Abstract
Introduction
Our phase I Hepatic Immunotherapy for Metastases (HITM) trial tested the safety of chimeric antigen receptor modified T cell (CAR-T) hepatic artery infusions (HAI) for unresectable CEA+ liver metastases (LM). High neutrophil:lymphocyte ratios (NLR) predict poor outcome in cancer patients and we hypothesized that NLR changes would correlate with early responses to CAR-T HAI.
Methods
Six patients completed the protocol. Three patients received CAR-T HAI in dose escalation (1 × 108, 1 × 109, and 1 × 1010cells) and the remainder received 3 doses (1 × 1010 cells) with IL2 support. Serum cytokines and NLR were measured at multiple time points.
Results
The mean NLR for all patients was 13.9 (range 4.8-38.1). NLR increased in four patients following treatment with a mean fold change of 1.9. Serum IL6 levels and NLR fold-changes demonstrated a trend towards a positive correlation (r=0.77, p=0.10). Patients with poor CEA responses were significantly more likely to have higher NLR level increases (p=0.048).
Conclusions
Increased NLR levels were associated with poor responses following CAR-T HAI. NLR variations and associated cytokine changes may be useful surrogates of response to CAR-T HAI.
Keywords: chimeric antigen receptor modified T cells, metastatic hepatic colorectal cancer, cytokines, neutrophil-lymphocyte ratio, immunotherapy
Introduction
Colorectal cancer (CRC) is the third most common malignancy and the second leading cause of cancer-related death worldwide.(1) Twenty five percent of patients with CRC present with stage IV disease involving the liver at the time of diagnosis and an additional 50% will develop metachronous colorectal liver metastases (CRLM).(2) For patients with CRLM, resection is the only curative option but 80% of these patients present with unresectable disease.(3) Moreover, only 17-25% of patients are free of disease 10 years after resection.(4, 5) Current systemic chemotherapy offers a median survival time of only 20-24 months.(6) Novel therapeutic options are needed and immunotherapy is a promising alternative.
We recently completed the phase I Hepatic Immunotherapy for Metastases (HITM, NCT01373047) trial evaluating the safety and tolerability of anti-CEA chimeric antigen receptor modified T cells (CAR-T). CAR-Ts are autologous lymphocytes engineered to target tumor antigens and have shown significant promise in recent years.(7, 8) We delivered CAR-T specific for carcinoembryonic antigen (CEA) by hepatic artery infusion (HAI) as we speculate that off-target toxicity from systemic infusion of immunotherapeutics can be minimized through use of regional delivery strategies.(9)
Systems to define tumor response based on cross sectional imaging lesion size changes, such as the response criteria in solid tumors (RECIST), were created primarily for cytotoxic chemotherapy studies.(10) Criteria based on changes in tumor size inadequately measure early tumor response after immunotherapy, as lesions may initially get larger due to inflammation and edema, in addition to response kinetics being prolonged in some cases.(11) As such, we are interested in studying alternative biological surrogates of early response to immunotherapy. We performed correlative work on HITM patient biospecimens to identify potential surrogates of early response to CAR-T HAI, as conventional imaging may not accurately characterize intrahepatic tumor response.(11)
We examined serum cytokine changes following CAR-T HAI to determine if peripheral fluctuations in markers of inflammation correlated with immune activity in the liver. Interleukin 6 (IL6) is an inflammatory cytokine that has been linked to disease status,(12-14) tumor size (15) and presence of liver metastases (LM).(16) CRC cells produce IL6 and increased IL6 levels correlate with progression and recurrence.(12) A decrease in serum IL6 levels following CRLM ablation or resection correlates with lower recurrence rates.(17, 18) IL6 has also been associated with the generation of pro-inflammatory IL17-producing CD4 T cells, or Th17 cells.(19) Serum levels of IL6 and IL17 not only serve as surrogates of tumor response,(20) but these cytokines also drive mobilization of neutrophils from the bone marrow, another correlate of outcome in solid tumor patients.(21, 22)
The neutrophil:lymphocyte ratio (NLR) has been used as an indicator of immunity and inflammation in the tumor microenvironment.(23-25) High NLR has been linked to tumor immune evasion in the liver.(26) Several groups have reported an association between high NLR and poor outcomes in patients with CRLM.(21, 27) NLR, which is in part governed by IL6 and IL17 concentrations in the serum, could serve as an early marker of the inflammatory response within the intrahepatic space following CAR-T HAI. The goal of our study was to determine if NLR and serum cytokine variations occurred following anti-CEA CAR-T HAI. Furthermore, we wished to assess if these variations in serum cytokines and NLR levels may reflect anti-tumor activity of CAR-T within the intrahepatic space.
