Abstract
Chronic hepatic diseases such as cirrhosis, hepatocellular carcinoma and virus mediated immunopathogenic infections are affecting billions of people worldwide. These diseases commonly initiate with fibrosis. Owing to the various side effects of anti-fibrotic therapy and the difficulty of diagnosing asymptomatic patients, suitable medication remains a major concern. To overcome this drawback, the use of cytokine-based sustained therapy might be a suitable alternative with minimal side effects. Here, we studied the therapeutic efficacy and potential mechanisms of IL30 as anti-fibrosis therapy in murine liver fibrosis models. Carbon tetrachloride (CCl4) mixed with corn oil at a ratio 1:3 was injected intraperitoneally (IP) 1µl/gm body weight twice per week for 1 month or 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC) 0.1% (wt/wt) Purnima 5015 Chow was fed for 3 weeks to induce liver fibrosis. Either control vector (pCtr) or pIL30 was injected hydrodynamically once per week. A significant decrease in collagen deposition and reduced expression of α-smooth muscle Actin (αSMA) protein indicated that IL30–based gene therapy dramatically reduced bridging fibrosis that was induced by CCl4 or DDC. Immunophenotyping and knockout studies showed that IL30 recruits NKT cells to the liver to decrease activated hepatic stellate cells (HSCs) significantly and ameliorate liver fibrosis. Both flow cytometric and antibody mediated neutralization studies showed NKT cells alleviate liver fibrosis in an NKG2D dependent manner. Furthermore, chronic treatment with CCl4 showed inducible surface expression of the NKG2D ligand Rae1 on activated HSCs as compared to quiescent ones.
Taken together, our results show that highly target specific liver NKT cells selectively remove activated HSCs via an NKG2D-Rae1 interaction to ameliorate liver fibrosis after IL30 treatment.
Keywords: IL30, NKT, NKG2D, Rae1, CCl4
Introduction
As the body’s principal detoxifying organ, the liver removes pathogens, toxic chemicals, antigens from the blood, and metabolic waste from the circulatory system. Continuous exposure of the liver to these products leads to apoptosis or necrosis of hepatocytes. To overcome these pathophysiological conditions, the liver’s resident immune cells infiltrate affected areas of this organ and initiate restoration of structural integrity. However, when the balance shifts toward injury, chronic inflammation of the liver occurs, which if prolonged can lead to liver fibrosis (1).
Liver fibrosis is a wound-healing response that occurs to maintain organ integrity after repetitive insults from various biochemical metabolites (2–4). The continued progression of liver fibrosis causes modulation of hepatic architecture and portal hypertension, which will ultimately lead to cirrhosis, organ failure and even cancer in some patients (5, 6). Cirrhosis is the twelfth leading cause of death in the United States, and hepatocellular carcinoma is ranked third among cancer-related deaths worldwide (7, 8). In humans, liver fibrosis progresses slowly and asymptomatically (9, 10). So it is often undetected until the cirrhosis stage, which is irreversible and lacks effective treatment. A major pathological feature is that activated HSCs undergo transdifferentiation to myofibroblastic cells to enhance synthesis and accumulation of collagen and extra cellular matrix (11). Even though both the cellular and molecular mechanisms of this disease have been delineated, effective anti-fibrotic therapy with minimal side effects remains (9).
The ideal therapy for liver fibrosis would be a naturally occurring biological inhibitor that minimizes off-target effects. The aim of our lab is to provide novel therapeutic options to tackle this long standing problem. One such biologically occurring cytokine IL30 is able to recover liver from necrotic lesions caused by acute hepatic inflammation (12, 13). However we know that these injuries are healed up due to efficient regeneration ability of this organ upon removal of toxic products. Thus area to be explored is the broader clinical application of IL30 against chronic liver diseases. Also we need to discover an in-depth mechanism of its action, as the limited knowledge of its modus operandi to inhibit interferon gamma (IFN-γ) secretion is not enough to explain its efficacy for chronic liver diseases which are highly complex in nature.
IL30, a subunit p28 of IL27, can signal closely related to the IL12 family (14), and several studies have shown that IL27 exerts both inflammatory and anti-inflammatory effects on T cells(15, 16). IL30 was found to inhibit STAT1 and STAT3 signaling to suppress T-cell differentiation into TH17 in autoimmune diseases (14). Also Oncomine database revealed that IL30 expression is low in hepatitis-infected liver tissues compared to normal ones. It suggests that overexpression of IL30 might be a suitable therapy against chronic liver diseases. With this rationale, we administered this cytokine as an anti-fibrotic therapy in a CCl4-treated liver fibrosis model. Our goal was to attenuate the severity of liver fibrosis, which is an important step toward avoiding the irreversible damage from cirrhosis and subsequent decomposition.
Evidence suggests that inhibiting activated HSCs by inducing apoptosis could have therapeutic potential for liver fibrosis (17). The liver is predominantly enriched with B, NK, NK-like T (NKT), dendritic, and macrophage immune cells (18). Interestingly, hepatic immune cells both promote and act against liver fibrosis, very dynamically as found in numerous animal models (1, 19–21). Several anti-fibrotic therapies have demonstrated that immune cells might remove excessive collagen-producing activated HSCs (22, 23). In this investigation, we discovered that IL30 could be a novel therapy for CCl4- or DDC-induced liver fibrosis. We also provided experimental evidence that delineates the dynamics in the liver’s immune system of removing activated HSCs and supporting the liver to regenerate from chemical-induced liver fibrosis after IL30 treatment.
Materials and Methods
Chemical reagents and antibodies
Carbon tetrachloride, corn oil, percoll, collagenase type I, DNase I and pronase were purchased from Sigma Aldrich. The anti-desmin and anti-αSMA antibodies were purchased from Abcam. For immunohistochemistry, the anti-αSMA antibody was purchased from DAKO. The anti-Rae1 and anti- NKG2D antibodies were purchased from R&D systems. NKT, NK, CD3, CD4 and CD8 T cells expression from liver MNCs and splenocytes were identified by using anti-NK1.1, anti-CD3, anti-CD4, anti-CD8, anti-NKG2D, and anti-CD8 antibodies using flow cytometry purchased from Ebiosciences. Anti-CD3, anti-CD4 and anti-CD8 antibodies were purchased from Abdserotec for immunohistochemistry application.
