Abstract
Objective
To test the hypothesis that the effect of red blood cell (RBC) transfusion on intermittent hypoxemia (IH) in extremely low birth weight (ELBW) infants is dependent on postnatal age.
Study Design
Oxygen saturation of 130 ELBW infants, who required transfusion, was monitored continuously for the first 8wks of life. We compared the characteristics of IH (SpO2 ≤ 80% for ≥4s and ≤3min), 24h before and both 24h and 24-48h after each RBC transfusion at three distinct time periods: Epoch 1, 1-7d; Epoch 2, 8-28d; and Epoch 3, >28d.
Results
In Epoch 1, the frequency and severity of IH events were not significantly different before and after transfusion. In both Epochs 2 and 3 there was a decrease in IH frequency and severity 24h after RBC transfusion that persisted for 48h. In addition, there was a decrease in the overall time spent with SpO2 ≤ 80% which persisted for 24h after transfusion in Epochs 1 and 3, and for 48h in Epoch 3.
Conclusion
The benefit of RBC transfusion on IH is age dependent as improvement in the frequency and severity of IH after transfusion only occurs beyond the first week of life. These observations will aid clinician’s decision making by clarifying the benefit of RBC transfusions on patterns of oxygenation in preterm infants.
Keywords: Red Blood Cell Transfusion, Intermittent Hypoxemia, Apnea of Prematurity, Oxygen Saturation
INTRODUCTION
Intermittent hypoxemia (IH) during early postnatal life is typically the result of impaired respiratory control often superimposed upon suboptimal lung function1. It may be associated with increased perinatal morbidities and poor neurodevelopmental outcome2-4. Red blood cell (RBC) transfusions may ameliorate IH events by enhancing oxygen carrying capacity with resultant improvement in respiratory control and/or oxygen reserves1. Previous studies have assessed the relationship between RBC transfusions and apnea of prematurity with and without desaturation events and have reported conflicting results5-16. Zagol et al. showed sustained improvement in apnea associated with oxygen desaturation after RBC transfusions6. Seidel et al also showed enhancement in oxygenation after RBC transfusions16. In contrast, Westkamp et al., Poets et al., and Stute et al. found no change in the incidence of IH events post RBC transfusion10, 13, 14. These contradictory results may be due to small sample size, lack of continuous saturation monitoring, or broad postnatal and gestational age ranges6, 10, 13, 14, 16.
Recently, we have documented that the incidence of IH changes over the first two months of life in a cohort of 79 preterm infants of 24-28 weeks gestation3. During the first week of life IH occurs infrequently; in weeks 2-3, IH progressively increases, plateaus around 4wks; and decreases thereafter3. Previous studies addressing the incidence of IH following RBC transfusion did not consider alterations in occurrence of IH with progressing postnatal age which may have influenced their findings. Therefore, we sought to characterize the association between the effect of RBC transfusions on IH and postnatal age by testing the hypothesis that the effect of transfusion on IH in ELBW infants is dependent on postnatal age.
MATERIALS AND METHODS
Study Design and Data Collection
To allow for a large sample size and, thus, an increased chance of detecting an effect of transfusion on the incidence of IH we examined two cohorts of preterm infants (24-27 6/7wks gestation) followed for the first 8wks of life. The cohorts were formed between 2005 and 2009 at Rainbow Babies and Children’s Hospital Neonatal Intensive Care Unit. The first cohort comprised 79 infants who received standard clinical care including the need for respiratory support, oxygen and caffeine3. Standard patient care included a target SpO2 between 85% and 95%. An additional cohort included 98 infants enrolled in the SUPPORT trial, a multi-centered randomized trial that compared two different target ranges of oxygen saturation ranges (85 - 89%, n=49 and 91- 95%, n=49)17, 18. Infants with congenital malformations were excluded.
