Abstract
Objectives
The serologic hallmark of primary Sjögren’s syndrome (pSS) is IgG antibodies specific for Ro (SSA) and La (SSB). Molecular characteristics of glandular-derived B cells at the site of pSS inflammation have been described; however, parallels between glandular antibody-secreting cells (ASC) and serologic antibody specificities have not been evaluated. We utilized recombinant monoclonal antibody (hmAb) technology to study salivary gland-(SG) derived ASC specificities, evaluating their molecular characteristics and identified IgG antibody specificity.
Methods
hmAbs were generated from glandular IgG ASC. Heavy and light chain usage and immunoglobulin subclass were analyzed by sequencing. ELISA, indirect immunofluorescence, enzyme immunoassay and 35S immunoprecipitation analysis were used to determine antibody specificity.
Results
Evaluation of single ASCs from SG biopsies of patients with primary SS or with SS and overlapping SLE revealed significant concordance between serum autoantibody and glandular ASC specificities. Glandular-derived ASC heavy and light chains were extensively somatically hypermutated, indicative of antigen-driven responses. Specifically, we produced the first fully human monoclonal autoantibodies derived from salivary glands in this study.
Conclusions
Salivary glands in SS patients are a site for antibody production, which extend beyond the canonical Ro and/or La SS specificities. Furthermore, we demonstrate that glandular antibody production strongly reflects the serological humoral response in the two patients studied herein.
Sjögren’s syndrome (SS) is a chronic, autoimmune disorder characterized by severe keratoconjunctivitis sicca and xerostomia, which can occur as a primary manifestation (pSS) or secondary to other rheumatic diseases including systemic lupus erythematosus (SS/SLE). The inflammatory and lymphoproliferative components of SS primarily target exocrine glands, though extra-glandular manifestations are not uncommon. Hallmarks of SS include serum antibodies to Ro (or SSA) and La (or SSB) and focal lymphocytic infiltration of lacrimal and SGs. Glandular infiltrates are comprised of antigen-experienced CD4+CD45RO+ T cells, CD27+ B cells and plasma cells (1–4).
The SGs contribute to mucosal autoimmunity by receiving antigen-specific cells from the nasal- and gastric-associated lymphoid tissue (5). Ro- and La-specific lymphocytes with a plasma cell-like morphology have been detected in glandular infiltrates, surrounding acini and along the basement membrane of salivary ducts of SS patients using biotinylated antigens and immunohistochemistry (6–8). Enriched levels of anti-Ro and/or anti-La in tears and saliva (IgG and IgA) correlate with higher titers of these Ab specificities (IgG and IgM) in SS patient serum (9–11). Thus, sites of mucosal immunity in SS patients could reveal critical features about development of the humoral immune response during disease progression.
B cell recruitment and overexpression of survival factors lead to enhanced migration and accumulation of polyclonal memory CD27+ B and CD27high Ab-secreting cells (ASCs) in inflamed salivary glands of SS patients (12). The SG microenvironments in SS, composed of aggregated networks of B and T lymphocytes, follicular dendritic cells and activated endothelial cells promote the survival of autoreactive B cells and plasma cells (6, 12–14). Jonsson et al. found that 28% of 269 pSS patients had germinal center (GC)-like architecture in SG biopsy samples. These structures were associated with higher titers of anti-Ro and anti-La, as well as higher focus scores (15).
Ro- and La-specific ASCs in SGs and peripheral blood of SS patients have been implicated in salivary gland dysfunction (16). Ab studies in SS patients have focused primarily on Ro and La because of their prominence in SS, whereas evaluation of other antigen specificities in glandular tissues has been limited. Besides anti-Ro and anti-La, antibodies to Sm and rheumatoid factor (RF) have been found in serum and saliva of SS patients (9, 17, 18), indicating that Abs secreted in saliva can be diverse. The purpose of our study was to interrogate the glandular ASC humoral immune response of a pSS and an SS/SLE patient by producing hmAbs, characterizing their molecular sequences, assessing clonal relatedness and determining their specificities. With this work, we show concordance between glandular and serum specificities, demonstrate that ASCs other than anti-Ro or anti-La are present in SS SGs and produce Ab in situ, and that the SGs are an active site of humoral memory.
MATERIALS AND METHODS
Human Subject Sample Collection
Samples and data were obtained from subjects following written, informed consent and evaluated in the OMRF Sjögren’s Research Clinic (OSRC). Studies were approved by the OMRF and University of Oklahoma Health Sciences Center Institutional Review Boards. Labial salivary glands were collected for histological analysis and single-cell sorting for hmAb production from two subjects positive for anti-Ro and anti-La (Table 1). Hematoxylin and eosin (H&E) stained SG sections were obtained from the OSRC repository for initial pathological evaluation (Fig 1). Both subjects met American/European combined (2) and American College of Rheumatology (1) criteria for SS. One subject met the American College of Rheumatology criteria for SLE (19), and the other self-reported Raynaud’s phenomenon. Data from the medical history as well as physical, ophthalmologic and dental exams were collected as previously reported (20). Serum antibodies were assessed by the Clinical Immunology Laboratory of the OMRF using double immunodiffusion, as well as indirect immunofluorescence for ANA and Crithidia luciliae anti-dsDNA. Autoantigen testing for 13 specificities was performed using BioRad BioPlex 2200™ ANA screening as previously described (21). Serum (IgG) and stimulated parotid saliva was tested for antibodies to Ro, La, Sm, SmRNP and PL12 by ELISA. PL12 ELISAs were performed on plasma and saliva from the SS/SLE and pSS patients was well as a cohort of pSS plasma samples from the OSRC collection identified with (n=72) and without (n=97) Raynaud’s. Raynaud’s determinations were made based on patient questionnaires completed at the time of sample collection. Patients with medical record documentation of other anti-PL12-associated disease Abs (anti-synthetase syndrome, scleroderma, interstitial lung disease, myositis, polymyositis or dermatomyositis) were excluded from analysis.
Table 1.