Mice
Eight- to 10-week old female C57BL/6J, CD1d−/− (C57BL/6 background), RAG2−/− were purchased from the Jackson Laboratory. They were maintained in a specific pathogen free facility and were treated in accordance with the guidelines approved by the I.A.C.U.C. at M.D. Anderson Cancer Center (Houston, TX). Cytokine-encoding and control plasmid DNA (5µg per mouse in 1.5 ml of 0.45% saline solution) were injected hydrodynamically through tail vein. Mice were received treatments once per week.
Alanine aminotransferase (ALT)
Serum ALT (Bioo Scientific) was measured by the standard colorimetric method at 510 nm.
Liver injury and fibrosis induced by Carbon Tetrachloride or 3,5 Diethoxycarbonyl-1,4 Dihydrocollidine (DDC) Diet
Carbon tetrachloride was mixed with corn oil at a ratio 1:3. Each mouse was injected intraperitoneally (IP) 1µl/gm body weight twice per week for 1 month. For DDC induced liver injury, mice were fed diet containing 0.1% (wt/wt) Purnima 5015 Chow (Lab supply) for 3 weeks.
Histological Analyses
10% buffered formalin fixed liver tissues were embedded in paraffin and stained with hematoxylin and eosin. Liver sections were scored by a pathologist according to histopathological standard scale for liver fibrosis(24). Masson’s Trichrome staining was performed according to the manufacturer’s instructions (Diagnostic Biosystems). Image J was used to quantitate the percentage of affected area on the micrographs.
Liver mononuclear cells preparation
Method for isolation of liver mononuclear cells was followed as previously published (25). Liver NKT or non NKT T cells were purified using α-CD3 and α-NK1.1 antibodies via the cell sorting method for the adoptive transfer experiment. Approximately, 80%–85% purity of the NKT population was obtained.
Cytotoxic assays
HSCs were freshly isolated from mouse liver using Histodenz (Sigma Aldrich) as described previously(26). Purity and viability of HSCs were measured as published(22). These cells were labeled with Calcein-AM dye (Life Technologies). Purified liver NKT cells using MACS kit (Miltenyi Biotech) were co-cultured with these HSCs for 4h as defined. Cytotoxicity was measured with an emission of 480 nm and an excitation of 530 nm.
Gene constructs
The gene clones used in this study include control vector pCtr and pIL30. The constructs of these clones were reported previously (12). The serum concentration of IL30 was measured using colorimetric ELISA (R&D Systems) from all types of treatments to validate that the plasmid is expressing in vivo (Supplementary Fig. 4).
Western blotting
Liver samples were homogenized by following a protocol as described previously to extract proteins (27). Western blotting was performed as described previously (28).
Statistical analyses
Animals were randomly selected for control and treatment groups. All data were calculated as the mean+/− the standard error of the mean using GraphPad Prism v5.00. One-way ANOVA with Tukey’s post-hoc test was used to analyze the data. Mann-Whitney’s rank sum test was used to calculate the statistical significance of the pathological scores of fibrosis.
Results
IL30 ameliorates liver fibrosis induced by CCl4
To investigate the biological function of IL30 as an anti-fibrotic cytokine, we used one of the well-established liver fibrosis models i.e. CCl4-induced liver injury. We administered CCl4 via intraperitoneal injection for 4 weeks, which caused chronic inflammation and bridging fibrosis, as shown by increased infiltration of immune cells surrounding the central vein areas (Fig. 1 a) and an unbiased pathological score report supports this severity of fibrosis (Fig. 1 d). To assess fibrosis, we evaluated type I collagen deposition and it’s quantitation by trichrome staining of liver tissue (Fig. 1, b and e) and activation of HSCs by staining for αSMA, a myogenic marker (Fig. 1, c and f). CCl4-treated tissue had significantly higher levels of collagen deposition and expression of αSMA than did control vector- or corn oil-treated liver tissue.
Figure 1. IL30 ameliorates CCl4-induced liver fibrosis.
(a–g) Two groups of wild-type mice (n=5 mice per group) were injected with CCl4 solution twice a week for 1 month. One group was given control vector (pCtr) and other pIL30 by hydrodynamic delivery once per week. Control groups such as pCtr, pIL30, Corn oil plus pCtr and Corn Oil plus pIL30 (n=4 mice per group) were treated in a similar regularity. (a) Infiltration of liver mononuclear cells was evaluated by H&E staining. Arrow marks are showing the bridging fibrosis. (b) Liver fibrosis was evaluated by Masson’s trichorme staining and immunohistochemistry of the expression of αSMA protein (c). (d) Pathological scores of liver fibrosis as defined in Materials and Methods were shown in bar graph. Masson’s trichrome and αSMA positive areas were quantified using image J as shown in panels (e) and (f) respectively. (g) Expression of αSMA protein in liver tissues was assessed by Western blot analysis. (h) Serum level of alanine aminotransferase (ALT) was shown. Representative data were shown as mean ± SEM from three independent experiments. One-way Anova analyses: *P < .05; **P < .01; ***P< .001.
To ensure the success of IL30–mediated protection against liver fibrosis, both histopathological and biochemical examinations were carried out. Histological examination of the liver sections revealed that IL30 treatment significantly reduces fibrosis as confirmed by the pathological score (Fig. 1, a and d). Similarly, collagen deposition around the central vein area (Fig. 1, b and e) and αSMA expression in HSCs (Fig. 1, c and f) showed significant decrease. Western blot analysis demonstrated that the levels of αSMA protein decreased after IL30 treatment (Fig. 1 g). Also IL30 treatment significantly decreased serum ALT level, which could explain IL30’s role in protecting the liver (Fig. 1 h). We validated the anti-fibrotic role of IL30 in another fibrosis model which was induced by a 0.1% DDC diet (Supplementary Fig. 1). Similar hepatic protection by IL30 was found in the DDC model as in the CCl4 model.
