Abstract
Hibernation is an energy saving adaptation that involves a profound suppression of physical activity that can continue for 6-8 months in highly seasonal environments. While immobility and disuse generate muscle loss in most mammalian species, in contrast, hibernating bears and ground squirrels demonstrate limited muscle atrophy over the prolonged periods of physical inactivity during winter suggesting that hibernating mammals have adaptive mechanisms to prevent disuse muscle atrophy. To identify common transcriptional programs that underlie molecular mechanisms preventing muscle loss, we conducted a large-scale gene expression screen in hind limb muscles comparing hibernating and summer active black bears and arctic ground squirrels using custom 9,600 probe cDNA microarrays. A molecular pathway analysis showed an elevated proportion of over-expressed genes involved in all stages of protein biosynthesis and ribosome biogenesis in muscle of both species during torpor of hibernation that suggests induction of translation at different hibernation states. The induction of protein biosynthesis likely contributes to attenuation of disuse muscle atrophy through the prolonged periods of immobility of hibernation. The lack of directional changes in genes of protein catabolic pathways does not support the importance of metabolic suppression for preserving muscle mass during winter. Coordinated reduction of multiple genes involved in oxidation reduction and glucose metabolism detected in both species is consistent with metabolic suppression and lower energy demand in skeletal muscle during inactivity of hibernation.
Keywords: protein biosynthesis, hibernation, black bear, arctic ground squirrel, functional genomics, gene expression
Introduction
Hibernation is an adaptive strategy of reduced metabolic demand and greatly limited mobility that conserves energy during periods of low food availability in highly seasonal or unpredictable environments (Boyer and Barnes, 1999; Carey et al., 2003). In humans and most mammals, physical inactivity leads to loss of muscle strength and mass. In contrast, hibernating bears and ground squirrels demonstrate very limited muscle atrophy over the prolonged periods (6-8 months) of physical inactivity of winter hibernation suggesting that hibernating mammals have evolved natural mechanisms that prevent disuse muscle atrophy. Although attenuation of atrophy in muscle over the hibernation season in bears and ground squirrels has been documented (Harlow et al. 2001; Lohuis et al. 2007a; Gao et al. 2012), the molecular mechanisms underlying this protective musculoskeletal adaptation are not known.
Genome-wide transcriptional screening provides a means for the identification of the transcriptional changes and pathways that are potentially involved in the molecular mechanisms preventing disuse muscle atrophy in hibernating mammals. The first study of transcriptional changes in skeletal muscle of black bears using a pilot version of a custom microarray revealed elevated expression of genes involved in protein biosynthesis during hibernation, but no coordinated directional changes were detected for genes within catabolic pathways (Fedorov et al. 2009). Although genome coverage was limited, these results led to the hypothesis that elevated expression of genes involved in protein biosynthesis contributes to molecular mechanisms preserving muscle mass during periods of physical inactivity. The energy cost of increased anabolism was suggested as an important trade-off with the adaptive mechanisms that allow bears to maintain full musculoskeletal function and preserve mobility during and immediately after hibernation, thus promoting survival (Fedorov et al. 2012). Decrease in protein catabolism during hibernation due to metabolic suppression for conserving energy is an alternative hypothesis to explain the attenuation of muscle atrophy (Lee et al. 2008). We expect from this hypothesis coordinated decrease in expression level of genes involved catabolic pathways during torpor of hibernation.
The black bear (Ursus americanus) and arctic ground squirrel (Urocitellus parryii) are evolutionary distant species that demonstrate different modes of hibernation. Both species remain sequestered within hibernacula overwinter and show little or no locomotion for 5-7 months (Daan et al. 1991; Tøien et al. 2011). However, unlike bears hibernating with core body temperature 30 - 36°C and metabolic rate reduced by 50-75% (Tøien et al. 2011), small hibernators (<1 kg) such as the arctic ground squirrels enter deep levels of torpor with body temperatures near 0 °C and 98% reduction of metabolic rate. Torpor in small hibernators is periodically interrupted by spontaneous arousal episodes when animals raise their metabolism and body temperature returns to euthermic levels (>30°C) for less than 24 hours (Boyer & Barnes 1999). Similar to black bears, preservation of muscle mass has been reported in several species of ground squirrels (Gao et al. 2012; Andres-Mateos et al. 2013). The distant phylogenetic relation between black bears and ground squirrels suggests that mechanisms regulating gene expression rather than evolution of specific genes underlay their ability to prevent disuse muscle atrophy. Comparison of transcriptional profiles and pathways significantly enriched by co-regulated genes between these two evolutionary distant hibernating species is expected to identify general and species specific transcriptional changes important in protection from disuse atrophy in muscle.
In the present study, we used a more representative set of 9,600 cDNA probes on a microarray custom to black bears (Fedorov et al. 2011) to identify additional co-regulated functional groups of differentially expressed genes in muscle of hibernating bears as compared to summer active animals. To obtain the first insight into transcriptional changes in muscle of hibernating ground squirrels, we developed the genomics resources to fabricate a 9,600 probe microarray custom to arctic ground squirrels. We focused on pathway analysis identifying functional groups of co-regulated genes rather than on expression of individual genes to assess the biological significance of transcriptional changes in skeletal muscle of both hibernating species. To reveal general patterns in transcriptional profiles during hibernation, we compared functional groups of differentially expressed genes detected in each species. In addition, we conducted pathway analysis of differentially expressed genes that are common in hibernating black bears and arctic ground squirrels. Transcriptional changes are discussed in light of how disuse muscle atrophy is prevented in hibernating mammals.
Material and Methods
Animals
Animal protocols were approved by the University of Alaska Fairbanks Institutional Animal Care and Use and USAMRMC Animal Care and Use Review Office.
We sampled the skeletal muscle from black bears including animals reported on in a previous study (Fedorov et al. 2009). Bears (51 -226 kg) were captured May-July (two bears sampled in hibernation were captured in October) from the field in Alaska and transferred to Fairbanks. These were “trouble bears” captured by the Alaska Department of Fish and Game that were scheduled for euthanasia. In order to minimize intra-group variation in gene expression, only males > 2 years old were used in these experiments. Summer active bears that were still feeding and active and housed in an outdoor enclosure were euthanized and sampled for tissues between late May - early July (n = 5) and on October 2 (n = 2). Food was withdrawn 24 hours before these animals were euthanized. Bears in the hibernating condition were without food since October 27 and euthanized for tissue sampling between March 1 - 26 (n = 6), about 1.5 months before expected emergence from hibernation. For monitoring of physiological conditions, bears were instrumented as previously described (Fedorov et al. 2009; Tøien et al. 2011). Briefly, core body temperature, ECG and EMG were monitored with radio telemetry. Oxygen consumption and respiratory quotient were monitored with open flow respirometry by drawing air from the closed dens (Tøien 2013). Bears were euthanized by an intravenous injection of pentobarbital with death assessed by termination of heart beats as determined with a stethoscope. Immediately prior to tissue sampling, the metabolic rate of anesthetized hibernating bears was 45.4 % of that of anesthetized summer bears, and body temperature was 3.5°C lower. Tissues collection from quadriceps followed immediately with samples frozen in liquid nitrogen within 10 minutes of death.
Arctic ground squirrels were trapped during July near Toolik Lake (68°N 149°W) and transported to the University of Alaska Fairbanks. Animals were housed at 20 ± 2°C with a 16:8-h light-dark photoperiod and provided with Mazuri rodent chow and water ad libitum. Hibernating animals were implanted abdominally with temperature-sensitive transmitters. Core body temperature (Tb) was monitored for stage of torpor by an automated telemetry system that records Tb every 10 min. Animals were transferred in September to a chamber with the temperature 2 ± 2°C and a 8:16-h light-dark photoperiod where they entered hibernation. Arctic ground squirrels in steady-state torpor and Tb 2.2+0.3°C (n = 10) were collected after 80-90% of the duration of the previous torpor bout (late torpor, 8-12 days). Squirrels sampled during hibernation had completed no less than two full-length torpor bouts and none were sampled after February (> 1 month of hibernation remained). Post reproductive, summer euthermic animals sampled in May and June 1-2 months after ending hibernation (n = 9) were used as non-hibernating controls. To decrease biological variation, all samples but one, were females in both groups. Torpid arctic ground squirrels were euthanized by decapitation without anesthesia, summer active animals were deeply anesthetized with isoflurane, euthanized with sodium pentobarbital and decapitated. Quadriceps skeletal muscle was rapidly dissected, frozen in liquid nitrogen and stored at −80°C until used.
