Abstract
Background
A valid biomarker is “an indicator of normal biologic or pathogenic processes, or pharmacological responses to a therapeutic intervention”. There is no validated biomarker for irritable bowel syndrome (IBS).
Aim
To assess ability of three quantitative traits to identify treatable processes to discriminate between IBS-diarrhea (IBS-D), IBS-constipation (IBS-C) and healthy volunteers (HV).
Methods
In 30 HV, 30 IBS-C and 64 IBS-D patients, we characterized bowel symptoms and quantitated pathophysiological mechanisms: bile acid (BA) synthesis (serum C4 and FGF19), fecal BA and fat, colonic transit (CT), and intestinal permeability (IP). We used multiple logistic regression and receiver-operating characteristic (ROCAUC) to appraise 3 factors (fecal BA, CT and IP) individually and in combination to identify discriminant targets for treatment in IBS.
Key Results
There were significant associations between the three subgroups and symptoms reflecting bowel function and the quantitative traits. There were significant associations between fecal BA and CT at 48h (r=0.43; p<0.001) and between fecal BA and IP (r=0.23; p=0.015). Individually, fecal BA and CT48 (but not IP) were significant independent predictors for distinguishing HV from IBS. In combination, they discriminated HV from IBS-D (ROCAUC 0.70), HV from IBS-C (ROCAUC 0.73), and IBS-C from IBS-D (ROCAUC 0.86). Colonic transit and fecal BA excretion together discriminate between health and IBS-C or IBS-D, or between the IBS subgroups with 75–90% specificity at 60% sensitivity.
Conclusion & Inferences
Colonic transit and fecal BA individually and together constitute useful biomarkers to identify treatable mechanisms in IBS and to differentiate subgroups of IBS.
Keywords: bile acid, permeability, transit, colon
INTRODUCTION
There is considerable overlap in stool frequency, consistency and ease of passage between health and irritable bowel syndrome (IBS); symptom-based criteria have reasonable sensitivity to identify IBS in primary care (1), but they have relatively low specificity (2) and are enhanced by incorporating basic investigations including erythrocyte sedimentation rate and blood count in order to exclude organic diseases (3). Existing diagnostic criteria perform modestly in distinguishing IBS from other diseases not associated with mucosal pathology such as celiac disease (4,5) or other organic diseases (6).
A number of pathophysiological mechanisms are recognized in IBS (7). A valid biomarker is defined as “a characteristic that is measured and evaluated as an indicator of normal biologic processes, pathogenic processes, or pharmacological responses to a therapeutic intervention (8).” Several potential markers have been proposed based on relatively small patient cohorts, including symptom responses to nutrient and lactulose challenge (9), breath methane (10), rectal hypersensitivity by balloon distension (11), circulating lymphocyte-stimulated expression of cytokines, circulating monocyte-inhibited expression of cytokines (12), decreased rectal mucosal IL-10 mRNA expression (13), fecal chromogranins and secretogranins (14), or density of duodenal chromogranin A expressing cells (15).
To date, studies proposing the biomarkers have not been generally replicated or proven useful to guide treatment. For example, a recent study of 2256 medical records of patients with 13 IBS-related diagnostic codes suggested that 82.8% of the patients had at least one abnormal fecal biomarker: quantitative stool culture for beneficial bacteria (Lactobacillus and Bifidobacterium) showed low growth (suggestive of intestinal dysbiosis) in 73.1%; abnormally elevated eosinophil protein X (suggestive of food allergy) in 14.3%; elevated calprotectin (suggestive of inflammation) in 12.1%; detection of parasites in 7.5%; and low pancreatic elastase (suggestive of exocrine pancreatic insufficiency) in 7.1% (16). These and other fecal biomarkers have been proposed for the differentiation of IBS from inflammatory bowel disease (17), though there clearly is overlap as exemplified by the 12.1% of patients with IBS codes who had elevated fecal calprotectin, and it has been suggested that, even in the primary care setting, the cut-off values of fecal calprotectin require revision to enhance the positive predictive value of the test for diagnosis (18).
Recently, the demonstration of a combination of 34 markers (24 genes demonstrated to have increased expression in IBS and 10 serological markers from pathways involved in pain, serotonin metabolism, mast cell activation and inflammation) able to differentiate IBS from health (ROCAUC = 0.81) suggests progress towards a diagnostic test for IBS compared with health, and for discrimination of IBS-C from IBS-D (19). However, both with fecal biomarkers and the 34-marker panel, there are, as yet, no outcome data from triaging patients for treatments.
We have extensively studied three quantitative, noninvasive traits that could potentially fulfill the definition of biomarker: colonic transit by scintigraphy, fecal bile acid excretion and intestinal permeability. Thus, among 119 patients with IBS, 48% with diarrhea-predominant IBS (IBS-D) had accelerated and 21% with constipation-predominant IBS (IBS-C) delayed transit (20), and among 286 patients with lower functional gastrointestinal disorders, 33.3% of IBS-D/functional diarrhea patients had accelerated colonic transit at 24 hours, and delayed colon transit at 48 hours was detected in 22.9% patients with IBS-C/functional constipation (21). Inter- and intra-subject variations of colonic transit in IBS-C or IBS-D have been well characterized. Colonic transit was significantly associated with stool form, frequency, and ease of stool passage (22), and clinical efficacy of a broad range of pharmacological agents was correctly predicted by colonic transit measured by scintigraphy (23).