Demographics, clinical laboratory and histological features of subjects within the study.
| Case A (SS/SLE) | Case B (pSS) | |
|---|---|---|
| AGE (YR) | 33 | 54 |
| SEX | F | F |
| RACE | African American | Caucasian/Hispanic |
| DIAGNOSIS | SLE, Secondary SS1 | Primary SS, Secondary Raynaud’s Syndrome8 |
| OBJECTIVE FEATURES | ||
| LG2 | Positive | Positive |
| Schirmers3 | Negative | Negative |
| Biopsy Focal Score4 | 5 | 2.8 |
| Biopsy Pathology Interpretation | Focal lymphocytic sialadenitis | Focal lymphocytic sialadenitis |
| Whole Unstimulated Salivary Flow5 | Negative | Positive |
| Serum Antibody Status6 | Ro52, Ro60, La, RNP, Sm, RF | Ro52, Ro60, PL12, RF |
| SLE Criteria7 | Leukopenia, Lymphopenia Anti-Sm, Pos ANA, Malar Rash, Oral ulcers, Pleuritis, Persistant proteinuria |
N/A |
| Medications | Hydroxychloroquine, Methotrexate, Pregabalin, Ergocalciferol | Hydroxychloroquine |
Sjögren’s syndrome (SS);
Positive ≥ 4 (AECG Criteria);
Positive ≤ 5mm/5 min;
Positive ≥ 1 per 4mm2;
Positive ≤ 1.5mL/15 min;
Antibodies evaluated: Centromere B (CenB), Histone, Jo-1, La, Rhematoid Factor (RF), Ribosomal P (Rib P); Ribonuclear Protein 70K, A and C (RNP70K, A, C), Ro52, Ro60, Smith B (SmB);
Tan et al, Arthritis Rheum 1982;25:1271;
Self-Reported.
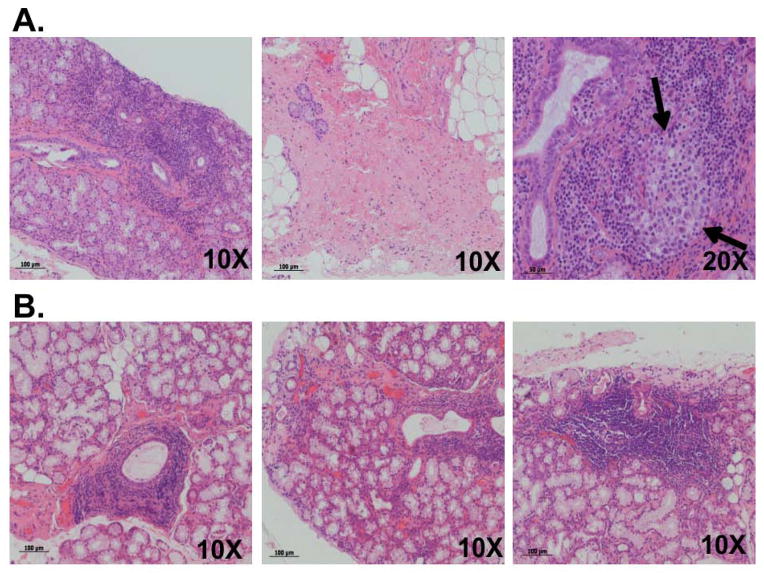
Figure 1. Histological evaluation of hematoxylin- and eosin-stained salivary gland biopsies from an SLE patient with secondary SS and a primary SS patient with Raynaud’s syndrome.
Diffuse periductal and perivascular lymphocytic infiltration (Fig 1A, left), sclerosis and fatty replacement (Fig 1A, center) can be seen in the biopsy of the SS/SLE patient. A structure resembling an ectopic germinal center was also detected within the gland (Fig 1A, right, black arrows). Significant perivascular and periductal lymphocytic infiltration (Figs 1B, left and center) can be observed in the pSS patient, along with periductal foci within the gland (Fig 1B, right).
Purification and Fluorescence-Activated Cell Sorting (FACS) of Mononuclear Cells from Labial SG Biopsies
Labial SG tissue consisting of 4–5 glands were mechanically separated and enzymatically digested in 10 mL of HBSS containing Ca2+ and Mg2+ (21-023-CV, Cellgro), 10 mg collagenase D and 1 mg DNase I (Roche Diagnostics) at 37°C in 5% CO2 for 30 min to release infiltrating cells. A single cell suspension was made by filtering material through a 40 μm cell strainer into RPMI with 10% FCS and washed with Special FACS Wash (SFW; Dulbecco’s PBS with 5% FCS and 0.1% sodium azide), suspended in blocking solution (SFW containing 10% human AB serum) and counted by trypan blue exclusion. Cells were blocked then stained with anti-CD3-PE, anti-CD4-PE-Cy5, anti-CD8-APC, anti-CD19-PE-Cy7, anti-IgG-FITC, anti-CD27-Alexa 750 and anti-CD38-APC-Cy5.5. The antibody-secreting population (CD3−CD4−CD8−CD19+CD27highCD38highIgG+) was bulk sorted (FACSAria, BD Biosciences, Inc), then single cell sorted (MoFlo, Beckman Coulter, Inc.) into 96-well PCR plates containing 10 μL/well of collection buffer (5 mL of RNase-free water, 10 mM Tris, pH 8.0 and 3750 U of RNasin (Promega, Inc.)).
Production of Recombinant Monoclonal Antibodies
hmAbs were produced from single-sorted cells by the OMRF Human Recombinant Monoclonal Antibody Core Facility as described (21, 22).
Analysis of Heavy and Light Chain Gene Usage
Variable (V), diversity (D) and joining (J) domains, along with complementarity determining regions (CDR) of the heavy and light chains from each hmAb were identified using the International Immunogenetics Information System database (IMGT/V-Quest) (21, 22).
Specificity Determination for Recombinant Monoclonal Antibodies, Saliva and Plasma Samples
Various assays were employed to determine the specificities of the hmAbs. Reactivity to an antigen was considered “positive” if the hmAb was reactive to that antigen by at least one assay. A hmAb was considered “polyreactive” if it was capable of binding multiple, structurally unrelated Ags (23).
Nuclear Antigen Screening ELISAs
All hmAbs (1 μg/ml) were initially screened for reactivity to 1 unit of Ro, La, Sm or SmRNP (Immunovision) as previously described (21). hmAbs having positive ELISA screens were then titered for affinity using a 14-point dilution series ELISA and analyzed by non-linear regression analysis using Prism 5 software (GraphPad Software, Inc.). Stimulated saliva samples were screened for IgG and IgA Abs to Ro, La, Sm, the SmRNP complex, and PL12 (Syd Labs) by ELISA. Antigens (0.2 μg/well) were coated onto plates and samples and controls screened at a 1:25 dilution. Binding was detected with anti-human IgG (Jackson ImmunoResearch) and IgA (Sigma) AP-conjugated secondary Abs and PNPP substrate (Sigma), then the absorbance determined at 405 nm. Samples were considered positive if the average absorbance was greater than the mean ± 2SD of stimulated saliva samples from 3 healthy subjects.
