Abstract
The effect of hepatitis B virus (HBV) co-infection in patients with hepatitis C virus (HCV) remains unclear. We used the National Veterans Affairs HCV Clinical Case Registry to identify patients with confirmed HCV viremia during 1997–2005. We defined HBV co-infection as a positive test for hepatitis B surface antigen, HBV DNA, or hepatitis B e antigen. We defined cirrhosis and HCC based on the validated ICD9 codes and determined mortality through the end of 2009. We performed Cox proportional hazard regression analyses to examine the effect of HBV co-infection stratified by HBV DNA status (positive or negative) on the risk of cirrhosis, HCC, and death adjusting for patients’ age, gender, race, HIV infection, alcohol or drug use, Deyo Score, and antiviral treatment. Among 99,548 patients with HCV infection, 1370 patients (1.4%) had HBV co-infection. Of the co-infected patients, 677 (49.4%) patients had at-least 1 HBV DNA test done and 303 patients (44.7%) tested positive for HBV DNA. The incidence rates of cirrhosis, HCC, and death were significantly higher in patients with HBV co-infection and detectable HBV DNA compared to HCV mono-infection (36.8, 6.9, and 41.7 versus 17.4, 3.6, and 31.4 per 1,000 person-years, respectively; p<0.05 for all comparisons). After adjustment for demographic, clinical, and treatment factors, patients with detectable HBV DNA had a significantly higher risk for cirrhosis, (hazard ratio [HR] =1.89 95% CI=1.46–2.45), HCC (HR=2.12, 95%CI=1.26–3.60), and death (HR=1.62, 95%CI=1.33–1.99), respectively, compared to HCV mono-infected patients. There were no differences in the risk of cirrhosis, HCC, or overall mortality between co-infected patients with undetectable HBV DNA and those with HCV mono-infection (HRs=1.18, 95% CI=0.90–1.55; 1.54, 95% CI=0.93–2.56; 1.08, 95% CI=0.88–1.33, respectively). In conclusion, we found that while only a small number of HCV patients were co-infected with HBV, patients with documented HBV viremia were at a significantly higher risk for cirrhosis, HCC, and overall death than HCV mono-infected patients. Absence of HBV replication was associated with a clinical course similar to that of HCV mono-infected patients.
Keywords: HBV, HCV, Viral hepatitis, Cirrhosis, HCC
Introduction
Chronic infection with hepatitis B virus (HBV) and hepatitis C virus (HCV) are the most common causes of chronic liver disease worldwide. In the U.S., approximately 2.7–3.9 million people have HCV and 800,000–1.4 million people have chronic HBV infection (1, 2). Data suggest that ~ 2–10% of patients with chronic HCV may indeed be co-infected with HBV (2, 3, 4).
Many studies have examined the clinical outcomes of chronic HBV and HCV separately. However, less is known about the clinical course and outcomes of patients with HCV-HBV co-infection (5, 6). Most of the available data on clinical outcomes in HCV-HBV co-infected patients come from Southeast Asia and southern Europe (7). HBV is acquired in the perinatal period or early childhood in these regions, whereas in the U.S. it is often acquired much later in life (8). There are also racial differences that further limit the generalizability of existing, albeit scant, data to the U.S. patients with HCV-HBV co-infection. Moreover, most studies have evaluated the clinical outcomes of HCV co-infection in cohorts of patients with HBV (9) and not vice-versa. There are also several reports on the effects of HBV-HCV co-infection in HIV+ populations, where increased morbidity is observed (10, 11, 12). In summary, the effect of HBV co-infection on clinical outcomes in U.S. patients with HCV remains unclear.
Data show that HCV and HBV can modulate each other when co-infecting the same host. Although viral dominance patterns are unpredictable, it is believed that HCV is dominant in the majority of HCV-HBV co-infected patients as evidenced by low to absent HBV DNA levels in these patients (13). However, most of the previous studies did not have the ability to examine the impact of HBV viral dominance (i.e., detectable HBV DNA) on the clinical course of HCV-HBV co-infected patients. Information about the clinical course of HCV-infected patients with HBV co-infection is important for providing evidence based prognosis estimates that are needed for counseling patients regarding intensity of follow-up as well as urgency of receiving antiviral treatment.
In a national cohort of Veterans with HCV, we examined whether clinical outcomes such as cirrhosis, HCC and death differ between patients with HCV-HBV co-infection versus those with HCV mono-infection. Specifically, we examined the effect of active HBV replication as evidenced by positive HBV DNA on clinical outcomes in the co-infected patients—data that may help stratify risks and treatment recommendations for this unique patient population.