A meta-analysis showed bile acid malabsorption (BAM) in up to 50% of patients with chronic functional diarrhea or IBS-D (24). About one-third of patients with IBS-D have increased and 10% of IBS-C decreased fecal bile acid (BA) excretion (25,26) and hepatic BA synthesis rates, estimated indirectly by serum 7α-hydroxy-4-cholesten-3-one (C4) measurements (27). Patients with BAM have excellent responses to BA sequestrants therapy (24), and bile acid supplementation accelerates colonic transit and improves bowel function in female patients with IBS-C (28).
Several groups have reported increased intestinal or colonic permeability in patients with IBS relative to control groups, as summarized elsewhere (29). However, the relationships of BA malabsorption or BA deficiency in IBS to quantitative traits such as bowel transit and permeability are unclear, and there are presently no approved treatments aimed at restoring normal permeability, though there is evidence that gluten withdrawal can restore normal barrier function in patients with IBS-D, especially if they are carriers of HLA-DQ2/8 (30).
The aim of our study was to assess the ability of three quantitative traits to identify treatable processes to discriminate between IBS-D, IBS-C and healthy volunteers. There were two sub-aims: first, to characterize symptoms and a range of quantitative traits in IBS-D, IBS-C and healthy volunteers to prove that the groups of participants were different in these subjective and objective measures; and second, to develop individual or composite biomarkers to identify treatable processes in IBS, specifically in IBS-D and IBS-C.
METHODS
Participants: Eligibility of Patients with IBS and Healthy Volunteers
From a database of ~1000 patients with functional gastrointestinal diseases who reside within ~150 miles of Mayo Clinic in Rochester, Minnesota, and healthy volunteers from the same region, we invited participation and enrolled subjects who volunteered for the study until the quota required by the protocol was filled (in accordance with NIH DK92179). Participants completed the validated bowel disease questionnaires corresponding to Rome criteria [including Somatic Symptom Checklist (31)] and the Hospital Anxiety and Depression Scale [HAD (32)]. The participants were 30 healthy controls, 30 patients with IBS-C, and 64 patients with IBS-D [by Rome III criteria (33)], prospectively studied over ~24 months. The demographics, symptoms, and gastrointestinal and colonic transit measurements were highly comparable to the data in a previous cohort of patients published in 2008 (20). The data in the two cohorts are provided in Appendix Table 1, and demonstrate that the current cohort is representative of IBS at our center.
This study was approved by Mayo Clinic Institutional Review Board.
Measurements of Quantitative Traits
Quantitative traits were measured by extensively validated methods in our laboratory.
A. Scintigraphic Gastrointestinal and Colonic Transit
To measure gastrointestinal and colonic transit, we used the dual isotope method which has been extensively used and validated, including studies of 287 patients with lower functional gastrointestinal diseases (21) and the performance characteristics of the method in health and IBS (22).
B. Bile Acid Malabsorption (BAM) and Synthesis
We used two methods to document diarrhea related to bile acid:
-
Serum 7α-hydroxy-4-cholesten-3-one (C4) is a measurement of hepatic cholesterol synthesis and is closely related to the fecal loss of bile acids. Serum C4 is a validated method for BAM. In head-to-head comparisons with the 75SeHCAT retention test, increased serum C4 had sensitivity of 90% and specificity of 79% in diagnosing BAM (34), where shorter retention half-time of 75SeHCAT is associated with increased level of C4. Serum C4 also had 98% negative predictive value and 74% positive predictive value for diagnosis of BAM (35).
We based our serum C4 assay on the method [adapted from Galman et al. (36)] using HPLC/tandem mass spectrometry (27). Thus, serum C4 was used to screen for high bile acid synthesis (27).
Fecal bile acid excretion: Using HPLC/tandem mass spectrometry, we have adapted a method used with serum samples (37) to measure fecal total and individual bile acids (26) in a 48-hour collection of stools collected while ingesting a 100g fat per day diet, measured by HPLC/tandem mass spectrometry.
C. Serum Fibroblast Growth Factor 19 (FGF19)
Serum FGF19, a measure of feedback regulation of bile acid synthesis (38), was measured by ELISA (FGF19 Quantikine Enzyme-Linked Immunosorbent Assay Kit, R&D Systems, Minneapolis, MN), as in previous studies (39).
D. Fecal Fat
Daily fecal fat excretion, while ingesting a 100g fat diet daily, was measured by nuclear magnetic resonance spectrometry at Mayo Clinic’s Department of Laboratory Medicine and Pathology.
E. Colonic Permeability by Urinary Excretion of Lactulose and Mannitol after Oral Ingestion
We used orally administered lactulose and mannitol in aqueous solution to study intestinal permeability (40). We had previously identified increased small bowel permeability in IBS-D compared to healthy controls (41). The 0–2 hour urine reflects most closely small intestinal permeability, and the 8–24 hour urine reflects colonic permeability. The validated HPLC-tandem mass spectrometric method was used (40).
Statistical Analysis
We used Spearman correlations to explore the associations among total fecal BA, colonic transit and intestinal permeability.