Methods
Patients and clinical intervention
The six patients who completed the phase I HITM (NCT01373047) clinical trial were the subjects of our study. Eligibility criteria included documented CEA+ liver metastases, elevated serum CEA levels, life expectancy greater than four months, adequate organ function and presence of unresectable disease progressive on standard systemic chemotherapy. The three patients in cohort 1 were treated with three anti-CEA CAR-T HAI doses in escalation fashion (1 × 108, 1 × 109, and 1 × 1010 cells) without IL2 (IL2-). For cohort 2, the three patients received three HAI CAR-T doses at the maximum tolerated level (1 × 1010 cells) in addition to continuous systemic IL2 (IL2+) infusion at 75,000 IU/kg/day via an ambulatory infusion pump. CAR-T doses were given two weeks apart and IL2 was administered from the time of the first dose until two weeks following the final CAR-T HAI. All patients received a small amount of IL2 (approximately 6 × 105IU) with each CAR-T infusion, as IL2 was present in all cell preparations. All clinical doses of CAR-Ts were prepared at Roger Williams Medical Center Cancer Immunotherapy GMP facility. HAI were delivered percutaneously via a femoral arterial approach in the Roger Williams Medical Center Interventional Radiology Suite.
Cytokines and Neutrophil Lymphocyte Ratios
We measured serum levels of IL6 and IL17, in addition to peripheral blood NLR, in all patients at day 0 or baseline and days 1, 2, 4 and 7 after each CAR-T HAI, which were given at 2-week intervals. NLR and cytokines were measured up to 7 days following the final CAR-T infusion, with the exception of patient 4, who had no samples taken one day after his final infusion due to disease progression. ELISA (eBioscience, San Diego, CA) was used to measure cytokines IL6 and IL17 concentrations in serum. We calculated NLR from the absolute values of neutrophils and lymphocytes measured in complete blood count (CBC) performed at the same time points. Fold changes were determined using the baseline sample and the final sample taken following the CAR-T HAI. Comparing pre-treatment levels to the final level in cohort 1 or the final level before IL2 discontinuation or dose reduction in cohort 2 determined CEA responses.(28)
Statistical analysis
Prism from GraphPad Software Inc. (La Jolla, California) was used for statistical analysis, Spearman test was used for receiver operating characteristics (ROC) curves. A two-tailed T-test was used to compare means. A p value of ≤ 0.05 was considered statistically significant. The NLR median for all patients was 8.6 and we chose 10 as the cut off value to classify NLR as high or low for simplicity and ease of clinical use. For analyses stratified by CEA response, a positive response was considered to be at least a 10% decrease from baseline CEA level.
Results
Patient Characteristics
Eight patients were enrolled in the phase I HITM trial, of which six received three hepatic artery anti-CEA CAR-T doses and were eligible for this study. The average age was 57 years old (range 51-66), and two patients were females and four were males (Table 1). Five patients had been diagnosed with unresectable CEA+ CRLM and one with CEA+ ampullary adenocarcinoma. The first three patients received anti-CEA CAR-T HAI as an intrapatient escalation dose of 1 × 108, 1 × 109 and 1 × 1010 cells. The second cohort received 3 HAI at the 1 × 1010 dose level with systemic IL2 infusions. Details pertaining to adverse events and response data are reported elsewhere.(28)
TABLE 1. Patient characteristics and sample data.