Methods
Data Source
We used data from the VA HCV Clinical Case Registry (CCR), which contains health information for all known HCV-infected patients from 128 VA facilities nationwide. The CCR automatically identifies patients with positive HCV antibody tests as well as HCV-related ICD-9 codes. Data elements in the CCR include demographics; laboratory test results; outpatient and inpatient VA pharmacy data; and inpatient and outpatient diagnoses codes. Additional details of the CCR data are published elsewhere (14). We examined data sets obtained from the VA HCV CCR database for patients diagnosed with HCV in the VA between October 1, 1997 and September 31, 2005 with follow up through December 31, 2009.
Study Population
Patients had to have at least one positive HCV RNA test or HCV genotype test result between FY 1997 and 2005, at least one visit at a VA facility, and a minimum of one year of follow up after the HCV index date to be included in the study. The HCV index date was defined as the first occurrence of a positive HCV test or HCV-related ICD-9 code.
Study Exposure
HBV co-infection was defined as a positive test for hepatitis B surface antigen (HBsAg), HBV DNA, or hepatitis B e antigen (HBeAg) within 1 year before or after the HCV index date. HBV DNA status was determined by the presence of a positive or negative HBV DNA test anytime during the study period. Patients without a HBV DNA test were excluded from the primary analyses. The date of HCV diagnosis was used as the index date for both the mono-infected and co-infected groups regardless of the HBV diagnosis date.
Study Outcomes
Cirrhosis was defined by ICD-9 codes 571.2, 571.5 or 571.6. HCC was defined by ICD-9 code 155.0 and the absence of code 155.1. Both of these definitions have been validated against detailed chart reviews and shown to have a high positive predicative value in previous studies (15, 16). Prevalent or coexisting cases of cirrhosis or HCC were defined as those recorded any time before or within one year after the HCV index date. Incident or newly diagnosed cirrhosis or HCC were defined as any cirrhosis or HCC present after one year following the HCV index date. Patients were followed up until their last VA visit, date of death, or December 31, 2009, whichever occurred first.
Potential confounders included demographic (age at the time of HCV index date, gender, and race/ethnicity) and clinical factors (HIV, diabetes, alcohol abuse, drug abuse, antiviral treatment, and Deyo comorbidity index)(26). HIV and alcohol abuse were defined by positive laboratory testing or ICD-9 code recorded any time prior to 1 year after the HCV index date. Drug abuse was defined by ICD-9 code that were recorded any time prior to or 1 year after the HCV index date. Antiviral treatment was defined as any prescription for HBV or HCV medication, including interferon and nucleos(t)ide analogues. We also estimated the total number of outpatient visits per year to account for utilization of healthcare.
Statistical Analysis
We calculated incidence rates per 1000 person-years for newly diagnosed cirrhosis and HCC as well as overall mortality rate in HBV co-infected (stratified by HBV DNA status) and HCV mono-infected groups. We generated Kaplan-Meier curves to illustrate and compare the cumulative incidence of cirrhosis, HCC, and mortality in the co-infected (stratified by HBV DNA status) versus mono-infected patients beginning at HCV index date till the end of follow up period. We used the log rank test to evaluate the differences among these rates.
We performed three separate multivariable Cox proportional hazards regression models. In the first model, we examined the risk of cirrhosis in each of the HBV co-infected patients with detectable HBV DNA and HBV co-infected patients without detectable HBV DNA versus the risk in HCV mono-infected patients. For this model, we excluded patients with prevalent cirrhosis and HCC from all comparison groups and adjusted for age, gender, race, alcohol abuse, drug abuse, antiviral treatment, and Deyo comorbidity index. In the second model, we compared the risk of HCC in the three study groups. We excluded prevalent cases of HCC from each group for this model. In the third model, we compared the risk of death in the three study groups. This model included all patients in our study cohort. Potential confounders were as detailed above.
All statistical analyses were performed using SAS 9.1 (SAS Institute, Cary, NC).
Results
Patient Characteristics
There were 98,178 patients with HCV mono-infection and 1,370 with HCV-HBV co-infection. Of the co-infected patients, 677 (49.4%) patients had ≥ 1 HBV DNA test done and 303 patients (44.7%) tested positive for HBV DNA. Table 1 displays the baseline characteristics of our study sample for the HCV mono-infected patients and HBV-co-infection patients stratified by serum DNA status. Co-infected patients with undetectable DNA were younger and more likely to be male compared to HCV mono-infected patients. Co-infected patients with detectable DNA were more likely to be Caucasian, have a higher comorbidity score, and more likely to have prevalent cirrhosis compared with mono-infected patients. Patients with HBV co-infection (regardless of DNA status) were also more likely to be HIV positive and to have received antiviral treatment for either HCV or HBV.