We next investigated whether IL30 protects the liver by eliminating activated HSCs or by preventing CCl4 from activating quiescent HSCs. Two groups of mice were pretreated with CCl4 for 2 weeks followed by treatment of one with a control vector and other with pIL-30. Because we obtained sufficient control data during the first experiment presented in Figure 1, most of the controls were eliminated from this second experiment to reduce unnecessary use of mice. We found similar results in this post-injury model as in the prevention model following IL30 treatment: they showed reduction in ALT level, inflammation area, collagen deposition and αSMA expression (Fig. 2). These findings clearly demonstrate that IL30 administration ameliorates severe liver fibrosis by significantly decreasing the collagen deposition and activated HSCs.
Figure 2. IL30 depleting activated HSCs in CCl4 induced post-injury model.
(a–g) Two groups of wild-type mice (n=5 mice per group) were injected with CCl4 solution twice a week for 1 month. One group was given control vector (pCtr) and other pIL30 by hydrodynamic delivery on 3rd and 4th weeks along with CCl4 treatment. (a) Fixed liver tissues were stained with H&E. (b) Liver fibrosis was evaluated by trichrome staining of liver sections and immunohistochemistry of the expression of αSMA protein (c). (d) % Affected area shown in the bar graph signifies the area infiltrated by immune cells to the total area in each view field × 100. Trichrome staining and αSMA positive areas were quantified using image J as shown in panels (e) and (f) respectively. (g) Serum ALT was measured. Representative data were shown as mean ± SEM from two independent experiments. One-way Anova analyses: *P < .05; **P < .01; ***P< .001.
NKT cells are required to protect the liver from CCl4-induced fibrosis treated with IL30
Several studies have shown that resident immune cells play important roles to protect the liver from various toxic biochemical metabolites (22, 29–32). With this rationale, we next explored the underlying immune cell dynamics in injured liver tissue after IL30 treatment. Notably, immunohistochemistry staining showed that CD4 and CD8 T cells did not significantly infiltrate the liver after IL30 treatment (Supplementary Fig. 2). Unpublished studies from our lab found that blocking of CD8 T cells using antibody did not have any effect on IL30 mediated protection. Also, CD4 receptor is expressed on liver NKT cells which ruled out the possibility of CD4 depletion studies (33). Intrahepatic B (1), macrophages (20) and dendritic cells (34) are profibrogenic. Interestingly, NK and NKT cells were characterized as hepatoprotective immune cells against fibrosis or acute inflammation in poly I: C or IL15 treatment, respectively (22, 29, 32, 33). Additionally, iNKT cells have been found to prevent liver fibrosis naturally for the first 2 weeks of CCl4 treatment (35). From these studies, we concluded that the resident NK or NKT populations or both might help to attenuate liver fibrosis after IL30 therapy.
In agreement with these observations, flow cytometric analysis revealed that number of liver NKT cells significantly increased in CCl4-induced liver toxicity, but the NK population did not significantly increase upon IL30 administration (Fig. 3, a and b). Furthermore, chronic CCl4 toxicity with control vector treatment depleted resident liver NKT cells, which were rescued by IL30 treatment to act against fibrosis (Fig. 3, a and b). Concordant to this, NKT population decreased significantly in spleen in CCl4 plus IL30 treated mice compared to CCl4 plus control vector ones (Supplementary Fig. 3). It suggests that the increase of NKT population in the liver after IL30 administration might be coming from secondary lymphoid organs or circulation.
Figure 3. IL30 requires NKT cells to improve CCl4-induced liver fibrosis.
(a–b) Two groups of wild-type mice (n=5 mice per group) were injected with CCl4 solution twice a week for 1 month. One group was given control vector (pCtr) and other pIL30 by hydrodynamic delivery once per week. Control groups-pCtr and pIL30 (n=4 mice per group) were treated in a similar regularity. Liver mononuclear cells were analyzed by flow cytometry. The percentage of cells within selected gates is indicated. (a) Density plots of liver mononuclear cells from pCtr, IL30, CCl4 plus pCtr and CCl4 plus pIL30 treated mice. NKT (CD3+ NK1.1+), NK (CD3+ NK1.1−), Non NKT T (CD3+NK1.1−) and double-negative (CD3-NK1.1−) populations were depicted in the scatter plot. (b) Bar graphs showed the percentage of liver NK or NKT cells to total cell population counted. Student t test was performed to compare between CCl4 plus pCtr and CCl4 plus pIL30. (c–g) Two groups of CD1d−/− mice CCl4 plus pCtr and CCl4 plus pIL30 were treated similarly as stated above. (c) Serum level of ALT was calculated. (d) Infiltration of liver mononuclear cells was evaluated by H&E staining. (e) Bar graphs showed % Affected area. (f) Liver sections were stained with trichrome staining to determine collagen deposition. (g) Collagen deposition was quantified by calculating the percentage of area affected on the micrographs using image J. Representative data were shown as mean ± SEM from two independent experiments. One-way Anova analyses: ns, not significant,*P < .05; **P < .01; ***P< .001.
To corroborate the involvement of NKT cells but not NK cells, we used CD1d−/− mice. Results indicated that IL30 treatment did not significantly decrease the serum ALT level upon CCl4-induced liver injury (Fig. 3 c). H&E staining revealed that IL30 failed to decrease immune cells infiltration (Fig. 3, d and e). Trichrome staining showed similar results (Fig. 3, f and g). The treatment with IL30 in CD1d−/− mice suggests that NKT cells play a major role in IL30–mediated protection against liver fibrosis while NK cells have no such role.
