Abstract
Background
This study compared the rate and extent of recovery on measures of learning and memory, processing speed, and working memory in treatment-seeking alcohol dependent individuals (ALC) who were never-smokers (nvsALC), former-smoker (fsALC), and active smokers (asALC), over the first 8 months of sustained abstinence from alcohol. Assessments after 1 week, 1 month, and 8 months of abstinence in ALC enabled a comparison of the rates of neurocognitive changes from 1 to 4 weeks versus 1 to 8 months of abstinence.
Methods
ALC and never-smoking controls were administered standardized measures of auditory-verbal and visuospatial learning and memory, processing speed, and working memory. Controls completed a baseline assessment and a follow-up approximately 9-months later.
Results
Over 8 months of abstinence, asALC showed poorer recovery than nvsALC on visuospatial learning, and both fsALC and asALC recovered less than nvsALC on processing speed measures. The corresponding recovery rates for the ALC group, as a whole, were greater from 1 to 4 weeks than from 1 to 8 months of abstinence; these findings were largely driven by improvements in nvsALC. The recovery levels for fsALC on most measures were similar to those in asALC. Additionally, over 8 months, asALC showed significantly less improvement with increasing age than nvsALC on measures of processing speed and learning and memory than nvsALC. At 8 months of abstinence, asALC were inferior to controls and nvsALC on multiple measures, fsALC performed worse than nvsALC on several tests, but nvsALC were not different from controls on any measure.
Conclusions
Overall, ALC showed rapid improvement on measures of visuospatial learning and processing speed during the first month of abstinence from alcohol. Results also provide robust evidence that smoking status influenced the rate and level of neurocognitive recovery over 8 months of abstinence in this ALC cohort.
Keywords: alcohol dependence, cigarette smoking, cognition, recovery, longitudinal
INTRODUCTION
Numerous studies report that individuals with an alcohol use disorder perform more poorly than healthy controls on multiple neurocognitive domains of function following acute detoxification from alcohol, although the nature and level of impairment show considerable variability across individuals [for review see (Durazzo and Meyerhoff, 2007; Oscar-Berman, 2000; Rourke and Grant, 2009; Stavro et al., 2012)]. Approximately 55% of those with an alcohol use disorder manifest clinically significant neurocognitive deficits after acute detoxification, and variable levels of recovery are apparent with short-term (i.e., ≤1 month), intermediate-term (i.e., 1–12 months) and long-term (i.e., >1 year) abstinence from alcohol (Rourke and Grant, 2009). However, dysfunction in several abilities has been reported to persist after intermediate and long-term abstinence, particularly in the domains of executive skills, learning and memory, visuospatial skills, and postural stability (Durazzo and Meyerhoff, 2007; Rourke and Grant, 2009; Stavro et al., 2012). Multiple factors, including, but not limited to age, sex, treatment history, alcohol consumption level, number of detoxifications, nutritional status, comorbid psychiatric and biomedical conditions, and concurrent substance use disorders may affect both the magnitude of neurocognitive dysfunction exhibited following detoxification, as well as the rate and level of recovery demonstrated with sustained abstinence from alcohol (Durazzo and Meyerhoff, 2007; Oscar-Berman and Marinkovic, 2007; Rourke and Grant, 2009).
Chronic cigarette smoking is the most prevalent comorbidity in those with an alcohol use disorder (Durazzo and Meyerhoff, 2007), and cigarette smoking is associated with significant neurocognitive deficiencies in non-clinical cohorts and individuals with alcohol use disorders (Durazzo et al., 2010; Durazzo et al., 2014). Recently, we observed in treatment-seeking alcohol dependent individuals (ALC) with 1 month of abstinence, that smoking status interacted with age, where actively smoking ALC demonstrated poorer neurocognition with increasing age than never-smoking ALC (Durazzo et al., 2013). Actively smoking ALC also showed less neurocognitive recovery during short-term (1 month) (Pennington et al., 2013) and intermediate-term (8 months) (Durazzo et al., 2007) abstinence than never-smoking ALC. Additionally, the performance of former-smoking ALC was intermediate to that of never-smoking ALC and actively-smoking ALC on multiple neurocognitive measures (Durazzo et al., 2013; Pennington et al., 2013).
Investigations of neurocognitive recovery in abstinent ALC have typically employed two assessment points involving a baseline study after approximately 1 month of sobriety and a follow-up study after 6–12 months of abstinence (Durazzo and Meyerhoff, 2007; Rourke and Grant, 2009; Stavro et al., 2012). In these studies, only linear rates of change in neurocognitive recovery could be assessed. Consequently, it is unclear if the actual trajectory of neurocognitive changes in ALC during the first year of sustained abstinence is strictly linear. Differential rates of neurocognition change over the first year of abstinence may be clinically relevant, given ALC with the lowest processing speed at 1 month of abstinence showed a significantly increased risk for relapse following treatment (Durazzo et al., 2008).
The goal of this longitudinal study was to compare the rate and degree of neurocognitive recovery in never-smoking (nvsALC), former-smoking (fsALC) and actively-smoking ALC (asALC) over approximately 8 months of sustained abstinence from alcohol. Assessment points after approximately 1 week, 1 month, and 8 months of abstinence enabled a comparison of the rates of change on measures of learning and memory, processing speed, and working memory during short-term abstinence (i.e., 1 to 4 weeks) versus changes occurring over an intermediate period of abstinence (i.e., 1 to 8 months). We also examined the effects of comorbid medical, psychiatric and substance use comorbidities on longitudinal neurocognitive changes in ALC. We predicted that:
Over 8 months of abstinence, asALC and fsALC demonstrate a lower rate of recovery than nvsALC on measures of learning and memory, processing speed, and working memory;
Age interacts with smoking status (asALC vs. fsALC, vs. nvsALC), where with increasing age, asALC and fsALC show poorer neurocognitive recovery than nvsALC.
The rate of change in ALC is greater during short-term (1 to 4 weeks) abstinence than during intermediate duration abstinence (1 to 8 months).
After 8 months of abstinence, nvsALC perform equivalent to never-smoking controls (CON), but fsALC and asALC are inferior to nvsALC and CON on all measures.
For asALC, greater lifetime years of smoking is related to poorer recovery over 8 months of abstinence on all measures.