Oxygen saturation was recorded continuously using high-resolution (2s averaging time and a 0.5Hz sampling rate) pulse oximetery (Radical; Masimo, Irvine, CA) from day 1 to 8wks postnatal age. Intermittent hypoxemia was defined as a drop in SpO2 ≤ 80% for ≥ 4s and ≤ 3min duration. The lower threshold of 4s was based on previous data by Poets et al.13. The upper limit in duration (3min) was chosen arbitrarily to distinguish intermittent hypoxemia from sustained changes in baseline oxygenation. In addition, to account for all periods of hypoxemia, the total time of SpO2 ≤ 80% was calculated which included both intermittent and sustained (>3min) hypoxemic events.
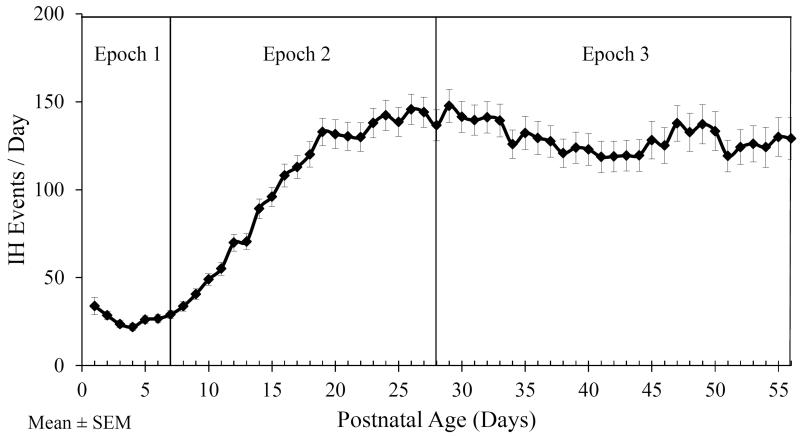
For each RBC transfusion, we documented the timing, volume and change in hematocrit. To avoid the potential effect of multiple transfusions, the RBC transfusion was considered eligible for analysis if there was no other transfusion within the 24h before and 48h after the transfusion. The 8 week monitoring period was stratified into three epochs based on the frequency of IH for all 177 infants (Figure 1). As previously published by Di Fiore et al.3, we identified a low incidence of IH during the first week of postnatal age, followed by a progressive increase over weeks 2-3, a plateau around 4wks, and a decrease thereafter. Epoch 1 was defined as the first week of life when there are few IH events. Epoch 2 was defined as 8d to 4wks of life when there is an increase in the number of IH events. Epoch 3 was defined as beyond 4wks of life when the IH frequency plateaus and begins to decrease. For each infant only one RBC transfusion in each epoch was analyzed. In infants with multiple transfusions, the first eligible transfusion in each epoch was selected.
Figure 1.
The frequency of intermittent hypoxemia (IH) events during the first 8wks of postnatal life. The 8wk postnatal period was stratified into 3 epochs. Epoch 1 was defined as 1-7d of life, Epoch 2 between 8 – 28d of life, Epoch 3 after 28d of life.
Statistical Analysis
One way analysis of variance (ANOVA) with repeated measures was performed separately for each epoch to compare the incidence of hypoxemic events in the 24h period before, and 24h and 24-48h after the transfusion. Post hoc comparisons of pre and post transfusion time points (24h pre, 24h post and 24-48h post) were performed by the Tukey-Kramer method. The oxygen saturation target range (wide (85-95%), high (91-95%) and low (85-89%)) was included as a covariate in the ANOVA model to control for a potential effect of baseline oxygen saturation target. Generalized Estimating Equations (GEE) logistic model or ANOVA were used to compare baseline characteristics among epochs for categorical and continuous data, respectively.
Sample Size
Based on previous data suggesting a 40% decrease14 in the number of IH events with an average standard deviation of 80 IH per day (Figure 1), we calculated a sample size of 24 infants would be needed in each epoch to detect a change of this magnitude, (α=0.05, 1-β=0.80). Accessibility to our large infant cohort enabled us to include 78 infants in Epoch 1, 109 infants in Epoch 2, and 75 infants in Epoch 3. The increased number of infants enrolled in this study enabled us to detect a smaller change of 22% in the incidence of IH (α=0.05, 1-β=0.80).