IL30 is able to protect against liver fibrosis in RAG2−/− mice upon adoptive transfer of NKT cells
To further investigate the involvement of NKT cells in IL30 mediated protection against liver fibrosis, we transferred purified liver NKT population from wild type mice to liver of RAG2−/− mice. RAG2−/− mice were selected as they have no B or T but have functional NK cells. These mice were also helpful to understand whether NKT cells need any other subset of T lymphocytes to attenuate liver fibrosis. Both biochemical and histochemical examinations showed that IL30 was unable to protect the RAG2−/− mice from liver fibrosis (Fig.4, a–e). Figure 4f shows the schematic representation of the adoptive transfer experiment. Purified liver NKT (CD3+ NK1.1+) and non NKT T (CD3+ NK1.1−) cells from naïve mice were adoptively transferred to RAG2−/− mice livers once per week for 3 times.
Figure 4. Adoptive transfer of NKT but not non-NKT T cells improves liver fibrosis in RAG2−/− mice after IL30 treatment.
(a–e) Two groups of RAG2−/− were injected with CCl4 solution twice a week for 1 month. One group was inoculated with pCtr and other pIL30 once per week along with CCl4 treatment. (a) Serum level of ALT was calculated. (b) Infiltration of liver mononuclear cells was evaluated by H&E staining. (c) Trichrome staining in liver tissues determined collagen deposition. (d) Bar graphs showed % Affected area. (e) Collagen deposition was quantified by calculating the percentage of area affected on the micrographs using image J. (f–h) Four groups of RAG2−/− mice (n=3~5 mice per group) were injected with CCl4 solution twice a week for 1 month. Two groups were administered with pCtr and other two with pIL30 once per week from 2nd week onwards along with CCl4 treatment. FACS sorted 150,000 cells/mouse liver NKT or non NKT T population was inoculated in liver of both pCtr or pIL30 treated groups once per week for 3 times. (f) Schematic representation of the experimental design. (g) Collagen deposition was measured by trichrome staining. (h) Quantification of collagen deposition was done similarly as stated above. Representative data were shown as mean ± SEM from two independent experiments. One-way Anova analyses: *P < .05; **P < .01; ***P< .001.
Trichrome staining showed that mice that received NKT cells after in vivo therapy with IL30 had significantly less hepatic accumulation of collagen than did mice that received non NKT T cells (Fig. 4, g and h). This finding clearly demonstrates that the NKT cells actively decrease degree of liver fibrosis in the RAG2−/− mice. Interestingly, other populations of T cells, excluding NKT, did not have any significant role in IL30–mediated protection against liver fibrosis.
NKT cells require NKG2D surface expression to inhibit liver fibrosis
Our results corroborate that IL30–mediated liver protection depends on liver NKT cells (Fig. 3 and 4). We next explored the mechanism of the NKT cells’ ability to affect activated HSCs to protect against liver fibrosis. Activated NKT cells secrete IL-4, IL-5, IFN, and TNFα (32); however, these cells also act through an NKG2D-mediated pathway in hepatitis and to alleviate fibrosis (35, 36).To support it, we gated the NKT population from liver mononuclear cells by flow cytometry. Interestingly, we found that NKT cells from IL30–treatment expressed higher levels of NKG2D than did from control vector-treatment (Fig. 5 a, left panel). However, T cells excluding NKT population, did not show any induction of surface NKG2D after IL30 treatment (Fig. 5 a, right panel).
Figure 5. IL30 facilitated hepatic protection is through NKG2D dependent mechanism.
(a) Two groups of wild-type mice were injected with CCl4 solution twice a week for 1 month. One group was injected hydrodynamically with pCtr and other pIL30 once per week along with CCl4 treatment. CD3+NK1.1+ gated liver NKT population by FACS were analyzed for NKG2D expression. Histogram for NKG2D on liver NKT and Non NKT T cells were illustrated by: CCl4 plus pCtr treated as dotted, CCl4 plus IL30 treated as solid and isotype control as thin. (b–f) Wild-type mice were treated as previously however 250 µg NKG2D neutralizing or control IgG antibody was injected intraperitoneally before and after pCtr or pIL30 treatment per week for 1 month. (b) Serum level of ALT was measured. (c) H& E staining of the livers revealed infiltration of immune cells and modification of hepatic architecture. (d) Trichrome staining highlighted the collagen deposition surrounding the central vein area. (e) Bar graphs showed % affected area. (f) Collagen deposition was quantified by calculating the percentage of area affected on the micrographs using image J. (g) Freshly isolated activated HSCs were co-cultured with liver mononuclear cells enriched from CCl4 plus pIL30 treated mice and preincubated with different doses of α-NKG2D for 4 h at an effector-target ratio of 25:1. Representative data were shown as mean ± SEM from three independent experiments. One-way Anova analyses: *P < .05; **P < .01; ***P< .001.
Subsequently, we treated mice in vivo with NKG2D blocking antibody, which efficiently inhibits NKG2D and its ligand interaction. CCl4-treated mice were treated with α-NKG2D or control IgG twice per week before and after IL30 therapy. Inhibiting the functional activity of the NKG2D receptor caused failure of the IL30 treatment to protect against liver fibrosis, whereas the control antibody did not affect the IL30 treatment as revealed by the reduced serum ALT level (Fig. 5 b). Histological examination of the liver sections showed that mice treated with NKG2D-blocking antibody showed signs of massive inflammation, hepatocellular damage, and loss of tissue architecture (Fig. 5, c and e). These histological abnormalities were significantly absent in mice treated with control IgG. Moreover, collagen deposition was significantly higher in NKG2D-blocking antibody-treated livers than in the control IgG-treated ones (Fig. 5, d and f). Similarly pre-blocking the liver mononuclear cells with α-NKG2D reduced cytolytic activity against activated HSCs (Fig. 5 g).
CCl4 treatment induces NKG2D ligand Rae1 expression on activated HSCs
Our findings clearly show that IL30-mediated inhibition of liver fibrosis is NKT cell dependent (Fig. 3, 4) for preventing fibrosis because the activated HSCs were reduced by IL30 treatment (Fig. 1 c and 5 g). From the above mentioned results we found that NKT cells upregulate NKG2D on their surface.