MATERIALS AND METHODS
Participants
ALC were recruited from the VA Medical Center (SFVAMC) Substance Abuse Day Hospital and the Kaiser Permanente Chemical Dependence Recovery Program outpatient clinics in San Francisco, and CON were recruited from the local community (See Durazzo et al., 2008 for details regarding the outpatient treatment received by ALC). Participants provided written informed consent before engaging in study procedures, which conformed to the Declaration of Helsinki, and was approved by the University of California San Francisco and SFVAMC. A total of 133 unique ALC participants were enrolled (30 nvsALC, 28 fsALC, and 75 asALC). Ninety-three (25 nvsALC, 15 fsALC, and 53 asALC) were first studied after 7±4 days of abstinence (assessment point 1=AP1) and 75 of these participants were re-assessed after approximately 1-month of abstinence (AP2). An additional 58 ALC presented for treatment with 2 to 3 weeks of sobriety, because they underwent detoxification at other facilities; these 58 participants completed their first assessment after approximately 1-month of abstinence and were included at AP2 for a total sample of 133 ALC. Of the total sample, 17 ALC relapsed between AP1 and AP2, 55 relapsed between AP2 and AP3, and 7 participants were lost to follow-up (e.g., moved out-of-state or no longer interested in participating). Fifty-four ALC (14 nvsALC, 16 fsALC, 24 asALC) maintained continuous sobriety from alcohol for at least 6-months and were studied again after 232±56 days of abstinence (AP3). Data analyses for all cross-sectional and longitudinal analyses were confined to those ALC who had not relapsed at the specific AP. fsALC had not smoked for 13±10 years before study, smoked for 16±12 years over lifetime, with 16±19 pack-years. Demographic and alcohol consumption variables for ALC subgroups studied at AP1, AP2, or AP3 were not significantly different, and ALC subgroups did not differ in length of abstinence at any AP. Most ALC in this study participated in our previous research (Durazzo et al., 2013; Pennington et al., 2013). Thirty-nine CON completed a baseline study, and 19 CON were re-assessed after 264±45 days. Table 1 provides demographic and clinical information for the 93 ALC participants studied at AP1 (representative of the total sample) and 39 CON studied at baseline.
Table 1.
Demographics and Clinical Measures for ALC Subgroups at Assessment Point 1 (AP1) and CON at Baseline
| Measure | CON n = 39 |
nvsALC n = 25 |
fsALC n = 15 |
asALC n = 53 |
|---|---|---|---|---|
| Days abstinent AP1 AP2 AP3 |
NA |
7 (4) 33 (10) 232 (59) |
7 (4) 34 (8) 231 (40) |
7 (5) 33 (8) 233 (55) |
| Agea | 47 (9) | 50 (10) | 55 (13) | 50 (9) |
| Educationb | 16.0 (2.0) | 14.5 (2.3) | 14.2 (2.4) | 13.7 (1.9) |
| Male (%) | 89 | 84 | 85 | 96 |
| Caucasian (%) | 81 | 75 | 86 | 72 |
| Benzodiazepine usage for ALC (%) | NA | 16 | 13 | 11 |
| AMNARTc | 120 (7) | 114 (10) | 115 (7) | 113 (8) |
| Beck Depression Inventoryd | 3 (2) | 16 (10) | 13 (8) | 15 (10) |
| State-Trait Anxiety Inventory – Traite | 32 (9) | 47 (11) | 47 (11) | 50 (13) |
| 1-year average drinks per monthf | 14 (15) | 371 (209) | 257 (129) | 417 (213) |
| Lifetime average drinks per monthg | 15 (14) | 189 (112) | 141 (99) | 266 (147) |
| History of comorbid substance abuse/dependence (%) | NA | 30 | 15 | 28 |
| History of comorbid psychiatric disorder (%) | NA | 59 | 50 | 54 |
| History of comorbid medical disorder (%) | NA | 41 | 50 | 52 |
| Fagerstrom Test of Nicotine Dependence | NA | NA | NA | 5 (2) |
| Pack-years | NA | NA | 16 (19) | 25 (19) |
| Lifetime years of smoking | NA | NA | 16 (12) | 25 (12) |
Note. Mean (standard deviation).
nvsALC > CON;
CON > nvsALC = fsALC = asALC.
CON > nvsALC = fsALC = asALC.
CON < nvsALC = fsALC = asALC.
CON < nvsALC = fsALC = asALC.
CON < all ALC; fsALC < asALC.
CON < all ALC; fsALC = nvsALC < asALC.
p < .05 for all above group differences. NA = not applicable.
Primary inclusion criteria for ALC were a current Diagnostic and Statistical Manual of Mental Disorders-4th Edition [DSM-IV; APA (1994)] diagnosis of alcohol dependence, fluency in English, consumption of >150 alcohol-containing drinks/month (one drink equivalent=13.6 g pure alcohol) for at least 8 years before enrollment for males, and consumption of >80 drinks per month for at least 6-years before enrollment for females. Primary exclusion criteria for ALC and CON are fully detailed elsewhere (Durazzo et al., 2004). Briefly, all participants were free of psychiatric, neurological, physical, and medical conditions known/suspected to influence brain neurobiology and/or neurocognition, with the exceptions of anxiety disorders (except post traumatic stress disorder), major depressive disorders, hepatitis C, hypertension, and type-2 diabetes in ALC; these conditions are highly prevalent in ALC (Mertens et al., 2003; Mertens et al., 2005; Moss et al., 2010). In ALC, dependence on any substance (other than or nicotine) within the 5 years preceding study enrollment was exclusionary. Participants were screened for recent alcohol and illicit substances at all APs.
Clinical Assessment
At their first assessment, all participants completed the Structured Clinical Interview for DSM-IV-Axis I Disorders, Patient Edition, Version 2.0, and standardized questionnaires assessing depressive (Beck Depression Inventory [BDI]) and anxiety (State-Trait Anxiety Inventory, for Y-2 [STAI]) symptomatology, lifetime alcohol consumption [Lifetime Drinking History (LDH)], lifetime substance use [a questionnaire assessing substance type, and quantity and frequency of use based on the NIDA Addictive Drug Survey] and level of nicotine dependence via the Fagerstrom Tolerance Test for Nicotine Dependence. From the LDH, average number of drinks/month over 1 year prior to enrollment and average number of drinks/month over lifetime were calculated. At all APs, the total number of cigarettes smoked per day and lifetime years of smoking were recorded for sALC. For ALC, at AP3, the Timeline Follow-Back Interview was administered to assess any alcohol consumption and the quantity/frequency of any other substance use, and review of electronic medical records, and/or telephone interview of collateral sources (i.e., family or friends) were used to verify their abstinence/relapse status. See (Durazzo et al., 2011) for corresponding references for the above measures and cohort-maintenance details.
ALC with a current/past history of a non-exclusionary medical condition that may have influenced brain morphology or neurocognition were considered to be positive for the medical comorbidity factor; the most common medical comorbidities were current hypertension and hepatitis C seropositivity. ALC were considered positive for the substance use disorder comorbidity if they met DSM-IV criteria for current or lifetime substance abuse or past dependence (>5 years prior to enrollment); most met criteria for cocaine or methamphetamine abuse/dependence. ALC were considered to be positive for a psychiatric comorbidity if they met current or lifetime criteria for a unipolar mood or anxiety disorder; the majority met criteria for major depressive disorder, recurrent or substance (alcohol)-induced mood disorder, with depressive features.
Neurocognitive Assessment
At all APs, participants completed standardized measures of working memory, processing speed, and auditory-verbal and visuospatial learning and memory. The following measures were administered: Brief Visual Memory Test Revised (BVMT) -Total Recall, trials 1–3 (visuospatial learning measure) and Delayed Recall (visuospatial memory measure); California Verbal Learning Test-II (CVLT) – immediate Total Recall, trials 1–5 (auditory-verbal learning measure), average of Short-and-Long Delay Free Recall (auditory-verbal memory measure); Wechsler Adult Intelligence Scale 3rded. Digit Span (working memory measure), Symbol Search, and Digit Symbol (measure of processing speed). Alternate forms for the CVLT and BVMT were used at follow-up assessments. Premorbid verbal intelligence was estimated with the American National Adult Reading Test (AMNART). See (Pennington et al., 2013) for corresponding references for the above measures. The asALC were allowed to smoke ad libitum before and during neurocognitive testing to reduce the potential confound of nicotine withdrawal (Sacco et al., 2004).