Plasma samples were diluted 1:100 and tested in duplicate on recombinant human PL12-coated plates. Binding was detected using anti-human IgG-AP and PNPP substrate then the absorbance determined at 405 nm. Samples were standardized for intra-plate variation by normalization to a positive control included on each plate. Statistical analysis was completed using Mann-Whitney testing.
IgG Rheumatoid Factor ELISA
hmAbs were screened for rheumatoid factor (RF) activity using purified human IgG Fc as antigen (Jackson ImmunoResearch). Binding was detected with F(ab′)2-specific goat anti-human IgG-AP (Jackson ImmunoResearch) and PNPP substrate (Sigma). Duplicate samples were considered positive if the average absorbance (405 nm) was greater than the mean absorbance ± 3SD of 4 pneumovax-specific hmAbs, developed in a separate study, which were negative for all other immunoassays (not polyreactive).
Other Methods of Detecting hmAb Reactivity to Nuclear Antigens
hmAb reactivity to nuclear antigens and dsDNA was evaluated using four methods including indirect immunofluorescence (HEp-2 and Crithidia luciliae; Alpha Diagnostic International, Inc.), Bioplex 2200™ ANA screening (dsDNA, chromatin, Ribosomal P, Ro, La, Centromere B, Sm, SmRNP, RNP, Scl-70, Jo-1; Bio-Rad Laboratories) and INNO-LIA™ ANA Update EIA testing (SmB, SmRNP, RNP-A, RNP-C, Ro52, Ro60, La, Centromere Protein-B, Scl-70, Jo-1, Ribosomal P, histones; Innogenetics). To further identify anti-Ro52 and anti-Ro60-specific antibodies, an in vitro transcription and translation immunoprecipitation assay developed by Dr. Umesh Deshmukh was performed using Ro52 and Ro60 labeled with 35S-methionine and biotinylated-Lys (TNT-Quick coupled transcription/translation system, Promega). hmAb specificities were confirmed using 35S protein and RNA immunoprecipitation followed by Western blotting as previously described (24, 25).
RESULTS
Serologic and histologic features of SS/SLE and pSS subjects selected for hmAb production
Two subjects from the Oklahoma Sjögren’s Research Clinic (OSRC), one with pSS (and Raynaud’s) and the other with SLE and SS, were selected for inclusion in our study. Table 1 outlines the demographic, clinical, laboratory and histologic features of each subject. H&E-stained labial SG biopsy sections of each patient revealed significant focal lymphocytic infiltration (Fig 1). Extensive lymphocytic and periductal infiltration (Fig 1A left; focus score = 5), glandular fibrosis with fatty replacement (Fig 1A center) and a germinal center-like structure (Fig 1A right, arrows) was observed in the SS/SLE patient. The pSS subject had a focus score of 2.8 with periductal lymphocytic infiltration, limited fibrosis and fatty replacement but no GC-like structures in the sections examined (Fig 1B, left, center and right).
Peripheral blood analysis indicated that both patients had slight leukopenia, extensive IgG hypergammaglobulinemia (SS/SLE=2458 mg/dL and pSS=1676 mg/dL), rheumatoid factor IgG antibodies (SS/SLE=420 U/mL and pSS=25 U/mL) and a positive ANA (SS/SLE=1:9720 nuclear speckled; pSS=1:40 nuclear speckled). To observe differences due to disease complications secondary to SS (SLE), we evaluated the serum Ab profiles of both study subjects. Serum IgG antibodies to Ro, La, SmB and RNP-A were detected in the SS/SLE patient by Oüchterlony double immunodiffusion, INNO-LIA™ and/or BioPlex2200™ assays. We found IgG antibodies to Ro and La in the pSS patient by INNO-LIA™ and BioPlex2200™ assays, as well as PL-12-binding IgG antibodies by ELISA.
Molecular analysis of recombinant monoclonal antibodies derived from salivary glands of a primary SS and a SS/SLE patient
The molecular characteristics of isolated IgG ASCs derived from SGs were evaluated from each of our case subjects. The ASC B cell compartment comprised approximately 0.1% of the total purified glandular cells in both case subjects. Twenty-one productive heavy and light chain pairs were amplified from the individually sorted IgG-expressing ASC cells (CD3−CD4−CD8−CD27hiCD38hiIgG+) of the SS/SLE (n=4) and pSS (n=17) patients. We amplified those with kappa light chain sequences only due to the limited number of ASC cells obtained from the SGs of the two patients. By comparing experimentally determined sequences with published immunoglobulin isotype sequences, we identified the primary isotype for 17 of the 21 productive Ab pairs. Of those Abs with identified isotypes, 88% were IgG1 (15/17), and either IgG2 (1/17) or IgG4 (1/17) for the remaining 12%. hmAb and germline heavy and light chain sequences were aligned to determine V-, D- and J- gene usage (Table S1). All 21 hmAbs used either the VH3 or VH4 variable heavy chain genes (43% and 57%, respectively). The most commonly observed D and J heavy chain genes were DH4 and JH4, occurring in 47% and 62% of the hmAbs, whereas, Vκ1 and Jκ4 were the most commonly observed light chain genes (both 52%, respectively). These observations are likely due to the clonality of amplified sequences from the pSS patient (5/17) and normal recombinatory bias (26, 27). Further analyses of these sequences revealed extensive somatic hypermutation in all of the hmAb sequences produced from both the SS/SLE and pSS subjects (Table S1). Neither disease status nor Ab specificity had apparent effects on the total number of synonymous or non-synonymous somatic hypermutations (Table S1).
