Abstract
Aims
To test whether changes in the subcellular localization of maspin parallel morphological progression in pulmonary adenocarcinoma, we compared its expression between lepidic and invasive growth patterns.
Methods
Applying immunohistochemistry, we compared maspin expression in lepidic and invasive growth patterns occurring in different tumours (series #1, n = 86) as well as within the same tumour and in the same section (series #2, n = 29).
Results
In both series, the lepidic growth pattern (n = 45) was significantly associated with nuclear maspin, while the invasive (n = 70) with combined nuclear and cytoplasmic maspin (P < 0.05). In the second series, transition from a lepidic to an invasive pattern in the same tumour was associated predominantly with a shift respectively from a nuclear to a combined nuclear and cytoplasmic maspin (15/29) or preservation of nuclear expression (8/29). A shift from nuclear maspin to negative expression (3/29) or other patterns (3/29) were also observed.
Conclusions
Nuclear maspin is a typical but not exclusive feature of the lepidic growth pattern of pulmonary adenocarcinoma, whereas combined nuclear and cytoplasmic maspin characterizes invasion. These data show that changes of expression and subcellular localization of maspin may constitute an important biological end point of tumour progression and aid in the classification of lung adenocarcinoma.
Keywords: invasive adenocarcinoma, lepidic growth pattern, maspin, pulmonary adenocarcinoma progression, subcellular localization
Introduction
Despite the advances brought forth by mass screening,1 and targeted molecular therapy,2 lung cancer retains the highest cancer mortality.3
A major challenge is that primary lung cancers constitute a heterogeneous group of diseases, with a wide range of biological, and clinical differences.4–7 However, many of these are reflected by histological features, betraying differences in cellular origin.5 Furthermore, lung cancers, more frequently than other tumours, display multiple growth patterns,8 which may impact prognosis in early stage disease.9
The revised classification of pulmonary adenocarcinoma10 recognizes this phenomenon and emphasizes the decisive role of the lepidic growth pattern. Its absolute size and the size of an associated invasive component dictate histological classification. By specifically endorsing the term adenocarcinoma in situ (AIS) for tumours with no invasion, measuring between 0.5 and 3 cm, the revised classification indirectly supports a model of histogenesis of adenocarcinoma where the lepidic growth pattern is a pre-invasive phase in the history of adenocarcinoma. This model is consistent with previous data, including seminal morphological studies on small peripheral adenocarcinoma.11 It justifies studying the lepidic growth patterns to unveil the molecular basis of progression in lung adenocarcinoma. A similar approach indeed has yielded historical insights on tumour progression in other anatomical sites.12 However, an additional pathway to invasive adenocarcinoma, in which this develops from normal peripheral airways, without a transition from a lepidic pattern, has also been postulated.13
A better understanding of the different growth patterns of lung cancer and their molecular underpinnings may help devise more accurate, histology-based models of tumour progression and drug-response. Yet, our knowledge of the differential molecular changes underlying these two types of progression is sketchy.13 K-Ras,14 EGFR mutations,14–16 and p16 loss,16 which show similar frequencies in non invasive and invasive patterns, are not likely to play a role. In contrast, p53 mutations,15,16 EGFR amplification,14 and allelic losses in multiple chromosomes, including the p53 locus on chromosome 17,16 have been associated with invasion. Myc amplification has been linked with poor prognosis in both small (<2 cm) and stage I adenocarcinoma.17
Maspin is a tumour suppressor protein with structural homology to serine protease inhibitors. A large host of clinical and in vitro evidence highlights maspin as playing an important role in inhibiting tumour progression, in many anatomical sites, including the lung.18–21 A unique feature of maspin is that it is epithelial specific. Further, its tumour suppressor activity is linked to its nuclear localization,22 possibly mediated by maspin's inhibition of histone deacethylase.23
We hypothesized that changes in the subcellular localization of maspin expression may parallel the morphological progression of adenocarcinoma. We addressed this question by comparing maspin's cellular expression pattern, between lepidic and invasive growth patterns. Our results suggest that changes of expression and subcellular localization of maspin may constitute an important biological end point of tumour progression and in addition may provide a diagnostic aid in the classification of lung adenocarcinoma.