Institutional Review Board Approval
The University Hospitals Institutional Review Board approved the study. Since this is an expanded analysis of a previously obtained data set, a waiver of consent was granted.
RESULTS
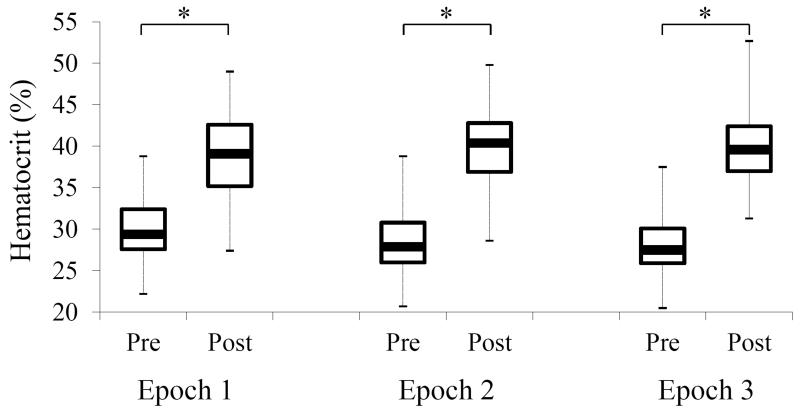
Of the 177 preterm infants included in the cohorts 130 had eligible RBC transfusions in the first 8wks of life. Characteristics of the study population are presented in Tables 1 and 2 based on the epoch in which they were transfused. A total of 262 RBC transfusions met criteria for analysis. There was no difference in gestational age, birth weight, race or gender across epochs (Table 1). More infants in Epoch 1 were on ventilator support compared to Epochs 2 and 3 (Table 2). The majority of infants received supplemental oxygen encompassing approximately 90% of infants during each time period (Table 2). The administration of caffeine was significantly lower in Epoch 1 compared to Epochs 2 and 3 (Table 2). The hematocrit increased comparably after RBC transfusion in each of the three epochs (Figure 2). The median RBC transfusion volume was 20ml/kg administered over a duration of four hours.
Table 1.
Demographic Data of the Studied Infants Based on Transfusion Epoch
| Epoch 1 | Epoch 2 | Epoch 3 | |
|---|---|---|---|
| N | 78 | 109 | 75 |
| Gestational Age | 26wks (IQR 25 - 26) | 26wks (IQR 25 - 27) | 26wks (IQR 25 - 27) |
| Birth Weight | 730g (IQR 655 - 806) | 770g (IQR 667 - 870) | 730g (IQR 635 - 840) |
| Male | 51% | 47% | 48% |
| Black | 58% | 58% | 60% |
| Post-Natal Age | 4d (IQR 2 - 5) | 13d (IQR 10 - 18) | 33d (IQR 31 - 38) |
| Weight at Transfusion | 665g (IQR 610 - 760) | 798g (IQR 670 - 927) | 1030g (IQR 898 - 1260) |
Table 2.
Percent of Infants Receiving Respiratory Support
| Epoch 1 | Epoch 2 | Epoch 3 | |
|---|---|---|---|
| Ventilator Support | 72% | 58%* | 57%* |
| Non Invasive Ventilation | 27% | 35% | 31% |
| Other (Nasal Cannula, O2 Hood) | 1% | 3% | 7% |
| No Respiratory Support | 0% | 3% | 5% |
| Respiratory Support Data Not Available | 0% | 1% | 0% |
| Supplemental Oxygen | 91% | 92% | 89% |
| Caffeine | 62% | 81%* | 79%* |
p<0.05 versus Epoch 1
Figure 2.
Hematocrit data presented in box-and-whisker diagram. There was no difference in pre and post RBC transfusion hematocrit level across epochs. There was a significant increase in the hematocrit level post RBC transfusion in all three epochs (*p<0.001). Box plot represent median and interquartile ranges. Whiskers represent the hematocrit range (minimum – maximum).