| P#1 | P#4 | P#5 | P#6 | P#7 | P#8 | ||
|---|---|---|---|---|---|---|---|
| Age | 54 | 55 | 63 | 51 | 53 | 66 | |
| Sex | F | M | M | M | F | M | |
| Diagnosis | Colon | Ampullary | Colon | Colon | Colon | Colon | |
| CAR-T doses | 108, 109, and 1010 | 108, 109, and 1010 | 108, 109, and 1010 | (1010) × 3 | (1010) × 3 | (1010) × 3 | |
| Adjuvant IL2 | No | No | No | Yes | Yes | Yes | |
| NLR | Mean | 9.3 | 38.1 | 4.8 | 6.6 | 13.5 | 11.6 |
| Peak | 14.3 | 89.0 | 13.3 | 19.6 | 43.0 | 31.3 | |
| Δ | 2.1 | 5.5 | 1.3 | -1.3 | 1.3 | -5.8 | |
| IL6 | Mean | 22.6 | 86.4 | 15.8 | 78.4 | 83.4 | 103.7 |
| Peak | 41.2 | 119.9 | 35.5 | 135.4 | 287.9 | 200.3 | |
| Δ | 2.3 | 1.6 | 3.6 | 2.4 | 6.6 | 1.2 | |
| IL17 | Mean | 42.4 | 1134.7 | 226.4 | 325.4 | 13.6 | N/Aˆˆ |
| Peak | 89.2 | 1289.2 | 266.2 | 673.3 | 228.6 | 0 | |
| Δ | -2.0 | -1.4 | 1.0 | 4.6 | N/Aˆ | N/Aˆˆ |
All mean and peak serum cytokine concentrations expressed in pg/ml. Δ denotes fold-change in parameter from baseline value before T cell infusions to final value.
= IL17 detected on only one measurement.
= IL17 not detected in any samples.
Neutrophil:lymphocyte ratios following hepatic artery CAR-T infusions
The mean NLR for the HITM trial patients ranged between 4.8 and 38.1 (Table 1). Patients 4, 7 and 8 had a mean NLR > 10, while patients 1, 5 and 6 had mean NLR ratios < 10. Each patient demonstrated an increase in NLR at some point in response to CAR-T infusion when comparing baseline to peak values (Figure 1A). We analyzed NLR fold change among patients treated with or without systemic IL2 to determine if cytokine support impacted this parameter. The mean NLR fold-change was 1.39 for cohort 1 (-IL2) and 1.36 for cohort 2 (+IL2) (p=0.48). However, the only two NLR decreases seen were in the +IL2 group (Figure 1B). Importantly, these patients also demonstrated responses in terms of decreased serum CEA and increased fibrosis or necrosis in tumor biopsy specimens (not shown).(28)
Figure 1. Neutrophil:lymphocyte ratio changes in response to CAR-T hepatic artery infusions.
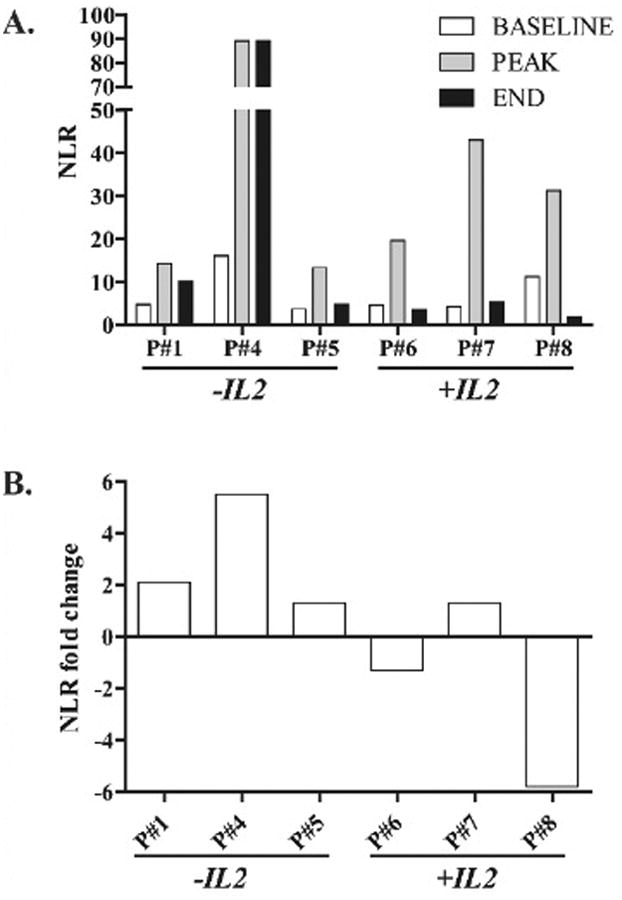
(A) NLR serum levels for patients in the HITM trial who did or did not receive continuous low-dose systemic IL2 infusions. (B) Fold-changes in the NLR for each patient. NLR values were calculated from complete blood counts taken at multiple time points in relation to each CAR-T infusion.