For all groups, two methods were used to convert raw scores for each measure to z-scores: (1) based on the baseline performance of CON (n = 39); (2) based on age-adjusted standardized scores via the normative data accompanying the particular measure. Z-scores based on CON were used in all cross-sectional and longitudinal analyses.
Data Analyses
Cross-sectional analyses
Comparisons among ALC groups and CON on demographic and clinical variables were conducted with multivariate analysis of variance and Fisher’s Exact Test where appropriate. Comparisons of ALC and CON on neurocognitive measures at each AP were conducted with generalized linear modeling with group (nvsALC, fsALC, asALC, and CON), age, education, and AMNART as predictors. Significant main effects for group (p<.05) were followed-up with pairwise t-tests. In pairwise comparisons between nvsALC, fsALC, and asALC, lifetime average number of drinks/month and 1-year-average drinks/month were also separately employed as covariates because of the significantly greater alcohol consumption in asALC (see Table 1). Despite our a priori predictions, a modified Bonferroni procedure (Sankoh et al., 1997) adjusted significance level (p=.05) for pairwise comparisons (1-tailed for a priori predictions) for each neurocognitive measure; the procedure’s adjustment was based on the average intercorrelation among neurocognitive measures for all groups (r=.49) and the number of pairwise comparisons (n=6), and produced an adjusted alpha p<.020 for pairwise comparisons among nvsALC, fsALC, asALC, and CON. Effect sizes for pairwise group comparisons were calculated with Cohen’s d (Cohen, 1988) (see Supplementary Table 1).
Table 3.
CON and ALC Subgroup Performance (z-scores) on Neurocognitive Measures at AP1, AP2, and AP3.
| Measure | AP1 | AP2 | AP3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CON n = 39 |
nvsALC n = 25 |
fsALC n = 15 |
asALC n = 53 |
CON n = 39 |
nvsALC n = 30 |
fsALC n = 28 |
asALC n = 75 |
CON n = 39 |
nvsALC n = 14 | fsALC n = 16 |
asALC n = 24 |
|
| BVMT Total Recall | −0.23 (1.09) | −0.79 (1.03) | −1.21& (1.04) | −1.06# (1.05) | −0.25 (1.04) | −0.15 (1.00) | −0.58 (1.01) | −0.63* (1.01) | −0.08 (1.10) | 0.31 (1.05) | −0.43+ (1.03) | −0.76#* (1.06) |
| BVMT Delayed Recall | −0.17 (1.22) | −0.46 (1.15) | −1.19& (1.17) | −0.85#* (1.18) | −0.19 (1.09) | −0.31 (1.05) | −0.84& (1.06) | −0.69# (1.06) | −0.07 (1.07) | 0.33 (1.04) | −0.58+ (1.02) | −0.69#* (1.08) |
| CVLT Total Recall | −0.21 (1.06) | −0.04 (1.02) | −0.73 (1.04) | −0.86#* (1.05) | −0.20 (1.17) | 0.10 (1.09) | −1.04&+ (1.14) | −1.15#* (1.14) | −0.15 (1.09) | 0.71 (1.12) | −0.52+ (1.03) | −0.51* (1.10) |
| CVLT Delayed Recall | −0.15 (1.14) | 0.24 (1.09) | −0.69 (1.11) | −0.72* (1.13) | −0.20 (1.14) | 0.09 (1.06) | −0.69+ (1.10) | −0.79#* (1.10) | −0.10 (1.02) | 0.54 (1.02) | −0.32+ (1.03) | −0.49* (1.05) |
| Digit Span | −0.29 (0.95) | −0.97^ (0.92) | −0.90& (0.67) | −0.82 (0.95) | −0.28 (0.98) | −0.44 (0.96) | −0.81& (0.96) | −0.55 (0.97) | −0.27 (0.87) | 0.18 (0.86) | −0.22 (0.85) | −0.25 (0.89) |
| Digit Symbol | −0.20 (0.90) | −0.90^ (0.85) | −0.79& (0.86) | −1.11 # (0.88) | −0.18 (0.83) | −0.17 (0.80) | −0.43 (0.80) | −0.81#* (0.82) | −0.08 (0.88) | 0.12 (0.86) | −0.17 (0.82) | −0.74#* (0.92) |
| Symbol Search | −0.13 (0.93) | −0.35 (0.88) | −0.86& (0.63) | −0.84# (0.93) | −0.10 (1.02) | 0.02 (0.97) | −0.39 (0.98) | −0.60* (0.99) | −0.13 (0.99) | 0.48 (0.96) | −0.64+ (0.95) | −0.35* (1.00) |
Note. Mean (standard deviation), z-scores for all groups are based on standardization to CON; BVMT: Brief Visuospatial Memory Test-Revised; CVLT: California Verbal Learning Test-II;
asALC < CON;
fsALC < CON;
nvsALC < CON;
asALC < nvsALC;
fsALC < nvsALC;
all group differences p < .020.
Longitudinal analyses (see Supplementary information for details)
ALC
Change on neurocognitive measures across the AP1-AP2-AP3 interval for ALC were evaluated with linear mixed modeling. Main effects and interactions for all analyses were considered statistically significant at p<.05. Smoking status (nvsALC vs. fsALC vs. asALC), age, education, AMNART, lifetime average drinks/month (or 1-year-average drinks/month), and abstinence duration (months) served as predictors in all models. In all longitudinal analyses described below, when significant effects were observed for smoking status, abstinence duration, and/or their interactions, medical, psychiatric, and substance abuse comorbidity (binary factors–positive vs. negative history) were added separately as secondary predictors.
ALC Analysis 1 tested for linear and non-linear trajectory changes in each neurocognitive measure for ALC over the AP1-AP2-AP3 interval. For each measure, a base model with smoking status, age, education, AMNART, and lifetime average drinks/month (or 1-year average drinks/month), abstinence duration (linear), smoking status × abstinence duration, or smoking status × age was statistically compared to a second model containing the foregoing predictors plus a quadratic term for abstinence duration (i.e., months abstinent2).
ALC Analysis 2 examined rates of change in ALC on neurocognitive measures between AP1-AP2, AP2-AP3, and AP1-AP3. This analysis also tested for differences in rates of change (i.e., slopes) for AP1-AP2 versus AP2-AP3 for each neurocognitive measure.
ALC Analysis 3 tested for associations between smoking severity variables (i.e., lifetime years smoking, pack-years) and change in neurocognitive measures over the AP1-AP2-AP3 interval, separately for asALC and fsALC. The neurocognitive measure (e.g., BVMT Total Recall) was the dependent measure, and age, education, AMNART, abstinence duration (linear), lifetime average drinks/month, and the smoking exposure measure (lifetime years smoking or pack-years) served as predictors. False discovery rate (FDR) (Benjamini and Hochberg, 1995) was used to control for multiplicity of associations.