Table 1.
Characteristics of HCV mono-infected patients compared with patients with HBV co-infection by serum DNA status
| HBV co-infection | |||||
|---|---|---|---|---|---|
| Characteristics (%) | HCV Mono-infection (n=98,178) | HBV DNA + (n=303) | P-value | HBV DNA − (n=374) | P-value |
| Age (years) | 0.16 | 0.004 | |||
|
| |||||
| ≤ 40–49 | 51.8 | 54.8 | 59.4 | ||
| 50–59 | 39.6 | 39.6 | 35.4 | ||
| ≥ 60 | 8.6 | 5.6 | 5.1 | ||
|
| |||||
| Gender | 0.62 | 0.02 | |||
|
| |||||
| Female | 2.8 | 3.3 | 0.8 | ||
| Male | 97.2 | 96.7 | 99.2 | ||
|
| |||||
| Race/Ethnicity | 0.02 | 0.13 | |||
|
| |||||
| Caucasian | 54.3 | 60.1 | 51.6 | ||
| African-American | 31.9 | 31.3 | 36.9 | ||
| Other | 5.5 | 2.0 | 5.4 | ||
| Missing | 8.3 | 6.6 | 6.1 | ||
|
| |||||
| Alcohol abuse | 46.7 | 47.9 | 0.68 | 49.7 | 0.24 |
|
| |||||
| Drug abuse | 41.6 | 45.9 | 0.13 | 45.5 | 0.13 |
|
| |||||
| Deyo Score | 0.001 | 0.17 | |||
|
| |||||
| 0 | 73.9 | 71.0 | 77.5 | ||
| 1 | 18.5 | 15.8 | 14.7 | ||
| 2+ | 7.6 | 13.2 | 7.8 | ||
|
| |||||
| Prevalent cirrhosis | 5.6 | 15.2 | <0.0001 | 6.7 | 0.38 |
|
| |||||
| HIV | 6.1 | 18.8 | <0.0001 | 9.4 | 0.008 |
|
| |||||
| Antiviral Treatment | 18.1 | 49.2 | <0.0001 | 27.0 | <0.0001 |
The p-values are derived from two-group comparisons between patients with HCV mono-infection and HBV co-infected patients with and without detectable HBV DNA, respectively.
The characteristics of the co-infected patients who did not undergo a HBV DNA test compared with the other groups are displayed in Appendix Table. In general, patients without a HBV DNA test were more likely to abuse alcohol and drugs, and less likely to have baseline cirrhosis and receive antiviral treatment compared to co-infected patients who underwent HBV DNA testing.
Clinical Outcomes
As detailed in Table 2, during 652,344 person-years of follow up (median 7.4 years), cirrhosis developed at an incidence rate of 36.8/1,000 patient-years (95% CI=28.46–47.62) and 21.4/1,000 patient-years (95%CI=16.42–27.86) for co-infected patients with and without detectable DNA compared to the rate of 17.4/1,000 patient-years (95% CI= 17.11 – 17.75) in the HCV mono-infected patients. The rate of HCC was also higher in co-infected patients with and without detectable HBV DNA (6.9 and 5.2/1,000 patient-years, respectively) compared with HCV mono-infected patients (3.6/1,000 patient-years) in patients with HCV mono-infection. Similarly, all-cause mortality was higher in co-infected patients (41.7 and 30.2/1,000 patient-years for patients with and without detectable HBV DNA) deaths in the mono-infected group (31.47 per 1,000 patient-years).
Table 2.