Methods
Tissue Collection Series and Classification
Archived resections of pulmonary adenocarcinoma were collected and studied with the full institutional approval of our internal ethics committee, the Institutional Review Board (study 056809MP4E[R]/protocol 1011009032, approved February 1, 2012). All the cases represented, at the best of clinical and pathological information available to us (including positive TTF-1 stain in the majority of cases), primary lung cancers. Specimens studied were either whole tissue block tumour sections or 1 mm cores of representative tumour tissue, composing tissue multi arrays (TMA). The hematoxylin and eosin (H&E) slides for all these cases were reviewed and the cases classified first, according to the 2011 recommendations.10 It is commonly noted that sampling8 and serial sectioning may contribute to adenocarcinoma heterogeneity. To circumvent this problem and tightly associate each morphological pattern (i.e. lepidic versus invasive), with a specific immunohistochemistry (IHC) result, we used both TMA and whole tissue sections. Further, each sample used in the study was re-classified on the basis of the pattern present in the H&E stained level matching the one used for IHC, regardless of the original diagnosis of the case. The histological distinction of invasion from lepidic patterns was carried out on the basis of current guidelines. These include: the presence of a growth pattern considered per se to represent invasion,10 or of histological hall marks of stromal invasion, i.e. the presence of marked tissue remodeling, deemed to represent ‘foci of active fibroblastic proliferation’, rather than stromal collapse.11 The presence of high grade cytological features or complex architecture10 within an intra-alveolar proliferation, prompted us to render a diagnosis of invasive adenocarcinoma. Cases with such features were deemed to represent intra-alveolar colonization by adjacent invasive adenocarcinoma, and not lepidic patterns.
We divided our adenocarcinoma samples into two series. The first series compared isolated lepidic or invasive growth patterns.
The cases of lepidic growth patterns in this series were 16:8 from whole block sections and 8 from TMAs. The 8 whole block sections of lepidic pattern were from cases diagnosed as AIS (n = 3); Lepidic Predominant Adenocarcinoma (LPA) (n = 2); Minimally Invasive Adenocarcinoma (MIA) (n = 2); Acinar (n = 1). The 8 lepidic patterns from TMAs were from cases diagnosed as: Acinar (n = 3); AIS (n = 2); MIA (n = 1); LPA (n = 1); Adenosquamous carcinoma (n = 1).
The source of the invasive patterns in the first series was: 16 cases from whole sections, 54 cases from TMAs. The 16 whole sections were from cases diagnosed as: Acinar (n = 9); Solid (n = 2); Mucinous (MUC) (n = 2), LPA (n = 1); Papillary (n = 1); Micropapillary (n = 1). The 54 invasive patterns studied as TMAs were from cases diagnosed as Acinar, (n = 43); Solid, (n = 4); MUC (n = 6) and Micropapillary (n = 1).
The second series included 29 whole tissue sections of cases showing both lepidic and invasive growth patterns in the same section. None of these cases was included in the first series. The original diagnoses of these cases were: Acinar (n = 14); LPA (n = 9); MIA (n = 5); Muc (n = 1).
Immunohistochemistry
Slides were stained using standard IHC methods, after antigen retrieval. In brief, slides were deparaffinized through xylene and alcohol, rinsed in distilled H2O. Heat-induced epitope retrieval was accomplished using an EDTA buffer at pH 8.0, in a decloaking chamber (Biocare medical, Concord, CA, USA). Endogenous peroxidase was blocked using 3% H2O2. Non-specific staining was blocked using the universal blocking agent CAS (Invitrogen Carlsbad, CA, USA). Primary antibody monoclonal mouse anti-human Maspin from BD Pharmingen (San Diego, CA, USA; catalog #554292; clone G167-70) was used at a 1/100 overnight @ 4°C. Biotinylated secondary anti-mouse 1/200 was applied for 30 minutes (Vector Labs, Burlingame, CA, USA). Detection was carried out with an ABC/DAB system (Vector Labs). Slides were counterstained using hematoxylin QS (Vector Labs).