Analysis of hmAb heavy and light chain usage and CDR3 region sequences revealed that all of the glandular-derived hmAb sequences from the SS/SLE patient were not clonally related, whereas both clonally related (n=5) and non-clonally related (n=12) hmAbs were produced by the pSS patient (Table S1). The amino acid length (ranging from 7–19 amino acids) and the number of charged residues (arginine, lysine, histidine, aspartic acid or glutamic acid) in the CDR3 regions were normally distributed in both subjects (Table S1). CDR3 regions were not conserved, with the exception of the clonally-related Abs.
hmAbs produced from salivary gland ASCs of pSS and SS/SLE patients target multiple autoantigens in addition to Ro and La
To evaluate the hmAb characteristics and specificities produced by individual glandular-derived ASCs, we cloned and produced hmAbs in vitro then evaluated them using ELISA, indirect immunofluorescence, line blot, and BioPlex2200™ ANA (results summarized in Tables 2 and 3). Of the 4 hmAbs derived from the SS/SLE subject, 3 targeted common nuclear autoantigens, including the SmRNP complex, Ro and SmB (Fig 2 & Tables 2 and 3). The A3-E03 antibody demonstrated high-affinity for the SmRNP complex by ELISA (Fig 2A, right panel; Kd=9.0×10−11M). BioPlex2200™ ANA analysis of this hmAb confirmed high-level reactivity to the SmRNP complex (8 AI; positive ≥ 1 AI; range 0–8 AI) and low-level reactivity to chromatin (1.9 IU/mL; positive ≥ 1.0 IU/mL). HEp-2 analysis of A3-E03 showed coarse nuclear speckled ANA pattern consistent with reactivity to RNP (Fig 2C, lower panel), while anti-dsDNA (by Crithidia) was negative. BioPlex2200™ and INNO-LIA™ analyses for A3-E03 were negative for the RNP antigen (data not shown).
Table 2.
Human recombinant monoclonal antibodies from salivary gland plasmablasts of SS patients recognize diverse autoantigens and are often polyreactive
| Case | hmAb Name | Antigen Reactivity |
|---|---|---|
|
SS/SLE ”Case A” |
||
| A2-G03 | Ro2 (ELISA, BPX), Ro52/Ro60 (INNO), Ro60 (35S IP, IV-IP), hYRNAs 3,4,& 5 (RNA IP), HEp2+/Cyto&NS | |
| A2-G05 | SmB (INNO) | |
| A3-E03 | RNP (ELISA, RNA IP), SmRNP (BPX), Sm (35S IP, BPX), Chromatin (BPX), HEp2+/NS | |
| A3-A05 | Unknown | |
|
pSS Case B |
B1-A05 | Ro60 (IV-IP) |
| B1-E01 | SmB (INNO) | |
| B2-D02 | ACL (ELISA) | |
| B2-E041 | PL122 (35S IP), Ro (ELISA), HEp2+/Cyto | |
| B2-G05 | Ro60 (35S IP), SmB (INNO), RF (ELISA), hYRNAs 1-5 (RNA IP) | |
| B3-C061 | Sm/SmRNP/Ro/La (ELISA), Ro52 (IVT-IP), HEp2+/Cyto | |
| B4-A011 | Sm/SmRNP/Ro/La (ELISA), HEp2+/Cyto | |
| B4-B05 | RF (ELISA), HEp2+/Cyto | |
| B4-C03 | ACL/Sm/SmRNP/Ro/La (ELISA), HEp2+/Cyto, dsDNA (Crithidia) | |
| B4-E06 | Ro60 (35S IP, IVT-IP), hYRNAs 1–5 (RNA IP) | |
| B4-G01 | PL12 (35S IP), TRNAs (RNA IP), RF (ELISA), HEp2+/Cyto | |
| B4-G031 | ACL/Sm/SmRNP/Ro/La/Sm (ELISA), HEp2+/Cyto&NS | |
| B4-G04 | RF (ELISA) | |
| B4-B031 | Unknown (HEp2+/Cyto) | |
| B1-A01 | Unknown | |
| B4-A05 | Unknown | |
| B4-F03 | Unknown |
Indicates clonally-related antibodies
Abbreviations: INNO - INNO-LIA, ELISA - Enzyme-linked immunosorbent assay, ACL - anti-cardiolipin, BPX - Bioplex ANA 2200, S35IP - S35-labeled Protein Immunoprecipitation, RNA IP – RNA Co-Immunoprecipitation, IV-IP - Invitro transcribed and translated protein immunoprecipitation, HEp2+ - positive by HEp2 indirect immunofluorescence assay, NS - nuclear speckled, Cyto - cytoplasmic, RF - rheumatoid factor
Table 3.
Summary of Patient antibody specificities in serum, saliva and single-cell sorted salivary gland derived hmAbs
| Assay | Serum Specificities | Saliva Specificities | hmAb Specificities |
|---|---|---|---|
| Patient: SS/SLE, “Case A” | |||
| ANA | ANA Pos/NS | NP | ANA Pos/NS and Cyto |
| Crithidia | Negative | NP | Negative |
| DID | Ro, La, SmRNP | NP | NP |
| ELISA-IgG | RF | Ro60, La, SmRNP, Sm | Ro, RNP, RF |
| ELISA-IgA | NP | SmRNP | NP |
| Bio-Plex2200 | Ro, La | NP | Ro, SmRNP, Sm, Chromatin |
| INNO-LIA | RNP-A, Ro52, Ro60, La | NP | Ro52, Ro60, SmB |
| 35S-IP | NP | NP | Ro60, Sm |
| IVT-IP | NP | NP | Ro60 |
| RNA-IP | NP | NP | hYRNAs 3, 4, 5/RNP |
| Patient: pSS, “Case B” | |||
| ANA | ANA Pos/NS | NP | ANA Pos/NS/Cyto |
| Crithidia | Negative | NP | Positive (B2-C03) |
| DID | Negative | NP | NP |
| ELISA-IgG | PL12, RF | Ro60, RF | ACL, Ro, RF, Sm/SmRNP/Ro/La |
| ELISA-IgA | NP | Ro60,PL12 | NP |
| Bio-Plex2200 | Ro | NP | Negative |
| INNO-LIA | Ro52, Ro60 | NP | SmB |
| 35S-IP | NP | NP | Ro60, PL12 |
| IVT-IP | NP | NP | Ro52, Ro60 |
| RNA-IP | NP | NP | hYRNAs 1-5/tRNAs |
Abbreviations: NP-Not performed, ANA-anti-nuclear antibody, NS-Nuclear Speckled, Cyto-Cytoplasmic, ELISA-Enzyme linked immunosorbent assay, DID-Öuchterlony double-immunodiffusion, IP-Immunoprecipitation, RNA-IP-RNA Co-Immunoprecipitation, IVT-IP-In vitro transcription/translation immunoprecipitation assay, ACL-anti-cardiolipin, RF-rheumatoid factor
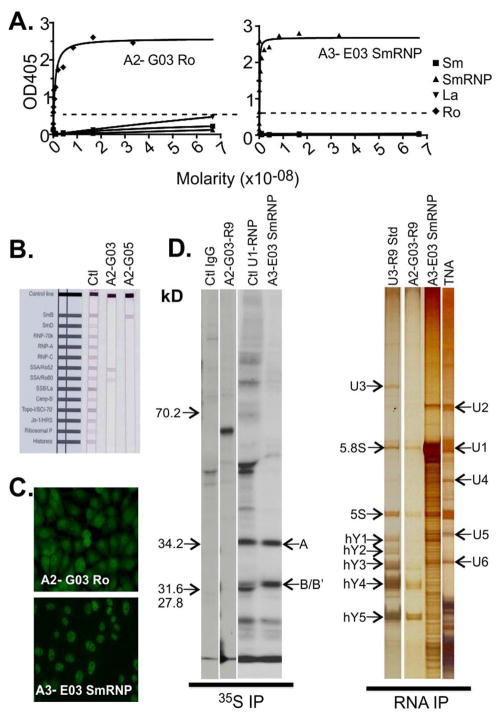
Figure 2. Labial salivary gland-derived human recombinant antibodies produced by an SS/SLE Patient have specificity to Ro, La and SmRNP.