RNA preparation
Skeletal muscle samples (approximately 250 mg) were homogenized in 2 ml Lysing Matrix D tubes containing specialized beads and RLT buffer using a mini-beater (FastPrep-FP120, Qbiogene, Inc., Carlsbad, CA, USA) for 1 min at 4800 rpm. Total RNA was isolated from the tissues using an RNeasy midi kit (Qiagen Inc., Valencia, CA, USA). All RNA samples were processed by DNase I (Qiagen) treatment. RNA quality was evaluated with an Agilent 2100 Bioanalyzer and concentration was measured by using Nanodrop ND-1000.
Developing genomic resources
Gene discovery and custom microarrays fabrication for the black bear are described in detail in Fedorov et al. (2009, 2011) as well as genomic analysis of the expressed sequencing tags (Zhao et al. 2010). We used the same procedure for the arctic ground squirrel. In short, normalized, subtracted cDNA libraries were constructed from heart, brain, brown fat, skeletal muscle, and liver of hibernating and euthermic arctic ground squirrels. We used SMART template-switching protocol and primer extension PCR, normalization and subtraction as was previously described (Fedorov et al. 2009). Expressed sequencing tags (EST) were sequenced from the 5’-end with the universal M13 forward primer. After filtering off vector contamination and mtDNA inserts, a total of 24,371 high quality ESTs were mapped to the genome of the hibernating 13-lined ground squirrel, Ictidomys tridecemlineatus (Shao et al. 2010), a close relative of the arctic ground squirrel. Among 15,639 mapped sequences, 10,252 ESTs were annotated with 3,883 unique genes. All 24,371 ESTs were re-annotated by searching against human RefSeq sequences (http://www.ncbi.nlm.nih.gov/RefSeq/) (e-value <= 1E-10). For 20,477 ESTs with human accession number, we were able to annotate 6,647 sequences with Ensembl/biomart database (http://www.biomart.org/index.html) obtaining 5,944 different genes. A total of 9,600 cDNA inserts were PCR amplified and printed on nylon membrane with a Biorobotics arrayer in the Microarray core facility of the Wistar institute.
Hybridization
Hybridization of black bear and ground squirrel samples was carried out with a species specific 9,600 cDNA custom microarrays. Samples of total RNA were linearly amplified with Illumina TotalPrep RNA Amplification Kit (Ambion), and 1.6 μg of the amplified RNA was labeled with 65 μCi of [33P]dCTP as previously described (Kari et al. 2003). All RNA samples were amplified, labeled and hybridized in the same batch. The hybridization was carried out for 18 hours at 42°C in 3 ml of a hybridization buffer (6× SSC, 0.5% SDS, 6M Urea). Filters were rinsed at room temperature with 2× SSC/1% SDS to remove residual probe and MicroHyb solution and then transferred to preheated wash solutions in a temperature-controlled shaking water bath. Filters were washed twice for 30 min in 1.5 l of 2× SSC/1% SDS at 50°C and then once for 30 min in 1.5 l of 0.5× SSC/1% SDS at 55°C and once for 30 min in 1.5 l of 0.1× SSC/0.5% SDS at 55°C. Filters were then exposed to phosphorimager screens for four days and scanned at 50-μm resolution in a Storm Phosphorimager. Image analysis was performed with the ImaGene program (Biodiscovery).
Microarray data analysis
Only non-empty spots with less than four flags across the samples were included into the analysis, the flagged signals were omitted. The background corrected signal was obtained by subtracting local background median density from signal median density. Background corrected signals were divided by their median on the array to obtain the normalized median densities representing the normalized expression values. The array data were transformed with quantile normalization (Bolstad et al. 2003) and a one-way ANOVA test was used to select genes that exhibited significant differences between hibernating and summer active animals. Similar to experiments with other tissues (Fedorov et al. 2011), a p-value<0.05 and |log2 FC |>0.5 were set as cutoffs for significant differences in expressed genes, where FC is fold change (the mean expression value in the hibernating animals divided by the mean expression value in the summer active animals as the criteria for differentially expressed genes as previously reported for other tissues (Fedorov et al. 2009, 2011). The false discovery rate (FDR) was calculated using random permutation as described by Storey & Tibshirani (2003). The FDR was defined as the number of significant selected genes divided by the average of the number of significant genes under permutations. The genes demonstrating significant hybridization signal on the arrays were classified according to their Gene Ontology (GO) categories of the biological processes. If one gene had multiple probes on the array, we selected the probe with the smallest ANOVA p-value to represent the gene. Lists of all significant genes on the array and differentially expressed genes with cutoffs of p-value<0.05 and |log2 fold change|>0.5 were uploaded to GO miner (http://discover.nci.nih.gov/gominer/index.jsp). The enrichment in each GO category was calculated as the proportion of differentially expressed genes relative to the expected proportion on the array. The significance of enrichment for each GO category was estimated by one-sided Fisher exact test. The false discovery rate was assessed by resampling all the significant genes on the array (Zeeberg et al. 2003; Zeeberg et al. 2005)
In addition to GO miner analysis, we verified enrichment in significant GO categories of the biological processes by using Gene Set Enrichment Analysis (http://www.broad.mit.edu/gsea/index.jsp). There are two advantages of this method. First, unlike GO miner, GSEA estimates enrichment by taking into consideration all of the genes with significant hybridization signals in an experiment, not only those above arbitrary cutoffs for significance of expression differences, false discovery rate and fold-change (Subramanian et al. 2005). Second, both over-expressed and under-expressed genes are analyzed in the same run to obtain integrative estimate of enrichment by the use of the following algorithm. Genes were ranked according to the correlation between their expression values and the phenotype class (hibernating and summer active phenotypes) distinction by using the signal to noise ratio. An enrichment score (ES) that reflects the degree to which genes involved in a category are overrepresented at the extremes (over-expressed genes at the top and under-expressed genes at the bottom) of the entire ranked list of genes was calculated. The ES was normalized to account for the size of the category gene set presented in the experiment, yielding a normalized enrichment score (NES). The positive values of the NES indicate enrichment by over-expressed genes and the negative values suggest elevated proportion of under-expressed genes. The statistical significance of the NES was estimated by using phenotype-based permutation test. A cutoff of 25% for false discovery rate of gene set enrichment was used as this value was suggested appropriate for exploratory studies (Subramanian et al. 2005). We also used GSEA to test enrichment in selected gene sets that are expected to be important for muscle homeostasis. These gene sets were obtained from Molecular Signatures Database www.broadinstitute.org/gsea/msigdb/index.jsp).