One important question is how NKT cells specifically remove the activated HSCs without affecting the quiescent HSCs. So it is important to determine whether NKG2D ligands are expressed on the surface of activated HSCs. Previous studies have shown that the activation of HSCs induces the expression of Rae1 protein (22). The expression and quantitation of Rae1 protein in HSCs is predominantly induced around the central vein of hepatic tissue following CCl4 treatment (Fig. 6 a, d). The identity of those HSCs was validated by the positive staining with α-Desmin antibody. To corroborate that Rae1 expression occurs only on activated but not on quiescent HSCs, sections of liver tissue were stained with both Rae1 and αSMA (the HSC marker) antibody. As expected, a colocalization of both proteins was detected surrounding the central vein where CCl4 normally activates these cells (Fig. 6 b, e).
Figure 6. Activated hepatic stellate cells (HSCs) express inducible Rae1 in liver fibrosis.
(a–b) One group of wild-type mice (n=5) were injected with CCl4 solution twice a week for 1 month along with pCtr and other group only pCtr once per week. (a) Immunofluorescent confocal microscopy was utilized to detect Rae1 protein expression in HSCs that can be identified by staining of desmin and (b) to detect Rae1 protein expression in activated HSCs that express αSMA. (c) One group of wild-type mice was treated with CCl4 solution twice per week along with pCtr for 3 weeks and other group only pCtr once per week. Rae1 positive population was sorted by magnetic-activated cell sorting from a single cell suspension of liver tissue from both groups. Immunofluorescent confocal microscopy was used to detect HSCs by staining the protein Desmin. Bar graphs show the percentage quantitation of fluorescent images of Rae1 positive cells in total HSCs (d) and Rae1 positive cells in total activated HSCs (αSMA as marker) (e). Student t test was performed to compare between pCtr and CCl4 plus pCtr. (f) Freshly isolated quiescent or activated HSCs were lysed, and lysates were blotted with Rae1, Desmin and Actin antibodies. (g) Heptic NKT population was eluted from CCl4 and pCtr or CCl4 and pIL30 treated mice. Freshly isolated activated or quiescent HSCs were co-cultured at different effector to target ratios for 4h. The cytotoxicity assays were measured by the release of Calcein-AM dye. Representative data were shown as mean ± SEM from two independent experiments. *P < .05; **P < .01; ***P< .001.
To confirm this observation, the Rae1-positive cells were isolated from the livers of both control vector and CCl4 plus control vector treated mice. Staining the enriched HSC cells with Desmin antibody showed numerous HSCs in CCl4 plus control vector treated mice while none in the only control vector ones (Fig. 6 c). This study suggests that CCl4 induced the surface expression of Rae1 in the activated HSCs. Western blot analysis of these isolated HSCs lysates confirmed that only CCl4-treated mice, which have more activated HSCs, express a higher level of Rae1 (Fig. 6 f). This result further confirmed the immunohistochemistry data.
Next, we investigated whether IL30 treatment enhances the cytotoxic activity of the NKT cells toward activated HSCs or acts indirectly to alleviate liver fibrosis. We performed in vitro cytotoxicity assays by isolating liver NKT cells, which were pretreated with either CCl4 plus IL30 or CCl4 plus control vector. Purified liver NKT cells isolated from CCl4 plus control vector-treated mice showed similar basal level cytolytic activity, toward either the activated or quiescent HSCs (Fig. 6 g). However, the liver NKT cells from CCl4 plus IL30–treated mice presented a very high level of cytolytic activity toward the activated HSCs (approximately 62%) compared to quiescent HSCs (approximately 19%) (Fig. 6 g). Thus our results clearly showed that IL30 treatment enhances cytotoxic activity of NKT cells to ameliorate fibrosis.
In summary, this study characterizes IL30 as an anti-fibrotic cytokine in murine models of liver fibrosis. Also IL30 drives NKT cells to decrease activated HSCs, the principal collagen-producing cells in liver fibrosis. This IL30-induced NKT cells removed the collagen-producing activated HSCs via NKG2D-Rae1 interaction (Fig.7).
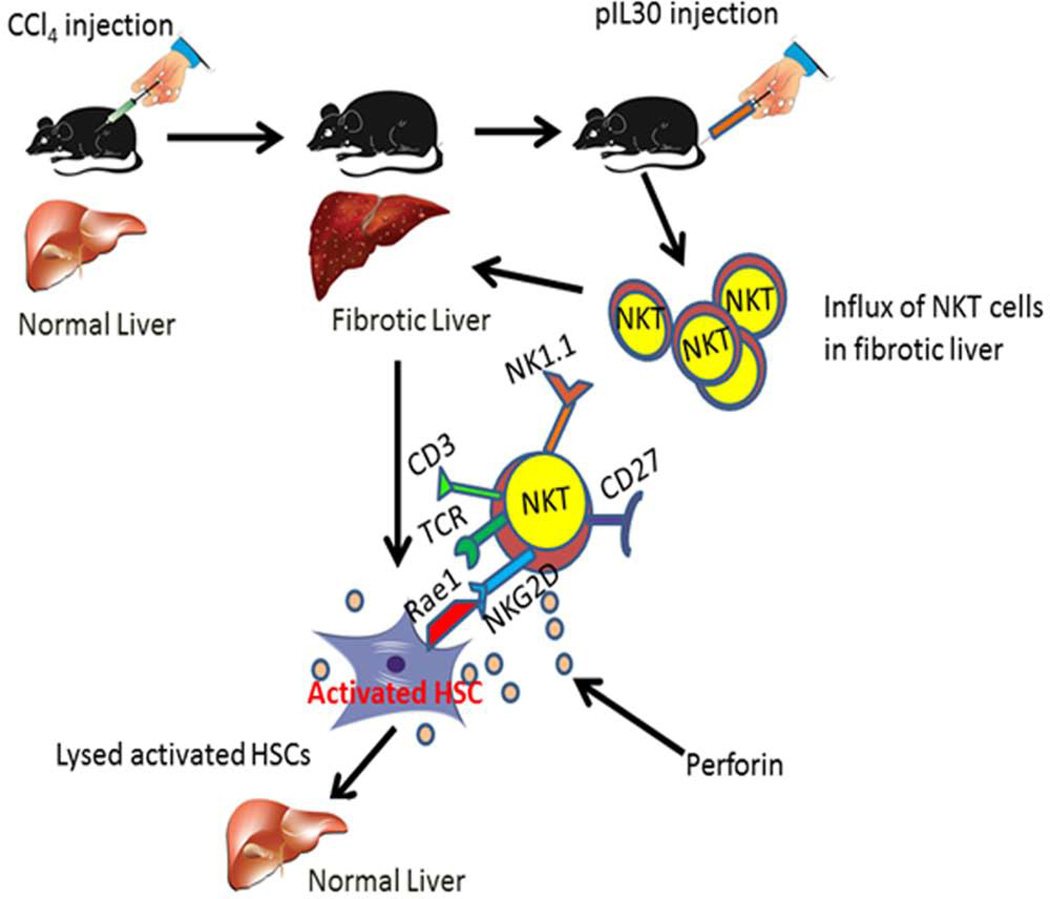
Figure 7. Schematic representation of IL30–mediated improvement of liver fibrosis.