CON
CON were assessed at baseline and follow-up. Linear mixed modeling was used to assess for change across measures in CON, and predictors included age, education, AMNART, and assessment interval.
RESULTS
Participant demographics and clinical measures
At AP1, there were no differences between nvsALC, fsALC, asALC, and CON on the percentage of males and Caucasians. CON were younger than fsALC, and CON had more years of formal education than all ALC subgroups (all p<.05). asALC had higher lifetime and 1-year-average drinks/month than fsALC, and higher lifetime average than nvsALC (all p<.05). No differences were apparent between nvsALC, fsALC, and asALC on the BDI, STAI, and frequency of psychiatric, medical and substance use comorbidies, or on frequency of benzodiazepine use to manage withdrawal symptoms (see Table 1).
Neurocognitive changes in ALC over 8 Months of Abstinence
Analysis 1 – ALC rates of change in neurocognition over all APs (AP1-AP2-AP3)
Measures showing significant linear and quadratic rates of change (SeeFigure 1, Table 2, and Supplementary Figure 1)
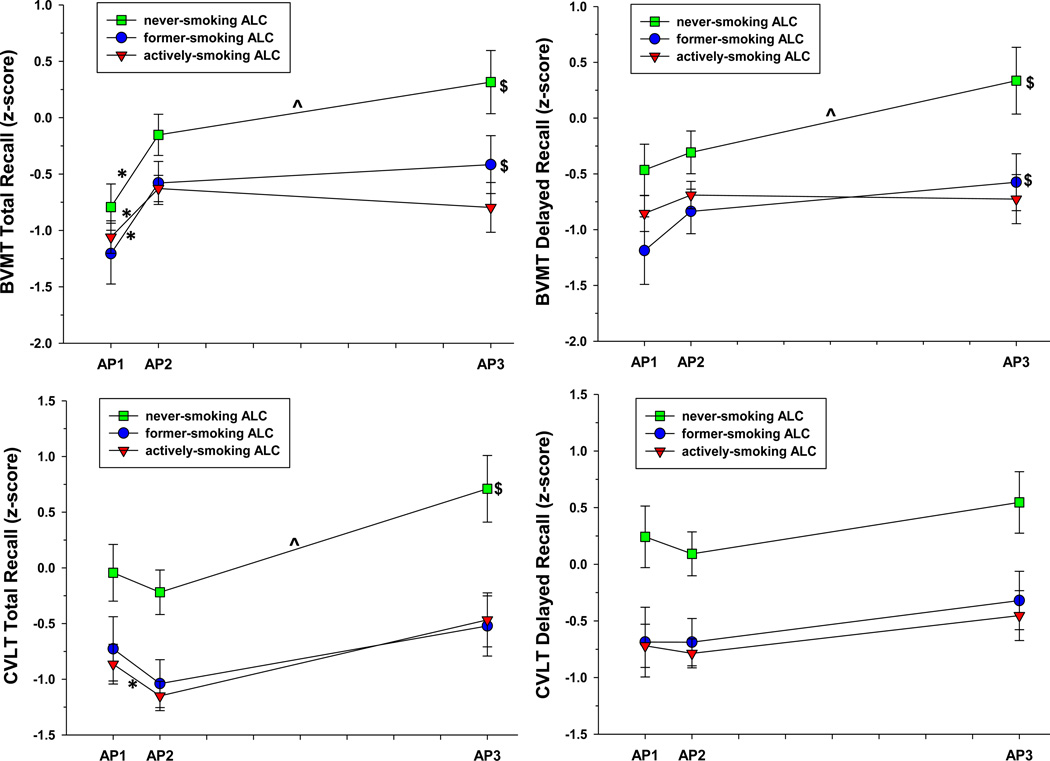
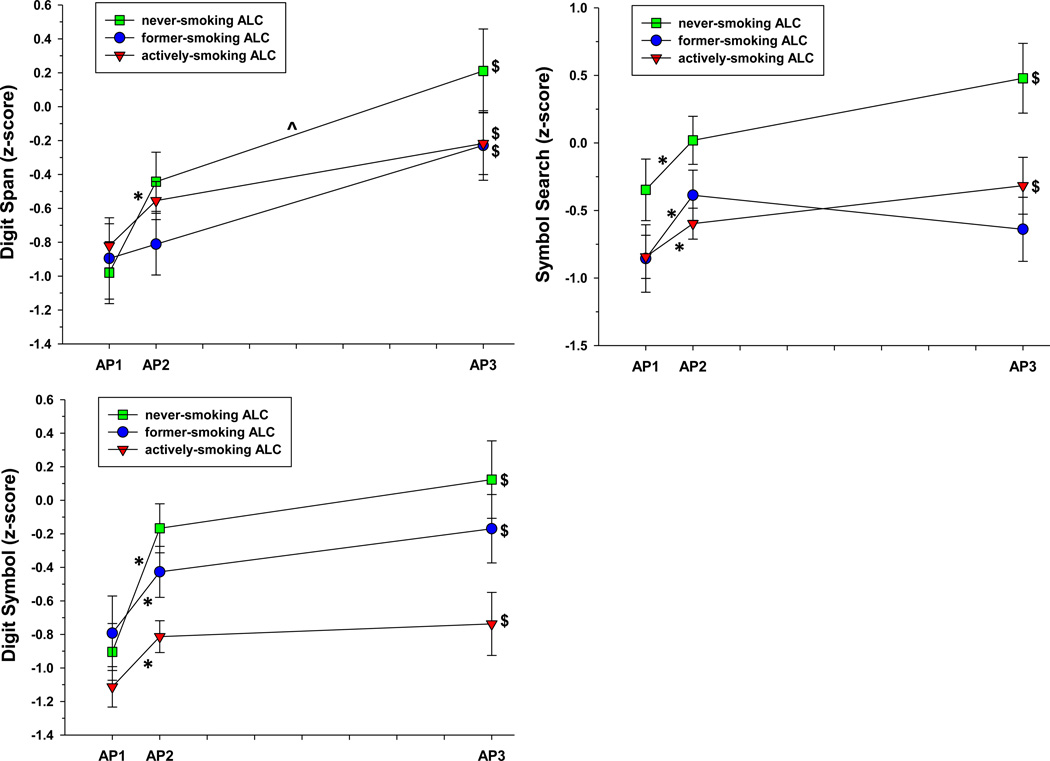
Figure 1.
a. ALC performance on BVMT and CVLT measures across assessment points. BVMT = Brief Visuospatial Memory Test-Revised. CVLT = California Verbal Learning Test-II. *significant change over AP1-AP2 interval. ^significant change over AP2-AP3 interval. $significant change over AP1-AP3 interval.
b. ALC performance on Digit Span, Digit Symbol, and Symbol Search across assessment points. *significant change over AP1-AP2 interval. ^significant change over AP2-AP3 interval. $significant change over AP1-AP3 interval.
Table 2.