Quantitative real- time PCR
We validated the microarray experiments by quantitative real-time PCR (RT PCR) tests using the same total RNA samples. Hint1 was selected as a reference gene for black bear skeletal muscle, and Ywhaz for ground squirrel, based on the stability of expression values across all samples obtained from the microarray experiments and then tested by RT PCR. All samples showed similar expression values for the correspondent reference genes with low standard deviation in multiple RT-PCR tests. Total RNA concentrations were measured with a NanoDrop ND-1000 spectrophotometer, and cDNA was synthesized from 0.5 μg of total RNA from each sample. The reverse transcription was carried out with MiltiScribeTM reverse transcriptase (Applied Biosystems) with oligo d(T)16 primer in 25 μl reactions at 25° C for 10 min, 48° C for 30 min, and at 95° C for 5 min. The synthesized cDNA was diluted 4x with RNase-free water, and 4 μl of diluted cDNA was used in the 20 μl-volume real-time PCR. Primers were designed with the Primer3 software (http://frodo.wi.mit.edu/primer3/) using the black bear and arctic ground squirrel EST sequences (Table S1). Real-time PCR was performed in triplicates with Power SYBR Green PCR Master Mix (Applied Biosystems) on an ABI-7900 HT. Cycling parameters were 50° C for 2 min of incubation, 95° C for 10 min of Taq activation, and 40 cycles of 95° C for 15 sec and 60° C for 1 min. Controls with no template were set to exclude contamination, and controls with no reverse transcriptase but all other components were taken to exclude nonspecific amplification from genomic DNA. Specificity of amplification was checked with the melting curve analysis and agarose gel electrophoresis. Four 10-fold dilutions of a sample with mixed cDNA from all samples were used for a standard curve for each primer set for calculating RT PCR efficiency. We tested a difference in gene expression between hibernating and summer active animals with P < 0.10 as cutoff according to Pfaffl (2001). We calculated the fold-change in level of expression of a target gene relative to a reference gene for each sample and then compared the values for each group using Student’s t-test as described by Livak & Schmittgen (2001).
Results
Differentially expressed genes in black bears
Signals from 9,093 of 9,600 (95%) probes across all samples showed median intensities that were above background. A total of 415 unique genes (4.6% of all genes with significant signals) were differentially expressed in the skeletal muscle tissue of bears sampled during hibernation compared with in bears sampled in summer (Table S2). All but three differentially expressed genes (Otud1, Col3a1, Ube2q2) demonstrated changes in expression that differed less than four-fold (|log2FC| < 2). Of the significantly differentially expressed genes, we identified 198 (47.5%) genes that were over-expressed and 217 (52.5%) genes that were under-expressed during hibernation.
Differentially expressed genes in arctic ground squirrels
Signals from 9,147 of 9,600 (95%) probes across all 19 samples showed median intensities that were above background. In total, 548 unique genes (6.0% of all genes with significant signals) were differentially expressed in arctic ground squirrel skeletal muscle tissue during late torpor (Table S4). All but three differentially expressed genes (Ankrd1, Acaa2 , Abra) demonstrated changes in expression that differed less than four-fold (|log2FC| < 2). Of the significantly differentially expressed genes, 349 (63.7%) genes were over-expressed and 199 (36.3%) genes were under-expressed in ground squirrel skeletal muscle sampled during late torpor compared to summer.
Quantitative RT PCR validation of microarray results
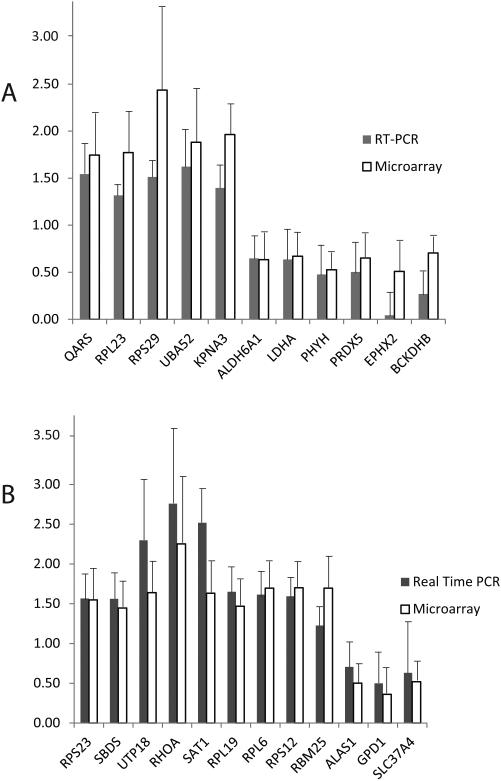
To validate results from microarray, we conducted quantitative real-time PCR tests for 30 randomly selected genes that showed differences in expression at P < 0.05 in the array hybridization for the black bear. Twenty-four (80%) tested genes showed significant changes in the same direction as the array results (Table S3, Fig. 1). Similar to in the bear, a total of 20 (80%) out of 25 tested genes showed significant changes consistent with the array results for the arctic ground squirrel (Table S5, Fig. 1). The observed experimental false discovery rate of 20% only slightly exceeds the experimental value (15%) for the commercial whole human genome microarray platform (Agilent; Wang et al. 2006), thus this is reasonable for an exploratory study in a non-model species. Further support for microarray results comes from the highly significant positive correlations (black bear: r = 0.93, P < 0.0001; ground squirrel: r = 0.84, P < 0.0001) between the amplitude of fold changes for true positive genes in RT PCR and microarray experiments.
Figure 1.
Differentially expressed genes in skeletal muscle tissue confirmed with real-time PCR: A – black bear, B - arctic ground squirrel. Solid and open bars with standard deviation bars show normalized expression values obtained in real-time PCR and microarray experiments, respectively. The values were normalized to the mean in summer active animals.
Differentially expressed genes common for both species
A total of 2138 genes with hybridization signals on the microarrays were shared between the two species. Among 66 differentially expressed genes shared between species, 46 genes changed in the same direction in both species (Table 1). In total 30 genes were over-expressed and 16 genes were under-expressed with a significant correlation (r = 0.72, P < 0.0001) between fold changes in both species during hibernation (Table 1). Notably, gene Ndrg2 was over-expressed during torpor of hibernation in both species (Table 1) which is consistent with elevated expression of this gene previously detected by RNA-seq in muscle of torpid 13-lined ground squirrels (Hampton et al. 2011).
Table 1. Differentially expressed genes changed in the same direction in skeletal muscle of both the black bear and the arctic ground squirrel during torpor of hibernation.