Serum IL6 and IL17 changes following hepatic artery CAR-T infusions
We measured serum IL6 and IL17 levels at baseline (day 0) and then at days 1, 2, 4 and 7 following each CAR-T HAI which were given 3 times every 2 weeks. The mean IL6 fold-change for all patients was 3.0 (range 1.2 – 6.6) (Table 1). Patients who received IL2 had the highest peak serum IL6 concentrations in response to CAR-T HAI, with levels of 287.9 pg/ml and 200.3 pg/ml for patients 7 and 8, respectively (Figure 2A). No significant differences in IL6 fold-change was seen in the group of patients receiving IL2 during the CAR-T HAI compared to the group not receiving IL2 (p=0.19). However, the highest IL6 mean fold-change of 6.6 occurred with patient 7, who did receive IL2 (Figure 2B). IL6 levels increased in all patients at variable time points after the administration of CAR-T HAI (Figure 2C).
Figure 2. Serum IL6 and IL17 responses to CAR-T hepatic artery infusions.
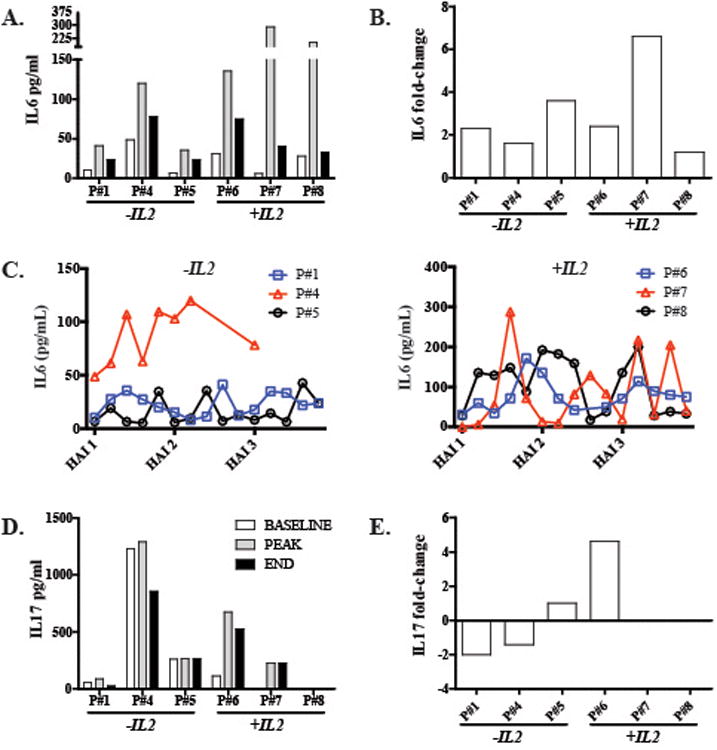
(A) IL6 peripheral blood concentrations for each patient are illustrated, along with fold-changes (B). (C) IL6 absolute values throughout CAR-T HAI treatments in IL2 - and IL2 + groups. (D) IL17 peripheral blood concentrations for each patient are illustrated, along with fold-changes (E). Fold-changes were calculated as the ratio of final to pre-treatment levels. Patient 7 had IL17 detected in only one sample, while patient 8 did not have detectable IL17 at any point.
We observed greater variability in post-infusion IL17 serum levels compared to IL6. The highest peak (1289.2 pg/ml) and average (1134.7 pg/ml) IL17 levels were found in patient 4 who did not receive IL2 and who progressed rapidly following his final CAR-T infusion (Figures 2D-E). Patient 7 had IL17 detectable at only one time point while patient 8 had no detectable serum IL17. Patients 1 and 4 experienced decreased IL17 levels following CAR-T HAI while IL17 levels increased for patients 5 (+3.9 pg/ml) and 6 (+413.0 pg/ml). Having determined that both IL6 or IL17 levels increased at various time points in all patients following HAI of CAR-T, we compared cytokine and NLR changes for each patient given the role of IL6 and IL17 in the regulation of peripheral neutrophil dynamics.
Correlation of serum IL6 and IL17 levels with neutrophil:lymphocyte ratio variations after hepatic artery CAR-T infusions
Serum IL6 concentrations and NLR variations trended toward a positive correlation (r=0.77, p=0.10, Figure 3A). The correlation between serum IL17 and NLR variations was weak (r=0.2, p=1.0, Figure 3B). Following stratification of patients based on their mean NLR values, we found a trend towards higher mean serum IL6 concentrations in patients with higher mean NLR (p=0.07, Figure 3C). We did not find a similar trend when comparing IL17 levels between those who did or did not receive systemic IL2 support (not shown).