Cases tallied as (N+C) showed nuclear and cytoplasmic stain in the great majority (≥80%) of tumour cells. Cases tallied as (N) showed sharp positive maspin nuclear stain in ≥80% cells, with negative cytoplasm. Cases tallied as negative showed no stain at all in tumour cells. Cases previously confirmed for (N) maspin, and (N+C) maspin, respectively were included in each run as positive controls. Internal positive controls were basal cells of the bronchial mucosa, reactive pneumocytes and stromal cells. A negative control was included in each run, constituted by a known maspin positive case not incubated with the primary antibody. Normal alveoli and bronchioles provided internal negative controls.
Statistics
Statistical analysis was performed by using GraphPad software (reference: http://www.graphpad.com/quickcalcs/contingency1/). Chi-square tests were used to compare the differences in maspin expression pattern between study groups, and P values were calculated with two tails. P values were considered significant if ≤0.05.
Results
Maspin expression in samples of isolated lepidic versus invasive adenocarcinoma growth patterns, across different tumours (1st series)
Our aim was to determine whether specific maspin expression patterns are linked to these growth patterns. For this purpose, we used samples from unselected, consecutively resected adenocarcinomas, collected over the previous years by one of us (F.L.). Great care was taken in classifying the patterns by current guidelines for distinguishing lepidic versus invasive patterns. Further, the classification was done on the H&E level matching the level used in IHC (see Methods). Representative micrographs of staining patterns are provided in Figure1. Comparison showed that, in both TMA and whole block cases, the lepidic growth pattern showed a statistically significant association with the (N) versus the (N+C) expression pattern while the (N+C) pattern segregated with invasion (summarized in Table1).
Figure 1.
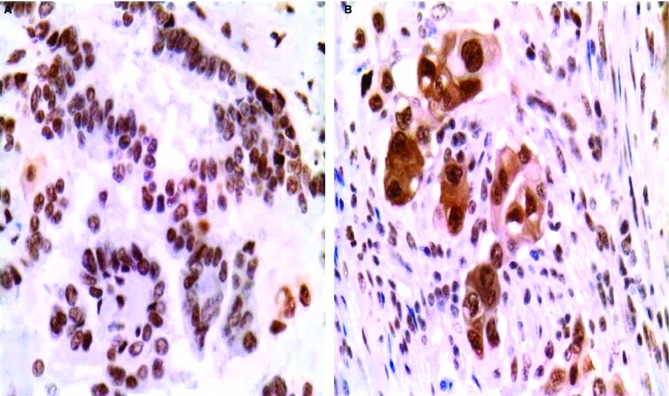
Representative micrographs of maspin in cases showing either the lepidic A, or the invasive B, growth pattern. Maspin subcellular localization is nuclear (N) or nuclear plus cytoplasmic (N+C).
Table 1.
Summary of maspin expression patterns in lepidic versus invasive adenocarcinoma in both the first and the second series of cases
| Maspin expression | First series | Second series | ||||||
|---|---|---|---|---|---|---|---|---|
| TMA | Whole sections | Whole sections | All cases | |||||
| Lepidic | Invasive | Lepidic | Invasive | Lepidic | Invasive | Lepidic | Invasive | |
| N* | 8/8 | 27/54 | 8/8 | 8/16 | 26/29 | 8/29 | 42/45 | 43/99 |
| N+C* | 0/8 | 26/54 | 0/8 | 8/16 | 2/29 | 17/29 | 2/45 | 51/99 |
| Negative | 0/8 | 1/54 | 0/8 | 0/16 | 1/29 | 4/29 | 1/45 | 5/99 |
|
P value N versus N+C |
0.0163 | 0.022 | <0.0001 | 0.0001 | ||||
N is defined as nuclear positivity, and N+C is defined as both nuclear and cytoplasmic positivity.
Maspin expression in samples showing both lepidic and invasive adenocarcinoma in the same section (2nd series)
Changes in the subcellular localization of maspin expression may parallel the morphological progression of adenocarcinoma. Accordingly a critical aspect of our design was to use a collection of cases showing both an in situ and an invasive pattern within the same tumour section.
Twenty nine such cases were identified, none of which had been included in the previous analysis. A comparison of the lepidic and invasive patterns, considered in isolation, confirmed a strong association of the lepidic pattern with nuclear maspin (Table1). The three lepidic cases that deviated from this predominant pattern were all of non-mucinous type. Two of them showed a (N+C) maspin expression pattern, whereas the third case was negative for maspin expression.