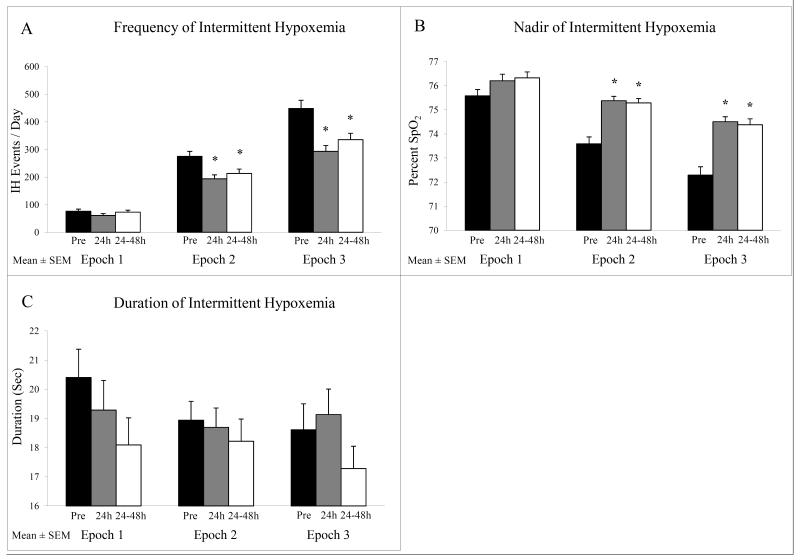
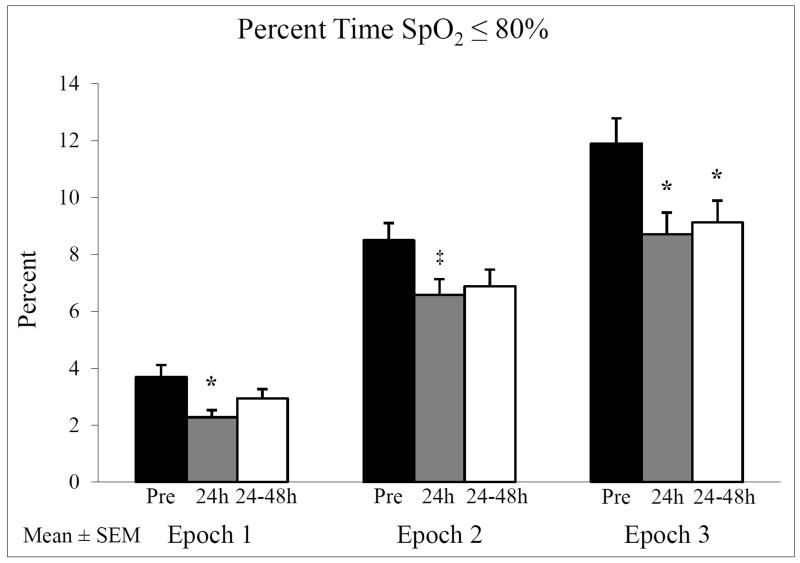
In Epoch 1, where minimal IH events occurred, there was no significant difference in the incidence, severity (nadir) and duration of IH before and after RBC transfusion (Figure 3). However, in both Epoch 2, in a postnatal age range where there is an increase in IH events, and Epoch 3, in a postnatal age range where the high incidence of IH events plateaus, there was a significant decrease in IH frequency 24h after RBC transfusion that persisted for 48h (all p<0.02) (Figure 3A). In addition, there was a decrease in the severity of IH (higher nadir) in Epochs 2 and 3 that persisted for 48h (all p<0.02) (Figure 3B). There was no significant change in duration of IH with RBC transfusion (Figure 3C). We also examined the percent time spent with SpO2 ≤ 80% in all three epochs. In both Epochs 1 and 3 there was a significant improvement (p<0.05) in the overall time spent with SpO2 ≤ 80% at 24h after RBC transfusion persisting for 48h in Epoch 3 only (Figure 4). In Epoch 2 the improvement in time with SpO2 ≤ 80% approached but did not reach statistical significance (p=0.058).