Number of cases, person-years, and incidence rates per 1,000 person-years in HCV patients with and without HBV co-infection by serum HBV DNA status
| Clinical Outcome | Infection Status | Cases | Person-Years | Incidence Rate (95% CI) | Unadjusted hazard ratio (95% CI) |
|---|---|---|---|---|---|
| Cirrhosis | HCV mono-infected | 11,369 | 652,344 | 17.43 (17.11 – 17.75) | 1.00 |
|
| |||||
| HBV co-infected, DNA + | 58 | 1,575 | 36.82 (28.46–47.62) | 2.15 (1.66–2.79) | |
| HBV co-infected, DNA − | 55 | 2,571 | 21.39 (16.42–27.86) | 1.22 (0.94–1.59) | |
|
| |||||
| HCC | HCV mono-infected | 2,613 | 717,052 | 3.64 (3.51 – 3.79) | 1.00 |
|
| |||||
| HBV co-infected, DNA + | 14 | 2,041 | 6.86 (4.06–11.58) | 1.97 (1.16–3.33) | |
| HBV co-infected, DNA − | 15 | 2,895 | 5.18 (3.12–8.59) | 1.38 (0.83–2.29) | |
|
| |||||
| Death | HCV mono-infected | 25,334 | 805,036 | 31.47 (31.08 – 31.86) | 1.00 |
|
| |||||
| HBV co-infected, DNA + | 95 | 2,277 | 41.72 (34.12–51.02) | 1.37 (1.12–1.67) | |
| HBV co-infected, DNA − | 97 | 3,214 | 30.18 (24.73–36.82) | 0.95 (0.77–1.15) | |
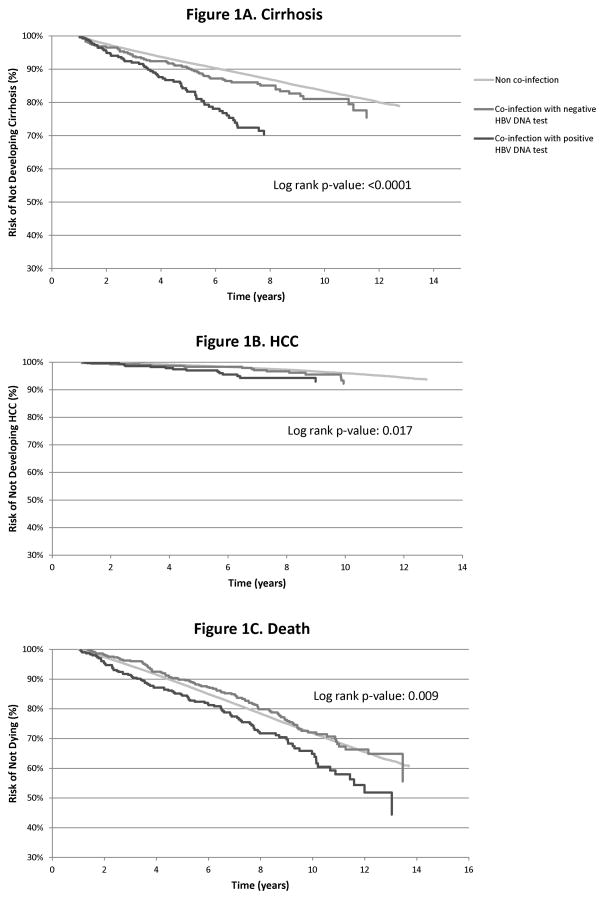
Figure 1 displays the relationship of co-infection further stratified by HBV DNA status for the cumulative incidence of cirrhosis, HCC, and overall mortality, respectively. HBV co-infection, specifically presence of detectable HBV DNA, was strongly associated with time until diagnosis of all three outcomes in patients with HCV (log rank test p value <0.02).
Figure 1.
Cumulative incidence of cirrhosis (1A), hepatocellular Cancer (1B), and death (1C) in HCV mono-infected patients compared to HCV-HBV co-infected patients with detectable (positive) and undetectable (negative) HBV DNA. We use the log rank test to test the differences among these rates. HCC-hepatocellular cancer
We examined the independent association between HBV co-infection and risk of incident cirrhosis, HCC, and death after adjusting for demographic, clinical, and treatment factors (Table 3). In the multivariable Cox proportional hazards models, the risk of cirrhosis in HCV-HBV co-infected patients with detectable HBV DNA (i.e. HBV dominance) was 89% greater, the risk of HCC was 112% greater, and risk of death was 62% greater compared with HCV mono-infected patients. However, while the adjusted HRs were slightly elevated, patients with HBV co-infection with negative DNA status were not an increased risk for cirrhosis, HCC, or overall death compared with mono-infected patients (adjusted HRs=1.18, 95% CI=0.90–1.55; 1.54, 95% CI=0.93–2.56; 1.08, 95% CI=0.88–1.33, respectively). (Table 3).
Table 3.