| Gene Symbol |
Gene Name | Black Bear | Arctic Ground Squirrel |
||
|---|---|---|---|---|---|
|
| |||||
| P | Log2FC | P | Log2FC | ||
| Translation (13 genes) | |||||
| Eif3k | Eukaryotic translation initiation factor 3 subunit K |
0.006 | 0.995 | 0.012 | 0.630 |
| Eif3m | Eukaryotic translation initiation factor 3 subunit M |
0.020 | 0.876 | 0.030 | 1.021 |
| Fxr1 | Fragile X mental retardation syndrome- related protein 1 |
0.008 | 1.649 | <0.001 | 0.871 |
| Rpl23 | 60S ribosomal protein L23 | 0.013 | 0.826 | <0.001 | 0.641 |
| Rpl27 | 60S ribosomal protein L27 | 0.011 | 0.990 | 0.012 | 0.485 |
| Rpl27a | 60S ribosomal protein L27a | 0.012 | 0.931 | <0.001 | 0.619 |
| Rpl8 | 60S ribosomal protein L8 | 0.021 | 0.389 | 0.005 | 1.354 |
| Rps12 | 40S ribosomal protein S12 | 0.008 | 1.534 | <0.001 | 0.767 |
| Rps13 | 40S ribosomal protein S13 | 0.010 | 0.856 | 0.036 | 0.335 |
| Rps15a | 40S ribosomal protein S15a | 0.011 | 0.713 | <0.001 | 0.553 |
| Rps23 | 40S ribosomal protein S23 | 0.008 | 1.099 | 0.004 | 0.630 |
| Rps24 | 40S ribosomal protein S24 | 0.012 | 1.738 | 0.034 | 0.495 |
| Rps27 | 40S ribosomal protein S27a | 0.008 | 1.592 | 0.003 | 0.831 |
| Oxidation Reduction (7 genes) | |||||
| Adh4 | Alcohol dehydrogenase 4 | 0.004 | −0.505 | 0.022 | −0.346 |
| Aldh9a1 | 4-trimethylaminobutyraldehyde dehydrogenase |
0.032 | −1.538 | 0.015 | −0.706 |
| Atp5f1 | ATP synthase subunit b, mitochondrial | 0.030 | −0.415 | 0.034 | −0.727 |
| L2hgdh | L-2-hydroxyglutarate dehydrogenase, mitochondrial |
0.011 | −0.622 | 0.003 | −0.832 |
| Ndufs1 | NADH-ubiquinone oxidoreductase 75 kDa subunit, mitochondrial |
0.009 | −0.514 | 0.041 | −0.450 |
| Nnt | NAD(P) transhydrogenase, mitochondrial | 0.007 | −0.756 | 0.007 | −0.873 |
| Phyh | Phytanoyl-CoA dioxygenase, peroxisomal | <0.001 | −0.926 | <0.001 | −0.802 |
| Other GO Categories (26 genes) | |||||
| Acyp1 | Acylphosphatase | 0.014 | 0.794 | 0.002 | 0.827 |
| Ankrd1 | Ankyrin repeat domain-containing protein 1 | 0.020 | 1.580 | 0.005 | 3.273 |
| Atp6v0d1 | V-type proton ATPase subunit d 1 | 0.015 | 1.505 | 0.008 | 0.665 |
| Caprin1 | Caprin-1 | 0.012 | 0.533 | 0.010 | 0.390 |
| Cebpz | CCAAT/enhancer-binding protein zeta | 0.021 | 0.477 | 0.017 | 0.760 |
| Cmya5 | Cardiomyopathy-associated protein 5 | 0.026 | 1.48 | 0.016 | 0.512 |
| Gbe1 | 1,4-alpha-glucan-branching enzyme | 0.027 | 0.698 | 0.004 | 1.189 |
| Gnb1 | Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 |
0.040 | 0.598 | 0.042 | 0.347 |
| Gnb2l1 | Guanine nucleotide-binding protein subunit beta-2-like 1 |
0.013 | 0.353 | <0.001 | 0.802 |
| Maged2 | Melanoma antigen, family D, 2 | 0.016 | 0.293 | 0.008 | 0.460 |
| Ndrg2 | NDRG family member 2 | <0.001 | 1.223 | <0.001 | 1.705 |
| Nolc1 | Nucleolar and coiled-body phosphoprotein 1 | 0.026 | 0.466 | 0.035 | 0.518 |
| Peal5 | Phosphoprotein enriched in astrocytes 15 | 0.029 | 0.342 | 0.014 | 1.202 |
| Psmd13 | Proteasome (prosome, macropain) 26S subunit, non-ATPase, 13 |
0.012 | 0.570 | 0.047 | 0.609 |
| Rb×1 | Ring-box 1, E3 ubiquitin protein ligase | 0.008 | 0.490 | 0.037 | 0.334 |
| Sptbn1 | Spectrin, beta, non-erythrocytic 1 | 0.008 | 1.318 | 0.027 | 0.608 |
| Tomm20 | Translocase of outer mitochondrial membrane 20 homolog (yeast) |
0.009 | 0.617 | 0.008 | 0.656 |
| App | Amyloid beta (A4) precursor protein | 0.006 | −0.556 | 0.042 | −0.489 |
| CdkS | Cyclin-dependent kinase 8 | 0.008 | −0.579 | 0.005 | −0.454 |
| Ckm | Creatine kinase, muscle | 0.029 | −0.575 | 0.015 | −0.889 |
| Coq10b | Coenzyme Q10 homolog B (S. cerevisiae) | 0.001 | −0.664 | 0.023 | −0.393 |
| Dnm11 | Dynamin 1-like | 0.003 | −0.483 | 0.013 | −0.372 |
| Kbtbd2 | Kelch repeat and BTB (POZ) domain containing 2 |
0.015 | −0.531 | 0.027 | −0.452 |
| Pdlim7 | PDZand LIM domain 7 | 0.008 | −0.642 | 0.006 | −1.192 |
| Tmeml26a | Transmembrane protein 126A | 0.001 | −0.436 | 0.004 | −0.671 |
| Usp10 | Ubiquitin specific peptidase 10 | <0.001 | −0.805 | 0.001 | −0.364 |
Functional gene sets enriched by differentially expressed genes
Genes with a significant hybridization signal on the arrays were classified according to their Gene Ontology (GO) categories of biological processes by using GO miner. GO categories with less than five differentially expressed genes detected were excluded from the analysis (Zeeberg et al. 2003). Significant enrichment of categories of biological processes by differentially expressed genes was validated by the results of gene set enrichment analysis (GSEA; Table 2, 3). To estimate enrichment, GSEA ranks all genes with significant hybridization signals in the experiment; thus, its results do not depend on the selection of genes above cutoffs for significance of expression differences and false discovery (Subramanian et al. 2005). For muscle in hibernating black bears, the proportion of over-expressed genes was significantly elevated for two related gene sets involved in translation and ribosome biogenesis (Table 2, Fig. 2). Biological process categories of glucose metabolism and oxidative reduction were significantly enriched by under-expressed genes (Table 2). The same GO categories with the addition of pyruvate metabolism and cellular respiration as part of oxidation reduction were enriched by under-expressed genes in skeletal muscle of the arctic ground squirrel during late torpor (Table 3, Fig 2).
Table 2.
Selected Gene Ontology categories of biological processes significantly enriched with differentially expressed genes in black bear skeletal muscle based on the data from two microarrays (Fedorov etal. 2009; this study). Arrows indicate direction of gene expression changes in hibernating animals. FDR is false discovery rate and NES is normalized enrichment score.
| GO Miner | GSEA | |||||
|---|---|---|---|---|---|---|
| GO Category | Total genes on array |
Changed genes |
|
|||
| Enrich- ment |
FDR | NES | FDR | |||
| Translation (GO: 0006412) | 365 | 47↑ | 2.999 | <0.001 | 1.59 | 0.037 |
| Ribosome biogenesis (GO: 0042254) |
134 | 16↑ | 2.781 | 0.006 | 1.54 | 0.035 |
| Glucose metabolic process (GO: 0006006) |
131 | 18↓ | 2.579 | 0.030 | −1.41 | 0.076 |
| Oxidation reduction (GO: 0055114) |
610 | 59↓ | 1.815 | 0.003 | −1.66 | <0.001 |
Table 3.
Gene Ontology categories of biological processes significantly enriched with differentially expressed genes in the arctic ground squirrel skeletal muscle. Arrows indicate direction of gene expression changes in torpid animals. FDR is false discovery rate.
| GO Miner | GSEA | |||||
|---|---|---|---|---|---|---|
| GO Category | Total genes on array |
Changed genes |
|
|||
| Enrich- ment |
FDR | NES | FDR | |||
| Translation (GO:0006412) |
343 | 581↑ | 2.548 | <0.001 | 1.81 | 0.006 |
| Ribosome biogenesis (GO:0042254) |
142 | 34↑ | 3.608 | <0.001 | 1.98 | <0.001 |
| Oxidation reduction (GO:0055114) |
589 | 59↓ | 2.657 | <0.001 | −1.24 | 0.123 |
| Glucose metabolic process (GO: 0006006) |
125 | 29↓ | 6.154 | <0.001 | −1.76 | 0.006 |
| Glucose catabolic process (GO:0006007) |
54 | 18↑ | 8.842 | <0.001 | −1.94 | <0.001 |
| Gluconeogenesis (GO:0006094) |
38 | 13↓ | 9.075 | <0.001 | −1.89 | 0.012 |
| Alcohol catabolic process (GO:0046164) |
64 | 19↓ | 7.875 | <0.001 | −1.31 | 0.133 |
| Pyruvate metabolic process (GO:0006090) |
52 | 15↓ | 7.652 | <0.001 | −1.69 | 0.009 |
| Cellular respiration (GO:0045333) |
141 | 16↓ | 3.010 | 0.002 | −1.55 | 0.063 |
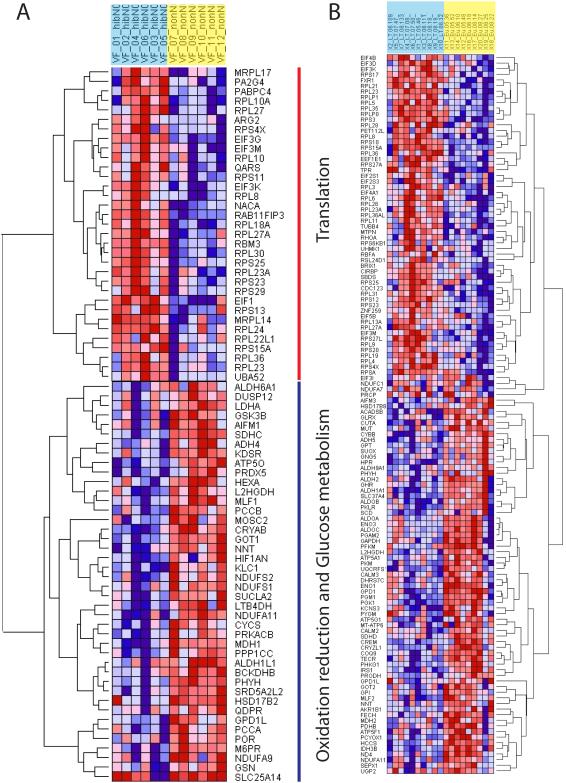
Figure 2.