The Kruskal Wallis test was used to assess univariate associations of quantitative traits among the three groups. Multiple logistic regression models were used to derive weighting factors for three primary measurements (total fecal BA, colonic transit at 48 hours, and urine mannitol excretion 0–2 hours). Receiver-operating characteristic (ROC) curves were plotted, and the areas under the curves (AUC) were estimated as a measure of the discriminatory value of the individual or combined traits, with the objective of identifying treatable biomarkers in IBS. Since small intestinal permeability was not a significant predictor for discriminating among the groups, ROC curves from the logistic models for two of the predictor variables (which reflect treatable mechanisms in IBS, that is, colonic transit and fecal BA excretion), as well as the overall model were used to illustrate the ability to discriminate between healthy volunteers (HV) and IBS-D, separately IBS-C, and between IBS-D and IBS-C. Restriction to three factors considered in the models was predicated by 30 participants each in HV and IBS-C groups.
RESULTS
Demographics and Psychosomatic Features of Participants
Demographics were similar in the three groups (Table 1), but there were higher psychosomatic symptom and depression scores in IBS patients. As previously documented in other studies of IBS, there was higher BMI among patients with IBS-D.
Table 1. Demographics, Psychosomatic Scores and Quantitative Measurements of the Three Groups of Participants.
Kruskal Wallis test was used to assess the associations of quantitative traits in the three groups.
| Data: mean ± SEM | IBS-C | Healthy Controls | IBS-D | p value |
|---|---|---|---|---|
| N | 30 | 30 | 64 | |
| Gender (F/M) | 30/0 | 22/8 | 59/5 | |
| Age, y | 44.6 ± 1.1 | 39.3 ± 2.1 | 41.9 ± 1.5 | 0.161 |
| BMI, kg/m2 | 26.6 ± 0.8 | 25.4 ± 0.7 | 29.7 ± 0.9 | 0.013 |
| Anxiety score (HAD) | 2.8 ± 0.5 | 2.1 ± 0.3 | 4.0 ± 0.5 | 0.117 |
| Depression score (HAD) | 0.9 ± 0.2 | 0.4 ± 0.1 | 1.6 ± 0.2 | 0.0005 |
| Psychosomatic symptom score | 0.4 ± 0.06 | 0.2 ± 0.04 | 0.5 ± 0.04 | <0.0001 |
| Bowel function | ||||
| # BM/day | 0.85± 0.1 | 1.18 ± 0.1 | 2.26 ± 0.1 | <0.0001 |
| BM form | 2.86 ± 0.2 | 3.77 ± 0.1 | 4.76 ± 0.1 | <0.0001 |
| Quantitative traits | ||||
| Fecal fat (g/day) | 5.1 ± 0.9 | 6.6 ± 0.8 | 9.1 ± 0.9 | 0.007 |
| Serum C4 (ng/mL) | 23.5± 4.4 | 25.3± 3.1 | 34.7± 3.6 | 0.019 |
| Serum FGF-19 (pg/mL) | 134.9 ± 18.4 | 171.6 ± 25.0 | 118.8 ± 10.8 | 0.051 |
| Total Fecal Bile Acid (μM/48h) | 579 ± 161 | 957 ± 185 | 2495 ± 382 | <0.0001 |
| Mean % fecal LCA/CDCA/DCA/CA | 49/1/48/2 | 39/1/59/1 | 30/6/53/8 | All <0.001, except DCA =0.033 |
| Gastric emptying T1/2 min | 117.3 ± 5.0 | 120.0 ± 5.7 | 124.7 ± 3.8 | 0.51 |
| Colonic filling at 6 h, % | 55.0 ± 5.5 | 54.1 ± 4.3 | 55.2 ± 3.4 | 0.92 |
| Colonic transit GC24 | 2.34± 0.2 | 2.41 ± 0.2 | 2.85 ± 0.2 | 0.13 |
| Colonic transit GC48 | 3.22± 0.17 | 3.86± 0.17 | 4.18± 0.12 | 0.0001 |
| Urine mannitol 0–2h | 264.8 ± 42.9 | 355.2 ± 49.5 | 444.3 ± 75.2 | 0.039 |
| Urine mannitol 8–24h | 65.8 ± 16.1 | 43.6 ± 4.2 | 45.5 ± 5.0 | 0.708 |
BM=bowel movements; LCA=lithocholic acid; CDCA=chenodeoxycholic acid; DCA=deoxycholic acid; CA=cholic acid; ns=not significant
Prior Abdominal Surgeries in the Three Groups
Among the 30 healthy participants, 2 had undergone cholecystectomy (6.7%) and 6 appendectomy. Among the 30 patients with IBS-C, 3 had undergone cholecystectomy (10%), 3 appendectomy, and 1 each rectocele repair, enterocele repair, and banding of internal hemorrhoids. Among the 64 with IBS-D, 10 had undergone cholecystectomy (15.6%), 1 sphincterotomy, 10 appendectomy, and 1 each enterocele repair, intra-abdominal and inguinal hernia repair, and sleeve gastrectomy. Thus, 54 of the patients with IBS-D had not undergone cholecystectomy.