Cox proportional hazard models of clinical outcomes between patients with HCV-HBV co-infection stratified by serum HBV DNA status (detectable or undetectable) versus patients with HCV mono-infection
| Characteristics | Adjusted Hazard Ratio (95% Confidence interval) | ||
|---|---|---|---|
| Cirrhosis | HCC | Death | |
|
Infection Status
| |||
| HCV mono-infected | 1.00 | 1.00 | 1.00 |
| HBV co-infected, DNA + | 1.89 (1.46–2.45) | 2.12 (1.26–3.60) | 1.62 (1.33–1.99) |
| HBV co-infected, DNA − | 1.18 (0.90–1.55) | 1.54 (0.93–2.56) | 1.08 (0.88–1.33) |
|
| |||
|
Demographics
| |||
| Gender | |||
|
| |||
| Female | 1.00 | 1.00 | 1.00 |
| Male | 1.39 (1.22–1.58) | 5.23 (2.96–9.22) | 1.59 (1.44–1.75) |
|
| |||
|
Age in years
| |||
| < 50 | 1.00 | 1.00 | 1.00 |
| 50–59 | 1.20 (1.15–1.25) | 2.13 (1.96–2.32) | 1.51 (1.46–1.55) |
| ≥ 60 | 1.15 (1.06–1.24) | 3.22 (2.83–3.65) | 2.97 (2.86–3.09) |
|
| |||
|
Race
| |||
| Caucasian | 1.00 | 1.00 | 1.00 |
| Black | 0.62 (0.60–0.65) | 0.83 (0.75–0.91) | 0.68 (0.66–0.70) |
| Other | 1.19 (1.11–1.28) | 1.41 (1.23–1.62) | 0.85 (0.81–0.90) |
| Missing | 0.72 (0.66–0.78) | 0.81 (0.68–0.97) | 1.24 (1.18–1.29) |
|
| |||
|
Clinical Factors
| |||
|
Alcohol abuse
| |||
| No | 1.00 | 1.00 | 1.00 |
| Yes | 1.39 (1.33–1.45) | 1.42 (1.29–1.55) | 1.43 (1.39–1.48) |
|
| |||
|
Drug abuse
| |||
| No | 1.00 | 1.00 | 1.00 |
| Yes | 0.74 (0.70–0.78) | 0.58 (0.52–0.65) | 0.85 (0.82–0.87) |
|
| |||
|
HIV
| |||
| No | 1.00 | 1.00 | 1.00 |
| Yes | 0.96 (0.88–1.04) | 0.95 (0.79–1.14) | 1.20 (1.14–1.26) |
|
| |||
|
Antiviral Treatment
| |||
| No | 1.00 | 1.00 | 1.00 |
| Yes | 1.84 (1.76–1.91) | 1.09 (0.99–1.21) | 0.46 (0.44–0.48) |
|
| |||
|
Deyo score
| |||
| 0 | 1.00 | 1.00 | 1.00 |
| 1 | 1.13 (1.08–1.19) | 1.11 (1.01–1.22) | 1.48 (1.44–1.53) |
| 2 or higher | 1.17 (1.09–1.26) | 0.94 (0.80–1.10) | 2.26 (2.18–2.35) |
|
| |||
| Outpatient visits | 1.00 (1.00–1.00) | 1.00 (1.00–1.01) | 1.00 (1.00–1.00) |
Discussion
The current report is the largest cohort study in the U.S. to examine the long-term clinical outcomes of HCV patients with HBV co-infection. We found that compared with patients with HCV mono-infection, those with HBV-HCV co-infection and positive HBV DNA had a significantly increased risk of cirrhosis (~89% increase), HCC (~112% increase) and death (~62% increase) over a median of 7.4 years of follow up. In contrast, absence of HBV replication was associated with a clinical course similar to that of HCV mono-infected patients. These differences persisted after adjusting for potential confounders including demographic, clinical, and antiviral treatment related factors.
Our study confirms the findings from previous studies, but extends the reach of these results for the first time to the U.S. population of HCV-HBV co-infected patients (17). The majority of these previous studies found a negative (i.e., adverse) effect of HBV-HCV co-infection on clinical outcomes including HCC and cirrhosis compared to mono-infection with either virus alone. In contrast, some reports (again from the non-US populations) report no additive effect of HBV and HCV on morbidity. For example, in a recent meta-analysis, Cho et al. found that HBV-HCV co-infection had only a sub-additive increased risk for HCC as compared to HBV or HCV mono-infection (18). However, use of disparate serological markers for HBV and HCV infection as well as over-reliance on HBsAg status might have underestimated the number of co-infected patients in these studies. Our results show that HCV and HBV may indeed have a synergistic effect on progression of liver disease in this large group of U.S. patients with confirmed HCV (defined on the basis of active HCV viremia) and well-defined HBV status. Furthermore, most of the HCV patients in the VA are diagnosed as a result of a system wide screening program, rather than after development of complications from liver disease; and majority of HCV patients had concurrent testing for HBV (19). Presence of this unique screening mechanism makes our sample a relatively unbiased cohort.