CCl4 1:3 ratio with corn oil was administered to mice i.p. injection once per week to develop liver fibrosis. HSCs undergo transdifferentiation owing to stimulation by various profibrogenic factors. Upon activation, NKG2D receptor target ligand Rae1 undergoes upregulation in these cells. IL30 treatment via hydrodynamic delivery induces the influx of NKT cells in the liver and showed induced NKG2D surface expression. These IL30 driven NKT cells lysed activated HSCs and removed from hepatic tissue to ameliorate liver fibrosis.
Discussion
Many chronic liver diseases begin with a common clinical manifestation- fibrosis. Though it initiates as a wound healing response, excessive accumulation of collagen and fibronectin around exacerbated tissue leads to permanent damage, organ failure and eventually liver-related mortality. Dysregulated immune cells, profibrogenic factors and aberrant functioning of myofibroblasts are considered to be the key therapeutic targets to attenuate liver fibrosis. However, there is no suitable medication proven to be effective to preclude liver fibrosis (37). Though certain cytokines showed hepatoprotection against alcoholic liver disease, clinical efficacy to protect from fibrosis, cirrhosis and end-stage liver diseases still remain unanswered.
IL30 inhibits inflammation in various autoimmune and infectious disease models (16, 38, 39). These published studies demonstrated that IL30 exerts its anti-inflammatory effects by inhibiting T cells differentiation to TH17 or antagonizing the proinflammatory cytokines by blocking the gp130 mediated signaling. As a principal site of inflammation, liver undergoes both acute and chronic toxicities due to exposure and duration to numerous biochemical products. Surprisingly, the hepatoprotective effect and mechanism of action of IL30 has not been extensively studied in murine models. Previously it was shown that, IL30 regulates CD4 T cells by inhibiting the secretion of IFN-γ to protect from acute inflammation (13).
To fill the gap in knowledge, here we have shown for the first time that IL30 requires NKT cells to ameliorate liver fibrosis. Flow cytometric data demonstrated an increase in NKT population in liver injury after IL30 administration. Liver NKT cells have been shown to facilitate hepatic inflammation by recruiting neutrophils and proinflammatory monocytes (33); however, these cells have also been shown to protect the liver from acute inflammation and to inhibit early phase fibrotic injury (32, 33, 35) as the NKT population is depleted upon chronic exposure of the liver to toxic metabolic products (35). Paradoxically, IL30 sustains the NKT population in the liver to alleviate fibrosis after 4 weeks of treatment. A possible explanation for the maintenance of NKT cells in the liver could be proliferation or influx from other secondary lymphoid organs or through the circulation. From our flow cytometric data, we found that IL30 facilitates the influx of NKT cells only and not proliferation, because treatment with IL30 alone did not show significant increase in the NKT population.
There are several mechanisms through which NKT cells exert cytotoxicity- either by secreting cytokines IL4, IL5, TNFα or NKG2D-Rae1 mediated pathway (32, 35, 36). One study demonstrated that a subpopulation of liver NKT cells protected the liver from fibrosis through an NKG2D-dependent mechanism (35). In our chronic disease model, flow cytometric analysis showed that liver NKT cells had increased NKG2D surface expression after IL30 treatment. Moreover, NKG2D-neutralizing antibody prevented IL30 from protecting the liver against injury in vivo. We investigated the underlying mechanism of IL30 mediated NKT cells induction to decrease activated HSCs rather than quiescent HSCs. Several studies showed that the activated HSCs, principal collagen producing cells, expressed NKG2D ligand Rae1 on their surface during the progression of fibrosis (22, 40). Similarly, CCl4 treatment enhances the expression of NKG2D ligand Rae1 on the surface of activated HSCs, which allows the NKT cells to identify the activated HSCs using the IL30-induced NKG2D receptor. These data explain why NKT cells from IL30–treated mice preferentially target activated HSCs rather than quiescent HSCs.
Though hepatic diseases are acute or chronic in nature, chronic liver diseases are progressive and multifactorial processes where resident immune cells, non-immune cells, growth factors and cytokines play multiple roles to support pro or anti-protective role toward healthy liver. Thus only inhibition of IFN-γ secretion by IL30 is unable to provide a comprehensive mechanism of its action to protect liver (12, 14). Here we showed for the first time that IL30 is able to protect liver from chronic liver diseases which are affecting billions of people worldwide. Also, IL30 treatment up regulates surface expression of NKG2D on liver NKT cells and enhances their cytotoxic properties to act on activated HSCs.
With our current findings, it suggests that IL30 can be utilized in hepatic protection against chronic liver diseases. Correlating with our results, clinical studies of patients with hepatitis C virus (HCV) and cirrhosis revealed that the infected liver tissue had significantly fewer hepatic NKT cells than did uninfected human liver samples (41). Additionally, as HCV or cirrhosis progressed, the cytotoxicity of NKT cells decreased. Therefore, IL30 treatment might enhance cytotoxic activity of resident NKT cells in human liver in protecting the liver from hepatitis-mediated injury and provide antitumor immunity toward cirrhosis and hepatocellular carcinoma.