Clustering and heat maps of expression values of genes involved in translation, glucose metabolism and oxidation-reduction GO category in skeletal muscle in black bears (A) and arctic ground squirrels (B). On the top hibernating animals are in blue and summer active individuals in yellow. Over-expressed genes are in red, under-expressed genes are in blue.
Apart from validation of the GO miner result, we also used GSEA to test enrichment in selected gene sets (Molecular Signature data base) known to be important for muscle homeostasis (Table 4). In both species, gene sets separately involved in three stages of translation (initiation, elongation and termination) were enriched by over-expressed genes during hibernation. Except for reduction of the ubiquitin proteasome pathway in the bear muscle, no protein catabolism categories (proteasome, proteolysis and lysosome) demonstrated enrichment by differentially expressed genes during hibernation. The muscle contraction gene set expression was decreased in both species during hibernation.
Table 4.
Gene Set Enrichment Analysis (GSEA) results for selected pathways. NES - normalized enrichment score, positive values indicate elevated expression and negative - under-expression in torpid muscle, FDR false discovery rate. Significant values are in bold.
| Black Bear | Arctic Ground Squirrel | |||
|---|---|---|---|---|
| Pathway |
|
|||
| NES | FDR | NES | FDR | |
| Translation initiation | 2.49 | <0.001 | 1.77 | 0.008 |
| Translation elongation | 2.83 | <0.001 | 1.91 | <0.001 |
| Translation termination | 2.78 | <0.001 | 1.93 | <0.001 |
| Proteosome | −0.85 | 0.691 | 1.03 | 0.503 |
| Proteolysis | −1.08 | 0.321 | −0.86 | 0.674 |
| Lysosome | −0.90 | 0.619 | 1.10 | 0.289 |
| Ubiquitin proteosome | −1.31 | 0.065 | −0.81 | 0.709 |
| Muscle contraction | −1.61 | 0.016 | −1.61 | 0.014 |
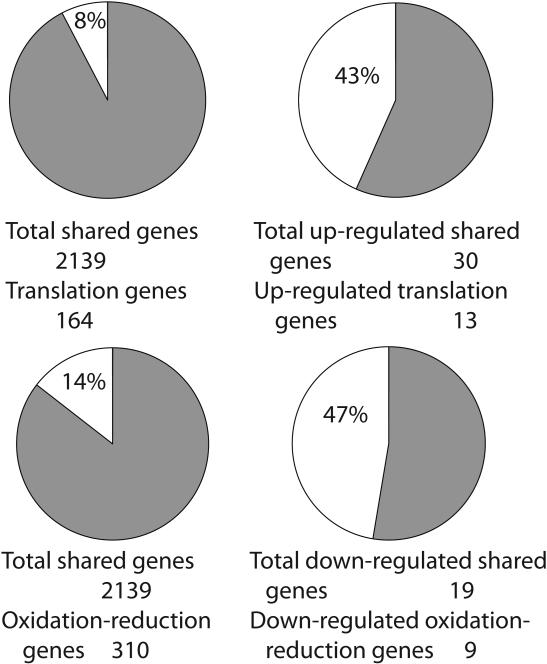
To directly compare results of differential gene expression analysis in the black bear and arctic ground squirrel, we conducted the GO miner pathway analysis of genes common for both species including results from the previous study in black bear muscle (Fedorov et al. 2009). A total of 2138 genes with significant signals on the microarrays were shared between the two species. Similar to species specific results, the two GO categories were enriched with over-expressed genes common for bears and ground squirrels: translation and ribosome biogenesis (Fig. 3). Under-expressed genes shared between the two species were over represented in oxidation reduction GO category (Fig. 3).
Figure 3.
Gene Ontology (GO) enrichment analysis of common genes differentially expressed in the black bear and arctic ground squirrel skeletal muscles during torpor of hibernation. A –translation GO category, enrichment is 5.625 (P<0.001), B – oxidation reduction GO category, enrichment is 2.904 (P<0.05). Numbers show a percent of genes in the correspondent GO category among the total number of common genes with significant hybridization signals on the microarrays for both species (left pie), and percent among the total differentially expressed genes shared between two species (right pie).
Discussion
Our comparative study reveals common transcriptional programming in skeletal muscle of two hibernating mammals that includes the coordinated induction of protein biosynthesis (translation) genes and suppression of genes involved in oxidation reduction and glucose metabolism. The relatively high proportion of over-expressed genes in muscle of torpid arctic ground squirrels (60.9%) and black bears (47.5%) suggests the importance of regulatory changes at the transcriptional level during torpor of hibernation. The similar proportion of over-expressed genes detected in muscle of two species with different modes of hibernation suggests that regulation at the transcriptional level is largely independent of Tb during hibernation, since bears maintain a relatively high Tb (30-36° C), compared to very low levels in torpid arctic ground squirrels (near 0° C) (Tøien et al. 2011; Barnes 1989).
Protein metabolism
Animals used as models of muscle atrophy due to disuse show a profound loss of protein from their muscle tissue (Lecker & Goldberg 2002). We recently showed that the coordinated induction of protein biosynthesis genes is a prominent feature of the transcriptome in liver, heart and muscle of hibernating black bears (Fedorov et al. 2009, 2011). Those results are now supported by the present wide-coverage study that identifies the elevated expression of 31 protein biosynthesis genes, in addition to the 16 translation genes over-expressed in skeletal muscle of hibernating bears (Fedorov et al. 2009). The coordinated over-expression of protein biosynthesis genes implies that translation of proteins is induced in skeletal muscle during hibernation, and this is supported by physiological evidence. Except for a moderate (10%) decrease in muscle protein concentration reported specifically for lactating female bears (Tinker et al. 1998), no muscle protein loss indicative of muscle atrophy was detected in hind limb muscles of black bears over prolonged periods of inactivity and fasting during hibernation (Lundberg et al. 1976; Koebel et al. 1991; Harlow et al. 2001; Lohuis et al. 2007a, b). It has been suggested that hibernating bears may maintain the potential for protein biosynthesis by recycling nitrogen lost in urea through hydrolysis by bacteria and hepatic synthesis of amino acids back into structural protein (Nelson 1980; Barboza et al. 1997). Transcriptome profiling revealed coordinated under-expression of genes involved in amino acid catabolism (Fedorov et al. 2011) and amino group utilization through the urea cycle (Fedorov et al. 2009) in the liver of hibernating bears. These findings imply reduction in amino acid catabolism and suggest, besides possible urea recycling, redirection of amino acids from catabolic pathways to the enhancement of protein biosynthesis.