HBV and HCV are known to modulate each other during chronic infection. HBV-HCV co-infection is associated with lower DNA and RNA levels for both viruses respectively, although HBV viremia is depressed to a larger extent (20). HCV decreases the expression of HBV proteins and inhibits HBV replication within livers of co-infected patients (21, 22). On the other hand, high HBV viral loads have been associated with an increased risk for the development of HCC in HBV mono-infected patients (23). In our study, we found that 44.7% of the HBV co-infected patients who underwent testing for HBV DNA had evidence of detectable DNA in the serum. Our results also confirmed our hypothesis that active HBV replication--measured by positive serum DNA levels –resulted in worse clinical outcomes for co-infected patients. Indeed, many previous studies are limited by the use of HBsAg, rather than serum DNA levels, as a marker for co-infection, and thus cannot distinguish between inactive versus active HBV. Active replication of HBV might induce HCC at a higher frequency in HCV patients for a number of possible reasons. While cirrhosis is the most important risk factor for HCC in patients with HCV, HBV in itself increases the risk of HCC. The X protein of HBV has been extensively studied as a potential oncogene precipitating HCC (24, 25), which could contribute to an increased incidence of HCC with HBV infection. This may explain the increased cancer risk and incidence rate observed in the co-infected cohort with detectable serum DNA levels in all patients as well as those with cirrhosis.
We also found that 50.6% of patients with HBV co-infected did not receive a test for HBV DNA despite a median of 7.4 years of follow up in the VA. This group tended to be more likely to have alcohol and drug abuse diagnoses. Furthermore, untested patients were also less likely to be treated with antivirals and less likely to have a diagnosis of cirrhosis. This constellation suggests under-surveillance in a high-risk population that may not have consistent contact with the health care system. On the other hand, collectively, our data underline the critical importance of screening co-infected patients for serum HBV DNA, since the presence of active HBV replication resulted in much worse outcomes compared to HCV mono-infection and co-infection with positive HBsAg. Our results also suggest that focusing on younger patients and those with comorbid alcohol and drug use may improve the rates of HBV viral load testing in patients with co-infection.
Our study is limited by the observational retrospective nature of its design and missing some variables such as family history of HCC (26). However, large prospective studies with sufficient long term follow up to document clinical outcomes in HCV-HBV co-infected patients are not likely to be forthcoming due to cost and feasibility issues. Several unmeasured patient characteristics could have also affected our results. Specifically, although we accounted for HBV or HCV antiviral treatment in our regression models, we did not fully examine the effectiveness of antiviral treatment in suppressing HCV or HBV in this study; future studies will focus on treatment effectiveness in co-infected patients. We also did not have information on adherence to antiviral medication; therefore, we were unable to account for differences in treatment adherence across patients groups. However, our observation underscores the importance of treating HCV-HBV co-infected patients given their poorer prognosis, especially with the advent of oral highly effective interferon free regimens (27). Our results are derived from diagnosed HCV infected patients who sought care in the VA healthcare system, and although the generalizability of the biologic process of cirrhosis progression probably extends from these veterans to other HCV-HBV infected individuals in the VA as well as nonveterans, further research would be needed to confirm that. We are also limited by the ICD-9 coding system’s sensitivities and specificities for our outcomes, which may vary between the VA and non-VA practitioners, thus limiting the generalizability of overall rates of cirrhosis and its complications to HCV-HBV co-infected patients outside of the VA. Finally, mutations within HBV have been shown to alter the course of co-infection. However, the majority of co-infected patients did not have these data available in their electronic medical records (28). In spite of these weaknesses, our study has several strengths including the long period of follow up, use of previously validated definitions of cirrhosis and HCC, and examination of a range of variables that may differentially impact clinically meaningful endpoints in HCV (with or without co-infection). Furthermore, most of our cohort likely acquired HCV (and HBV) as a result of injection drug use or sexual transmission (29, 30) and thus more closely represents the U.S. born patients who primarily acquire HBV later in life compared to immigrants from HBV endemic regions of the world (fewer than 4% of our HBV-HCV infected patients belonged to Asian race) where perinatal transmission remains the most prevalent source of infection.
In conclusion, we found that while only a small number of HCV patients (1.4%) were co-infected with HBV, patients with documented HBV viremia were at a significantly higher risk for cirrhosis, HCC, and overall mortality than HCV mono-infected patients. Patients with HBV and HCV co-infection, therefore, necessitate special attention by physicians and should be triaged for close monitoring and/or antiviral treatment. Our study also highlights the critical importance of testing co-infected patients for serum HBV DNA, as presence HBV viremia dramatically increased the risk of cirrhosis, HCC, and mortality in our cohort. Future studies are needed to confirm the safety and effectiveness of combination regimens including new direct acting antiviral agents for HCV with existing HBV medications.