In summary, IL30 alone or in combination may be an ideal therapy to treat chronic inflammatory liver diseases with the aid of the host immune system in human subjects. Future clinical studies with IL30 are warranted for the treatment of chronic liver diseases.
Supplementary Material
Acknowledgments
Work in the authors’ laboratory was supported by grants from the National Institutes of Health to Dr. Shulin Li (NIH R01CA120895). The University of Texas MD Anderson Cancer Center is supported in part by the NCI CCSG Core Grant CA16672.Special thanks to Prasad Phatarpekar, Dr. Reshmi Mitra and Dr. Srinivas Somanchi for valuable suggestions and contribution toward this article.
Abbreviation
- CCl4
carbon tetrachloride
- DDC
3,5-diethoxycarbonyl-1,4-dihydrocollidine
- HSCs
hepatic stellate cells
- αSMA
alpha smooth muscle actin
- MNCs
mononuclear cells
- NK
natural killer
- NKT
NK like T
- NKG2D
natural killer group 2, member D
- Rae1
retinoic acid early inducible 1
- CD
cluster of differentiation
- H&E
hematoxylin and eosin
Footnotes
Conflict of interest statement
We declare that none of the authors have a financial interest related to this work.
References
- 1.Bhogal RK, Bona CA. B cells: no longer bystanders in liver fibrosis. J Clin Invest. 2005;115:2962–2965. doi: 10.1172/JCI26845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kershenobich Stalnikowitz D, Weissbrod AB. Liver fibrosis and inflammation. A review. Ann Hepatol. 2003;2:159–163. [PubMed] [Google Scholar]
- 3.Iredale JP. Models of liver fibrosis: exploring the dynamic nature of inflammation and repair in a solid organ. J Clin Invest. 2007;117:539–548. doi: 10.1172/JCI30542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friedman SL. Liver fibrosis -- from bench to bedside. J Hepatol. 2003;38(Suppl 1):S38–S53. doi: 10.1016/s0168-8278(02)00429-4. [DOI] [PubMed] [Google Scholar]
- 5.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012;18:1028–1040. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Starr SP, Raines D. Cirrhosis: diagnosis, management, and prevention. Am Fam Physician. 2011;84:1353–1359. [PubMed] [Google Scholar]
- 8.Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485–1491. doi: 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Popov Y, Schuppan D. Targeting liver fibrosis: strategies for development and validation of antifibrotic therapies. Hepatology. 2009;50:1294–1306. doi: 10.1002/hep.23123. [DOI] [PubMed] [Google Scholar]
- 10.Schuppan D, Popov Y. Hepatic fibrosis: from bench to bedside. J Gastroenterol Hepatol. 2002;17(Suppl 3):S300–S305. doi: 10.1046/j.1440-1746.17.s3.18.x. [DOI] [PubMed] [Google Scholar]
- 11.Yang C, Zeisberg M, Mosterman B, Sudhakar A, Yerramalla U, Holthaus K, Xu L, et al. Liver fibrosis: insights into migration of hepatic stellate cells in response to extracellular matrix and growth factors. Gastroenterology. 2003;124:147–159. doi: 10.1053/gast.2003.50012. [DOI] [PubMed] [Google Scholar]
- 12.Dibra D, Cutrera J, Xia X, Kallakury B, Mishra L, Li S. Interleukin-30: a novel antiinflammatory cytokine candidate for prevention and treatment of inflammatory cytokine-induced liver injury. Hepatology. 2012;55:1204–1214. doi: 10.1002/hep.24814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang S, Liang R, Luo W, Liu C, Wu X, Gao Y, Hao J, et al. High susceptibility to liver injury in IL-27 p28 conditional knockout mice involves intrinsic interferon-gamma dysregulation of CD4+ T cells. Hepatology. 2013;57:1620–1631. doi: 10.1002/hep.26166. [DOI] [PubMed] [Google Scholar]
- 14.Wang RX, Yu CR, Mahdi RM, Egwuagu CE. Novel IL27p28/IL12p40 cytokine suppressed experimental autoimmune uveitis by inhibiting autoreactive Th1/Th17 cells and promoting expansion of regulatory T cells. J Biol Chem. 2012;287:36012–36021. doi: 10.1074/jbc.M112.390625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Batten M, Li J, Yi S, Kljavin NM, Danilenko DM, Lucas S, Lee J, et al. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol. 2006;7:929–936. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 16.Stumhofer JS, Laurence A, Wilson EH, Huang E, Tato CM, Johnson LM, Villarino AV, et al. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7:937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 17.Anan A, Baskin-Bey ES, Bronk SF, Werneburg NW, Shah VH, Gores GJ. Proteasome inhibition induces hepatic stellate cell apoptosis. Hepatology. 2006;43:335–344. doi: 10.1002/hep.21036. [DOI] [PubMed] [Google Scholar]
- 18.Crispe IN. The liver as a lymphoid organ. Annu Rev Immunol. 2009;27:147–163. doi: 10.1146/annurev.immunol.021908.132629. [DOI] [PubMed] [Google Scholar]
- 19.Kakimi K, Guidotti LG, Koezuka Y, Chisari FV. Natural killer T cell activation inhibits hepatitis B virus replication in vivo. J Exp Med. 2000;192:921–930. doi: 10.1084/jem.192.7.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu C, Tao Q, Sun M, Wu JZ, Yang W, Jian P, Peng J, et al. Kupffer cells are associated with apoptosis, inflammation and fibrotic effects in hepatic fibrosis in rats. Lab Invest. 2010;90:1805–1816. doi: 10.1038/labinvest.2010.123. [DOI] [PubMed] [Google Scholar]