Figure 3.
Characteristics of IH pre and post RBC transfusion including (A) frequency, (B) nadir, and (C) duration. Frequency significantly decreased and IH nadir increased at 24h and 24-48h post RBC transfusion only in Epochs 2 and 3 (*p<0.02 versus pre RBC transfusion). There was no change in IH duration.
Figure 4.
Percent time with SpO2 < 80% pre and post RBC transfusion. The percent time with SpO2 ≤ 80% significantly decreased in Epochs 1 and 3 at 24h post RBC transfusion persisting for 24-48h in Epoch 3 (*p<0.05). There was a trend towards a decrease in time with SpO2 ≤ 80% in Epoch 2 that did not reach statistical significance (‡p=0.058).
To achieve a large sample size to detect relatively small alterations in IH associated with transfusion, the cohort included three oxygen saturation target groups (wide; 85-95%, high; 91-95% or low; 85-89%). There was no significant effect of SpO2 target range on the benefit of RBC transfusion (incidence, severity and duration) in any epoch.
DISCUSSION
Intermittent hypoxemic episodes are generally attributed to unstable respiratory control in preterm infants resulting in respiratory pauses and apnea19. Two proposed mechanisms may underlie the beneficial effect of RBC transfusions on IH. The first suggests that anemia decreases oxygen delivery to the respiratory control network leading to hypoxic ventilatory depression7, 20-22. Thus, RBC transfusions may decrease apnea frequency by improving oxygen delivery to the immature brainstem. The second underlying mechanism is that RBC transfusions increase oxygen stores resulting in greater stability of oxygenation in the presence of apnea. This is consistent with a recent model analysis showing that the rate of arterial oxygen desaturation during apnea decreased with higher hemoglobin levels1. Therefore, the improvement in IH after RBC transfusions may be due to either reduction in the frequency of apnea or increased oxygen stores ameliorating IH in the presence of apnea.
Our results demonstrate a significant improvement in IH after RBC transfusion beyond the first week of life. The stratification of postnatal age into three developmental stages may partially explain the conflicting literature by minimizing the potential confounder of the dynamic natural history of IH. There was no benefit of RBC transfusion on frequency, severity or duration of IH in Epoch 1. This may be due to the already low incidence of IH before RBC transfusion in the first week of life. The mechanism underlying this lower incidence of IH in Epoch 1 is not clear although higher ventilator use may have stabilized both lung volume and alveolar PO2 , decreasing the likelihood of oxygen desaturation1. This explanation seems unlikely as addition of mechanical ventilator status to the statistical model did not affect the relationship between transfusion and IH events. Since caffeine therapy improves respiratory control in preterm infants, the lack of benefit of transfusion on IH in Epoch 1 is unlikely due to caffeine use as significantly fewer infants received caffeine compared to Epochs 2 and 3. Previous studies addressing the relationship between RBC transfusions and IH have primarily assessed infants with both a wide and/or older postnatal age6, 10, 13, 14, 16. The mean postnatal age in studies that showed improvement in IH frequency after RBC transfusion6, 16 corresponded to Epochs 2 and 3 in the current study. Apart from stratification by postnatal age our study had the additional advantage of a tight gestational age range (24-27 6/7 wks).
Use of high resolution (2s averaging time and 0.5Hz sampling rate) oxygen saturation monitoring optimized our ability to accurately identify IH events of both long and short duration. Vagedes et al have shown that detection of IH can vary up to fivefold with prolonged averaging times, as commonly used in the clinical setting, resulting in an artificial decrease in IH of 5s-10s and increase in IH of >20s23. This may partially explain discrepancies between studies. Although there was no change in IH duration following RBC transfusion, there was an improvement in the total percent of time with SpO2 ≤ 80%. This finding may be attributed to a decrease in the frequency of both IH and more prolonged periods (>3 min) of hypoxemia that were not included in the IH analysis. Given no improvement in IH after RBC transfusion in Epoch 1, the benefit in the percent of time with SpO2 ≤ 80% may be due to a decrease in prolonged periods (>3 min) of hypoxemia. Recent data indicate that RBC transfusion may improve myocardial performance and possibly cardiac output in preterm infants and we cannot exclude the possibility that this effect of simple volume expansion may have benefited oxygenation status of our infants5,24.