ALC Linear and Quadratic Rates of Change in Neurocognitive Measures over 8 Months of Abstinence from Alcohol
| Measure | Linear | Quadratic | ||||
|---|---|---|---|---|---|---|
| β months abstinent |
SE | p-value | β months abstinent |
SE | p-value | |
| BVMT Total Recall | 1.436 | 0.342 | <.001 | −0.00414 | 0.0012 | <.001 |
| BVMT Delayed Recall | 0.302 | 0.141 | .033 | −0.00071 | 0.0005 | .14 |
| CVLT Total Recall | 1.239 | 0.468 | .01 | −0.00267 | 0.0017 | .10 |
| CVLT Delayed Recall | 0.227 | 0.135 | .10 | −0.00001 | 0.1345 | .27 |
| Digit Span | 0.623 | 0.207 | .003 | −0.00154 | 0.0007 | .039 |
| Digit Symbol | 2.562 | 0.715 | .004 | −0.00685 | 0.0026 | .008 |
| Symbol Search | 2.591 | 0.734 | .003 | −0.00895 | 0.0029 | .001 |
Note. β = slope. BVMT: Brief Visuospatial Memory Test-Revised. CVLT: California Verbal Learning Test-II. SE: standard error of the estimate.
Both the linear term and quadratic terms for abstinence duration were significant for ALC, as a group, and indicated improvement over 8 months of abstinence on BVMT Total Recall [linear: t(190) = 4.20, p<.001; quadratic: t(189) = −3.52, p<.001)], Digit Span [linear: t(154) = 3.01, p=.003; quadratic: t(153) = −2.08, p=.039)], Digit Symbol [linear: t(154) = 3.59, p<.001; quadratic: t(153) = −2.68, p=.008)], and Symbol Search [linear: t(144) = 3.85, p<.001; quadratic: t(143) = −3.26, p=.002)]. For BVMT Total Recall, the linear and quadratic effects were significant for all groups (all p<.05). For Digit Span, linear and quadratic effects were significant for nvsALC (all p<.05); for Digit Symbol linear and quadratic effects were significant for nvsALC and asALC (all p<.05); for Symbol Search linear and quadratic effects were significant for all groups (all p<.05).
Measures showing only linear rates of change (see Supplementary Figure 1)
The linear term for abstinence duration was significant for BVMT Delayed Recall [t(190) = 2.15, p=.032] and CVLT Total Recall [t(180) = 3.70, p<.001]. nvsALC and fsALC demonstrated improvement on the BVMT Delayed Recall, and nvsALC improved on CVLT Total Recall (all p<.05).
Measures showing a smoking status × abstinence duration interaction
Significant smoking status × abstinence duration (linear) interactions were observed for BVMT Total Recall [t(178) = −2.21, p=.028)] and Digit Symbol [t(149) = −2.94, p=.004)]. asALC showed less recovery than nvsALC over 8 months on BVMT Total Recall, and fsALC and asALC demonstrated less recovery than nvsALC on Digit Symbol (all p<.05).
Measures showing a smoking status × age interaction
Smoking status × age interactions were observed for BVMT Total Recall [t(187) = −2.05, p=.041)], BVMT Delayed Recall [t(187) = −1.99, p=.048)], CVLT Total Recall [t(174) = −2.02, p=.045)], CVLT Delayed Recall [t(174) = −1.98, p=.049)], and Symbol Search [t(149) = −2.10, p=.037)]. On all of these measures, asALC exhibited poorer recovery with increasing age than nvsALC (all p<.05); asALC demonstrated less recovery with increasing age than fsALC on BVMT Delayed Recall and Symbol Search (p<.05); fsALC showed poorer recovery with increasing age than nvsALC on CVLT Total Recall (p<.05).
In the above analyses, AMNART and age were predictors of change for all neurocognitive measures (all p<.05). Education predicted CVLT Total and Delayed Recall change (p<.05). Medical, psychiatric, and substance abuse comorbidities, as well as lifetime and 1-year average drinks per month were not significant predictors of change for any neurocognitive measure (all p>.17).
Analysis 2 - ALC rates of change in neurocognition between AP1-AP2, AP2-AP3, and AP1-AP3
AP1-AP2 interval: slopes for nvsALC, fsALC, and asALC indicated significant improvement on BVMT Total Recall, Digit Symbol and Symbol Search (all p<.05); asALC demonstrated a significant decrease on CVLT Total Recall (p<.05). AP2-AP3 interval: nvsALC improved on BVMT Total Recall, BVMT Delayed Recall, CVLT Total Recall, and Digit Span (all p<.05). AP1-AP3 interval: slopes for all groups indicated significant improvements on Digit Span and Digit Symbol; both nvsALC and fsALC demonstrated improvements on BVMT Total Recall and BVMT Delayed Recall, and nvsALC and asALC improved on symbol search (all p<.05). There was no significant smoking status × abstinence duration interactions for any measure over the AP1-AP2 or AP2-AP3 intervals (See Figures 1a and 1b).
For ALC, as a whole, the rates of change over the AP1-AP2 interval were significantly greater than over the AP2-AP3 interval for BVMT Total Recall, Digit Span, Digit Symbol, and Symbol Search (See Supplementary Table 1). These results support the findings in Analysis 1 that indicated both a linear and quadratic term for abstinence duration were significant predictors of change in BVMT Total Recall, Digit Span, Digit Symbol, and Symbol Search over 8 months. Analysis 2 findings indicated the significant quadratic term for abstinence duration on the above tests was driven by steep increases over AP1-AP2 and a flatter positive trajectory over the AP2-AP3.
Analysis 3 - Relationships of smoking severity measures and neurocognitive recovery in asALC and fsALC
asALC
Greater lifetime years of smoking was related to poorer recovery over 8 months on CVLT-II Total Recall [β = −.041, standard error (SE) = .011, p=.007], and CVLT-II Delayed Recall (β = −.035, SE=.011, p=.01). The interaction between lifetime years smoking and lifetime average drinks/month was a significant predictor of change over 8 months of abstinence for Digit Symbol (β = −.0001, SE = .00004, p=.046), with a trend for BVMT total recall (β = −.0001, SE = .00005, p=.07), where greater lifetime years of smoking and greater lifetime average drinks/month were synergistically related to poorer recovery on these measures.
fsALC
There were no significant associations between smoking exposure variables, duration of smoking cessation, and changes on any neurocognitive measure for fsALC after FDR correction.
Longitudinal neurocognitive changes in CON
CON showed no significant changes between baseline and follow-up (approximately 8.8 months) on any measure (all p>.15).
Cross-sectional comparisons between ALC and CON on neurocognition at AP1, AP2, and AP3
Since CON showed no significant changes on any measure between baseline and follow-up, the larger baseline sample (n=39) was used in all cross-sectional comparisons to ALC. AP1: fsALC and asALC performed worse than CON on multiple measures, while nvsALC were inferior to CON on Digit Span and Digit Symbol (all p≤.020). AP2: fsALC performed worse than CON on BVMT Delayed Recall and CVLT Total and Delayed Recall, asALC were inferior to CON and nvsALC on multiple tests, but nvsALC and CON did not differ on any measure. AP3: asALC performed worse than CON and nvsALC on multiple tests, and fsALC were inferior to nvsALC on 4 of 7 measures; large effect sizes were apparent for these mean differences. No significant differences were apparent between nvsALC and nsCON at AP3. At all APs, higher AMNART was associated with better performance, while higher age was related to poorer performance across all neurocognitive measures (p < .05) (see Table 3 and Supplementary Table 1 for effect sizes for group comparisons).
