rhMFGE8 decreased brain water content and improved neurological functions both at 24 hours and 72 hours after SAH. rhMFGE8 reduced oxidative stress and enhanced the expression of ERK, Nrf2 and HO-1; and the effects were abolished by integrin β3 siRNA and HO inhibitor SnPP IX.
Conclusion
rMFGE8 attenuated oxidative stress which may be mediated by integrin β3/Nrf2/HO pathway after SAH. rMFGE8 may serve as an alternative treatment to ameliorate early brain injury for SAH patients.
Keywords: MFGE8, HO-1, oxidative stress, SAH
Introduction
Aneurysmal subarachnoid hemorrhage (SAH) is one of the most life-threatening diseases with high mortality and disability rates1. Early brain injury has been reported as the primary cause of mortality in SAH patients and has been considered as a primary target for treatment2,3, 4. One of the key factors involved in the pathogenesis of early brain injury is oxidative stress5, which is caused by free radicals, including excess production of reactive oxygen species (ROS) and reactive nitrogen species (RNS). Therefore, an antioxidative strategy has attracted attention in the treatment of SAH.
Milk fat globule–EGF factor-8 (MFGE8) is a multifunctional glycoprotein originally identified as part of the milk fat globule membrane. MFGE8 appears to be instrumental in cell-cell interactions and has been involved in diverse physiological and pathophysiological functions, including fertilization6, angiogenesis7, and phagocytosis of apoptotic cells8. More recent studies have shown that MFGE8 regulates adaptive immune responses9 and inflammation10. In the central nervous system, administration of recombinant human MFGE8 (rhMFGE8) has neuroprotection against cerebral ischemia through suppression of inflammation and apoptosis after brain ischemia10, 11. rhMFGE8 also exhibits significant anti-oxidative effects through increasing heme oxygenase-1 (HO-1) in Alzheimer's disease model12. However, the effects of MFGE8 in early brain injury after SAH have not been investigated. In the present work, we addressed the role of MFGE8 in SAH. We showed for the first time that rhMFGE8 attenuated early brain injury through suppression of the oxidative stress involving integrin β3-dependent pathway.
Materials and Methods
All experiments were approved by the Institutional Animal Care and Use Committee of Loma Linda University.
SAH Model and Experimental Protocol
Two hundred and ten male (280–320 g) Sprague-Dawley rats (Indianapolis, IN) were used. The endovascular perforation model of SAH in rats was performed as reported previously13. Briefly, with 3% isoflurane anesthesia, a sharpened 4-0 monofilament nylon suture was inserted rostrally into the right internal carotid artery from the external carotid artery stump and perforated the bifurcation of the anterior and middle cerebral arteries. Sham-operated rats underwent the same procedures except the suture was withdrawn without puncture.
Two dosages (1 µg and 3.3 µg) of rhMFGE8 were injected intracerebroventricularly at 1.5 hours after SAH. SAH grades, neurological scores and brain water content were measured at 24 hours and 72 hours. To study mechanisms, MFGE8 small interfering RNA (siRNA), integrin β3 siRNA and heme oxygenase (HO) inhibitor SnPP IX were used for intervention. The oxidative stress and expression of MFGE8, integrin β3, heme oxygenase-1 (HO-1), extracellular signal-regulated kinases (ERK) and nuclear factor erythroid 2–related factor 2 (Nrf2) were measured by western blots at 24 hours after SAH.
Intracerebroventricular Drug Administration
Intracerebroventricular drug administration was performed as previously described14. Briefly, rats were placed in a stereotaxic apparatus under 2.5% isoflurane anesthesia. The needle of a 10-µl Hamilton syringe (Microliter 701; Hamilton Company, Reno, NV) was inserted through a burr hole into the right lateral ventricles at the following coordinates relative to bregma: 1.5 mm posterior, 1.0 mm lateral, and 3.2 mm below the horizontal plane of the skull. rhMFGE8 (Sigma) and SnPP IX (Santa Cruz Biotechnology, 3.3 µg) were injected respectively at 1.5 hours after SAH induction by a pump at a rate of 0.5 µl/min respectively. MFGE8 siRNA, integrin β3 siRNA and scrambled siRNA (500 pmol/3 µl, Santa Cruz Biotechnology) were injected at 2 days before SAH induction in by a pump at a rate of 0.5 µl/min.
SAH Grade
The severity of SAH was blindly evaluated using the SAH grading scale at the time of euthanasia as previously reported15. Rats with mild SAH (SAH grades ≤ 7 at 24 hours and SAH grades ≤ 5 at day 7) were excluded from the study16. A total of 16 animals were excluded due to mild SAH (SAH, 2; SAH+vehicle, 3; SAH+10µg/kg rhMFGE8, 2; SAH+3µg/kg rhMFGE8, 1; SAH+MFEG8 siRNA, 2; SAH+Scramble siRNA, 2; SAH+rhMFGE8+Scramble+siRNA, 1; SAH+rhMFGE8+SnPP IX, 1; SAH+rhMFGE8+integrin β3 siRNA, 2).