A limitation of this study was the lack of accurate long term apnea documentation which continues to be a challenge in the clinical setting. Bedside nursing documentation has been shown to underreport apnea events6 and impedance monitoring, the current clinical mode of monitoring respiration, cannot distinguish obstructive apnea from normal respiration. Therefore, long term airflow measurements are needed to differentiate these two potential mechanisms whereby RBC transfusion benefits IH episodes which is impractical in the clinical setting. However, we feel we have documented the concerning clinical component of apnea namely resultant IH19. Unfortunately available data do not allow us to document the response to IH (ie supplemental oxygen) which may have affected our findings. The inclusion of three different oxygen saturation target groups may also have affected our study findings although the effect of RBC transfusions on the hypoxemic events was not altered after adjusting for the oxygen saturation target. Including these multiple oxygen saturation target groups provided a large sample size that presented the ability to stratify the postnatal period and to detect more subtle alterations in IH patterns after RBC transfusion. Finally, we were unable to determine the precise indication for blood transfusion in many of the infants in this retrospective review, although anemia was the most common reason for transfusion. While the hematocrit threshold for transfusing preterm infants remains controversial11, 25, 26, the hematocrit at time of transfusion in this study (range 20.7% - 38.8%) is comparable to the two largest randomized trials (hematocrit range 22% - 46%) assessing transfusion thresholds11, 25. The decision to transfuse varies among physicians and may also be influenced by the patient’s daily condition. As the number of IH events was elevated during all epochs when compared to previous published data 3, it is conceivable that more patients may have been transfused on sick days with high number of IH events, followed by random improvement over the subsequent 48 hours that is not related to the transfusion, however, this cannot explain the lack of benefit in Epoch 1.
In summary, this study has shown a clear benefit of RBC transfusion on the incidence and severity of IH episodes in preterm infants beyond the first week of life. These beneficial effects of RBC transfusion in neonates should be weighed against potential risks such as transmitted infections27,28, intraventricular hemorrhage29,30, and necrotizing enterocolitis28, 31. Future studies may allow us to determine whether there is a threshold of IH that is associated with neonatal morbidity.
ACKNOWLEGEMENTS
We are thankful to the Neonatal Research Network SUPPORT trial.
Funding Source: Supported by NINDS/NIH Grant R01 NS069220 (T.E.D.)
Abbreviations
- IH
Intermittent Hypoxemia
- PNA
Postnatal Age
- RBC
Red Blood Cell
- SpO2
Oxygen Saturation
Footnotes
CONFLICT OF INTEREST All authors have no financial relationships and no conflict of interests relevant to this article.