Clinical Impairment across APs for ALC
Although there is no universally accepted cutoff for where clinically significant impairment begins, 1.5 standard deviations (STD) below the mean (approximately the 7th%ile) is typically designated as the lower boundary for the mildly impaired range of functioning [see (Heaton et al., 1991; Lezak et al., 2004)]. Table 4 provides z-scores for ALC derived from the normative data accompanying each measure. Using ≥ 1.5 STD below the mean as a cutoff for clinically significant impairment, z-scores based on test norms for the ALC group (i.e., nvsALC+fsALC+asALC) indicated the following: AP1: 41% of ALC were clinically impaired on BVMT Total Recall, 35% on BVMT Delayed Recall, and 15% on Digit Symbol; a low frequency (1–5%) of impairment was apparent on the other measures. AP2: 23% and 22% of ALC were impaired on BVMT Total and Delayed Recall, respectively, and a low frequency (0–5%) of clinical impairment was apparent on the other measures. AP3: 22% and 24% of ALC showed impairment on BVMT Total and Delayed Recall, respectively, 4% (driven by asALC) on Digit Symbol, and 0% on the remaining measures. Overall, nvsALC exhibited the lowest frequency of impairment across measures and APs (see Table 4).
Table 4.
ALC z-scores based on Test Norms for Neurocognitive Measures at AP1, AP2, and AP3.
| Measure | AP1 | AP2 | AP3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| nvsALC n = 25 |
fsALC n = 15 |
asALC n = 53 |
All ALC n = 93 |
nvsALC n = 30 |
fsALC n = 28 |
asALC n = 75 |
All ALC n = 133 |
nvsALC n = 14 |
fsALC n = 16 |
asALC n = 24 |
All ALC n = 54 |
|
| BVMT Total Recall | −0.96 (1.09) [35%] | −1.49 (1.10) [43%] | −1.16 (1.11) [42%] | −1.05 (1.14) [41%] | −0.37 (1.05) [10%] | −0.83 (1.09) [26%] | −0.65 (1.08) [27%] | −0.57 (1.10) [23%] | 0.05 (1.10) [7%] | −0.57 (1.08) [19%] | −0.73 (1.12) [33%] | −0.33 (1.14) [22%] |
| BVMT Delayed Recall | −0.44 (1.26) [32%] | −1.34 (1.27) [43%] | −0.85 (1.28) [39%] | −0.73 (1.32) [35%] | −0.31 (1.12) [23%] | −0.91 (1.17) [27%] | −0.59 (1.15) [21%] | −0.52 (1.16) [22%] | 0.32 (1.19) [5%) | −0.61 (1.17) [25%] | −0.53 (1.08) [33%] | −0.23 (1.20) [24%] |
| CVLT Total Recall | 1.00 (0.83) [0%] | 0.36 (0.92) [0%] | 0.33 (0.86) [2%] | 0.72 (0.99) [2%] | 0.89 (0.87) [0%] | 0.24 (0.94) [0%] | 0.26 (0.93) [3%] | 0.55 (0.98) [2%] | 1.62 (1.01) [0%] | 0.71 (1.03) [0%] | 0.83 (1.06) [0%] | 1.16 (1.12) [0%] |
| CVLT Delayed Recall | 0.81 (0.81) [0%] | 0.21 (0.53) [2%] | 0.15 (1.13) [2%] | 0.48 (0.92) [1%] | 0.66 (0.84) [3%] | −0.01 (0.91) [7%] | 0.08 (0.90) [5%] | 0.32 (0.94) [5%] | 1.06 (0.76) [0%] | 0.56 (0.76) [0%] | 0.48 (0.79) [0%] | 0.73 (0.79) [0%] |
| Digit Span | −0.24 (0.91) [8%] | −0.06 (0.90) [0%] | −0.01 (0.91) [3%] | 0.05 (0.95) [5%] | 0.26 (0.89) [0%] | −0.01 (0.93) [0%] | 0.30 (0.93) [0%] | 0.32 (0.94) [0%] | 0.63 (0.86) [0%] | 0.52 (0.85) [0%] | 0.46 (0.89) [0%] | 0.66 (0.92) [0%] |
| Digit Symbol | −0.48 (0.78) [12%] | −0.39 (0.79) [7%] | −0.61 (0.80) [19%] | −0.36 (0.86) [15%] | 0.15 (0.81) [0%] | −0.12 (0.85) [0%] | −0.29 (0.85) [10%] | 0.06 (0.91) [5%] | 0.61 (0.86) [0%] | 0.50 (0.85) [0%] | 0.21 (0.90) [9%] | 0.43 (0.91) [4%] |
| Symbol Search | 0.47 (0.63) [0%] | −0.12 (0.66) [0%] | −0.02 (0.65) [3%] | 0.16 (0.70) [2%] | 0.55 (0.83 [0%]) | 0.36 (0.86) [3%] | 0.23 (0.86) [3%] | 0.47 (0.88) [2%] | 1.00 (0.96) [0%] | 0.23 (0.80) [0%] | 0.38 (0.85) [0%] | 0.59 (0.88) [0%] |
Note. Mean (standard deviation); [% clinically impaired (≥ 1.5 STD below the mean) on the measure]; BVMT: Brief Visuospatial Memory Test-Revised; CVLT: California Verbal Learning Test-II
DISCUSSION
The main findings are as follows: (1) Over 8 months of abstinence, ALC, as a group, showed significant improvements on BVMT Total and Delayed Recall (measures of visuospatial learning and memory, respectively), Digit Symbol and Symbol Search (measures of processing speed), and Digit Span (measure of working memory); these improvements were largely driven by nvsALC. The recovery rates for ALC were non-linear with time on most of these tests, being greater between 1 and 4 weeks than between 1 and 8 months of abstinence. (2) Over 8 months of abstinence, asALC showed poorer recovery than nvsALC on BVMT Total Recall and Digit Symbol, and fsALC recovered less than nvsALC on Digit Symbol. (3) Over 8 months of abstinence, asALC exhibited significantly less recovery with increasing age than nvsALC on BVMT Total and Delayed Recall, CVLT Total and Delayed Recall (measures of auditory-verbal learning and memory, respectively), as well as on Symbol Search; asALC demonstrated less recovery with increasing age than fsALC on BVMT Delayed Recall and Symbol Search, and fsALC showed poorer recovery with increasing age than nvsALC on CVLT Total Recall. (4) Across ALC groups, medical, psychiatric, and substance abuse comorbidities, as well as lifetime and 1-year-average drinks per month did not predict change on any neurocognitive measure; however, in asALC, greater lifetime years of smoking were related to poorer recovery over 8 months on CVLT Total and Delayed Recall, (5) At 8 months of abstinence (i.e., at AP3), nvsALC were not significantly different from CON on any cognitive measure, whereas asALC remained inferior to CON on several tests, and asALC and fsALC performed significantly worse than nvsALC on multiple tests,
In this ALC cohort, smoking status interacted with both abstinence duration and age to robustly moderate recovery on measures of auditory-verbal and visuospatial learning and memory, and processing speed over 8 months of abstinence. These interactions were significant while controlling for education, estimated premorbid verbal intelligence, drinking severity, and medical, psychiatric and substance misuse comorbidities, and were primarily driven by poorer recovery in asALC compared to nvsALC, particularly with increasing age. Overall, fsALC demonstrated a pattern of change that was more similar to asALC than that of nvsALC. This assertion is supported by the inferior performance of both fsALC and asALC relative to nvsALC on measures of processing speed, learning and memory after 8 months of abstinence (i.e., AP3); however, fsALC was not statistically different from CON on any measure after 8 months. These findings provide robust evidence that smoking status influenced the rate and degree of neurocognitive change over 8 months of abstinence in this ALC cohort. See (Durazzo et al., 2010; Durazzo et al., 2014) for discussion of potential smoking-related biological mechanisms contributing to the differential recovery observed in these ALC subgroups.