Recombinant human monoclonal antibody screening for affinity to Sm (■), SmRNP (▲), Ro (◆) and La (▼) by ELISA (Positive cutoff ≥ 0.5 OD405). High affinity binding of A2-G03 anti-Ro by ELISA (A, left panel; Kd=7×10−10M) and A3-E03 to SmRNP (A, right panel; Kd=9.0×10−11M). INNO-LIA™ analysis of A2-G03 anti-Ro antibody to both 52 kD and 60kD Ro antigens (B, center strip), and SmB by A2-G05 antibody (B, right strip). Anti-nuclear antibody staining for A2-G03 (C, top panel) and A3-E03 anti-SmRNP antibody (C, bottom panel). 35S protein immunoprecipitation (35S IP) and RNA co-immunoprecipitation (RNA IP) of HeLa cell lysate (D) for A2-G03 to 60 kD Ro (35S IP, 3rd from left) and proteins from the SmRNP complex by A3-E03 (35S IP, 4th from left, arrows A and B/B′). RNA IP analysis showed co-precipitation of hYRNA3, hYRNA4 and hYRNA5 by A2-G03 (RNA IP, 2nd from left) and of multiple U-Small nuclear RNP complexes, particularly U1RNP by A3-E03 (RNA IP, 3rd from left). A U3-Ro standard (RNA IP, left) serum and total nucleic acids (TNA) (RNA IP, right) were included as controls.
A2-G03, derived from the SS/SLE patient, reacted strongly with Ro60 and weakly bound La by ELISA (Fig 2A, left panel; Kd=7×10−10M). A2-G03 also bound Ro60 and Ro52 by INNO-LIA (Fig 2B). HEp-2 ANA testing of A2-G03 showed both a nuclear speckled pattern and diffuse cytoplasmic staining (Fig 2C, upper panel). A2-G05 bound the SmB antigen on INNO-LIA™ (Fig 2B). None of the 4 hmAbs from the SS/SLE patient were reactive to RF by ELISA (data not shown). A3-A05 was negative in all testing panels and its antigen specificity remains unknown (Table 2).
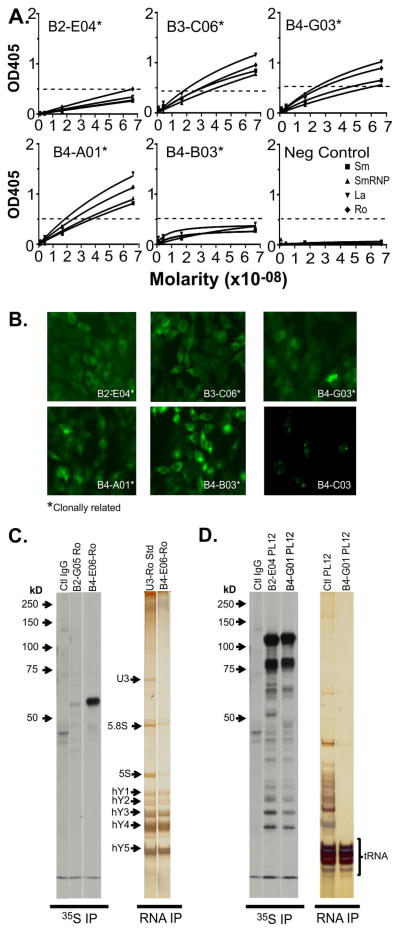
The Ab specificities were successfully identified for 71% (12 of 17) of the hmAbs derived from SG of the pSS patient. A group of 5 clonally-related hmAbs were identified (B2-E04, B3-C06, B4-A01, B4-B03 and B4-G03) that bound multiple antigens by ELISA (Fig 3A, Table 2) Of those crossing the positive threshold, B3-C06, B4-G03 and B4-A01 demonstrated a weak, common polyreactivity to Ro, La, Sm and RNP and B2-E04 bound Ro only. All 5 antibodies had non-specific cytoplasmic/cytoplasmic fiber patterns or low titer (1:40-1:120 endpoint) nuclear speckled patterns (Fig 3B) by immunofluorescent ANA testing. The non-clonally related hmAbs derived from the pSS patient, B2-D02, B2-G05, B4-C03, B4-E06 and B4-G01, had independently arising specificities to some of the same antigens seen in the polyreactive, clonally-related hmAbs (Table 2). hmAbs B4-G01, B2-G05, B4-B05 and B4-G04 were all reactive to IgG-RF ELISA (Table 2) and B4-C03 bound dsDNA by immunofluorescent Crithidia luciliae testing (Fig 3B).
Figure 3. Labial salivary gland-derived human recombinant antibodies produced by a primary SS patient can be polyreactive, clonal and bind multiple antigens.