Quantitative Traits and Relationships of Bile Acid, Colonic Transit and Intestinal Permeability
Table 1 shows differences in IBS-C, IBS-D and HV groups in number of bowel movements, stool consistency, colonic transit (GC48h), intestinal permeability, fecal fat, serum FGF19 and C4, and total fecal BA. There were significant associations between total fecal bile acid and colonic transit at 24 hours (rS=0.25, p=0.008) and at 48 hours (rS =0.43; p<0.001) and intestinal permeability (rS =0.23; p=0.015).
Discriminating among Healthy and IBS Groups
Using logistic regression analysis, the pre-specified three variables [total fecal BA, colonic transit at 48 hours, and a measure of small intestinal permeability (Figure 1)] showed: (a) total fecal BA was a significant predictor for HV vs. IBS-D (p=0.025, ROCAUC 0.70), and IBS-C vs. IBS-D (p=0.024, ROCAUC 0.81); (b) colonic transit GC48 was significant (p=0.03, ROCAUC 0.70) in discriminating HV from IBS-C, and IBS-C from IBS-D (p<0.001, ROCAUC=0.78). Small intestinal permeability was not a significant predictor. The overall model (fecal BA, colonic transit and small intestinal permeability) showed that the combination had greater AUC (discriminatory value) than each item individually for HV from IBS-C (ROCAUC=0.73) and for IBS-C from IBS-D (ROCAUC=0.86). Moreover, colonic transit at 24 hours also modestly increased (in the 2-item model with total fecal BA) the ability of total fecal BA to differentiate health from IBS-D (ROCAUC from 0.72 to 0.73).
Figure 1.
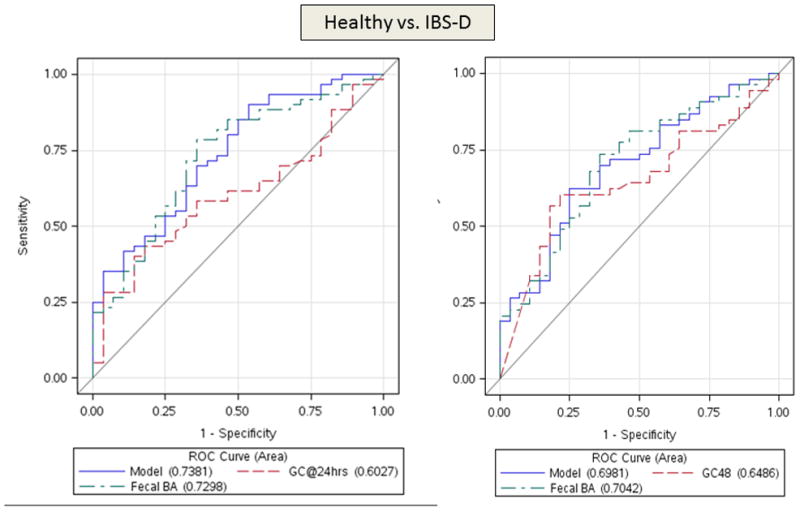
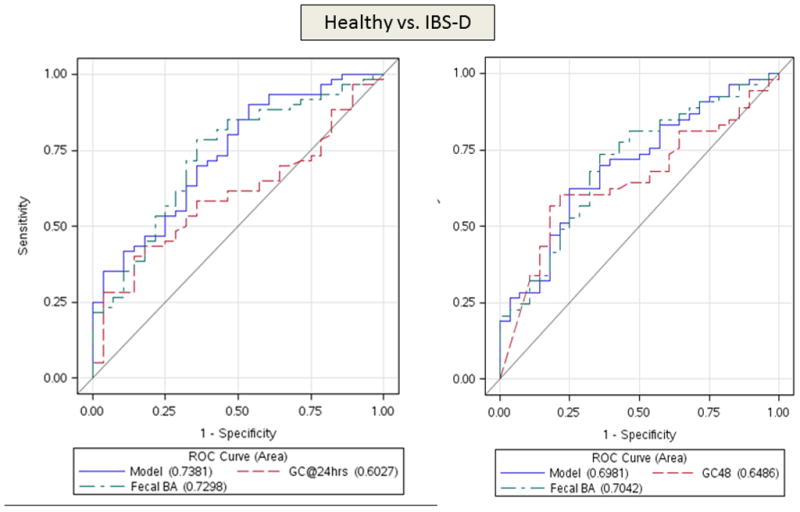
ROC curves demonstrating ability to differentiate IBS-D and healthy volunteers (top panel) using both colonic transit measurements at 24 hours and 48 hours. In the lower panel, ROC curves are based on colonic transit at 48 hours to discriminate IBS-C and healthy volunteers (left) and IBS-D and IBS-C (right). Urine mannitol (0–2 hours) was not significant in any of the logistic models. Note that the two factor model is generally superior to the individual factors, and that colonic transit (GC48h) has a greater influence on the ROC curves in IBS-C compared to health, and approximately equivalent impact to that of total fecal bile acid excretion in discriminating IBS-D and health or IBS-D and IBS-C. Colonic transit GC24h contributes modestly to the discrimination between IBS-D and healthy controls.
Serum C4 and FGF19 measurements did not augment the utility of total fecal bile acids for discriminating among groups.
Estimated Specificity at Different Levels of Sensitivity
Given the relatively high prevalence of IBS in the community, it is most relevant to achieve high specificity at reasonable levels of sensitivity. Thus, we estimated the specificity of the individual traits or models at 60% sensitivity (Table 2) for discriminating between the groups, with specificity ranging from 75% for IBS-D versus health, to 90% for IBS-D versus IBS-C.