Similar to in the black bear, protein biosynthesis and ribosome biogenesis categories are significantly enriched by over-expressed genes in muscle of arctic ground squirrels during torpor. There are several lines of evidence for inhibition of translation, however, in liver of hibernating ground squirrels at the low Tb of torpor. Radioactively-labeled amino acids show low rates of incorporation, and a significant portion of polyribosomes necessary for translation of mRNA are disassembled during torpor (Knight et al. 2000; van Breukelen & Martin 2001; Carey et al. 2003). Depression of protein biosynthesis in the liver is also indirectly supported by a large scale proteomic study that revealed no over-expressed proteins during torpor, as compared to summer active arctic ground squirrels (Shao et al. 2010). Protein synthesis in hibernating ground squirrels does occur during the brief (10-18 h) but regular interbout arousals when ground squirrels return to high, euthermic Tb each 1–3 weeks during the hibernation season (Knight et al. 2000; van Breukelen & Martin 2001; Carey et al. 2003). It has been shown that during torpor mRNA transcripts in liver of arctic ground squirrels are resistant to degradation, and this makes stabilized translatable transcripts available for elevated protein synthesis during the short arousal episodes (Knight et al. 2000; Pan & van Breukelen 2011). Transcriptional elevation of protein biosynthesis genes detected here in torpid squirrels likely facilitates induction of translation in muscle during arousals. Elevated expression of proteins involved in translation and ribosome biogenesis (Shao et al. 2010) as well as increases in incorporation rates of labeled amino acids (Zhegunov et al. 1988) during interbout arousals further support induction of protein biosynthesis at this stage of hibernation. There is a need for proteomic study in muscle of ground squirrels during interbout arousals to validate induction of protein biosynthesis during these episodes of hibernation. Elevated expression at protein level is expected for multiple translation genes that demonstrated transcriptional induction reported here in torpid muscle. Similar to bears, small mammalian hibernators such as ground squirrels and prairie dogs show resilience to muscle atrophy over prolonged periods of immobility and fasting as compared to non-hibernating mammals (Cotton & Harlow 2009; Gao et al. 2011; Andres-Mateos et al. 2013). Although moderate decreases in mass and protein concentration in some hind limb muscles but not others has been reported (Steffen et al. 1991; Wickler et al. 1991; Gao et al. 2011), no changes in mass or structure of quadriceps were detected over hibernation in ground squirrels (Andres-Mateos et al. 2013). Transcriptional induction of multiple protein biosynthesis genes reported here suggests that elevated translation during arousals likely contributes to protective mechanisms against muscle loss. Although decrease in protein synthesis is a prominent feature of muscle atrophy in mammalian disuse models (Rennie et al. 2010; Marimuthu et al. 2011), some increase in expression of several genes involved in ribosome biogenesis was detected in unloaded soleus of old rats (Wittwer et al. 2002; Dupont-Versteegden et al. 2008) and healthy women after 60 days of bed rest (Chopard et al. 2009). This increase in expression of genes encoding ribosomal proteins was considered as compensatory response to disuse atrophy (Dupont-Versteegden et al. 2008).
Under fasting conditions, elevated protein biosynthesis in skeletal muscle of hibernating mammals may be supplied with amino acids resulting from the catabolism of non-myofibrilar protein stored in other tissues (Harlow 1995). Collagen is an important source of essential amino acids, and increase in concentrations of metabolites of collagen catabolism in the blood plasma of hibernating bears implies its breakdown (Hissa et al. 1998; Lohuis et al. 2005). Small hibernating mammals such as prairie dogs demonstrate a 23% reduction in protein content of the liver overwinter (Cotton & Harlow 2009). Amino acids made available from the breakdown of labile protein reserves may support enhanced levels of protein biosynthesis that prevent skeletal muscle loss and, thus, preserves locomotion ability through 6 months of winter hibernation.
In addition to the decrease in protein synthesis, induction of protein catabolism is an important mechanism that contributes to disuse muscle atrophy in non-hibernating mammals (Lecker & Goldberg 2002; Jackman & Kandarian 2004; Marimuthu et al. 2011). Consistently, a coordinated increase in expression of genes involved in proteolysis, ubiquitin-proteasome pathway, and lysosome proteolysis was detected in muscles of different mammalian models of disuse (Wittwer et al. 2002; Chopard et al. 2009; Bialek et al. 2011). In contrast, our study revealed no increase in transcriptional levels of proteolysis genes in muscle of hibernating mammals. The lack of induction of protein degradation pathways may be an additional factor contributing to preservation of muscles mass during hibernation. However, molecular mechanisms preventing elevation of muscle proteolysis remain obscure. The importance of suppression of protein catabolism was suggested by Fuster et al. (2007) who showed that hibernating bears likely produce a proteolytic inhibitor that is released into plasma that blocks proteolysis of skeletal muscle.
Decrease in protein catabolism during hibernation due to metabolic suppression for conserving energy has also been suggested to contribute to the attenuation of muscle atrophy (Lee et al. 2008). Our expectation from this hypothesis is a coordinated decrease in expression levels of genes involved in protein catabolic pathways. This decrease is specifically expected to be clearly defined in muscles of ground squirrels with a large (98%) reduction of metabolic rate during torpor. We did not find a reduction in the transcription levels of proteolysis genes, except for some down-regulation of the ubiquitin-proteasome pathway in bears.
Metabolic processes
A coordinated under-expression of genes involved in the broad category of oxidation reduction and glucose metabolism was found in skeletal muscle of both torpid arctic ground squirrels and black bears. The decrease in energy producing oxidation reduction processes is consistent with metabolic suppression during hibernation which overall is an energy saving physiological adaptation. Transcriptional reduction of multiple genes responsible for key oxidation reduction processes such as glycolysis, cellular respiration and electron transport was previously reported for the liver of hibernating bears (Fedorov et al. 2011) and arctic ground squirrels (Yan et al. 2008). Although transcriptional induction of several genes involved in glycogenesis in the liver of both hibernating species may act to provide a source for glucose that is likely an important metabolic fuel for the brain, heart, and other glucose dependent tissues and functions (Fedorov et al. 2011; Yan et al. 2008; Buck & Barnes 2000), glycogenic genes were under-expressed in skeletal muscle. Consistent with this, decreased quantities of proteins involved in glucose metabolism in skeletal muscle of torpid ground squirrels (Hindle et al. 2011) implies a limited role of carbohydrate fuel in muscle metabolism during hibernation. Notably, under-expression of oxidation reduction and glucose metabolism genes was also detected in several mammalian disuse models (Calura et al. 2008; Bialek et al. 2011), corresponding to lower energy demand in skeletal muscle during physical inactivity.
Conclusions
Our comparative study represents the first research effort to elucidate transcriptional changes for thousands of genes in skeletal muscle during torpor of hibernation in two evolutionary distant mammalian species. The novelty of our study is the use of two diverse hibernating models to identify common gene expression programs underlying natural adaptation to musculoskeletal disuse with potential relevance to muscle atrophy prevention in non-hibernating mammals.
The common transcriptional program includes a coordinated increase in transcriptional levels of anabolic genes involved in protein biosynthesis that implies an induction of translation at different hibernation states. Anabolic transcriptional changes likely contribute to the attenuation of disuse muscle atrophy through prolonged periods of immobility and fasting. This adaptive mechanism allows hibernating mammals to maintain full musculoskeletal function and preserve mobility during and immediately after hibernation, thus promoting survival. The lack of directional changes in genes of protein catabolic pathways does not support the importance of metabolic suppression for preserving muscle mass.
The comparative transcriptome analyses reported here identify candidate pathways and generate an impetus for follow-up studies that include validation of expressional changes of translation genes at protein level in hibernating mammals. Candidate genes showing differences in expression consistent at transcriptional and protein levels in hibernating mammals need to be selected for protein expression analysis in non-hibernating mammalian models of disuse. It is expected that genes important for muscle loss prevention in hibernating mammals will demonstrate expression changes to opposite direction or no changes under conditions of disuse in non-hibernating models as compared to hibernating black bears and ground squirrels. The next step will be to test whether experimental altering of candidate gene expression is sufficient to reduce muscle atrophy in non-hibernating mammals.
Supplementary Material
Acknowledgements
We thank the Alaska Department of Fish and Game for supplying bears and Franziska Kohl for collecting physiological data and tissue sampling from arctic ground squirrels. This work was supported by USAMRMC (05178001) and by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institute of Health (Award Number R21AR064995). The content is solely responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health.