Recombinant human monoclonal antibody screening for affinity to Sm (■), nuclear RNP (▲), Ro (◆) and La (▼) by ELISA (Positive cutoff ≥ 0.5 OD405). An antibody lacking binding was included as a negative control (A, Neg Control). Anti-nuclear antibody staining by HEp-2 indirect immunofluorescence analysis showing diffuse cytoplasmic/cytoplasmic fiber staining (such as B2-E04 and B3-C06) and, in some cases, cytoplasmic and low titer nuclear speckled staining (such as B4-G03, B4-A01 and B4-B03). Crithidia kinetoplast immunofluorescence testing for B4-C03 (B, lower right panel). 35S protein immunoprecipitation of HeLa cell lysate for B2-G05 and B4-E06 bound a single band consistent with 60 kD Ro (C). RNA IP showed co-precipitation of hYRNAs1-5 by B4-E06 compared to a U3-Ro standard control (D). The antibodies B2-E04 and B4-G01 showed co-precipitation of a band consistent with PL12 around 100kD (D, 2nd and 3rd from left) and verified by RNA IP for B4-G01 shown next to a PL12 control serum (D, right panel).
Protein and RNA immmunoprecipitation identify autoantibodies to Ro and PL12 undetectable by other screening methods
Basic immunoassays such as ELISA are valuable for screening autoreactive hmAbs specificity. However, they cannot identify Abs directed toward unknown antigens and may miss some conformational epitopes. We incorporated radioactive immunoprecpitation (35S IP), in vitro transcribed and translated immunoprecipitation (IVT-IP) and RNA immunoprecipitation (RNA-IP) as additional screening methods to identify alternate hmAb specificities. For the SS/SLE patient-derived hmAbs, A3-E03 bound Sm core proteins 68kDa, A, B/B′, C, D, E and F by 35S IP. This binding was validated by the co-precipitation of the U-series, SnRNP RNAs U1, U2, U5 and U6 by RNA IP (Fig 2D), confirming BioPlex2200™ ANA (SmRNP) and ELISA (SmRNP) results (Fig 2A, second panel). A2-G03 Ro60 binding was confirmed by 35S IP (Fig 2D, left panel) and IVT-IP (data not shown), and was further validated by the co-precipitation of Ro60-binding human YRNAs (hYRNAs) 3, 4 and 5 by RNA-IP (Fig 2D, right panel).
These methods validated and/or identified six additional hmAb specificities in the pSS patient-derived hmAbs. Anti-Ro reactivity of B3-C06 (previously identified by ELISA) was confirmed by immunoprecipitation of Ro52 by IVT-IP. Three of the hmAbs bound Ro60 by 35S IP (B2-G05 and B4-E06, Fig 3C, left panel) or IVT-IP (B4-E06 and B1-A05, not shown). B4-E06 also co-precipitated Ro60-binding hYRNAs1-5 by RNA-IP (Fig 3C, left panel). Two hmAbs, B2-E04 and B4-G01, immunoprecipitated alanyl-tRNA synthetase (PL12) by 35S IP (Fig. 3C, right panel). Furthermore, B4-G01 co-precipitated PL12-binding tRNAs by RNA-IP, matching a control PL12 profile (Fig 3C, right panel). A review of the pSS patient medical records indicated no diagnosis of anti-synthetase syndrome or other PL12-associated diseases; however, this subject self-reported Raynaud’s. Raynaud’s is often seen concomitantly with PL12-associated diseases. IgG Abs to PL12 were detected in the plasma of the pSS patient by ELISA using purified recombinant antigen (Table 3). Five of the 17 hmAbs from the pSS patient were not reactive to any antigen testing panels and the specificities are unknown (Table 2)
Autoantibodies produced in SS patient saliva and serum mirror the SG ASC humoral immune response
Whether the specificities of secreted IgG and IgA autoantibodies of SS patients in tears and saliva are similar to that of glandular ASC is unknown (9, 28). To evaluate this, we screened stimulated saliva samples from the pSS and SS/SLE patients for IgG and IgA Abs to Ro60, La, SmRNP, Sm and PL12 by ELISA (Summarized in Table 3). Showing correlation to the hmAb specificities, the saliva of the SS/SLE patient had strong IgG reactivity to Ro60 and the SmRNP complex and was weakly reactive for IgG antibodies to La and Sm. We found IgA activity only against SmRNP. No IgA or IgG binding was detected to PL12 (Table 3). Some concordance was observed between the salivary and glandular ASC specificities in the pSS patient. Saliva from the pSS patient was strongly reactive for IgA reactivity to Ro60 and PL12, and weakly reactive for IgG antibodies to Ro60, showing no IgG or IgA reactivity to La, SmRNP or Sm (Table 3).
Comparison of antibody specificities in both the SS and SS/SLE patient revealed concordance between the antibody profiles in serum and glandular-derived ASCs, suggesting that the glandular repertoire is representative of and may contribute to the peripheral immune response. Each of the specificities identified in ASC-derived antibodies were also detected in the serum for both patients, with the exception of the polyreactive and anti-cardiolipin antibodies in the SS and chromatin-specific antibodies in the SS/SLE patients, respectively.
PL12 Does Not Associate with Raynaud’s in Patients with Primary Sjögren’s Syndrome
Since we had identified anti-PL12 antibodies in the serum, saliva and SG-derived hmAbs from the pSS patient who had Raynaud’s but no other manifestations that are known to associate with anti-PL12 Abs, we hypothesized that anti-PL12 Abs may also associate with Raynaud’s in pSS. We screened plasma from cohorts of pSS patients with and without Raynaud’s for PL12 binding by ELISA. No significant difference in the frequency of anti-PL12 antibodies was observed between the two groups (p=0.40, data not shown).
DISCUSSION
We have for the first time produced hmAbs from ASCs infiltrating SGs of humans with SS. We sought to produce hmAbs from SG-derived ASCs to better understand the humoral immune response at the site of inflammation in SS patients. Characterization and analysis of these hmAbs will shed light on the unifying feature of systemic rheumatic diseases, namely, the production of autoreactive Abs. The primary goal of this study was to produce hmAbs from salivary gland-derived ASCs to better understand the humoral immune response at the site of inflammation in SS patients.
The salivary gland is a mucosal inductive site that clonally selects and expands antigen-stimulated cells in ectopic GCs, in addition to serving as an effector site that recruits antigen-stimulated cells from other inductive sites (5). Ectopic GCs have been defined by others as aggregates of B cells surrounded by T cells, but usually lacking the classical GC structure described in lymphoid organs (29, 30). A similar structure was observed in the SG section of the SS/SLE patient (Fig 1A, right panel-black arrows). We did not have serial sections of the paraffin-embedded SG tissue from the SS/SLE patient; therefore, we were unable to do multi-color staining of the structure described herein to confirm its ectopic GC-like identity.