The z-scores for measures in cross-sectional and longitudinal analyses were based on the locally recruited CON. To assist with interpretation of the clinical relevance of findings in ALC, the standardized scores based on the corresponding normative data for each measure must also be considered. At AP1, visuospatial learning and memory showed the greatest level of clinical impairment across the ALC cohort (approximately 38%), with a low frequency of impairment on other measures (see Table 4). In fact, at AP1, the performance of nvsALC on measures of auditory-verbal learning (84th%ile) and memory (79th%ile) were in the high-average range of functioning. At AP2, visuospatial learning and memory again manifested the greatest level of clinical impairment in the total ALC cohort (23%), but only 10% of nvsALC showed impaired visuospatial learning. This is consistent with findings from a smaller sample of this cohort (Durazzo et al., 2013). At AP3, only 6% of nvsALC, but 22% of fsALC and 33% of asALC remained clinically impaired on visuospatial learning and memory measures. With the exception of 9% asALC impaired on Digit Symbol, no ALC participant was clinically impaired on other measures at AP3, and the means for nvsALC, fsALC, and asALC were in the average to above-average range on processing speed and auditory-verbal learning and memory measures. Factors contributing to the consistently higher level of clinical impairment on visuospatial learning and memory in ALC at AP2 and AP3 were not likely related to deficient basic or complex visuospatial processing, given most participants in this ALC cohort previously showed a low frequency of impairment on measures of visuospatial integration/synthesis and visuospatial problem-solving at 1-month of abstinence (Durazzo et al., 2013). We recently observed in the non-smoking ALC from this cohort that increasing regional gray and white matter volumes over approximately 8 months of sustained abstinence were associated with improving performance on processing speed and visuospatial learning and memory measures over the same time period; however, these structure-function relationships were not apparent in actively-smoking ALC (Durazzo et al., in press). Further assessment of the correspondence between changes in markers of neuronal integrity, perfusion, and white matter microstructure, and changes in visuospatial learning and memory during sustained abstinence may provide insights into the biological basis for the greater level of deficits in visuospatial learning and memory observed in ALC across APs.
Overall, ALC showed greater improvement rates on measures of visuospatial learning, processing speed, and working memory over the first month of abstinence than over 1–8 months of abstinence. The foregoing combined with the low frequency of clinical impairment on auditory-verbal learning and memory, processing speed and working memory measures in ALC, and the differential level and pattern of recovery demonstrated by nvsALC, fsALC and asALC, may have significant implications for cognitive remediation approaches in treatment-seeking ALC (e.g., Rupp et al., 2012), particularly for interventions employed soon after detoxification.
In this study, smoking status, but not alcohol consumption variables or medical, psychiatric, and substance misuse comorbidities, was associated with neurocognitive change in ALC. Other cross-sectional and longitudinal studies in ALC also reported weak or non-significant associations of alcohol consumption level and medical, psychiatric and substance misuse comorbidities with neurocognition [see (Durazzo et al., 2008, 2013) and references therein]. Additionally, minimally alcohol exposed, pre-adolescent/adolescent offspring of alcohol dependent individuals demonstrated multiple neurobiological abnormalities (e.g., lower regional brain volumes, altered BOLD response in limbic structures during presentation of emotional stimuli) and poorer performance on several neurocognitive domains than offspring of non-alcohol dependent individuals (Hill, 2010; Tessner and Hill, 2010). Taken together, in addition to cigarette smoking, it is highly likely that premorbid factors (i.e., genetic vulnerability or resiliency to the effects of excessive alcohol consumption), or comorbid factors not assessed in this study (e.g., diet/nutrition, exercise, subclinical liver, pulmonary, cardiac, or cerebrovascular dysfunction), influenced the baseline neurocognitive function, as well as the rate and magnitude of neurocognitive changes in ALC. In other words, any neurocognitive and neurobiological abnormalities observed in dependent individuals following detoxification or after a protracted period of abstinence are multifactorial and not simply a function of chronic and excessive alcohol consumption. Finally, the modest number of observations at TP3 increased the risk of model over-fitting and corresponding Type I error, despite all critical model assumptions being met in all analyses.
In conclusion, ALC, as a group, improved significantly on visuospatial learning, processing speed and working memory measures, and recovery rates on these tests were non-linear (i.e., greater between 1 and 4 weeks than between 1 and 8 months of abstinence). Importantly, nvsALC showed a greater level of recovery than both fsALC and asALC on most measures over 8 months. Therefore, failure to consider the common comorbidity of cigarette smoking in this ALC cohort would have resulted in an incomplete assessment and inaccurate conclusions regarding the rate and extent of longitudinal neurocognitive change observed in ALC. Overall, the findings indicate that a history of chronic smoking in ALC contributes to the substantial heterogeneity in neurocognitive recovery during abstinence from alcohol. Additional longitudinal studies, which include ALC participants with a greater variety and severity of the biomedical, psychiatric, and substance misuse comorbidities that commonly accompany alcohol use disorders, are required to understand more fully the mechanisms by which this clinical syndrome affects neurobiology and neurocognition, and their changes during abstinence. The information generated from such studies will have greater generalizability, and will be more relevant to clinical researchers and treatment providers who strive to improve the efficacy of pharmacological and behavioral treatments for those affected by alcohol use disorders.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health (DA24136 to TCD and AA10788 to DJM) administered by the Northern California Institute for Research and Education, and by the use of resources and facilities at the San Francisco Veterans Administration Medical Center.
ABBREVIATIONS
- AP
assessment point
- asALC
actively-smoking alcohol dependent participants
- CON
never-smoking control participants
- fsALC
former-smoking alcohol dependent participants
- nvsALC
never-smoking alcohol dependent participants
Footnotes
The authors have no disclosures and no conflicts of interest to report.