Figure 3. Correlation of HITM patient neutrophil:lymphocyte ratios with serum IL6 and IL17 levels.
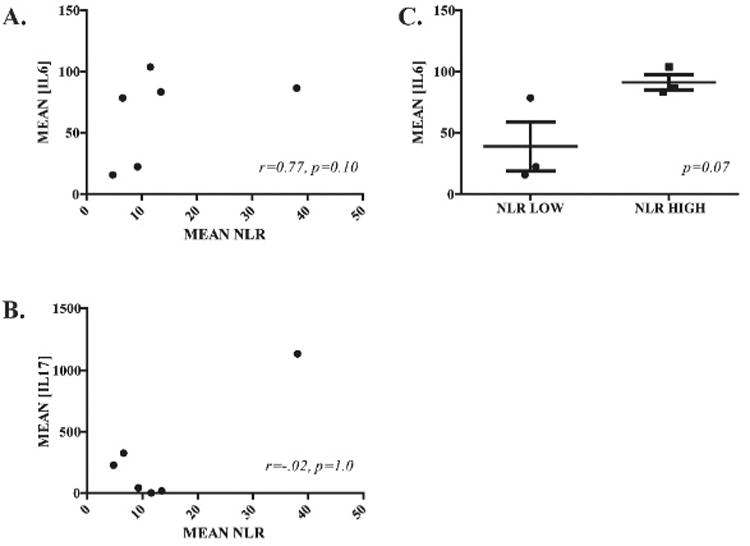
ROC plots illustrate the correlations between mean NLR values and mean serum IL6 (A) and IL17(B). (C) IL6 mean serum concentrations stratified and compared based on the mean NLR values.
Neutrophil:lymphocyte ratio correlates with serologic response after hepatic CAR-T infusions
To assess the correlation of NLR variations and anti-tumor activity of CAR-T HAI, CEA level was used as a surrogate of tumor response. We found a trend towards a correlation between NLR variations and CEA decreases (r=0.71, p=0.11) (Figure 4A). We also performed a stratified analysis to compare NLR fold change in patients with or without favorable CEA responses to CAR-T infusion. A 10% decrease in the absolute value of CEA serum levels was considered a favorable response. We found that patients who had a favorable CEA response to CAR-T HAI were significantly more likely to have had lower NLR fold changes (p=0.048, Figure 4B).
Figure 4. Neutrophil:lymphocyte ratio changes following CAR-T HAI correlates with serologic response to treatment.
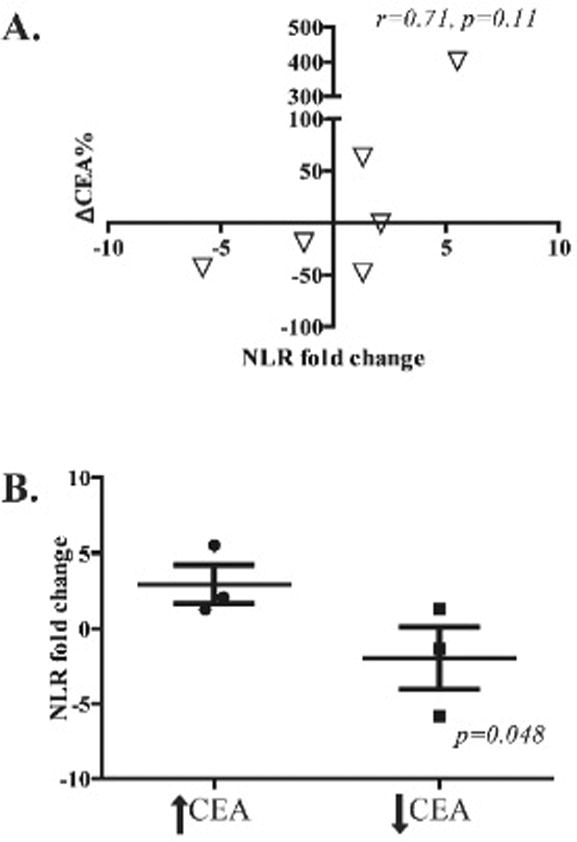
(A) ROC plot to demonstrate the NLR fold-change and percentage change in serum CEA for each patient following completion CAR-T HAI. Percentage CEA change from baseline is presented on the x-axis while NLR fold change is illustrated in the Y-axis. (B) NLR fold-change stratification by level of serum CEA following treatment, a decrease of at least 10% in CEA levels was considered a positive response.