The CDR3 regions of the hmAbs were studied to evaluate whether our ASC populations consisted primarily of recruited or expanded cell populations, and whether other diseases influenced these populations. The observations that CDR3 sequences from the SS/SLE patient were not clonally related (n=4), while those from the pSS patient were both clonally related (n=5) and not clonally related (n=12) (Table S1), are consistent with reports that SG immunoglobulin repertoires are likely polyclonal, recruited to the site by chemokines and then clonally expanded at the site of inflammation through chronic antigen exposure (31). Based on the association of ectopic GCs with clonal expansion of antigen-stimulated cells, we expected to find clonally related hmAbs in the SS/SLE patient. Perhaps the lack of this finding is due to low numbers of hmAbs isolated from this patient. Conversely, clonal Abs were detected in the pSS patient lacking GC-like structures. It is possible that the glands pooled for hmAb production did have ectopic GCs (and were the source of the clonally-related hmAbs), but the single SG chosen for histological analysis lacked them.
We recognize our study has limitations, especially regarding immunoglobulin gene family repertoires, due to low subject and hmAb numbers. We can, however, report that VH3 and VH4 heavy chain gene families were commonly and equally used by glandular derived-hmAbs isolated from both patients (Table S1). In contrast to reports of increased gene usage of Jκ2 and low frequencies of Jκ4 (≤2%) in IgG-producing B cells from parotid glands of SS patients, we observed increased usage of IgκV1 (55%) and IgκJ4 (55%) light chain genes (Table S1) (13). Interestingly, SS heavy and light chain gene usage in our hmAbs is consistent with that of SLE patient-derived B cells (14). A complete gene family repertoire analysis would be very informative and may be possible in the future.
To characterize the specificity of our hmAbs, we screened them against panels of known autoantigens. A majority of the gland-derived hmAbs (15/21) bound diverse autoantigens and demonstrated concordance with serum antibody profiles in both patients (Table 3), supporting the hypothesis that SS SGs are a site of antigenically diverse autoantibody production. Detection of IgG and IgA Abs sharing these specificities in the saliva further supports this conclusion (Table 3). Detection of hmAb reactivity varied amongst assays likely due to differences between assay sensitivities, antigen sources (native versus denatured proteins) and epitope presentation (sequential versus conformational).
The so-called atypical SS autoantibodies (other than Ro and La) occur in ~20% of pSS patients and associate with systemic autoimmune manifestations (32). Although rarely detected in pSS patients without overlapping SLE, nRNP and Sm antibodies have rarely been observed in (≤5%) of pSS serum samples (32–34). As expected, the SS/SLE patient sera demonstrated Abs to Ro and La, as well as the SLE-associated autoantigens Sm and nRNP (Table 3). In addition to anti-Ro, the pSS patient had antibodies binding the SLE-associated antigens Sm, nRNP and ACL, as well as alanyl-tRNA-synthetase (PL12) without known concurrent SLE or an anti-PL12-associated disease (Tables 2 and 3). However, the patient did have secondary Raynaud’s, which occurs in about 27% of our pSS patient cohort (unpublished data). Since Raynaud’s also often accompanies the PL12-associating diseases, we hypothesized that PL12 may directly associate with Raynaud’s in pSS patients. ELISA screening of pSS patient plasma samples with and without Raynaud’s for anti-PL12 did not support this hypothesis. SLE diagnosis sometimes follows SS diagnosis by several years (33), and SLE autoAbs have been reported to occur years prior to diagnosis (35). The incidence of autoAbs not commonly associated with pSS, such as anti-PL12 and the SLE-associated autoAbs we observed in the pSS patient without SLE, was unexpected. The significance of these results remains unknown, but we speculate that they may be indicative of future disease development.
In conclusion, we demonstrate the utility of single cell analysis in evaluating IgG specificities for glandular-derived ASCs of pSS patients. We have shown that ASCs specific for atypical SS autoantigens in addition to Ro and La, including those indicative of other rheumatic complications are present in SGs of pSS patients. Finally, we show that the SG is a vital Ab production site and is a site of humoral memory in pSS patients.
Supplementary Material
Supplemental Table 1. Human recombinant monoclonal antibody gene usage, mutational analysis and CDR3 sequence
Acknowledgments
The authors acknowledge the medical personnel and staff of the OMRF SSRC, Angie Duke and Jennifer Muther of the OMRF Human Recombinant Antibody Core Facility, Craig Wasson and Jody Gross of the OMRF Clinical Myositis Laboratory, Christina Lawrence of the Farris Laboratory, Jacob Bass and Diana Hamilton of the OMRF Flow Cytometry Core Facility, Cathy Velte and Camille Anderson of the Clinical Immunology Laboratory, Jourdan Anderson, Tim Gross and Wendy Klein with the James Laboratory, Julie Maier and the OMRF Imaging Core Facility and Sherry Hubbell with the Scofield Laboratory for their expert clinical and technical assistance. We also acknowledge Dr. Don Capra for critical review of the manuscript and Mollie Rangnow for her superb administrative support. The authors declare no competing financial interests. This publication was made possible by grants P50 AR060804 (KLS, CJL, RHS and ADF), 5U19 AI 082714 (KLS, CJL and JAJ), T32 AI 007633-09 (JSM), P30 GM103510 (JAJ) and P30 AR053483 (JAJ, JSM) from the NIH.
Footnotes
Conflict of Interest: The authors have no conflicts of interest to report.
Contributions: JSM, KAK, ADF, JAJ, KS, CJL, USD, KLS and RHS wrote and critically reviewed the manuscript. JSM, KAK, ADF, JAJ, USD, BK and KS designed the experiments. JSM, KAK, ADF, GH, DL, JAJ, NW, USD, LR, BK and KS performed experiments and analysis.
The contents are the sole responsibility of the authors and do not necessarily represent the official views of the NIH.