REFERENCES
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Statistical Society. 1995;57:289–300. [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- Diagnostic and statistical manual of mental disorders. 4th ed. Washington, D.C.: American Psychiatric Association; 1994. [Google Scholar]
- Durazzo TC, Gazdzinski S, Banys P, Meyerhoff DJ. Cigarette smoking exacerbates chronic alcohol-induced brain damage: a preliminary metabolite imaging study. Alcohol Clin Exp Res. 2004;28:1849–1860. doi: 10.1097/01.alc.0000148112.92525.ac. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Gazdzinski S, Yeh PH, Meyerhoff DJ. Combined neuroimaging, neurocognitive and psychiatric factors to predict alcohol consumption following treatment for alcohol dependence. Alcohol and Alcoholism. 2008;43:683–691. doi: 10.1093/alcalc/agn078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Mattsson N, Weiner MW. Smoking and increased Alzheimer’s disease risk: A review of potential mechanisms. Alzheimer's and Dementia. 2014;10:S122–S145. doi: 10.1016/j.jalz.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Meyerhoff DJ. Neurobiological and neurocognitive effects of chronic cigarette smoking and alcoholism. Front Biosci. 2007;12:4079–4100. doi: 10.2741/2373. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Meyerhoff DJ, Nixon SJ. Chronic cigarette smoking: implications for neurocognition and brain neurobiology. Int J Environ Res Public Health. 2010;7:3760–3791. doi: 10.3390/ijerph7103760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Mon A, Gazdzinski S, Yeh PH, Meyerhoff DJ. Serial longitudinal MRI data indicates non-linear regional gray matter volume recovery in abstinent alcohol dependent individuals. Addict Biol. doi: 10.1111/adb.12180. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Pennington DL, Schmidt TP, Mon A, Abe C, Meyerhoff DJ. Neurocognition in 1-Month-Abstinent Treatment-Seeking Alcohol-Dependent Individuals: Interactive Effects of Age and Chronic Cigarette Smoking. Alcohol Clin Exp Res. 2013;37:1794–1803. doi: 10.1111/acer.12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Rothlind JC, Gazdzinski S, Banys P, Meyerhoff DJ. Chronic smoking is associated with differential neurocognitive recovery in abstinent alcoholic patients: a preliminary investigation. Alcohol Clin Exp Res. 2007;31:1114–1127. doi: 10.1111/j.1530-0277.2007.00398.x. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Rothlind JC, Gazdzinski S, Meyerhoff DJ. The relationships of sociodemographic factors, medical, psychiatric, and substance-misuse co-morbidities to neurocognition in short-term abstinent alcohol-dependent individuals. Alcohol. 2008;42:439–449. doi: 10.1016/j.alcohol.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Tosun D, Buckley S, Gazdzinski S, Mon A, Fryer SL, Meyerhoff DJ. Cortical Thickness, Surface Area, and Volume of the Brain Reward System in Alcohol Dependence: Relationships to Relapse and Extended Abstinence. Alcohol Clin Exp Res. 2011;35:1187–1200. doi: 10.1111/j.1530-0277.2011.01452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Grant I, Matthews CG. Comprehensive norms for an expanded Halstead-Reitan Battery Demographic Corrections, Research Findings, and clinical applications. Odessa, FL: Psychological Assessment Resources, Inc.; 1991. [Google Scholar]
- Hill S. Neural plasticity, human genetics, and risk for alcohol dependence. Int Rev Neurobiol. 2013;91:53–94. doi: 10.1016/S0074-7742(10)91003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak MD, Howieson DB, Loring DW, Hannay HJ, Fischer JS. Neuropsychological Assessment. 4th ed. New York, NY: Oxford University Press; 2004. [Google Scholar]
- Mertens JR, Lu YW, Parthasarathy S, Moore C, Weisner CM. Medical and psychiatric conditions of alcohol and drug treatment patients in an HMO: comparison with matched controls. Arch Intern Med. 2003;163:2511–2517. doi: 10.1001/archinte.163.20.2511. [DOI] [PubMed] [Google Scholar]
- Mertens JR, Weisner C, Ray GT, Fireman B, Walsh K. Hazardous drinkers and drug users in HMO primary care: prevalence, medical conditions, and costs. Alcohol Clin Exp Res. 2005;29:989–998. doi: 10.1097/01.alc.0000167958.68586.3d. [DOI] [PubMed] [Google Scholar]
- Moss HB, Chen CM, Yi HY. Prospective follow-up of empirically derived Alcohol Dependence subtypes in wave 2 of the National Epidemiologic Survey on Alcohol And Related Conditions (NESARC): recovery status, alcohol use disorders and diagnostic criteria, alcohol consumption behavior, health status, and treatment seeking. Alcohol Clin Exp Res. 2010;34:1073–1083. doi: 10.1111/j.1530-0277.2010.01183.x. [DOI] [PubMed] [Google Scholar]
- Oscar-Berman M. NIAAA Research Monograph No. 34: Neuropsychological vulnerabilites in chronic alcoholism. In: Noronha A, Eckardt M, Warren K, editors. Review of NIAAA's Neuroscience and Behavioral Research Portfolio. Bethesda, MD: NIAAA; 2000. pp. 437–472. [Google Scholar]
- Oscar-Berman M, Marinkovic K. Alcohol: effects on neurobehavioral functions and the brain. Neuropsychol Rev. 2007;17:239–257. doi: 10.1007/s11065-007-9038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington DL, Durazzo TC, Schmidt T, Mon A, Abe C, Meyerhoff DJ. The Effects of Chronic Cigarette Smoking on Cognitive Recovery during Early Abstinence from Alcohol. Alc Clin Exp Research. 2013;37:1220–1227. doi: 10.1111/acer.12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rourke SB, Grant I. The Neurobehavior Correlates of Alcoholism. In: Grant I, Adams K, editors. Neuropsychological Assessment of Neuropsychiatric and Neuromedical Disorders. 3rd ed. New York, NY: Oxford University Press; 2009. pp. 398–454. [Google Scholar]
- Rupp CI, Kemmler G, Kurz M, Hinterhuber H, Fleischhacker WW. Cognitive remediation therapy during treatment for alcohol dependence. J. Stud. Alcohol Drugs. 2012;73:625–634. doi: 10.15288/jsad.2012.73.625. [DOI] [PubMed] [Google Scholar]
- Sacco KA, Bannon KL, George TP. Nicotinic receptor mechanisms and cognition in normal states and neuropsychiatric disorders. J Psychopharmacol. 2004;18:457–474. doi: 10.1177/0269881104047273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankoh AJ, Huque MF, Dubey SD. Some comments on frequently used multiple endpoint adjustment methods in clinical trials. Stat Med. 1997;16:2529–2542. doi: 10.1002/(sici)1097-0258(19971130)16:22<2529::aid-sim692>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Stavro K, Pelletier J, Potvin S. Widespread and sustained cognitive deficits in alcoholism: a meta-analysis. Addict Biol. 2012;18:203–213. doi: 10.1111/j.1369-1600.2011.00418.x. [DOI] [PubMed] [Google Scholar]
- Tessner KD, Hill SY. Neural circuitry associated with risk for alcohol use disorders. Neuropsychol Rev. 2010;20:1–20. doi: 10.1007/s11065-009-9111-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.