Neurological Score
Neurological scores were evaluated at 1 hour before euthanization by a blinded observer according to the 21-point scoring system described by Garcia et al., with modifications17.
Brain Water Content
Brains were removed at 24 or 72 hours after surgery and separated into left hemisphere, right hemisphere, cerebellum and brain stem. Each part was weighed immediately after removal (wet weight) and once more after drying in 105°C for 72 hours. The percentage of water content was calculated as (wet weight–dry weight)/wet weight13.
Immunofluorescence staining
Double-fluorescence labeling was performed as previously described13. Sections were incubated overnight at 4°C with goat anti–MFGE8 (Santa Cruz Biotechnology), rabbit anti-Ionized calcium binding adaptor molecule 1 (Iba1, Abcam), goat anti-glial fibrillary acidic protein (GFAP, Santa Cruz Biotechnology) and mouse anti-Neuronal nuclei (NeuN, EMD Millipore) primary antibodies. The expression of HO-1was detected by fluorescence labeling with goat anti-HO-1 (Santa Cruz Biotechnology). Appropriate fluorescence dye–conjugated secondary antibodies (Jackson Immunoresearch, West Grove, PA) were applied in the dark for 1 hour at 21°C. For negative controls, the primary antibodies were omitted and the same staining procedures were performed. The sections were visualized with a fluorescence microscope, and the photomicrographs were saved and merged with Image Pro Plus software (Olympus, Melville, NY).
Western Blot
The brain samples were collected at 24 hours after SAH. Western blotting was performed as described previously18. Primary antibodies used were: MFGE8 (Santa Cruz Biotechnology), HO-1, Nrf2 (Santa Cruz Biotechnology), 4-Hydroxynonenal (4-HNE, Abcam Biotech Company), Nitrotyrosine (Santa Cruz Biotechnology), integrin β3, extracellular-signal-regulated kinase (ERK) and phosphorylated ERK (p-ERK).
Statistical Analysis
Data are expressed as a mean±SEM. Mortality data were analyzed by Fisher exact test. All other data were analyzed by one-way ANOVA followed by Tukey post hoc test. P value of <0.05 was considered statistically significant. All statistical analyses were performed using GraphPad Prism for Windows.
Results
The expression of MFGE8 and HO-1 increased and peaked at 24 hours after SAH
After SAH, there were clear blood clots around basal cistern and the SAH grades decreased gradually as time passed. There were significant differences of SAH grades between sham and SAH animals up to day 7 (Figure 1A). Rather, the neurological deficits relived gradually and the animals showed no obvious difference on the neurological scores at 7 days after SAH (Figure 1B).
Figure 1.
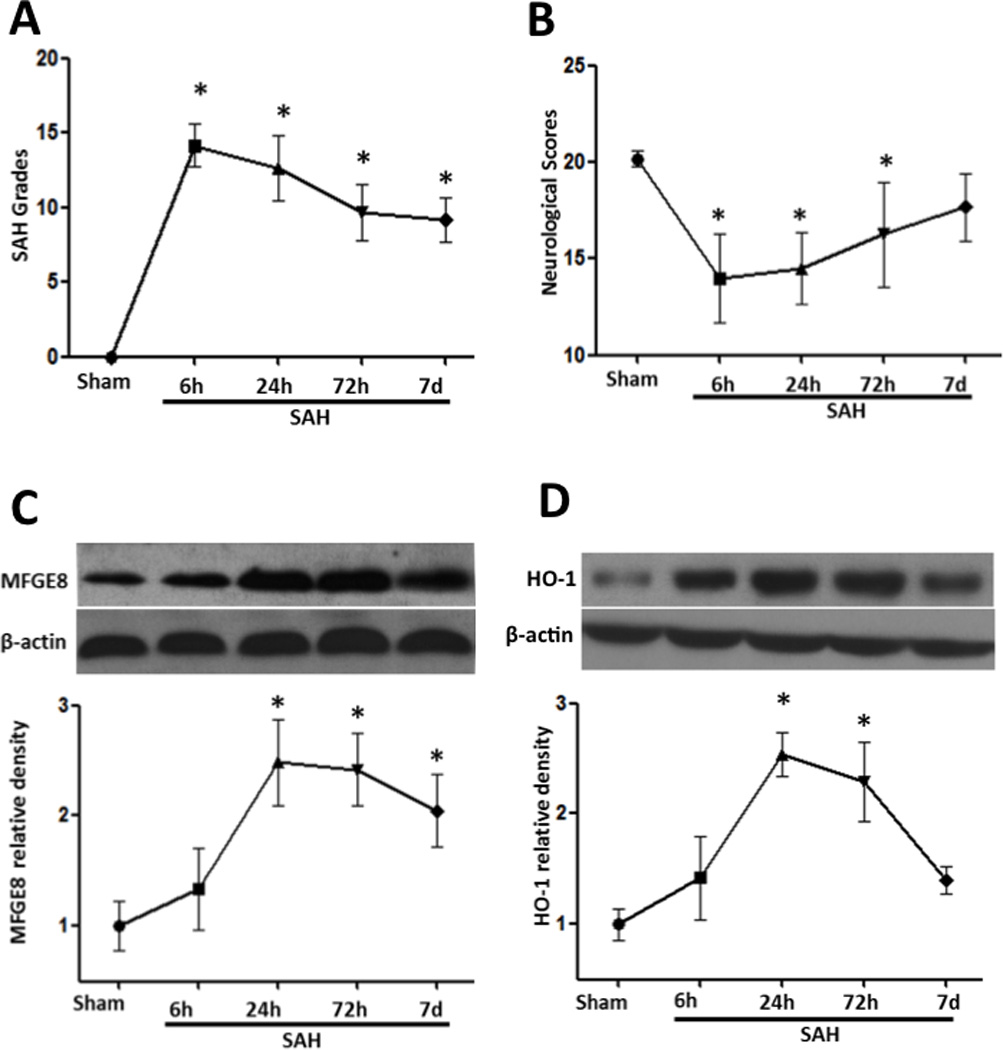