We then analyzed the change in maspin expression pattern occurring in each tumour between the two growth patterns. As summarized in Table2 the predominant observation was a shift of maspin expression from an (N) pattern in the lepidic foci to an (N+C) pattern (15/29) in the invasive foci. The second most frequent pattern observed was the retention of the (N) pattern in invasive (8/29), followed by a loss of nuclear maspin (3/29). The remaining cases of lepidic pattern retained an (N+C) (2/29) or a negative pattern (1/29) in the invasive component. Representative micrographs are provided in Figures2 and 3.
Table 2.
Maspin in the transition from lepidic to invasive adenocarcinoma in the second series of adenocarcinoma cases
| N in lepidic and (N+C) in invasive | 15/29 |
| N in both lepidic and invasive | 8/29 |
| N in lepidic but lost in invasive | 3/29 |
| (N+C) in both lepidic and invasive | 2/29 |
| Negative in both lepidic and invasive | 1/29 |
Figure 2.
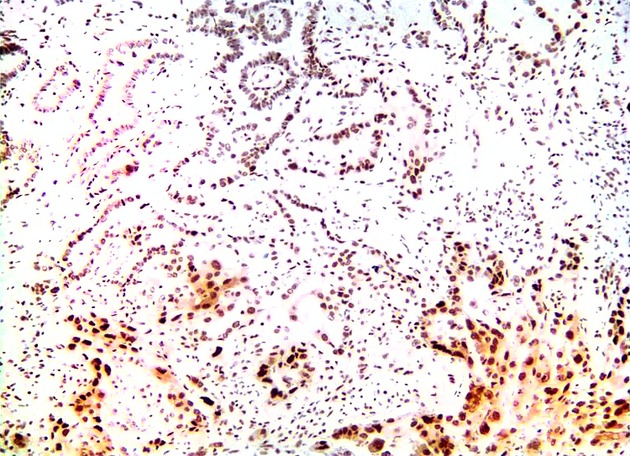
Low magnification micrograph of IHC of maspin, showing a change of maspin subcellular localization from an (N) pattern in the lepidic foci to an (N+C) pattern in the invasive focus in an adenocarcinoma specimen comprising both lepidic and invasive growth patterns.
Figure 3.
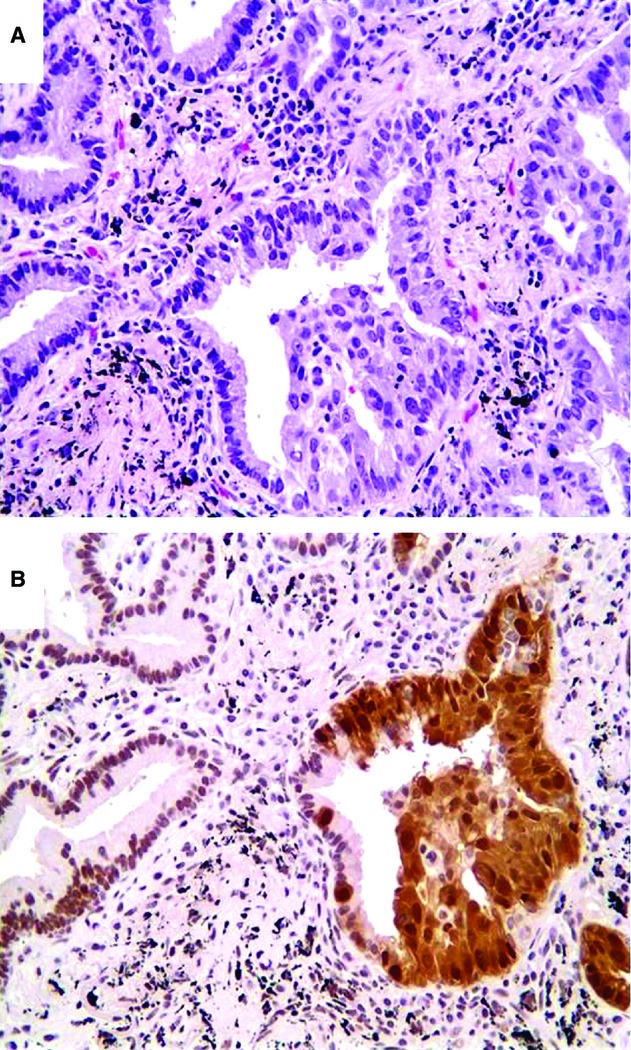
High power view shows a change from (N) to (N+C) maspin staining pattern occurring from lepidic to invasive adenocarcinoma, in parallel to increasing cell stratification and cytological atypia. A, H&E stain; B, IHC of maspin.