REFERENCES
- 1.Sands SA, Edwards BA, Kelly VJ, Davidson MR, Wilkinson MH, Berger PJ. A model analysis of arterial oxygen desaturation during apnea in preterm infants. PLoS Comput Biol. 2009;5:e1000588. doi: 10.1371/journal.pcbi.1000588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin RJ, Wang K, Koroglu O, Di Fiore J, Kc P. Intermittent hypoxic episodes in preterm infants: do they matter? Neonatology. 2011;100:303–310. doi: 10.1159/000329922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Fiore JM, Bloom JN, Orge F, Schutt A, Schluchter M, Cheruvu VK, et al. A higher incidence of intermittent hypoxemic episodes is associated with severe retinopathy of prematurity. J Pediatr. 2010;157:69–73. doi: 10.1016/j.jpeds.2010.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Janvier A, Khairy M, Kokkotis A, Cormier C, Messmer D, Barrington KJ. Apnea is associated with neurodevelopmental impairment in very low birth weight infants. J Perinatol. 2004;24:763–768. doi: 10.1038/sj.jp.7211182. [DOI] [PubMed] [Google Scholar]
- 5.Bifano EM, Smith F, Borer J. Relationship between determinants of oxygen delivery and respiratory abnormalities in preterm infants with anemia. J Pediatr. 1992;120:292–296. doi: 10.1016/s0022-3476(05)80447-0. [DOI] [PubMed] [Google Scholar]
- 6.Zagol K, Lake DE, Vergales B, Moorman ME, Paget-Brown A, Lee H, et al. Anemia, apnea of prematurity, and blood transfusions. J Pediatr. 2012;161:417–421. e411. doi: 10.1016/j.jpeds.2012.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joshi A, Gerhardt T, Shandloff P, Bancalari E. Blood transfusion effect on the respiratory pattern of preterm infants. Pediatrics. 1987;80:79–84. [PubMed] [Google Scholar]
- 8.DeMaio JG, Harris MC, Deuber C, Spitzer AR. Effect of blood transfusion on apnea frequency in growing premature infants. J Pediatr. 1989;114:1039–1041. doi: 10.1016/s0022-3476(89)80459-7. [DOI] [PubMed] [Google Scholar]
- 9.Sasidharan P, Heimler R. Transfusion-induced changes in the breathing pattern of healthy preterm anemic infants. Pediatr Pulmonol. 1992;12:170–173. doi: 10.1002/ppul.1950120308. [DOI] [PubMed] [Google Scholar]
- 10.Stute H, Greiner B, Linderkamp O. Effect of blood transfusion on cardiorespiratory abnormalities in preterm infants. Arch Dis Child Fetal Neonatal Ed. 1995;72:F194–196. doi: 10.1136/fn.72.3.f194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bell EF, Strauss RG, Widness JA, Mahoney LT, Mock DM, Seward VJ, et al. Randomized trial of liberal versus restrictive guidelines for red blood cell transfusion in preterm infants. Pediatrics. 2005;115:1685–1691. doi: 10.1542/peds.2004-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blank JP, Sheagren TG, Vajaria J, Mangurten HH, Benawra RS, Puppala BL. The role of RBC transfusion in the premature infant. Am J Dis Child. 1984;138:831–833. doi: 10.1001/archpedi.1984.02140470031010. [DOI] [PubMed] [Google Scholar]
- 13.Poets CF, Pauls U, Bohnhorst B. Effect of blood transfusion on apnoea, bradycardia and hypoxaemia in preterm infants. Eur J Pediatr. 1997;156:311–316. doi: 10.1007/s004310050607. [DOI] [PubMed] [Google Scholar]
- 14.Westkamp E, Soditt V, Adrian S, Bohnhorst B, Groneck P, Poets CF. Blood transfusion in anemic infants with apnea of prematurity. Biol Neonate. 2002;82:228–232. doi: 10.1159/000065891. [DOI] [PubMed] [Google Scholar]
- 15.Keyes WG, Donohue PK, Spivak JL, Jones MD, Jr., Oski FA. Assessing the need for transfusion of premature infants and role of hematocrit, clinical signs, and erythropoietin level. Pediatrics. 1989;84:412–417. [PubMed] [Google Scholar]
- 16.Seidel D, Blaser A, Gebauer C, Pulzer F, Thome U, Knupfer M. Changes in regional tissue oxygenation saturation and desaturations after red blood cell transfusion in preterm infants. J Perinatol. 2012;33:282–287. doi: 10.1038/jp.2012.108. [DOI] [PubMed] [Google Scholar]