Time course of SAH grade, neurological scores, and expression of MFGE8 and HO-1 after SAH surgery. (A) SAH grades were decreased with time from 6 hours to day 7 after SAH. (B) Animals showed severely neurological deficits from 6 hours after SAH, improved as time passed and showed no significant difference with sham at day 7. (C) and (D), the temporal expression of MFGE8 and HO-1 after SAH. Both of them increased after SAH and peaked at 24 hours. n=6 for each group. *p<0.05 vs. Sham.
Western blots showed MFGE8 was seldom expressed in sham animals and increased from 6 hours after SAH. The increase of MFGE8 reached peak at 24 hours lasting to day 7 (Figure 1C). The protein expression of HO-1 also increased after SAH and peaked at 24 hours, and almost back to baseline at 7 days after SAH (Figure 1D).
Double immunostaining of MFGE8 with Iba1 (marker for microglia), GFAP (marker for astrocyte), and NeuN (marker for neuron) showed that MFGE8 is intensely expressed in the brain after SAH and highly co-localized with Iba1 at 24 hours (Figure 2). MFGE8 is not co-localized with GFAP and NeuN after SAH (Figure 2A). In brain tissue, the expression of HO-1 was low in shamed animals and remarkably increased at 24 hours after SAH (Figure 2B).
Figure 2.

Spatial expression of MFGE8 after SAH. MFGE8 is seldom expressed in brain in sham animals, and increased in microglials, but not in neurons and astrocytes at 24 hours after SAH. Scale bar=30 µm in Figure 2A and =100 µm in Figure 2B.
Administration of rhMFGE8 decreased brain water content and improved neurological functions after SAH
Two dosages of rhMFGE8 (1 µg and 3.3 µg) were administrated intracerebroventricularly 1.5 hours after SAH. Brain water content and neurological scores were measured. The data showed both high dosage and low dosage decreased the brain water content at 24 hours (Figure 3A and 3C), but only the high dosage improved the neurological deficits at 72 hours after SAH (Figure 3B and 3D). The results indicated that the high dosage is more effective, so we chose the high dosage for the following mechanism studies.
Figure 3.

rMFGE8 decreased brain water content and improved neurological functions at 24 and 72 hours after SAH. Both low dosage and high dosage decreased brain edema at 24 hours after SAH (A), but only high dosage improved the neurobehavioral deficits (B). High dosage of rMFGE8 decreased brain water content (C) and improved neurological functions (D) at 72 hours after SAH. n=6 for each group. *p<0.05 vs. Sham; #p<0.05 vs. SAH.
Knockdown integrin β3 and inhibition of HO abolished the beneficial effects of rhMFGE8 at 24h after SAH
To investigate the potential mechanisms of rMFGE8, we administrated integrin β3 siRNA or HO inhibitor SnPP IX with rhMFGE8 treatment. Both integrin β3 siRNA and SnPP IX significantly increased brain water content (Figure 4A p<0.05 vs. SAH+rhMFGE8), and decreased the neurological scores at 24 hours after SAH (Figure 4B, p<0.05 vs. SAH+rhMFGE8).
Figure 4.