- 21.Connolly MK, Bedrosian AS, Mallen-St Clair J, Mitchell AP, Ibrahim J, Stroud A, Pachter HL, et al. In liver fibrosis, dendritic cells govern hepatic inflammation in mice via TNF-alpha. J Clin Invest. 2009;119:3213–3225. doi: 10.1172/JCI37581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Radaeva S, Sun R, Jaruga B, Nguyen VT, Tian Z, Gao B. Natural killer cells ameliorate liver fibrosis by killing activated stellate cells in NKG2D-dependent and tumor necrosis factor-related apoptosis-inducing ligand-dependent manners. Gastroenterology. 2006;130:435–452. doi: 10.1053/j.gastro.2005.10.055. [DOI] [PubMed] [Google Scholar]
- 23.Gao B, Jeong WI, Tian Z. Liver: An organ with predominant innate immunity. Hepatology. 2008;47:729–736. doi: 10.1002/hep.22034. [DOI] [PubMed] [Google Scholar]
- 24.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 25.Liu ZX, Govindarajan S, Okamoto S, Dennert G. NK cells cause liver injury and facilitate the induction of T cell-mediated immunity to a viral liver infection. J Immunol. 2000;164:6480–6486. doi: 10.4049/jimmunol.164.12.6480. [DOI] [PubMed] [Google Scholar]
- 26.Nakamura T, Arii S, Monden K, Furutani M, Takeda Y, Imamura M, Tominaga M, et al. Expression of the Na+/Ca2+ exchanger emerges in hepatic stellate cells after activation in association with liver fibrosis. Proc Natl Acad Sci U S A. 1998;95:5389–5394. doi: 10.1073/pnas.95.9.5389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cutrera J, Dibra D, Xia X, Hasan A, Reed S, Li S. Discovery of a linear peptide for improving tumor targeting of gene products and treatment of distal tumors by IL-12 gene therapy. Mol Ther. 2011;19:1468–1477. doi: 10.1038/mt.2011.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mitra A, Ross JA, Rodriguez G, Nagy ZS, Wilson HL, Kirken RA. Signal transducer and activator of transcription 5b (Stat5b) serine 193 is a novel cytokine-induced phospho-regulatory site that is constitutively activated in primary hematopoietic malignancies. J Biol Chem. 2012;287:16596–16608. doi: 10.1074/jbc.M111.319756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hosoya S, Ikejima K, Takeda K, Arai K, Ishikawa S, Yamagata H, Aoyama T, et al. Innate immune responses involving natural killer and natural killer T cells promote liver regeneration after partial hepatectomy in mice. Am J Physiol Gastrointest Liver Physiol. 2013;304:G293–G299. doi: 10.1152/ajpgi.00083.2012. [DOI] [PubMed] [Google Scholar]
- 30.Ito H, Ando K, Nakayama T, Taniguchi M, Ezaki T, Saito K, Takemura M, et al. Role of Valpha 14 NKT cells in the development of impaired liver regeneration in vivo. Hepatology. 2003;38:1116–1124. doi: 10.1053/jhep.2003.50471. [DOI] [PubMed] [Google Scholar]
- 31.Graubardt N, Fahrner R, Trochsler M, Keogh A, Breu K, Furer C, Stroka D, et al. Promotion of liver regeneration by natural killer cells in a murine model is dependent on extracellular adenosine triphosphate phosphohydrolysis. Hepatology. 2013;57:1969–1979. doi: 10.1002/hep.26008. [DOI] [PubMed] [Google Scholar]
- 32.Li B, Sun R, Wei H, Gao B, Tian Z. Interleukin-15 prevents concanavalin A-induced liver injury in mice via NKT cell-dependent mechanism. Hepatology. 2006;43:1211–1219. doi: 10.1002/hep.21174. [DOI] [PubMed] [Google Scholar]
- 33.Wondimu Z, Santodomingo-Garzon T, Le T, Swain MG. Protective role of interleukin-17 in murine NKT cell-driven acute experimental hepatitis. Am J Pathol. 2010;177:2334–2346. doi: 10.2353/ajpath.2010.100028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiao J, Sastre D, Fiel MI, Lee UE, Ghiassi-Nejad Z, Ginhoux F, Vivier E, et al. Dendritic cell regulation of carbon tetrachloride-induced murine liver fibrosis regression. Hepatology. 2012;55:244–255. doi: 10.1002/hep.24621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park O, Jeong WI, Wang L, Wang H, Lian ZX, Gershwin ME, Gao B. Diverse roles of invariant natural killer T cells in liver injury and fibrosis induced by carbon tetrachloride. Hepatology. 2009;49:1683–1694. doi: 10.1002/hep.22813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vilarinho S, Ogasawara K, Nishimura S, Lanier LL, Baron JL. Blockade of NKG2D on NKT cells prevents hepatitis and the acute immune response to hepatitis B virus. Proc Natl Acad Sci U S A. 2007;104:18187–18192. doi: 10.1073/pnas.0708968104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gao B. Hepatoprotective and anti-inflammatory cytokines in alcoholic liver disease. J Gastroenterol Hepatol. 2012;27(Suppl 2):89–93. doi: 10.1111/j.1440-1746.2011.07003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fitzgerald DC, Zhang GX, El-Behi M, Fonseca-Kelly Z, Li H, Yu S, Saris CJ, et al. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat Immunol. 2007;8:1372–1379. doi: 10.1038/ni1540. [DOI] [PubMed] [Google Scholar]
- 39.Pearl JE, Khader SA, Solache A, Gilmartin L, Ghilardi N, deSauvage F, Cooper AM. IL-27 signaling compromises control of bacterial growth in mycobacteria-infected mice. J Immunol. 2004;173:7490–7496. doi: 10.4049/jimmunol.173.12.7490. [DOI] [PubMed] [Google Scholar]
- 40.Gao B, Radaeva S, Park O. Liver natural killer and natural killer T cells: immunobiology and emerging roles in liver diseases. J Leukoc Biol. 2009;86:513–528. doi: 10.1189/jlb.0309135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kawarabayashi N, Seki S, Hatsuse K, Ohkawa T, Koike Y, Aihara T, Habu Y, et al. Decrease of CD56(+)T cells and natural killer cells in cirrhotic livers with hepatitis C may be involved in their susceptibility to hepatocellular carcinoma. Hepatology. 2000;32:962–969. doi: 10.1053/jhep.2000.19362. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.