Footnotes
Data Accessibility
All microarray data series were submitted to NCBI Gene Expression Omnibus (GEO) with accession number GSE17154 at http://www.ncbi.nlm.nih.gov/geo/.
References
- Andres-Mateos E, Brinkmeier H, Burks TN, Mejias R, Files DC, Steinberger M, Soleimani A, Marx R, Simmers JL, Lin B, Finanger Hedderick E, Marr TG, Lin BM, Hourdé C, Leinwand LA, Kuhl D, Föller M, Vogelsang S, Hernandez-Diaz I, Vaughan DK, Alvarez de la Rosa D, Lang F, Cohn RD. Activation of serum/glucocorticoid-induced kinase 1 (SGK1) is important to maintain skeletal muscle homeostasis and prevent atrophy. EMBO Molecular Medicine. 2013;5:80–91. doi: 10.1002/emmm.201201443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barboza PS, Farley SD, Robbins CT. Whole-body urea cycling and protein turnover during hyperphagia and dormancy in growing bears (Ursus americanus and U. arctos) Canadian Journal of Zoology. 1997;75:2129–2136. [Google Scholar]
- Barnes BM. Freeze avoidance in a mammal - body temperatures below 0-degrees-c in an arctic hibernator. Science. 1989;244:1593–1595. doi: 10.1126/science.2740905. [DOI] [PubMed] [Google Scholar]
- Bialek P, Morris C, Parkington J, St.Andre M, Owens J, Yaworsky P, Seeherman H, Jelinsky SA. Distinct protein degradation profiles are induced by different disuse models of skeletal muscle atrophy. Physiological Genomics. 2011;43:1075–1086. doi: 10.1152/physiolgenomics.00247.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolstad BM, Irizarry RA, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics. 2003;19:185–193. doi: 10.1093/bioinformatics/19.2.185. [DOI] [PubMed] [Google Scholar]
- Boyer BB, Barnes BM. Molecular and metabolic aspects of mammalian hibernation. Bioscience. 1999;49:713–724. [Google Scholar]
- Buck CL, Barnes BM. Effects of ambient temperature on metabolic rate, respiratory quotient, and torpor in an arctic hibernator. American Journal of Physiology, Regulatory Integrative Comparative Physiology. 2000;279:255–262. doi: 10.1152/ajpregu.2000.279.1.R255. [DOI] [PubMed] [Google Scholar]
- Calura E, Cagnin S, Raffaello A, Laveder P, Lanfranchi G, Romualdi C. Meta-analysis of expression signatures of muscle atrophy: gene interaction networks in early and late stages. BMC Genomics. 2008;9:630. doi: 10.1186/1471-2164-9-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey HV, Andrews MT, Martin SL. Mammalian hibernation: cellular and molecular responses to depressed metabolism and low temperature. Physiological Reviews. 2003;83:1153–1181. doi: 10.1152/physrev.00008.2003. [DOI] [PubMed] [Google Scholar]
- Chopard A, Lecunff M, Danger R, Lamirault G, Bihouee A, Teusan R, Jasmin BJ, Marini JF, Leger JJ. Large-scale mRNA analysis of female skeletal muscles during 60 days of bed rest with and without exercise or dietary protein supplementation as countermeasures. Physiological Genomics. 2009;38:291–302. doi: 10.1152/physiolgenomics.00036.2009. [DOI] [PubMed] [Google Scholar]
- Cotton CJ, Harlow HJ. Avoidance of skeletal muscle atrophy in spontaneous and facultative hibernators. Physiological Biochemical Zoology. 2009;83:551–560. doi: 10.1086/650471. [DOI] [PubMed] [Google Scholar]
- Daan S, Barnes BM, Strijkstra AM. Warming up to sleep? Hibernating arctic ground squirrels sleep during arousals. Neuroscience Letters. 1991;128:265–268. doi: 10.1016/0304-3940(91)90276-y. [DOI] [PubMed] [Google Scholar]
- Dupont-Versteegden EE, Nagarajan R, Beggs ML, Bearden ED, Simpson PM, Peterson CA. Identification of cold-shock protein RBM3 as a possible regulator of skeletal muscle size through expression profiling. American Journal of Physiology. Regulatory Integrative and Comparative Physiology. 2008;295:R1263–R1273. doi: 10.1152/ajpregu.90455.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorov VB, Goropashnaya AV, Tøien Ø , Stewart NC, Gracey AY, Chang CL, Qin SZ, Pertea G, Quackenbush J, Showe LC, Showe MK, Boyer BB, Barnes BM. Elevated expression of protein biosynthesis genes in liver and muscle of hibernating black bears (Ursus americanus) Physiological Genomics. 2009;37:108–118. doi: 10.1152/physiolgenomics.90398.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorov VB, Goropashnaya AV, Tøien Ø , Stewart NC, Chang C, Wang H, Yan J, Showe LC, Showe MK, Barnes BM. Modulation of gene expression in heart and liver of hibernating black bears (Ursus americanus) BMC Genomics. 2011;12:171. doi: 10.1186/1471-2164-12-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorov VB, Goropashnaya AV, Tøien Ø , Stewart NC, Chang C, Wang H, Yan J, Showe LC, Showe MK, Donahue SW, Barnes BB. Preservation of bone mass and structure in hibernating black bears (Ursus americanus) through elevated expression of anabolic genes. Functional & Integrative Genomics. 2012;12:357–365. doi: 10.1007/s10142-012-0266-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster G, Busquets S, Almendro V, López-Soriano FJ, Argileés JM. Antiproteolytic effects of plasma from hibernating bears: a new approach for muscle wasting therapy? Clinical Nutrition. 2007;26:658–661. doi: 10.1016/j.clnu.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Gao Y-F, Wang J, Wang H-P, Feng B, Dang K, Wang Q, Hinghofer-Szalkay HG. Skeletal muscle is protected from disuse in hibernating dauria ground squirrels. Comparative Biochemistry and Physiology. A. 2012;161:296–300. doi: 10.1016/j.cbpa.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Hampton M, Melvin RG, Kendall AH, Kirkpatrick BR, Peterson N, Andrews MT. Deep sequencing the transcriptome reveals seasonal adaptive mechanisms in a hibernating mammal. PLoS ONE. 2011;6:e27021. doi: 10.1371/journal.pone.0027021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow HJ. Fasting biochemistry of representative spontaneous and facultative hibernators: the white-tailed prairie dog and the black-tailed prairie dog. Physiological Zoology. 1995;68:1262–1278. [Google Scholar]
- Harlow HJ, Lohuis T, Beck TD, Iaizzo PA. Muscle strength in overwintering bears. Nature. 2001;409:997. doi: 10.1038/35059165. [DOI] [PubMed] [Google Scholar]
- Hissa R, Puukka M, Hohtola E, Sassi ML, Risteli J. Seasonal changes in plasma nitrogenous compounds of the European brown bear (Ursus arctos arctos) Annales Zoologici Fennici. 1998;35:205–213. [Google Scholar]
- Hindle AG, Karimpour-Fard A, Epperson LE, Hunter LE, Martin SL. Skeletal muscle proteomics: carbohydrate metabolism oscillates with seasonal and torpor-arousal physiology of hibernation. American Journal of Physiology. Regulatory Integrative and Comparative Physiology. 2011;301:R1440–R1452. doi: 10.1152/ajpregu.00298.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman RW, Kandarian SC. The molecular basis of skeletal muscle atrophy. American Journal of Physiology. Cell Physiology. 2004;287:C834–C843. doi: 10.1152/ajpcell.00579.2003. [DOI] [PubMed] [Google Scholar]