The protective effects of rMFGE8 depend on integrin β3 and HO-1. Silencing MFG-E8 or its receptor integrinβ3 by siRNA, or block the activity of HO-1 by its inhibitor SnPP IX abolished the effects of rhMFGE8 on brain edema (A) and neurobehavioral deficits (B). n=6 for each group. #p<0.05 vs. SAH+PBS, & p<0.05 vs. SAH+ rhMFGE8.
Administration of rhMFGE8 enhanced the expression of HO-1 and decreased oxidative stress at 24 hours after SAH
Administration of rhMFGE8 significantly increased the protein level of MFGE8 in the brain, and MFGE8 siRNA effectively decreased it at 24 hours after SAH (Figure 5A). Consistent with MFGE8, the protein level of HO-1 was intensely increased by rhMFGE8 administration (Figure 5B). After SAH, oxidative stress had been generated and western blots revealed a significant increase of 4-HNE (Figure 5C) and nitrotyrosine (Figure 5D). Administration of rhMFGE8 suppressed the expression of 4-HNE and nitrotyrosine, and MFGE8 siRNA prevented the effects of rhMFGE8 (Figure 5). HO inhibitor SnPP IX did not change the protein level of HO-1 (Supplement Figure IA), but increased the expression of 4-HNE and nitrotyrosine in rhMFGE8 treated animals (Supplement Figure IB and IC).
Figure 5.
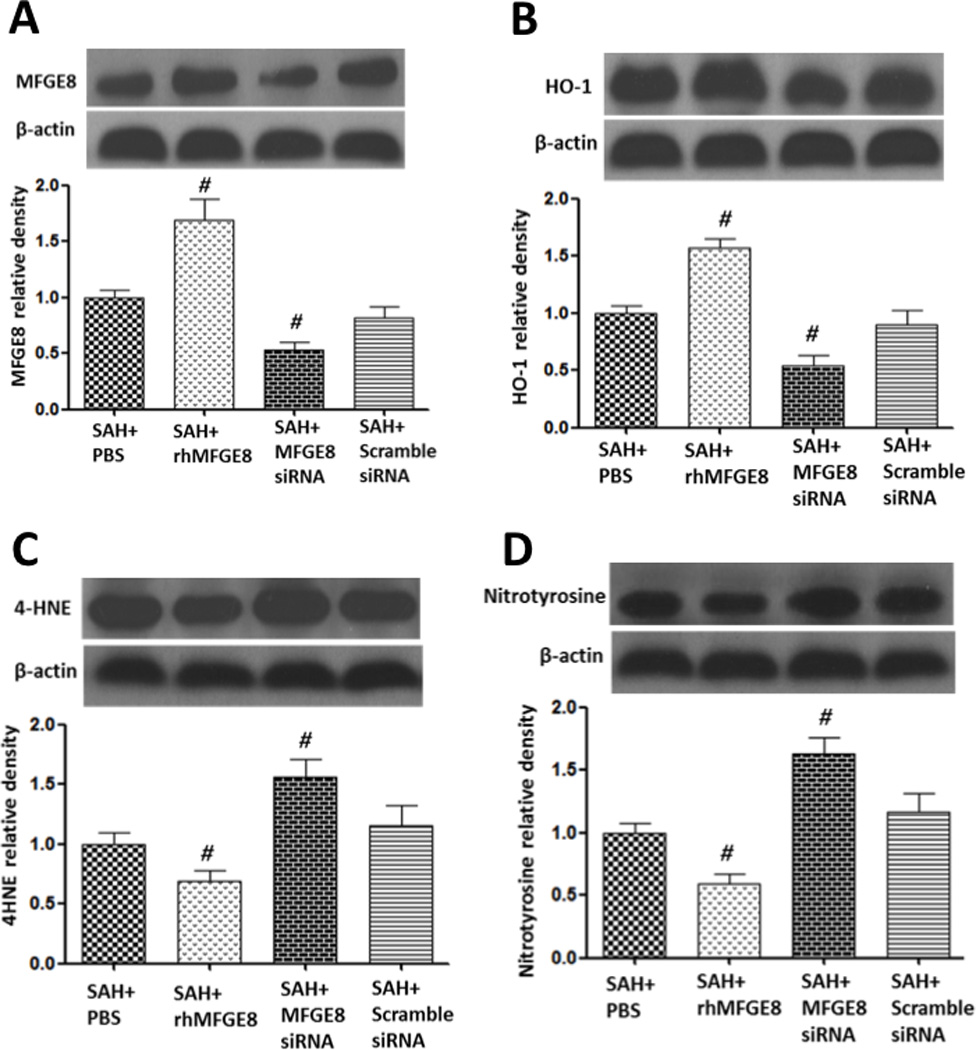
rMFGE8 modulated the expression of HO-1 and oxidative stress. Administration of rhMFGE8 increased the cellular level of MFGE8 (A) and HO-1 (B), and attenuated lipid peroxidation (C) and protein nitrification (D) at 24 hours after SAH. Silencing MFG-E8 by siRNA reversed the results. n=6 for each group. #p<0.05 vs. SAH+vehicle.