Discussion
We have demonstrated an increase in both serum IL6 and IL17 concentrations in patients with CEA+ LM following CAR-T HAI, accompanied by variations in NLR. To our knowledge, this is the first report to examine systemic inflammatory changes following regional hepatic infusion of a cellular immunotherapeutic product in patients with LM. While our data are limited by the small number of patients in the phase I HITM trial, the findings presented herein provide evidence that HAI of CAR-T induced an inflammatory response within the intrahepatic space and beyond. Decreases in NLR correlated with CEA responses to treatment. As such, increases in NLR levels after CAR-T HAI may predict lack of response to CAR-T HAI.
Recently, several biomarkers have been studied as surrogates of inflammatory responses that favor tumor progression. The most commonly used have been C-reactive protein, absolute or static NLR, and platelet-lymphocyte ratio. High levels of these biological markers have been associated with poor prognosis in colorectal, gastro-esophageal and renal cell cancers.(24) A NLR of 5 or more has been significantly associated with poor prognosis in metastatic colorectal adenocarcinoma.(23) Several studies have focused on NLR and its role as a prognostic factor in patients undergoing loco-regional therapy for various solid tumors, including CRLM.(25, 29, 30) To our knowledge this is the first publication examining NLR variations from baseline following treatment with genetically modified T cells. High levels of IL6 and IL17 promote NLR increases and have been associated with an immunosuppressive inflammatory state.(19) NLR might not only play a role as a prognostic marker as shown in prior studies, but as a surrogate of response in patients receiving immunotherapy. Patients with high NLR fold changes showed poor CEA responses. The correlation between NLR variations and CEA levels after CAR-T HAI suggest that NLR variations may be useful as a surrogate marker of tumor response.
It is not clear if IL2 had direct effects on IL6, NLR, and CEA levels, or if IL2 promoted a more robust anti-tumor immune response by CAR-T with subsequent decrease of NLR and CEA levels.(31) IL2 is known to promote expansion of peripheral lymphocyte populations, and as such may have expanded peripheral lymphocytes and hence lowered the NLR in cohort 2. Systemic cytokine and NLR responses were seen both in subjects who did and did not receive systemic IL2 support. The highest IL6 responses were seen in cohort 2, but IL17 fluctuations did not seem to relate to IL2 infusion. This suggests that IL2 contributes to the systemic cytokine response to CAR-T HAI, but the response is not dependent on IL2. Interestingly, NLR fold-changes in cohort 2 were negative in two of three patients (-1.3 and -5.8). We cannot determine if the IL2 effects noted in this study were a consequence of enhanced CAR-T activity within the liver or were peripheral effects of IL2 independent of CAR-T function. We speculate that following CAR-T HAI, NLR is not only dependent on IL6 and IL17, but is positively impacted by both IL2 infusion and the effect of IL2 on CAR-T function. Lower NLR may also be indicative a more favorable immunologic context within the intrahepatic space, thereby promoting CAR-T anti-tumor activity independent of IL2 infusions.
The use of NLR variations to assess early immunotherapy responses adds to the existing literature that has established the role of static NLR as a prognostic factor in patients with CRLM.(21, 26, 27, 32) Our data must be interpreted within the context of the study design limitations. The primary objective of the HITM trial was to evaluate the safety of CAR-T HAI, and so it was not powered to draw conclusions about the use of cytokines or NLR as predictors of long-term outcome. Our data suggest a role for NLR variation as a predictor of response to CAR-T HAI but not as a prognostic factor. We studied a small number of patients with unresectable CRLM, which may limit our ability to draw conclusions and allow us to identify only large differences with very small standard deviations. Additionally, our patients presented with advanced disease following heavy pre-treatment with cytotoxic therapy.(28) Further work is required to determine the utility of NLR and inflammatory cytokine measurements following CAR-T HAI in patients with lower disease burdens.