Supplementary Material
Acknowledgments
Funding: This work is partly funded by NIH grant T32 DK083266-01A1, NIH grant R01-CA-125487, NIH/National Institute of Diabetes and Digestive and Kidney Disease, Center Grant P30 DK56338 and the Center for Innovations in Quality, Effectiveness and Safety (#CIN 13-413).
Abbreviations
- HBV
Hepatitis B virus
- HCV
hepatitis C virus
- IDU
injection drug use
- VA
Veterans Affairs
- CCR
Clinical Case Registry
- ICD-9
International Classification of Disease, 9th Revision
- HBcAb
hepatitis B core antibody
- HBsAg
hepatitis B surface antigen
- HBeAg
hepatitis Be antigen
- HBeAb
hepatitis Be antibody
Footnotes
Disclosures: No conflicts of interest exist for Drs. Tyson, Kramer, Duan, Chen, Kruse, Kanwal, or El-Sarag.
Disclaimer: The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
Reference List
- 1.Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705–714. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 2.Hepatitis B virus infection among American patients with chronic hepatitis C virus infection: prevalence, racial/ethnic differences, and viral interactions. Hepatology. 2010;51(3):759–66. doi: 10.1002/hep.23461. [DOI] [PubMed] [Google Scholar]
- 3.Saravanan S, Velu V, Nandakumar S, et al. Hepatitis B virus and hepatitis C virus dual infection among patients with chronic liver disease. J Microbiol Immunol Infect. 2009;42(2):122–8. [PubMed] [Google Scholar]
- 4.Chu CJ, Lee SD. Hepatitis B virus/hepatitis C virus coinfection: epidemiology, clinical features, viral interactions and treatment. J Gastroenterol Hepatol. 2008;23(4):512–20. doi: 10.1111/j.1440-1746.2008.05384.x. [DOI] [PubMed] [Google Scholar]
- 5.Van der meer AJ, Veldt BJ, Feld JJ, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308(24):2584–93. doi: 10.1001/jama.2012.144878. [DOI] [PubMed] [Google Scholar]
- 6.Zhou JY, Zhang L, Li L, Gu GY, Zhou YH, Chen JH. High hepatitis B virus load is associated with hepatocellular carcinomas development in Chinese chronic hepatitis B patients: a case control study. Virol J. 2012;9:16. doi: 10.1186/1743-422X-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raimondo G, Cacciamo G, Saitta C. Hepatitis B virus and hepatitis C virus co-infection: additive players in chronic liver disease? Ann Hepatol. 2005;4(2):100–6. [PubMed] [Google Scholar]
- 8.Carey WD. The prevalence and natural history of hepatitis B in the 21st century. Cleve Clin J Med. 2009;76 (Suppl 3):S2–5. doi: 10.3949/ccjm.76.s3.01. [DOI] [PubMed] [Google Scholar]
- 9.Oh JK, Shin HR, Lim MK, et al. Multiplicative synergistic risk of hepatocellular carcinoma development among hepatitis B and C co-infected subjects in HBV endemic area: a community-based cohort study. BMC Cancer. 2012;12:452. doi: 10.1186/1471-2407-12-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teira R VACH Study Group. Hepatitis-B virus infection predicts mortality of HIV and hepatitis C virus coinfected patients. AIDS. 2013 Mar 13;27(5):845–8. doi: 10.1097/QAD.0b013e32835ecaf7. [DOI] [PubMed] [Google Scholar]
- 11.Fernández-montero JV, Soriano V. Management of hepatitis C in HIV and/or HBV co-infected patients. Best Pract Res Clin Gastroenterol. 2012;26(4):517–30. doi: 10.1016/j.bpg.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 12.Mocroft A, Neuhaus J, Peters L, et al. Hepatitis B and C co-infection are independent predictors of progressive kidney disease in HIV-positive, antiretroviral-treated adults. PLoS ONE. 2012;7(7):e40245. doi: 10.1371/journal.pone.0040245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raimondo G, Brunetto MR, Pontisso P, et al. Longitudinal evaluation reveals a complex spectrum of virological profiles in hepatitis B virus/hepatitis C virus-coinfected patients. Hepatology. 2006;43(1):100–7. doi: 10.1002/hep.20944. [DOI] [PubMed] [Google Scholar]