MFGE8 decreased HO-1 dependent on integrin β3/ERK/Nrf2 pathway
At 24 hours after SAH, administration of rhMFGE8 has no significant effects on the protein level of its receptor integrin β3 (Figure 6A). Integrinβ3 siRNA knocked down the receptor efficiently (Figure 6A). rhMFGE8 treatment increased p-ERK, Nrf2 and HO-1 compared with vehicle group (Figures 6B, 6C and 6D). Integrin β3 siRNA remarkably abolished the up-regulation of p-ERK, Nrf2 and HO-1 after rhMFGE8 administration (Figure 6B, 6C and 6D).
Figure 6.
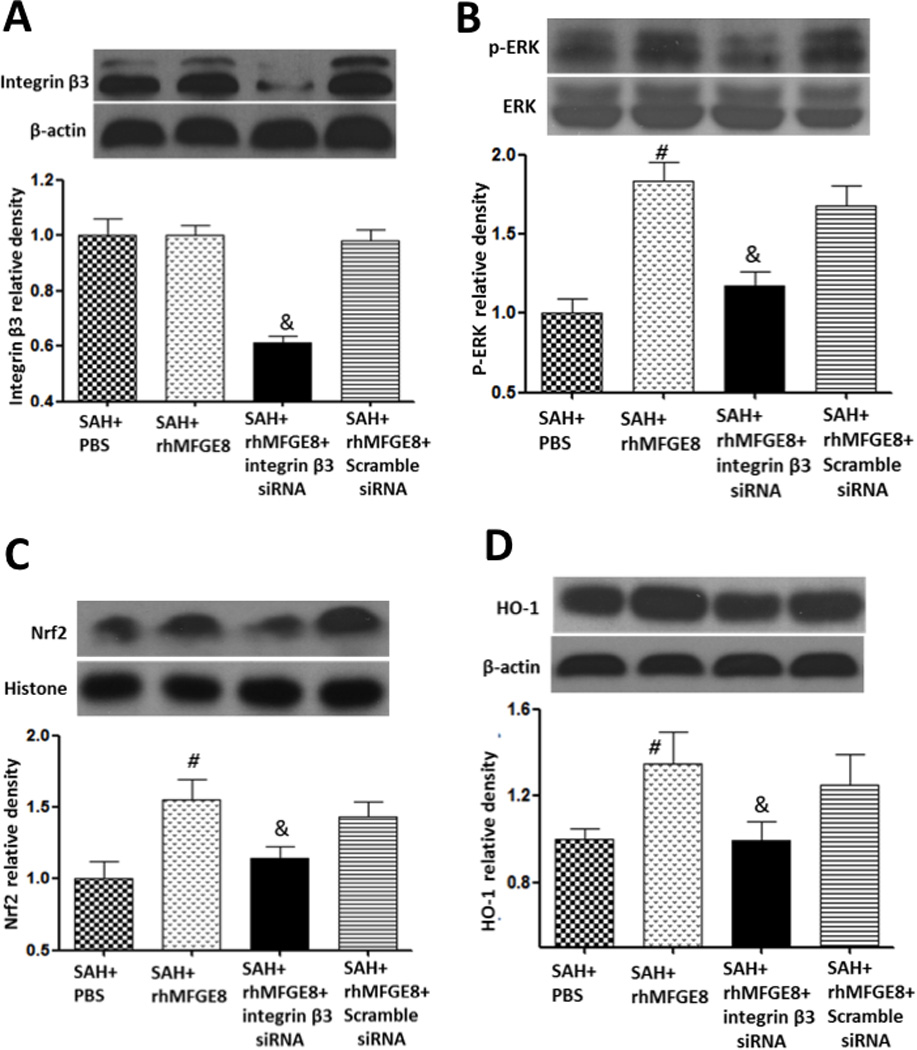
rhMFGE8 reduced oxidative stress dependent on integrin β3/ERK/HO-1 pathway. Administration of rhMFGE8 has no effects on protein level of integrin β3 (A), increased the expression of phosphorylated ERK (B), Nrf2 (C) and HO-1 (D). Silencing integrin β3 by siRNA removed the effects of rhMFGE8. n=6 for each group. #p<0.05 vs. SAH+PBS, &p<0.05 vs. SAH+ rhMFGE8.