Discussion
Our main/novel finding is that there is a tight association of (N) maspin with the lepidic growth pattern and that there is a strong association of (N+C) maspin with invasion.
The lepidic patterns we studied constituted different proportions of the overall tumour. In addition, they came from heterogenous tumours, covering virtually the whole spectrum of adenocarcinoma variants. Yet, from a morphological stand point, they had the histological features of a non invasive growth of pneumocytes that, within the indicated size limits, would qualify as AIS, by standard guidelines.10 We can confidently rule out, based on cyto-architectural features (see Methods) that they represented colonization by adjacent invasive carcinoma. Further, the fact that histologically indistinguishable lepidic patterns showed a very homogeneous staining expression pattern of maspin supports the assumption that they represent similar lesions. However, addditional molecular studies, specifically comparing lepidic patterns occurring in distinct histologic subgroups, i.e. AIS, MIA, LPA and other subtypes of adenocarcinoma, may clarify whether their molecular underpinnings differ in these different morphological settings.
Our results also showed a strong association of (N+C) maspin with invasion. This constituted a specific but not sensitive feature, since invasion also showed other maspin patterns. However, the consistency of this association, across unselected tumours, in separate series suggests that the acquisition of (N+C) maspin, by normal alveoli and bronchioles (which are normally negative for maspin) represents a significant, although not exclusive end-point in the progression of lung adenocarcinoma. This finding is consistent with our previous observation that upon chemical transformation a relative increase of cytoplasmic versus nuclear maspin, correlates with increased colony forming efficiency in vitro.24 Independently, a host of in vitro and in vivo data further link loss of maspin's tumour suppressor activity to a reduction in its nuclear to cytoplasmic levels.22 An association of (N) maspin with reduced angiogenesis has been reported in pulmonary24 and ovarian adenocarcinoma.25 We24 and others19 have described (N) maspin to be linked to improved prognosis in the lung. Thus, data support the hypothesis that preservation of nuclear maspin, even in invasive patterns, may be associated with favorable biological features. How changes in sub cellular localization of maspin occur is unknown. Interestingly, a high level of tyrosine phosphorylation has been recently shown to increase preferentially maspin's cytoplasmic localization.26 Recently, we identified a sequence, within the maspin gene which is crucial for determining nuclear localization,27 but whether maspin mutations occur in cancer is unknown.
The apparent dependence of its biological activity from the sub-cellular localization sets maspin apart from other tumour suppressor, but is not without precedents. p27 acts as a tumour suppressor in the nucleus, inhibiting cell cycle progression, but may function as an oncogene in the cytoplasm.28,29
We also noted that differential expression of maspin in lung adenocarcinoma seems not limited to changes of subcellular location. In our series, loss of maspin expression was more frequent in invasive (5/99) than in lepidic pattern (1/45). Future studies are needed to elucidate whether the loss of maspin also represents a distinct gain of function in tumour progression.
Overall, our data support a tentative model of tumour progression in pulmonary adenocarcinoma, where the lepidic phase is strongly linked to a nuclear expression pattern, while invasion is associated to (i)-a shift to a combined (N+C) pattern, (ii)-loss of expression, or (iii)-retention of nuclear maspin.
In this study, we found that that a (N+C) staining pattern is specific but not sensitive for invasive carcinoma, whereas a nuclear pattern is sensitive but not specific for the lepidic pattern. These findings may assist in the classification of lung adenocarcinoma. The distinction, particularly in tumours with a prominent lepidic growth pattern, of areas of stromal collapse or fibrosis, from true invasion is marred by marked inter-observer variability.30 Yet, the microscopic size of invasion dictates histological classification, and constitutes and independent predictor of prognosis in limited stage adenocarcinoma.9,31 Importantly, it is also significantly different from the tumour's gross measurement,9 in tumours with a prominent lepidic growth pattern.