- Kari L, Loboda A, Nebozhyn M, Rook AH, Vonderheid EC, Nichols C, Virok D, Chang C, Horng WH, Johnston J, Wysocka M, Showe MK, Showe LC. Classification and prediction of survival in patients with the leukemic phase of cutaneous T cell lymphoma. The Journal of Experimental Medicine. 2003;197:1477–1488. doi: 10.1084/jem.20021726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight JE, Narus EN, Martin SL, Jacobson A, Barnes BM, Boyer BB. mRNA stability and polysome loss in hibernating Arctic ground squirrels (Spermophilus parryii) Molecular and Cellular Biology. 2000;20:6374–6379. doi: 10.1128/mcb.20.17.6374-6379.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koebel DA, Miers PG, Nelson RA, Steffen JM. Biochemical changes in skeletal muscles of denning bears (Ursus americanus) Comparative Biochemistry and Physiology. B. 1991;100:377–380. doi: 10.1016/0305-0491(91)90390-y. [DOI] [PubMed] [Google Scholar]
- Lecker SH, Goldberg AL. Slowing muscle atrophy: putting the brakes on protein breakdown. Journal of Physiology. 2002;545:729. doi: 10.1113/jphysiol.2002.030429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Park JY, Yoo W, Gwag T, Lee JW, Byun MW, Choi I. Overcoming muscle atrophy in a hibernating mammal despite prolonged disuse in dormancy: proteomic and molecular assessment. Journal of Cellular Biochemistry. 2008;104:642–656. doi: 10.1002/jcb.21653. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lohuis TD, Beck TDI, Harlow HJ. Hibernating black bears have blood chemistry and plasma amino acid profiles that are indicative of long-term adaptive fasting. Canadian Journal of Zoology. 2005;83:1257–1263. [Google Scholar]
- Lohuis TD, Harlow HJ, Beck TDI. Hibernating black bears (Ursus americanus) experience skeletal muscle protein balance during winter anorexia. Comparative Biochemistry and Physiology. B. 2007a;147:20–28. doi: 10.1016/j.cbpb.2006.12.020. [DOI] [PubMed] [Google Scholar]
- Lohuis TD, Harlow HJ, Beck TDI, Iaizzo PA. Hibernating Bears Conserve Muscle Strength and Maintain Fatigue Resistance. Physiological and Biochemical Zoology. 2007b;80:257–269. doi: 10.1086/513190. [DOI] [PubMed] [Google Scholar]
- Lundberg DA, Nelson RA, Wahner HW, Jones JD. Protein metabolism in the black bear before and during hibernation. Mayo Clinic Proceedings. 1976;51:716–722. [PubMed] [Google Scholar]
- Marimuthu K, Murton AJ, Greenhaff PL. Mechanisms regulating muscle mass during disuse atrophy and rehabilitation in humans. Journal of Applied Physiology. 2011;110:555–560. doi: 10.1152/japplphysiol.00962.2010. [DOI] [PubMed] [Google Scholar]
- Nelson RA. Protein and fat metabolism in hibernating bears. Federation Proceedings. 1980;39:2955–2958. [PubMed] [Google Scholar]
- Pan P, van Breukelen F. Preference of IRES-mediated initiation of translation during hibernation in golden-mantled ground squirrels, Spermophilus lateralis. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2011;301:370–377. doi: 10.1152/ajpregu.00748.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Research. 2001;29:2001–2007. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennie MJ, Selby A, Atherton P, Smith K, Kumar V, Glover EL, Philips SM. Facts, noise and wishful thinking: muscle protein turnover in aging and human disuse atrophy. Scandinavian Journal of Medicine & Science in Sports. 2010;20:5–9. doi: 10.1111/j.1600-0838.2009.00967.x. [DOI] [PubMed] [Google Scholar]
- Shao C, Liu Y, Ruan H, Li Y, Wang H, Kohl F, Goropashnaya AV, Fedorov VB, Zeng R, Barnes BM, Yan J. Shotgun proteomics analysis of hibernating Arctic ground squirrels. Molecular & Cellular Proteomics. 2010;9:313–326. doi: 10.1074/mcp.M900260-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen JM, Koebel DA, Musacchia XJ, Milsom WK. Morphometric and metabolic indexes of disuse in muscles of hibernating ground squirrels. Comparative Biochemistry and Physiology. B. 1991;99:815–819. doi: 10.1016/0305-0491(91)90147-6. [DOI] [PubMed] [Google Scholar]
- Storey JD, Tibshirani R. Statistical significance for genome wide studies. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:9440–9445. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinker DB, Harlow HJ, Beck TD. Protein use and muscle-fiber changes in free-ranging, hibernating black bears. Physiological Zoology. 1998;71:414–424. doi: 10.1086/515429. [DOI] [PubMed] [Google Scholar]
- Tøien Ø . Automated open flow respirometry in continuous and long-term measurements: design and principles. Journal of Applied Physiology. 2013;114:1094–1107. doi: 10.1152/japplphysiol.01494.2012. 2013. [DOI] [PubMed] [Google Scholar]
- Tøien Ø , Blake J, Edgar DM, Grahn DA, Heller HC, Barnes BM. Hibernation in black bears: independence of metabolic suppression from body temperature. Science. 2011;331:906–909. doi: 10.1126/science.1199435. [DOI] [PubMed] [Google Scholar]
- van Breukelen F, Martin SL. Translational initiation is uncoupled from elongation at 18 degrees C during mammalian hibernation. American Journal of Physiology-Regulatory Integrative and Comparative Physiology. 2001;281:R1374–R1379. doi: 10.1152/ajpregu.2001.281.5.R1374. [DOI] [PubMed] [Google Scholar]
- Wang Y, Barbacioru C, Hyland F, Xiao W, Hunkapiller KL, Blake J, Chan F, Gonzalez C, Zhang L, Samaha RR. Large scale real-time PCR validation on gene expression measurements from two commercial long-oligonucleotide microarrays. BMC Genomics. 2006;7:59. doi: 10.1186/1471-2164-7-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickler SJ, Hoyt DF, van Breukelen F. Disuse atrophy in the hibernating golden mantled ground squirrel, Spermophilus lateralis. American Journal of Physiology. 1991;261:1214–1217. doi: 10.1152/ajpregu.1991.261.5.R1214. [DOI] [PubMed] [Google Scholar]
- Wittwer M, Flück M, Hoppeler H, Müller S, Desplanches D, Billeter R. Prolonged unloading of rat soleus muscle causes distinct adaptations of the gene profile. FASEB Journal. 2002;16:884–886. doi: 10.1096/fj.01-0792fje. [DOI] [PubMed] [Google Scholar]
- Zeeberg BR, Feng W, Wang G, Wang MD, Fojo AT, Sunshine M, Narasimhan S, Kane DW, Reinhold WC, Lababidi S, Bussey KJ, Riss J, Barrett JC, Weinstein JN. GoMiner: a resource for biological interpretation of genomic and proteomic data. Genome Biology. 2003;4:R28. doi: 10.1186/gb-2003-4-4-r28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeberg BR, Qin H, Narasimhan S, Sunshine M, Cao H, Kane DW, Reimers M, Stephens RM, Bryant D, Burt SK, Elnekave E, Hari DM, Wynn TA, Cunningham-Rundles C, Stewart DM, Nelson D, Weinstein JN. High-Throughput GoMiner, an 'industrial-strength' integrative gene ontology tool for interpretation of multiple-microarray experiments, with application to studies of Common Variable Immune Deficiency (CVID) BMC Bioinformatics. 2005;6:168. doi: 10.1186/1471-2105-6-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Shao CX, Goropashnaya AV, Stewart NC, Xu YC, Tøien Ø , Barnes BM, Fedorov VB, Yan J. Genomic analysis of expressed sequence tags in American black bear Ursus americanus. BMC Genomics. 2010;11:201. doi: 10.1186/1471-2164-11-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhegunov GF, Mikulinsky YE, Kudokotseva EV. Hyperactivation of protein synthesis in tissues of hibernating animals on arousal. Cryo-Letters. 1988;9:236–245. [Google Scholar]
- Yan J, Barnes BM, Kohl F, Marr TG. Modulation of gene expression in hibernating arctic ground squirrels. Physiological Genomics. 2008;32:170–181. doi: 10.1152/physiolgenomics.00075.2007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.