Discussion
Early brain injury, which occurs within 72 hours after cerebral aneurysm rupture, has been considered as a new target for improving the outcome after SAH. Recent studies reported that oxidative stress was involved in the pathogenesis of early brain injury and anti-oxidative treatment can be one of the therapeutic candidates for early brain injury after experimental SAH or in a clinical setting5. In this study, our goals were to test firstly whether rhMFGE8 administration suppressed oxidative stress and improved the outcome after SAH; and secondly, to investigate the potential mechanisms of rhMFGE8 in antioxidation. The results showed that MFGE8 was increased in the early stage of SAH and its expression was well consistent with the expression of HO-1, which is an essential cellular defense molecular of oxidative stress. Administration of rhMFGE8 significantly decreased brain edema and improved neurological deficits. rhMFGE8 up-regulated the expression of p-ERK, Nrf2 and HO-1, reduced lipid peroxidation and protein nitrification after SAH; and knockdown of its receptor integrin β3 by siRNA and inhibition of HO by SnPP IX abolished the anti-oxidation effects of rhMFGE8. This data indicated that rhMFGE8 might be an alternative therapy for early brain injury after SAH by alleviating oxidative stress though integrin β3/ERK/HO pathway.
MFGE8 plays important roles in several biological processes, including apoptotic cell clearance19, angiogenesis7, and anti-inflammation10. Recent studies showed that MFGE8-mediated potential therapeutic benefits in several models of central nervous system diseases. Li and his colleagues showed that MFGE8 was released from microglia and increased microglial neuroprotective activity against oligomeric amyloid β-induced neuronal cell death. This neuroprotection was mediated through integrin receptor and activation of Nrf2/HO-1 pathway12. In permanent middle cerebral artery occlusion (MCAO) rats, endogenous brain MFGE8 levels were decreased after cerebral ischemia, and exogenous rhMFGE8 was reported to reduce the infarct size and improve neurological function through suppression of inflammation and apoptosis11. In agreement with this study, MFGE8 knockout mice had significantly larger infarct size, and showed a marked increase in the expression of pro-inflammatory mediators IL-1β and TNF-α in the ischemic brain, which might be dependent on its receptor integrin β310. These results clearly indicated that endogenous MFGE8 is required for the neuroprotection against neurodegenerative disease and ischemic cerebral damage. In our study, we found that the level of MFGE8 was increased in SAH rats, and highly expressed in microglia. Administration of rhMFGE8 improved the neurological deficits and reduced brain edema via suppressing oxidative stress, while knockdown MFGE8 by siRNA exacerbated the outcomes and intensified oxidative stress. Our results were consistent with the previous studies to support the beneficial effects of MFGE8 in animal stroke models.
Oxidative stress became emerged as a key player in the development and progression of many pathological conditions including stroke. HO-1 is an essential cellular defense system against oxidant-induced injury during inflammatory processes20. HO-1 offers protection by catalyzing the first and rate-limiting step in the oxidative degradation of heme (Fe-protoporphyrin-IX) to carbon monoxide, ferrous iron (Fe2+), and biliverdin21. Studies have revealed that HO-1 played an important role in neuroprotection in ischemic stroke22, 23. In this study, we found that rhMFGE8 decreased the expressions of 4-HNE and nitrotyrosine, which are markers of lipid peroxidation (4-HNE) and protein nitrification (nitrotyrosine), when compared with the vehicle group. We further observed that the levels of HO-1 in ischemic brain tissue were up-regulated by rhMFGE8 after treatment, and HO inhibitor SnPP IX removed the anti-oxidation effects of rhMFGE8. Therefore, we conceptualize that rhMFGE8 improved the outcomes after SAH, possibly by suppressing oxidative stress via increasing HO-1.
How did rhMFGE8 decrease the expression of HO-1 and reduce oxidative stress after SAH? It is well known that integrin is a putative receptor of MFGE8 in various cell types10, 24–27. In vascular smooth muscle cells, Wang et al. demonstrated that MFGE8 facilitation of the cell cycle was mediated by integrins/ERK signaling. In diabetic mice, MFGE8 was found to coordinate fatty acid uptake through integrin avβ3 and integrin avβ5-dependent phosphorylation of Akt by phosphatidylinositide-3 kinase and mammalian target of rapamycin (mTOR) complex 227. Recently, Deroide and colleagues showed that MFGE8 attenuated inflammation in ischemic cerebral injury through integrin β3-dependent inhibition of NLRP3 inflammasome10. However, the precise mechanisms underlying its role in anti-oxidation after SAH are poorly understood. Increasing evidence suggests that HO-1 is up-regulated through the Nrf2 cascade28, 29. In Alzheimer’s disease model, MFGE8 was released from microglia, activated Nrf2/HO-1 pathway through integrin receptor, and suppressed oxidative stress12. In our experiment, we found the expression of HO-1 was coincident with that of MFGE-8 after SAH. Treatment with rhMFGE8 significantly increased the expression of p-ERK, Nrf2 and HO-1 at 24 hours after SAH and reduced oxidative stress; knockdown integrin β3 by siRNA deteriorated the neurological scores, increase brain edema, and decreased the expression of p-ERK, Nrf2 and HO-1. With the relevant cumulative findings, herein we concluded that MFGE8 improved the outcome of SAH via anti-oxidation which may depend on integrin β3/ERK/HO pathway.