Table 2.
Specificity (at 60% sensitivity) of Quantitative Traits to Differentiate Subgroups
| Quantitative Trait(s) | Discrimination of | Specificity |
|---|---|---|
| Total 48h fecal BA excretion | IBS-D and health | 75% |
| Model of fecal BA excretion and colonic transit GC48h | 75% | |
| Colonic transit GC48h | IBS-C and health | 85% |
| Model of fecal BA excretion and colonic transit GC48h | 85% | |
| Total 48h fecal BA excretion | IBS-C and IBS-D | 85% |
| Model of fecal BA excretion and colonic transit GC48h | 90% |
At 80% sensitivity, the 2-item model had 43% specificity to differentiate IBS-D from HV (Figure 1, top panel), 57% specificity for IBS-C from HV (Figure 1, lower left), and 81% specificity to differentiate IBS-C and IBS-D (Figure 1, lower right). For the latter differentiation, there was also 63% specificity at 90% sensitivity.
Overall, these data show that colonic transit and total fecal BA excretion constitute valid biomarkers that could be used for identifying treatable mechanisms in patients with IBS-C or IBS-D.
DISCUSSION
These data show that valid, noninvasive biomarkers of IBS (that is, colonic transit and total fecal BA excretion) constitute targets for treatment with available therapies in IBS, specifically in the IBS-D and IBS-C subgroups.
The objective of biomarkers is not to exclude organic disease, but to corroborate pathophysiological mechanisms that are amenable to therapy in the disease of interest. Exclusion of organic diseases remains essential in IBS, since organic disease may mimic IBS and affect the proposed biomarkers tested in this model. For example, colonic transit may be altered by colon cancer or inflammatory bowel disease, fecal BA excretion by ileal Crohn’s disease, and increased intestinal permeability is observed in small intestinal Crohn’s disease [both in macroscopically normal and abnormal small intestine (42–44)].
These biomarkers were evaluated with rigorously validated techniques for which gender-matched normal values are established. Therefore, colon transit and total fecal BA excretion are demonstrated by the analysis to be significant, either as single factors (colonic transit GC48 and fecal BA excretion for distinguishing health and IBS-C) or as combined factors validated in regression models (colon transit at 24 hours or 48 hours and total fecal BA excretion) for discrimination of treatable biological processes (with high specificity and reasonable sensitivity) in IBS-C from IBS-D, and to a lesser extent for each of the two subgroups of IBS in comparison to healthy controls.
The biomarkers of colonic transit and fecal bile acid excretion are valid and useful biomarkers for identifying treatable mechanisms in IBS because they provide quantitative information with well-established normal values [GC 24 of 1.3–4.4; GC48 1.9–5.0 (45)] for total fecal BA excretion [130–2337mM/48h (25)] and can be used to select pathophysiology-directed therapies for IBS. These include 5-HT4 agonists or secretagogues for delayed colonic transit, 5-HT3 antagonists or opioids for accelerated colonic transit, BA sequestrants (24) or farnesoid X receptor agonists (46) for BA diarrhea in IBS-D, and potentially BA supplementation or inhibition of ileal BA transporter for IBS-C associated with reduced fecal BA excretion (47).
Colonic transit and total fecal BA excretion fulfill criteria as valid biomarkers of the pathogenic processes and responsiveness to treatment, as required by the definition from the Biomarkers Definition Working Group (8). This contrasts with other proposed biomarkers, such as breath methane in IBS-C, fecal calprotectin or fecal granins in IBS-D, that do not necessarily identify a specific pathophysiology that can be addressed therapeutically. Though we have used scintigraphic measurements of colonic transit, similar observations of abnormal transit using radiopaque markers in patients with IBS with diarrhea or constipation (48) suggest that the latter measurements (that are more widely available) could potentially substitute for scintigraphic transit. Similarly, the availability in some countries of 75SeHCAT testing (49) or serum FGF19 (50) or C4 measurement for documentation of bile acid malabsorption suggests that these biomarker measurements may be adapted to suit the needs in clinical diagnosis (49) and research.
Potential Limitations
The quantitative traits measured are not completely independent. Thus, fast or slow colonic transit could contribute to the higher or lower total fecal bile acid excretion documented. However, they are statistically significant predictors of phenotype and, therefore, enhance the validity of the biomarkers separately or in combination. Similarly, alterations in transit (e.g. gastric emptying) may have contributed to the differences in the urinary excretion of mannitol from 0–2 hours; however, this is unlikely since there were no significant differences in the rates of gastric emptying or orocecal transit among the three groups. Moreover, the marker for small bowel permeability did not constitute an independent biomarker to differentiate the phenotypes of health and the subgroups of IBS, and therefore it was excluded from the 2-item model used to quantitate the AUC in discriminating health from IBS subgroups.
A second limitation of the current study is that we have addressed the biomarkers in the context of IBS-D and IBS-C, but did not study patients with IBS-mixed (IBS-M) in the current study. We have previously shown, in a prospective study, that IBS-M patients typically have accelerated colonic transit at 48 hours (20).
In conclusion, colonic transit and fecal BA excretion individually and together constitute useful IBS biomarkers to identify treatable pathophysiological processes in IBS, particularly in the subgroups of IBS-D and IBS-C. These data suggest that measuring colonic transit and fecal BA excretion could enhance the management of patients with IBS-D and IBS-C.