Immunotherapy has evolved considerably in the last decade and is becoming an integral component of the multidisciplinary management of solid organ tumors. Evaluation of tumor response after active immunotherapy for solid organ tumors is challenging, as the classic RECIST criteria are not reliable indicators in this setting.(11) We have shown that an increase in NLR after treatment correlates with increased CEA levels, a classic serological marker of tumor progression. (33) Our findings suggest that systemic variations of NLR and inflammatory cytokines can reflect the response to CAR-T activity within the intrahepatic space. Easily measured surrogates of immunologic or inflammatory activity such as NLR and serum cytokines deserve further study as indicators of immunotherapeutic agent activity.
Acknowledgments
Financial Support: Support for this work was provided by the National Institute of Health (1K08CA160662-01A1), the Society of Surgical Oncology Clinical Investigator Award supported by an education grant for Genentech, the Rhode Island Foundation, and Roger Williams Medical Center Graduate Medical Education Fund
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Ismaili N. Treatment of colorectal liver metastases. World J Surg Oncol. 2011;9:154. doi: 10.1186/1477-7819-9-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdalla EK, Vauthey JN, Ellis LM, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004;239:818–25. doi: 10.1097/01.sla.0000128305.90650.71. discussion 825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adam R, Wicherts DA, de Haas RJ, et al. Patients with initially unresectable colorectal liver metastases: is there a possibility of cure? Journal of Clinical Oncology. 2009;27:1829–1835. doi: 10.1200/JCO.2008.19.9273. [DOI] [PubMed] [Google Scholar]
- 5.Tomlinson JS, Jarnagin WR, DeMatteo RP, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol. 2007;25:4575–4580. doi: 10.1200/JCO.2007.11.0833. [DOI] [PubMed] [Google Scholar]
- 6.Falcone A, Ricci S, Brunetti I, et al. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the Gruppo Oncologico Nord Ovest. J Clin Oncol. 2007;25:1670–1676. doi: 10.1200/JCO.2006.09.0928. [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenberg SA. Cell transfer immunotherapy for metastatic solid cancer--what clinicians need to know. Nat Rev Clin Oncol. 2011;8:577–585. doi: 10.1038/nrclinonc.2011.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saied A, Pillarisetty VG, Katz SC. Immunotherapy for solid tumors-a review for surgeons. J Surg Res. 2013 doi: 10.1016/j.jss.2013.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishino M, Jagannathan JP, Krajewski KM, et al. Personalized tumor response assessment in the era of molecular medicine: cancer-specific and therapy-specific response criteria to complement pitfalls of RECIST. AJR Am J Roentgenol. 2012;198:737–745. doi: 10.2214/AJR.11.7483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolchok JD, Hoos A, O'Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 12.Knupfer H, Preiss R. Serum interleukin-6 levels in colorectal cancer patients--a summary of published results. Int J Colorectal Dis. 2010;25:135–140. doi: 10.1007/s00384-009-0818-8. [DOI] [PubMed] [Google Scholar]
- 13.Okugawa Y, Miki C, Toiyama Y, et al. Loss of tumoral expression of soluble IL-6 receptor is associated with disease progression in colorectal cancer. Br J Cancer. 2010;103:787–795. doi: 10.1038/sj.bjc.6605827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waldner MJ, Foersch S, Neurath MF. Interleukin-6--a key regulator of colorectal cancer development. Int J Biol Sci. 2012;8:1248–1253. doi: 10.7150/ijbs.4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ueda T, Shimada E, Urakawa T. Serum levels of cytokines in patients with colorectal cancer: possible involvement of interleukin-6 and interleukin-8 in hematogenous metastasis. J Gastroenterol. 1994;29:423–429. doi: 10.1007/BF02361238. [DOI] [PubMed] [Google Scholar]
- 16.Kinoshita T, Ito H, Miki C. Serum interleukin-6 level reflects the tumor proliferative activity in patients with colorectal carcinoma. Cancer. 1999;85:2526–2531. doi: 10.1002/(sici)1097-0142(19990615)85:12<2526::aid-cncr6>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 17.de Jong KP, von Geusau BA, Rottier CA, et al. Serum response of hepatocyte growth factor, insulin-like growth factor-I, interleukin-6, and acute phase proteins in patients with colorectal liver metastases treated with partial hepatectomy or cryosurgery. J Hepatol. 2001;34:422–427. doi: 10.1016/s0168-8278(00)00030-1. [DOI] [PubMed] [Google Scholar]
