Fig 2.
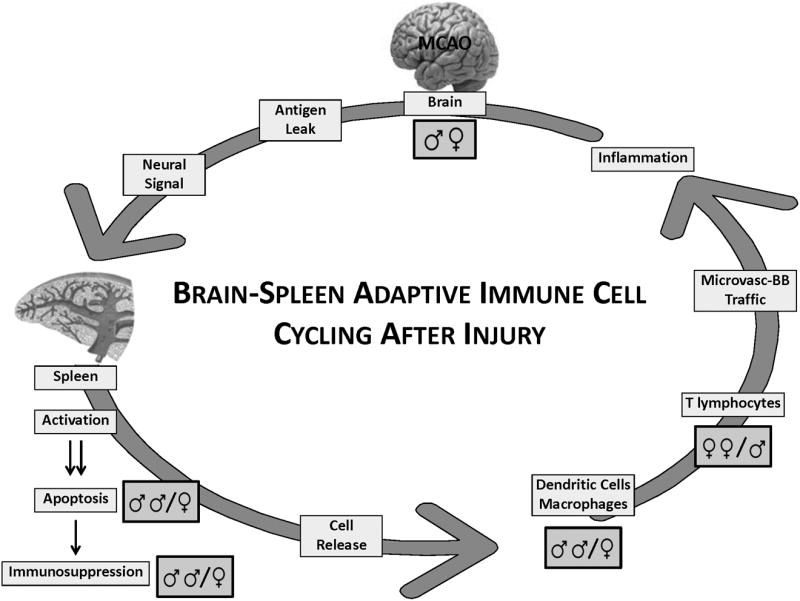
Brain-spleen immune cell cycling after experimental stroke. The evolving brain injury "signals" through the central nervous system for activation and apoptosis of the spleen, with consequent loss of many splenic immunocytes, leading to systemic immunosuppression. Remaining intra-splenic cell subsets (e.g. macrophages, lymphocytes and dendritic cells) are released into the blood, followed by trafficking across a cerebral microvasculature replete with inflammatory display of adhesion molecules and chemokines. The result is enhanced cerebral inflammation and damage, thus re-invigorating the cycle. Several points in the cycle are hypothesized to be sex-dependent. For example, splenic consequences post-experimental stroke have been observed to be more robust in male vs. female mice. Further, inflammatory cell trafficking is not identical in the male vs female post-ischemic brain. For example, macrophage infiltration into brain is particularly robust in male mice. Whether this observation is best explained by the typically larger infarct in male vs. female brain after experimental ischemia (middle cerebral artery occlusion), or by specific mechanisms of cell trafficking is unclear at present.