Supplementary Material
Acknowledgments
The authors thank Amy Boldingh, Deborah Rhoten and Michael Ryks for technical support, and Cindy Stanislav for secretarial assistance.
Funding
This study was supported by grants from National Institutes of Health (RO1-DK92179 to MC, and Mayo Clinic Center for Clinical and Translational Science grant UL1-TR000135).
Abbreviations
- AUC
area under the curve
- BA
bile acid
- BAM
bile acid malabsorption
- C4
7 α-hydroxy-4-cholesten-3-one
- FGF19
fibroblast growth factor 19
- GC
geometric center
- HAD
Hospital Anxiety and Depression Scale
- 5-HT
serotonin
- IBS
irritable bowel syndrome
- IBS-C
IBS-predominant constipation
- IBS-D
IBS-predominant diarrhea
- ROC
Receiver-operating characteristic curves
Footnotes
Disclosures
The authors have no potential conflicts of interest to disclose.
Authors’ Contributions
M. Camilleri: study concept and design; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript; obtained funding
A. Shin: fellow co-investigator; conduct of the study; critical revision of the manuscript
I. Busciglio: study coordinator
P. Carlson: technical support
A. Acosta: fellow co-investigator; conduct of the study; critical revision of the manuscript
A.E. Bharucha: staff investigator; critical revision of the manuscript
D. Burton: technical support; study supervision
J. Lamsam: technical support
A. Lueke: technical support
L.J. Donato: technical support
A.R. Zinsmeister: staff statistician; study design; analysis and interpretation of data; critical revision of the manuscript
References
- 1.Engsbro AL, Begtrup LM, Kjeldsen J, et al. Patients suspected of irritable bowel syndrome--cross-sectional study exploring the sensitivity of Rome III criteria in primary care. Am J Gastroenterol. 2013;108:972–80. doi: 10.1038/ajg.2013.15. [DOI] [PubMed] [Google Scholar]
- 2.Manning AP, Thompson WG, Heaton KW, et al. Towards positive diagnosis of the irritable bowel. Br Med J. 1978;277:653–54. doi: 10.1136/bmj.2.6138.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kruis W, Thieme C, Weinzierl M, et al. A diagnostic score for the irritable bowel syndrome. Its value in the exclusion of organic disease. Gastroenterology. 1984;87:1–7. [PubMed] [Google Scholar]
- 4.Camilleri M. Do the symptom-based, Rome criteria of irritable bowel syndrome lead to better diagnosis and treatment outcomes? The con argument. Clin Gastroenterol Hepatol. 2009;8:129. doi: 10.1016/j.cgh.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sainsbury A, Sanders DS, Ford AC. Prevalence of irritable bowel syndrome-type symptoms in patients with celiac disease: a meta-analysis. Clin Gastroenterol Hepatol. 2013;11:359–65. doi: 10.1016/j.cgh.2012.11.033. [DOI] [PubMed] [Google Scholar]
- 6.Ford AC, Bercik P, Morgan DG, et al. Validation of the Rome III criteria for the diagnosis of irritable bowel syndrome in secondary care. Gastroenterology. 2013;145:1262–70. doi: 10.1053/j.gastro.2013.08.048. [DOI] [PubMed] [Google Scholar]
- 7.Camilleri M. Peripheral mechanisms in irritable bowel syndrome. N Engl J Med. 2012;367:1626–35. doi: 10.1056/NEJMra1207068. [DOI] [PubMed] [Google Scholar]
- 8.Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69:89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- 9.Le Nevé B, Posserud I, Böhn L, et al. A combined nutrient and lactulose challenge test allows symptom-based clustering of patients with irritable bowel syndrome. Am J Gastroenterol. 2013;108:786–95. doi: 10.1038/ajg.2013.75. [DOI] [PubMed] [Google Scholar]
- 10.Hwang L, Low K, Khoshini R, et al. Evaluating breath methane as a diagnostic test for constipation-predominant IBS. Dig Dis Sci. 2010;55:398–403. doi: 10.1007/s10620-009-0778-4. [DOI] [PubMed] [Google Scholar]
- 11.Mertz H, Naliboff B, Munakata J, et al. Altered rectal perception is a biological marker of patients with irritable bowel syndrome. Gastroenterology. 1995;109:40–52. doi: 10.1016/0016-5085(95)90267-8. [DOI] [PubMed] [Google Scholar]
- 12.Kindt S, Van Oudenhove L, Broekaert D, et al. Immune dysfunction in patients with functional gastrointestinal disorders. Neurogastroenterol Motil. 2009;21:389–98. doi: 10.1111/j.1365-2982.2008.01220.x. [DOI] [PubMed] [Google Scholar]
- 13.Chang L, Adeyemo M, Karagiannides I, et al. Serum and colonic mucosal immune markers in irritable bowel syndrome. Am J Gastroenterol. 2012;107:262–72. doi: 10.1038/ajg.2011.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohman L, Stridsberg M, Isaksson S, et al. Altered levels of fecal chromogranins and secretogranins in IBS: relevance for pathophysiology and symptoms? Am J Gastroenterol. 2012;107:440–7. doi: 10.1038/ajg.2011.458. [DOI] [PubMed] [Google Scholar]