Taken together, our data show that changes of expression and subcellular localization of maspin constitute an important biological end point of tumour progression and may provide a diagnostic aid in the classification of lung adenocarcinoma.
Acknowledgments
The study was funded by NIH grant R21CA1543219-01 (SS and FL). All authors have participated in the design and/or performance and/or interpretation of the study. We acknowledge the expert editorial assistance of Dr. Amedeo Lonardo.
Conflict of interest
The authors disclose no conflicts of interests.
References
- 1.Bach PB, Mirkin JN, Oliver TK, et al. Benefits and harms of CT screening for lung cancer: a systematic review. JAMA. 2012;307:2418–2429. doi: 10.1001/jama.2012.5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reck M, Heigener DF, Mok T, Soria JC, Rabe KF. Management of non-small-cell lung cancer: recent developments. Lancet. 2013;382:709–719. doi: 10.1016/S0140-6736(13)61502-0. [DOI] [PubMed] [Google Scholar]
- 3.American Cancer Facts and Figures. 2014.
- 4.Goldstraw P, Ball D, Jett JR, et al. Non-small-cell lung cancer. Lancet. 2011;378:1727–1740. doi: 10.1016/S0140-6736(10)62101-0. [DOI] [PubMed] [Google Scholar]
- 5.Gazdar AF, Brambilla E. Preneoplasia of lung cancer. Cancer Biomark. 2010;9:385–396. doi: 10.3233/CBM-2011-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suda K, Tomizawa K, Yatabe Y, Mitsudomi T. Lung cancers unrelated to smoking: characterized by single oncogene addiction? Int. J. Clin. Oncol. 2011;16:294–305. doi: 10.1007/s10147-011-0262-y. [DOI] [PubMed] [Google Scholar]
- 7.Van Meerbeeck JP, Fennell DA, De Ruysscher DK. Small-cell lung cancer. Lancet. 2011;378:1741–1755. doi: 10.1016/S0140-6736(11)60165-7. [DOI] [PubMed] [Google Scholar]
- 8.Kerr KM. Pulmonary adenocarcinomas: classification and reporting. Histopathology. 2009;54:12–27. doi: 10.1111/j.1365-2559.2008.03176.x. [DOI] [PubMed] [Google Scholar]
- 9.Yoshizawa A, Motoi N, Riely GJ, et al. Impact of proposed IASLC/ATS/ERS classification of lung adenocarcinoma: prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases. Mod. Pathol. 2011;24:653–664. doi: 10.1038/modpathol.2010.232. [DOI] [PubMed] [Google Scholar]
- 10.Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J. Thorac. Oncol. 2011;6:244–285. doi: 10.1097/JTO.0b013e318206a221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noguchi M. Stepwise progression of pulmonary adenocarcinoma–clinical and molecular implications. Cancer Metastasis Rev. 2010;29:15–21. doi: 10.1007/s10555-010-9210-y. [DOI] [PubMed] [Google Scholar]
- 12.Vogelstein B, Fearon ER, Hamilton SR, et al. Genetic alterations during colorectal-tumour development. N. Engl. J. Med. 1988;319:525–532. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- 13.Yatabe Y, Borczuk AC, Powell CA. Do all lung adenocarcinomas follow a stepwise progression? Lung Cancer. 2011;74:7–11. doi: 10.1016/j.lungcan.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soh J, Toyooka S, Ichihara S, et al. Sequential molecular changes during multistage pathogenesis of small peripheral adenocarcinomas of the lung. J. Thorac. Oncol. 2008;3:340–347. doi: 10.1097/JTO.0b013e318168d20a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoo SB, Chung JH, Lee HJ, Lee CT, Jheon S, Sung SW. Epidermal growth factor receptor mutation and p53 overexpression during the multistage progression of small adenocarcinoma of the lung. J. Thorac. Oncol. 2010;5:964–969. doi: 10.1097/JTO.0b013e3181dd15c0. [DOI] [PubMed] [Google Scholar]