- 18.Evrard S, Menetrier-Caux C, Biota C, et al. Cytokines pattern after surgical radiofrequency ablation of liver colorectal metastases. Gastroenterol Clin Biol. 2007;31:141–145. doi: 10.1016/s0399-8320(07)89344-4. [DOI] [PubMed] [Google Scholar]
- 19.Hammerich L, Heymann F, Tacke F. Role of IL-17 and Th17 cells in liver diseases. Clin Dev Immunol. 2011;2011:345803. doi: 10.1155/2011/345803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vizio B, Novarino A, Giacobino A, et al. Potential plasticity of T regulatory cells in pancreatic carcinoma in relation to disease progression and outcome. Exp Ther Med. 2012;4:70–78. doi: 10.3892/etm.2012.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kishi Y, Kopetz S, Chun YS, Palavecino M, Abdalla EK, Vauthey JN. Blood neutrophil-to-lymphocyte ratio predicts survival in patients with colorectal liver metastases treated with systemic chemotherapy. Ann Surg Oncol. 2009;16:614–622. doi: 10.1245/s10434-008-0267-6. [DOI] [PubMed] [Google Scholar]
- 22.Suwa T, Hogg JC, English D, Van Eeden SF. Interleukin-6 induces demargination of intravascular neutrophils and shortens their transit in marrow. Am J Physiol Heart Circ Physiol. 2000;279:H2954–H2960. doi: 10.1152/ajpheart.2000.279.6.H2954. [DOI] [PubMed] [Google Scholar]
- 23.Paramanathan A, Saxena A, Morris DL. A systematic review and meta-analysis on the impact of pre-operative neutrophil lymphocyte ratio on long term outcomes after curative intent resection of solid tumours. Surg Oncol. 2013 doi: 10.1016/j.suronc.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 24.Roxburgh CSD, McMillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future oncology. 2010;6:149–163. doi: 10.2217/fon.09.136. [DOI] [PubMed] [Google Scholar]
- 25.Wang DS, Ren C, Qiu MZ, et al. Comparison of the prognostic value of various preoperative inflammation-based factors in patients with stage III gastric cancer. Tumour Biol. 2012;33:749–756. doi: 10.1007/s13277-011-0285-z. [DOI] [PubMed] [Google Scholar]
- 26.Halazun KJ, Aldoori A, Malik HZ, et al. Elevated preoperative neutrophil to lymphocyte ratio predicts survival following hepatic resection for colorectal liver metastases. European Journal of Surgical Oncology (EJSO) 2008;34:55–60. doi: 10.1016/j.ejso.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 27.Walsh SR, Cook EJ, Goulder F, Justin TA, Keeling NJ. Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. Journal of surgical oncology. 2005;91:181–184. doi: 10.1002/jso.20329. [DOI] [PubMed] [Google Scholar]
- 28.Katz SC. Hepatic Immunotherapy for Liver Metastases phase I trial. Society of Surgical Oncology 67th Cancer Symposium. 2014;40:52. [Google Scholar]
- 29.Azab B, Bhatt VR, Phookan J, et al. Usefulness of the neutrophil-to-lymphocyte ratio in predicting short-and long-term mortality in breast cancer patients. Annals of surgical oncology. 2012;19:217–224. doi: 10.1245/s10434-011-1814-0. [DOI] [PubMed] [Google Scholar]
- 30.Ding PR, An X, Zhang RX, et al. Elevated preoperative neutrophil to lymphocyte ratio predicts risk of recurrence following curative resection for stage IIA colon cancer. Int J Colorectal Dis. 2010;25:1427–1433. doi: 10.1007/s00384-010-1052-0. [DOI] [PubMed] [Google Scholar]
- 31.Lotze MT, Matory YL, Ettinghausen SE, et al. In vivo administration of purified human interleukin 2. II. Half life, immunologic effects, and expansion of peripheral lymphoid cells in vivo with recombinant IL 2. J Immunol. 1985;135:2865–2875. [PubMed] [Google Scholar]
- 32.Neal CP, Mann CD, Sutton CD, et al. Evaluation of the prognostic value of systemic inflammation and socioeconomic deprivation in patients with resectable colorectal liver metastases. Eur J Cancer. 2009;45:56–64. doi: 10.1016/j.ejca.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 33.Hammarstrom S. The carcinoembryonic antigen (CEA) family: structures, suggested functions and expression in normal and malignant tissues. Semin Cancer Biol. 1999;9:67–81. doi: 10.1006/scbi.1998.0119. [DOI] [PubMed] [Google Scholar]