- 17.Finer NN, Carlo WA, Walsh MC, Rich W, Gantz MG, Laptook AR, et al. Early CPAP versus surfactant in extremely preterm infants. N Engl J Med. 2010;362:1970–1979. doi: 10.1056/NEJMoa0911783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carlo WA, Finer NN, Walsh MC, Rich W, Gantz MG, Laptook AR, et al. Target ranges of oxygen saturation in extremely preterm infants. N Engl J Med. 2010;362:1959–1969. doi: 10.1056/NEJMoa0911781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abu-Shaweesh JM, Martin RJ. Neonatal apnea: what’s new? Pediatr Pulmonol. 2008;43:937–944. doi: 10.1002/ppul.20832. [DOI] [PubMed] [Google Scholar]
- 20.Cross KW, Oppe TE. The effect of inhalation of high and low concentrations of oxygen on the respiration of the premature infant. J Physiol. 1952;117:38–55. [PMC free article] [PubMed] [Google Scholar]
- 21.Rigatto H, Brady JP. Periodic breathing and apnea in preterm infants. II. Hypoxia as a primary event. Pediatrics. 1972;50:219–228. [PubMed] [Google Scholar]
- 22.Rigatto H, Brady JP, de la Torre Verduzco R. Chemoreceptor reflexes in preterm infants: I. The effect of gestational and postnatal age on the ventilatory response to inhalation of 100% and 15% oxygen. Pediatrics. 1975;55:604–613. [PubMed] [Google Scholar]
- 23.Vagedes J, Poets CF, Dietz K. Averaging time, desaturation level, duration and extent. Arch Dis Child Fetal Neonatal Ed. 2013;98:F265–266. doi: 10.1136/archdischild-2012-302543. [DOI] [PubMed] [Google Scholar]
- 24.Saleemi MS, El-Khuffash A, Kirkham C, Franklin O, Corcoran JD. Myocardial assessment using tissue doppler imaging in preterm very low-birth weight infants before and after red blood cell transfusion. J Perinatol. 2013;33:681–686. doi: 10.1038/jp.2013.39. [DOI] [PubMed] [Google Scholar]
- 25.Kirpalani H, Whyte RK, Andersen C, Asztalos EV, Heddle N, Blajchman MA, et al. The Premature Infants in Need of Transfusion (PINT) study: a randomized, controlled trial of a restrictive (low) versus liberal (high) transfusion threshold for extremely low birth weight infants. J Pediatr. 2006;149:301–307. doi: 10.1016/j.jpeds.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 26.Crowley M, Kirpalani H. A rational approach to red blood cell transfusion in the neonatal ICU. Curr Opin Pediatr. 2010;22:151–157. doi: 10.1097/MOP.0b013e328336eb3e. [DOI] [PubMed] [Google Scholar]
- 27.Martin RJ, Fanaroff AA, Walsh MC. Fanaroff and Martin’s neonatal-perinatal medicine : diseases of the fetus and infant. 9th ed Mosby/Elsevier; St. Louis, Mo.: 2011. [Google Scholar]
- 28.Christensen RD, Ilstrup S. Recent advances toward defining the benefits and risks of erythrocyte transfusions in neonates. Arch Dis Child Fetal Neonatal Ed. 2012;98:365–372. doi: 10.1136/archdischild-2011-301265. [DOI] [PubMed] [Google Scholar]
- 29.Baer VL, Lambert DK, Henry E, Snow GL, Butler A, Christensen RD. Among very-low-birth-weight neonates is red blood cell transfusion an independent risk factor for subsequently developing a severe intraventricular hemorrhage? Transfusion. 2011;51:1170–1178. doi: 10.1111/j.1537-2995.2010.02980.x. [DOI] [PubMed] [Google Scholar]
- 30.Christensen RD. Associations between “early” red blood cell transfusion and severe intraventricular hemorrhage, and between “late” red blood cell transfusion and necrotizing enterocolitis. Semin Perinatol. 2012;36:283–289. doi: 10.1053/j.semperi.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 31.Kirpalani H, Zupancic JA. Do transfusions cause necrotizing enterocolitis? The complementary role of randomized trials and observational studies. Semin Perinatol. 2012;36:269–276. doi: 10.1053/j.semperi.2012.04.007. [DOI] [PubMed] [Google Scholar]