- 16.Nakanishi H, Matsumoto S, Iwakawa R, et al. Whole genome comparison of allelic imbalance between noninvasive and invasive small-sized lung adenocarcinomas. Cancer Res. 2009;69:1615–1623. doi: 10.1158/0008-5472.CAN-08-3218. [DOI] [PubMed] [Google Scholar]
- 17.Iwakawa R, Kohno T, Kato M, et al. MYC amplification as a prognostic marker of early-stage lung adenocarcinoma identified by whole genome copy number analysis. Clin. Cancer Res. 2011;17:1481–1489. doi: 10.1158/1078-0432.CCR-10-2484. [DOI] [PubMed] [Google Scholar]
- 18.Berardi R, Morgese F, Onofri A, et al. Role of maspin in cancer. Clin. Transl. Med. 2013;2:8. doi: 10.1186/2001-1326-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berardi R, Santinelli A, Onofri A, et al. Maspin expression is a favorable prognostic factor in non-small cell lung cancer. Anal. Quant. Cytol. Histol. 2012;34:72–78. [PubMed] [Google Scholar]
- 20.Bodenstine TM, Seftor RE, Khalkhali-Ellis Z, Seftor EA, Pemberton PA, Hendrix MJ. Maspin: molecular mechanisms and therapeutic implications. Cancer Metastasis Rev. 2012;31:529–551. doi: 10.1007/s10555-012-9361-0. [DOI] [PubMed] [Google Scholar]
- 21.Lonardo F, Li X, Kaplun A, et al. The natural tumour suppressor protein maspin and potential application in non small cell lung cancer. Curr. Pharm. Des. 2010;16:1877–1881. doi: 10.2174/138161210791208974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goulet B, Chan G, Chambers AF, Lewis JD. An emerging role for the nuclear localization of maspin in the suppression of tumour progression and metastasis. Biochem. Cell Biol. 2012;90:22–38. doi: 10.1139/o11-053. [DOI] [PubMed] [Google Scholar]
- 23.Li X, Yin S, Meng Y, Sakr W, Sheng S. Endogenous inhibition of histone deacetylase 1 by tumour-suppressive maspin. Cancer Res. 2006;66:9323–9329. doi: 10.1158/0008-5472.CAN-06-1578. [DOI] [PubMed] [Google Scholar]
- 24.Lonardo F, Li X, Siddiq F, et al. Maspin nuclear localization is linked to favorable morphological features in pulmonary adenocarcinoma. Lung Cancer. 2006;51:31–39. doi: 10.1016/j.lungcan.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 25.Solomon LA, Munkarah AR, Schimp VL, et al. Maspin expression and localization impact on angiogenesis and prognosis in onvarian cancer. Gynecol. Oncol. 2006;101:385–389. doi: 10.1016/j.ygyno.2005.11.049. [DOI] [PubMed] [Google Scholar]
- 26.Tamazato Longhi M, Cella N. Tyrosine phosphorylation plays a role in increasing maspin protein levels and its cytoplasmic accumulation. FEBS Open Bio. 2012;2:93–97. doi: 10.1016/j.fob.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dzinic SH, Kakplun A, Li X, et al. Identification of an intrinsic determinant critical for maspin subcellular localization and function. PLoS One. 2013;8:e74502. doi: 10.1371/journal.pone.0074502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Besson A, Dowdy SF, Roberts JM. CDK inhibitors: cell cycle regulators and beyond. Dev. Cell. 2008;14:159–169. doi: 10.1016/j.devcel.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 29.Serres MP, Zlotek-Zlotkiewicz E, Concha C, et al. Cytoplasmic p27 is oncogenic and cooperates with Ras both in vivo and in vitro. Oncogene. 2011;30:2846–2858. doi: 10.1038/onc.2011.9. [DOI] [PubMed] [Google Scholar]
- 30.Thunnissen E, Beasley MB, Borczuk A, et al. Reproducibility of histopathological subtypes and invasion in pulmonary adenocarcinoma. An international interobserver study. Mod. Pathol. 2012;25:1574–1583. doi: 10.1038/modpathol.2012.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Borczuk AC, Qian F, Kazeros A, et al. Invasive size is an independent predictor of survival in pulmonary adenocarcinoma. Am. J. Surg. Pathol. 2009;33:462–469. doi: 10.1097/PAS.0b013e318190157c. [DOI] [PMC free article] [PubMed] [Google Scholar]