References
- 1.Shiboski SC, Shiboski CH, Criswell L, Baer A, Challacombe S, et al. Arthritis Care Res (Hoboken) 2012;64:475–87. doi: 10.1002/acr.21591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, et al. Ann Rheum Dis. 2002;61:554–8. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramos-Casals M, Nardi N, Brito-Zeron P, Aguilo S, Gil V, et al. Semin Arthritis Rheum. 2006;35:312–21. doi: 10.1016/j.semarthrit.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Hansen A, Odendahl M, Reiter K, Jacobi AM, Feist E, et al. Arthritis Rheum. 2002;46:2160–71. doi: 10.1002/art.10445. [DOI] [PubMed] [Google Scholar]
- 5.Grewal JS, Pilgrim MJ, Grewal S, Kasman L, Werner P, et al. FASEB J. 2011;25:1680–96. doi: 10.1096/fj.10-174656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tengner P, Halse AK, Haga HJ, Jonsson R, Wahren-Herlenius M. Arthritis Rheum. 1998;41:2238–48. doi: 10.1002/1529-0131(199812)41:12<2238::AID-ART20>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 7.Aqrawi LA, Skarstein K, Bredholt G, Brun JG, Brokstad KA. Scand J Immunol. 2012;75:61–8. doi: 10.1111/j.1365-3083.2011.02625.x. [DOI] [PubMed] [Google Scholar]
- 8.Halse A, Wahren-Herlenius M, Jonsson R. Clin Exp Immunol. 1999;115:208–13. doi: 10.1046/j.1365-2249.1999.00779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ben-Chetrit E, Fischel R, Rubinow A. Clin Rheumatol. 1993;12:471–4. doi: 10.1007/BF02231773. [DOI] [PubMed] [Google Scholar]
- 10.Toker E, Yavuz S, Direskeneli H. Br J Ophthalmol. 2004;88:384–7. doi: 10.1136/bjo.2003.028340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Busamia B, Gonzales-Moles MA, Mazzeo M, Linares J, Demarchi M, et al. Med Oral Patol Oral Cir Bucal. 2010;15:e437–40. doi: 10.4317/medoral.15.e437. [DOI] [PubMed] [Google Scholar]
- 12.Szyszko EA, Brokstad KA, Oijordsbakken G, Jonsson MV, Jonsson R, Skarstein K. Arthritis Res Ther. 2011;13:R2. doi: 10.1186/ar3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dorner T, Radbruch A, Burmester GR. Nat Rev Rheumatol. 2009;5:433–41. doi: 10.1038/nrrheum.2009.141. [DOI] [PubMed] [Google Scholar]
- 14.ter Borg EJ, Risselada AP, Kelder JC. Semin Arthritis Rheum. 2011;40:547–51. doi: 10.1016/j.semarthrit.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 15.Jonsson MV, Skarstein K, Jonsson R, Brun JG. The Journal of rheumatology. 2007;34:2044–9. [PubMed] [Google Scholar]
- 16.Ramos-Casals M, Font J. Rheumatology. 2005;44:1354–67. doi: 10.1093/rheumatology/keh714. [DOI] [PubMed] [Google Scholar]
- 17.Bournia VK, Vlachoyiannopoulos PG. Journal of autoimmunity. 2012;39:15–26. doi: 10.1016/j.jaut.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Fauchais AL, Martel C, Gondran G, Lambert M, Launay D, et al. Autoimmunity reviews. 2010;9:595–9. doi: 10.1016/j.autrev.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, et al. Arthritis Rheum. 1982;25:1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 20.Rasmussen A, Ice JA, Li H, Grundahl K, Kelly JA, et al. Annals of the rheumatic diseases. 2014;73:31–8. doi: 10.1136/annrheumdis-2013-203845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith K, Garman L, Wrammert J, Zheng NY, Capra JD, et al. Nat Protoc. 2009;4:372–84. doi: 10.1038/nprot.2009.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wrammert J, Smith K, Miller J, Langley WA, Kokko K, et al. Nature. 2008;453:667–71. doi: 10.1038/nature06890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dimitrov JD, Planchais C, Roumenina LT, Vassilev TL, Kaveri SV, Lacroix-Desmazes S. Journal of immunology. 2013;191:993–9. doi: 10.4049/jimmunol.1300880. [DOI] [PubMed] [Google Scholar]
- 24.Hirakata M, Suwa A, Nagai S, Kron MA, Trieu EP, et al. J Immunol. 1999;162:2315–20. [PubMed] [Google Scholar]
- 25.Trieu EP, Targoff IN. Methods Mol Biol. 2012;869:215–33. doi: 10.1007/978-1-61779-821-4_18. [DOI] [PubMed] [Google Scholar]
- 26.Brezinschek HP, Brezinschek RI, Lipsky PE. Journal of immunology. 1995;155:190–202. [PubMed] [Google Scholar]
- 27.Brezinschek HP, Foster SJ, Brezinschek RI, Dorner T, Domiati-Saad R, Lipsky PE. The Journal of clinical investigation. 1997;99:2488–501. doi: 10.1172/JCI119433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iwasaki K, Okawa-Takatsuji M, Aotsuka S, Ono T. Nihon Rinsho Meneki Gakkai Kaishi. 2003;26:346–54. doi: 10.2177/jsci.26.346. [DOI] [PubMed] [Google Scholar]
- 29.Chang A, Henderson SG, Brandt D, Liu N, Guttikonda R, et al. Journal of immunology. 2011;186:1849–60. doi: 10.4049/jimmunol.1001983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Risselada AP, Looije MF, Kruize AA, Bijlsma JW, van Roon JA. Seminars in arthritis and rheumatism. 2013;42:368–76. doi: 10.1016/j.semarthrit.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 31.Dorner T, Lipsky PE. Arthritis Res. 2002;4:360–71. doi: 10.1186/ar603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramos-Casals M, Brito-Zeron P, Font J. Semin Arthritis Rheum. 2007;36:246–55. doi: 10.1016/j.semarthrit.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 33.Manoussakis MN, Georgopoulou C, Zintzaras E, Spyropoulou M, Stavropoulou A, et al. Arthritis Rheum. 2004;50:882–91. doi: 10.1002/art.20093. [DOI] [PubMed] [Google Scholar]
- 34.Nardi N, Brito-Zeron P, Ramos-Casals M, Aguilo S, Cervera R, et al. Clin Rheumatol. 2006;25:341–6. doi: 10.1007/s10067-005-0059-3. [DOI] [PubMed] [Google Scholar]
- 35.Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, et al. N Engl J Med. 2003;349:1526–33. doi: 10.1056/NEJMoa021933. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Human recombinant monoclonal antibody gene usage, mutational analysis and CDR3 sequence