In conclusion, our results demonstrate, for the first time, that MFGE8 signaling is linked to modulation of oxidative stress after SAH. Administration of rhMFGE8 improved neurological deficits and attenuated brain edema through decreasing oxidative stress; Silencing MFGE8 or its receptor integrin β3, or block the activity of HO-1 abolished the protective effects of rhMFGE8. MFGE8 attenuated oxidative stress may depend on integrin β3/ERK/HO pathway. Thus, targeting MFGE8 could be a novel strategy to decrease oxidative stress and ameliorate early brain injury after SAH.
Supplementary Material
Acknowledgments
Sources of Funding
This study was supported partially by a grant from NIH NS081740 and NS082184 to JHZ.
Footnotes
Disclosures
None.
Reference
- 1.Biller J, Godersky JC, Adams HP., Jr Management of aneurysmal subarachnoid hemorrhage. Stroke. 1988;19:1300–1305. doi: 10.1161/01.str.19.10.1300. [DOI] [PubMed] [Google Scholar]
- 2.Cahill J, Calvert JW, Zhang JH. Mechanisms of early brain injury after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2006;26:1341–1353. doi: 10.1038/sj.jcbfm.9600283. [DOI] [PubMed] [Google Scholar]
- 3.Kusaka G, Ishikawa M, Nanda A, Granger DN, Zhang JH. Signaling pathways for early brain injury after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2004;24:916–925. doi: 10.1097/01.WCB.0000125886.48838.7E. [DOI] [PubMed] [Google Scholar]
- 4. Fujii M, Yan J, Rolland WB, Soejima Y, Caner B, Zhang JH. Early brain injury, anevolving frontier in subarachnoid hemorrhage research. Transl Stroke Res. 2013;4:432–446. doi: 10.1007/s12975-013-0257-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ayer RE, Zhang JH. Oxidative stress in subarachnoid haemorrhage: Significance in acute brain injury and vasospasm. Acta Neurochir Suppl. 2008;104:33–41. doi: 10.1007/978-3-211-75718-5_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ensslin MA, Shur BD. Identification of mouse sperm sed1, a bimotif egf repeat and discoidin-domain protein involved in sperm-egg binding. Cell. 2003;114:405–417. doi: 10.1016/s0092-8674(03)00643-3. [DOI] [PubMed] [Google Scholar]
- 7.Silvestre JS, Thery C, Hamard G, Boddaert J, Aguilar B, Delcayre A, et al. Lactadherin promotes vegf-dependent neovascularization. Nat Med. 2005;11:499–506. doi: 10.1038/nm1233. [DOI] [PubMed] [Google Scholar]
- 8.Hanayama R, Tanaka M, Miwa K, Shinohara A, Iwamatsu A, Nagata S. Identification of a factor that links apoptotic cells to phagocytes. Nature. 2002;417:182–187. doi: 10.1038/417182a. [DOI] [PubMed] [Google Scholar]
- 9.Hanayama R, Tanaka M, Miyasaka K, Aozasa K, Koike M, Uchiyama Y, et al. Autoimmune disease and impaired uptake of apoptotic cells in mfg-e8-deficient mice. Science. 2004;304:1147–1150. doi: 10.1126/science.1094359. [DOI] [PubMed] [Google Scholar]
- 10.Deroide N, Li X, Lerouet D, Van Vre E, Baker L, Harrison J, et al. Mfge8 inhibits inflammasome-induced il-1beta production and limits postischemic cerebral injury. J Clin Invest. 2013;123:1176–1181. doi: 10.1172/JCI65167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheyuo C, Jacob A, Wu R, Zhou M, Qi L, Dong W, et al. Recombinant human mfge8 attenuates cerebral ischemic injury: Its role in anti-inflammation and anti-apoptosis. Neuropharmacology. 2012;62:890–900. doi: 10.1016/j.neuropharm.2011.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li E, Noda M, Doi Y, Parajuli B, Kawanokuchi J, Sonobe Y, et al. The neuroprotective effects of milk fat globule-egf factor 8 against oligomeric amyloid beta toxicity. J Neuroinflammation. 2012;9:148. doi: 10.1186/1742-2094-9-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan J, Manaenko A, Chen S, Klebe D, Ma Q, Caner B, et al. Role of sch79797 in maintaining vascular integrity in rat model of subarachnoid hemorrhage. Stroke. 2013;44:1410–1417. doi: 10.1161/STROKEAHA.113.678474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen S, Ma Q, Krafft PR, Hu Q, Rolland W, 2nd, Sherchan P, et al. P2x7r/cryopyrin inflammasome axis inhibition reduces neuroinflammation after sah. Neurobiol Dis. 2013;58:296–307. doi: 10.1016/j.nbd.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sugawara T, Ayer R, Jadhav V, Zhang JH. A new grading system evaluating bleeding scale in filament perforation subarachnoid hemorrhage rat model. J Neurosci Methods. 2008;167:327–334. doi: 10.1016/j.jneumeth.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hasegawa Y, Suzuki H, Altay O, Zhang JH. Preservation of tropomyosin-related kinase b (trkb) signaling by sodium orthovanadate attenuates early brain injury after subarachnoid hemorrhage in rats. Stroke. 2011;42:477–483. doi: 10.1161/STROKEAHA.110.597344. [DOI] [PubMed] [Google Scholar]