- 14.Backus LI, Gavrilov S, Loomis TP, Halloran JP, Phillips BR, Belperio PS, et al. Clinical Case Registries: simultaneous local and national disease registries for population quality management. J Am Med Inform Assoc. 2009;16:775–783. doi: 10.1197/jamia.M3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davila JA, Weston A, Smalley W, El-Serag HB. Utilization of screening for hepatocellular carcinoma in the United States. J Clin Gastroenterol. 2007;41(8):777–782. doi: 10.1097/MCG.0b013e3180381560. [DOI] [PubMed] [Google Scholar]
- 16.Kramer JR, Davila JA, Miller ED, Richardson P, Giordano TP, El-Serag HB. The validity of viral hepatitis and chronic liver disease diagnoses in Veterans Affairs administrative databases. Aliment Pharmacol Ther. 2008;27(3):274–282. doi: 10.1111/j.1365-2036.2007.03572.x. [DOI] [PubMed] [Google Scholar]
- 17.Oh JK, Shin HR, Lim MK, et al. Multiplicative synergistic risk of hepatocellular carcinoma development among hepatitis B and C co-infected subjects in HBV endemic area: a community-based cohort study. BMC Cancer. 2012;12:452. doi: 10.1186/1471-2407-12-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cho LY, Yang JJ, Ko KP, et al. Coinfection of hepatitis B and C viruses and risk of hepatocellular carcinoma: systematic review and meta-analysis. Int J Cancer. 2011;128(1):176–84. doi: 10.1002/ijc.25321. [DOI] [PubMed] [Google Scholar]
- 19.Kanwal F, Hoang T, Kramer J, et al. The performance of process measures in hepatitis C. Am J Gastroenterol. 2012;107(10):1512–21. doi: 10.1038/ajg.2012.201. [DOI] [PubMed] [Google Scholar]
- 20.Jardi R, Rodriguez F, Buti M, et al. Role of hepatitis B, C, and D viruses in dual and triple infection: influence of viral genotypes and hepatitis B precore and basal core promoter mutations on viral replicative interference. Hepatology. 2001;34(2):404–10. doi: 10.1053/jhep.2001.26511. [DOI] [PubMed] [Google Scholar]
- 21.Guido M, Thung SN, Fattovich G, et al. Intrahepatic expression of hepatitis B virus antigens: effect of hepatitis C virus infection. Mod Pathol. 1999;12(6):599–603. [PubMed] [Google Scholar]
- 22.Torbenson M, Thomas DL. Occult hepatitis B. Lancet Infect Dis. 2002;2(8):479–86. doi: 10.1016/s1473-3099(02)00345-6. [DOI] [PubMed] [Google Scholar]
- 23.Chen CJ, Yang HI, Su J, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295(1):65–73. doi: 10.1001/jama.295.1.65. [DOI] [PubMed] [Google Scholar]
- 24.Becker SA, Lee TH, Butel JS, Slagle BL. Hepatitis B virus X protein interferes with cellular DNA repair. J Virol. 1998;72(1):266–72. doi: 10.1128/jvi.72.1.266-272.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hodgson AJ, Keasler VV, Slagle BL. Premature cell cycle entry induced by hepatitis B virus regulatory HBx protein during compensatory liver regeneration. Cancer Res. 2008;68(24):10341–8. doi: 10.1158/0008-5472.CAN-08-2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gordon SC, Lamerato LE, Rupp LB, Li J, Holmberg SD, Moorman AC, Spradling PR, Teshale EH, Vijayadeva V, Boscarino JA, Henkle EM, Oja-Tebbe N, Lu M CHeCS Investigators. Antiviral therapy for chronic hepatitis B virus infection and development of hepatocellular carcinoma in a US population. Clin Gastroenterol Hepatol. 2014;12:885–93. doi: 10.1016/j.cgh.2013.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmidt WN, Nelson DR, Pawlotsky JM, Sherman KE, Thomas DL, Chung RT. Direct-acting antiviral agents and the path to interferon independence. Clin Gastroenterol Hepatol. 2014;12:728–37. doi: 10.1016/j.cgh.2013.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hung CH, Chen CH, Lee CM, et al. Role of viral genotypes and hepatitis B viral mutants in the risk of hepatocellular carcinoma associated with hepatitis B and C dual infection. Intervirology. 2013;56(5):316–24. doi: 10.1159/000350738. [DOI] [PubMed] [Google Scholar]
- 29.Dominitz JA, Boyko EJ, Koepsell TD, et al. Elevated prevalence of hepatitis C infection in users of United States veteransmedical centers. Hepatology. 2005;41(1):88–96. doi: 10.1002/hep.20502. [DOI] [PubMed] [Google Scholar]
- 30.Forde KA, Tanapanpanit O, Reddy KR. Hepatitis B and C in African Americans: current status and continued challenges. Clin Gastroenterol Hepatol. 2014;12(5):738–48. doi: 10.1016/j.cgh.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.