- 15.El-Salhy M, Seim I, Chopin L, et al. Irritable bowel syndrome: the role of gut neuroendocrine peptides. Front Biosci (Elite Ed) 2012;4:2783–2800. doi: 10.2741/e583. [DOI] [PubMed] [Google Scholar]
- 16.Goepp J, Fowler E, McBride T, Landis D. Frequency of abnormal fecal biomarkers in irritable bowel syndrome. Glob Adv Health Med. 2014;3:9–15. doi: 10.7453/gahmj.2013.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Däbritz J, Musci J, Foell D. Diagnostic utility of faecal biomarkers in patients with irritable bowel syndrome. World J Gastroenterol. 2014;20:363–75. doi: 10.3748/wjg.v20.i2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pavlidis P, Chedgy FJ, Tibble JA. Diagnostic accuracy and clinical application of faecal calprotectin in adult patients presenting with gastrointestinal symptoms in primary care. Scand J Gastroenterol. 2013;48:1048–54. doi: 10.3109/00365521.2013.816771. [DOI] [PubMed] [Google Scholar]
- 19.Jones MP, Chey WD, Singh S, Gong H, Shringarpure R, Hoe N, Chuang E, Talley NJ. A biomarker panel and psychological morbidity differentiates the irritable bowel syndrome from health and provides novel pathophysiological leads. Aliment Pharmacol Ther. 2014;39:426–37. doi: 10.1111/apt.12608. [DOI] [PubMed] [Google Scholar]
- 20.Camilleri M, McKinzie S, Busciglio I, et al. Prospective study of motor, sensory, psychological and autonomic functions in 119 patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2008;6:772–81. doi: 10.1016/j.cgh.2008.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manabe N, Wong BS, Camilleri M, et al. Lower functional gastrointestinal disorders: evidence of abnormal colonic transit in a 287 patient cohort. Neurogastroenterol Motil. 2010;22:293–e82. doi: 10.1111/j.1365-2982.2009.01442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deiteren A, Camilleri M, Bharucha AE, et al. Performance characteristics of scintigraphic colon transit measurement in health and irritable bowel syndrome and relationship to bowel functions. Neurogastroenterol Motil. 2010;22:415–23. doi: 10.1111/j.1365-2982.2009.01441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Camilleri M. Scintigraphic biomarkers for colonic dysmotility. Clin Pharmacol Ther. 2010;87:748–53. doi: 10.1038/clpt.2010.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wedlake L, A’Hern R, Russell D, et al. Systematic Review: the prevalence of idiopathic bile acid malabsorption as diagnosed by SeHCAT scanning in patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2009;30:707–17. doi: 10.1111/j.1365-2036.2009.04081.x. [DOI] [PubMed] [Google Scholar]
- 25.Wong BS, Camilleri M, Carlson P, et al. Increased bile acid biosynthesis is associated with irritable bowel syndrome with diarrhea. Clin Gastroenterol Hepatol. 2012;10:1009–15. doi: 10.1016/j.cgh.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shin A, Camilleri M, Vijayvargiya P, et al. Bowel functions, fecal unconjugated primary and secondary bile acids, and colonic transit in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2013;11:1270–5. doi: 10.1016/j.cgh.2013.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Camilleri M, Nadeau A, Tremaine WJ, et al. Measurement of serum 7alpha-hydroxy-4-cholesten-3-one (or 7alphaC4), a surrogate test for bile acid malabsorption in health, ileal disease and irritable bowel syndrome using liquid chromatography-tandem mass spectrometry. Neurogastroenterol Motil. 2009;21:734–e43. doi: 10.1111/j.1365-2982.2009.01288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rao AS, Wong BS, Camilleri M, Odunsi-Shiyanbade ST, McKinzie S, Ryks M, Burton D, Carlson P, Lamsam J, Singh R, Zinsmeister AR. Chenodeoxycholate in females with irritable bowel syndrome-constipation: A pharmacodynamic and pharmacogenetic analysis. Gastroenterology. 2010;139:1549–58. doi: 10.1053/j.gastro.2010.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Camilleri M, Lasch K, Zhou W. Irritable bowel syndrome: methods, mechanisms, and pathophysiology. The confluence of increased permeability, inflammation, and pain in irritable bowel syndrome. Am J Physiol. 2012;303:G775–85. doi: 10.1152/ajpgi.00155.2012. [DOI] [PubMed] [Google Scholar]
- 30.Vazquez-Roque MI, Camilleri M, Smyrk T, Murray J, Marietta E, O’Neill J, Carlson P, Lamsam J, Janzow D, Eckert D, Burton D, Zinsmeister AR. A controlled trial of gluten-free diet in irritable bowel syndrome-diarrhea: effect on bowel frequency and intestinal functions. Gastroenterology. 2013;144:903–11. e3. doi: 10.1053/j.gastro.2013.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Talley NJ, Phillips SF, Wiltgen CM, et al. Assessment of functional gastrointestinal disease: the bowel disease questionnaire. Mayo Clin Proc. 1990;65:1456–79. doi: 10.1016/s0025-6196(12)62169-7. [DOI] [PubMed] [Google Scholar]