- 17.Garcia JH, Wagner S, Liu KF, Hu XJ. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke. 1995;26:627–634. doi: 10.1161/01.str.26.4.627. discussion 635. [DOI] [PubMed] [Google Scholar]
- 18.Hu Q, Ma Q, Zhan Y, He Z, Tang J, Zhou C, et al. soflurane enhanced hemorrhagic transformation by impairing antioxidant enzymes in hyperglycemic rats with middle cerebral artery occlusion. Stroke. 2011;42:1750–1756. doi: 10.1161/STROKEAHA.110.603142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lauber K, Keppeler H, Munoz LE, Koppe U, Schroder K, Yamaguchi H, et al. Milk fat globule-egf factor 8 mediates the enhancement of apoptotic cell clearance by glucocorticoids. Cell Death Differ. 2013;20:1230–1240. doi: 10.1038/cdd.2013.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharp FR, Zhan X, Liu DZ. Heat shock proteins in the brain: role of Hsp70, Hsp 27, and HO-1 (Hsp32) and their therapeutic potential. Transl Stroke Res. 2013;4:685–692. doi: 10.1007/s12975-013-0271-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Motterlini R, Foresti R. Heme oxygenase-1 as a target for drug discovery. Antioxid Redox Signal. 2014;20:1810–1826. doi: 10.1089/ars.2013.5658. [DOI] [PubMed] [Google Scholar]
- 22.Shah ZA, Li RC, Ahmad AS, Kensler TW, Yamamoto M, Biswal S, et al. The flavanol (−)-epicatechin prevents stroke damage through the nrf2/ho1 pathway. J Cereb Blood Flow Metab. 2010;30:1951–1961. doi: 10.1038/jcbfm.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang C, Zhang X, Fan H, Liu Y. Curcumin upregulates transcription factor nrf2, ho-1 expression and protects rat brains against focal ischemia. Brain res. 2009;1282:133–141. doi: 10.1016/j.brainres.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 24.Taylor MR, Couto JR, Scallan CD, Ceriani RL, Peterson JA. Lactadherin (formerly ba46), a membrane-associated glycoprotein expressed in human milk and breast carcinomas, promotes arg-gly-asp (rgd)-dependent cell adhesion. DNA and cell biol. 1997;16:861–869. doi: 10.1089/dna.1997.16.861. [DOI] [PubMed] [Google Scholar]
- 25.Yang C, Hayashida T, Forster N, Li C, Shen D, Maheswaran S, et al. The integrin alpha(v)beta(3–5) ligand mfg-e8 is a p63/p73 target gene in triple-negative breast cancers but exhibits suppressive functions in eR+) and erbb2(+) breast cancers. Cancer res. 2011;71:937–945. doi: 10.1158/0008-5472.CAN-10-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang M, Fu Z, Wu J, Zhang J, Jiang L, Khazan B, et al. Mfg-e8 activates proliferation of vascular smooth muscle cells via integrin signaling. Aging cell. 2012;11:500–508. doi: 10.1111/j.1474-9726.2012.00813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khalifeh-Soltani A, McKleroy W, Sakuma S, Cheung YY, Tharp K, Qiu Y, et al. Mfge8 promotes obesity by mediating the uptake of dietary fats and serum fatty acids. Nature med. 2014;20:175–183. doi: 10.1038/nm.3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Satoh T, Okamoto SI, Cui J, Watanabe Y, Furuta K, Suzuki M, et al. Activation of the keap1/nrf2 pathway for neuroprotection by electrophilic [correction of electrophillic] phase ii inducers. Proc Natl Acad Sci U S A. 2006;103:768–773. doi: 10.1073/pnas.0505723102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shah ZA, Li RC, Thimmulappa RK, Kensler TW, Yamamoto M, Biswal S, et al. Role of reactive oxygen species in modulation of nrf2 following ischemic reperfusion injury. Neuroscience. 2007;147:53–59. doi: 10.1016/j.neuroscience.2007.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