- 32.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 33.Longstreth GF, Thompson WG, Chey WD, et al. Functional bowel disorders. Gastroenterology. 2006;130:1480–91. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 34.Sauter GH, Münzing W, von Ritter C, et al. Bile acid malabsorption as a cause of chronic diarrhea: diagnostic value of 7alpha-hydroxy-4-cholesten-3-one in serum. Dig Dis Sci. 1999;44:14–9. doi: 10.1023/a:1026681512303. [DOI] [PubMed] [Google Scholar]
- 35.Brydon WG, Nyhlin H, Eastwood MA, et al. Serum 7 alpha-hydroxy-4-cholesten-3-one and seleno-homocholyltaurine (SeHCAT) whole body retention in the assessment of bile acid induced diarrhoea. Eur J Gastroenterol Hepatol. 1996;8:117–23. doi: 10.1097/00042737-199602000-00005. [DOI] [PubMed] [Google Scholar]
- 36.Gälman C, Arvidsson I, Angelin B, et al. Monitoring hepatic cholesterol 7alpha-hydroxylase activity by assay of the stable bile acid intermediate 7alpha-hydroxy-4-cholesten-3-one in peripheral blood. J Lipid Res. 2003;44:859–66. doi: 10.1194/jlr.D200043-JLR200. [DOI] [PubMed] [Google Scholar]
- 37.Tagliacozzi D, Mozzi AF, Casetta B, et al. Quantitative analysis of bile acids in human plasma by liquid chromatography-electrospray tandem mass spectrometry: a simple and rapid one-step method. Clin Chem Lab Med. 2003;41:1633–41. doi: 10.1515/CCLM.2003.247. [DOI] [PubMed] [Google Scholar]
- 38.Walters JR, Tasleem AM, Omer OS, et al. A new mechanism for bile acid diarrhea: defective feedback inhibition of bile acid biosynthesis. Clin Gastroenterol Hepatol. 2009;7:1189–94. doi: 10.1016/j.cgh.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 39.Odunsi ST, Camilleri M, McKinzie S, et al. Effects of chenodeoxycholate and a bile acid sequestrant, colesevelam, on intestinal transit and bowel function. Clin Gastroenterol Hepatol. 2010;8:159–65. doi: 10.1016/j.cgh.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Camilleri M, Nadeau A, Lamsam J, et al. Understanding measurements of intestinal permeability in healthy humans with urine lactulose and mannitol excretion. Neurogastroenterol Motil. 2010;22:e15–26. doi: 10.1111/j.1365-2982.2009.01361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rao AS, Camilleri M, Eckert DJ, et al. Urine sugars for in vivo gut permeability: validation and comparisons in irritable bowel syndrome-diarrhea and controls. Am J Physiol. 2011;301:G919–28. doi: 10.1152/ajpgi.00168.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bjarnason I, O’Morain C, Levi AJ, et al. Absorption of 51chromium-labeled ethylenediaminetetraacetate in inflammatory bowel disease. Gastroenterology. 1983;85:318–22. [PubMed] [Google Scholar]
- 43.Miki K, Moore DJ, Butler RN, et al. The sugar permeability test reflects disease activity in children and adolescents with inflammatory bowel disease. J Pediatr. 1998;133:750–4. doi: 10.1016/s0022-3476(98)70145-3. [DOI] [PubMed] [Google Scholar]
- 44.Peeters M, Ghoos Y, Maes B, et al. Increased permeability of macroscopically normal small bowel in Crohn’s disease. Dig Dis Sci. 1994;39:2170–6. doi: 10.1007/BF02090367. [DOI] [PubMed] [Google Scholar]
- 45.Kolar GJ, Camilleri M, Burton D, et al. Prevalence of colonic motor or evacuation disorders in patients presenting with chronic nausea and vomiting evaluated by a single gastroenterologist in a tertiary referral practice. Neurogastroenterol Motil. 2014;26:131–8. doi: 10.1111/nmo.12242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnston IM, Nolan JD, Dew T, et al. A new therapy for chronic diarrhea? A proof of concept study of the FXR agonist obeticholic acid in patients with primary bile acid diarrhea. Gastroenterology. 2013;144(Suppl 1):S60. [Google Scholar]
- 47.Camilleri M. Pharmacological agents currently in clinical trials for disorders of neurogastroenterology. J Clin Invest. 2013;123:4111–20. doi: 10.1172/JCI70837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Törnblom H, Van Oudenhove L, Sadik R, et al. Colonic transit time and IBS symptoms: what’s the link? Am J Gastroenterol. 2012;107:754–60. doi: 10.1038/ajg.2012.5. [DOI] [PubMed] [Google Scholar]
- 49.Gracie DJ, Kane JS, Mumtaz S, Scarsbrook AF, Chowdhury FU, Ford AC. Prevalence of, and predictors of, bile acid malabsorption in outpatients with chronic diarrhea. Neurogastroenterol Motil. 2012;24:983–e538. doi: 10.1111/j.1365-2982.2012.01953.x. [DOI] [PubMed] [Google Scholar]
- 50.Pattni SS, Brydon WG, Dew T, et al. Fibroblast growth factor 19 in patients with bile acid diarrhoea: a prospective comparison of FGF19 serum assay and SeHCAT retention. Aliment Pharmacol Ther. 2013;38:967–76. doi: 10.1111/apt.12466. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


