Abstract
Background
A low-level inflammation has been hypothesized to mediate visceral hypersensitivity in functional bowel disorders that persist after or even in the absence of gut inflammation. We aimed to test the efficacy of a steroidal anti-inflammatory treatment, and identify local inflammatory molecules mediating post- and non-inflammatory colorectal hypersensitivity using two mouse models.
Methods
Visceromotor responses to colorectal distension were quantified as a measure of colorectal sensitivity. On day 1, mice received intracolonic saline (control), trinitrobenzenesulfonic acid (post-inflammatory on day 15), or acidified hypertonic saline (non-inflammatory). Colorectal sensitivity before (day 10) and after (day 15) four-day dexamethasone treatment was compared, and colonic gene expression of inflammatory molecules was quantified.
Results
Dexamethasone effectively inhibited gene expression of inflammatory molecules such as interleukin (IL)-1β and mast cell protease-1 in the colon, but did not attenuate colorectal hypersensitivity in either model. Gene expression of inflammatory molecules in the colon did not differ between control and the non-inflammatory model, but the post-inflammatory model showed increased IL-10 and tight junction protein 2, and decreased IL-6, transforming growth factor (TGF)-β, a precursor of β-endorphin, occludin, and mucin 2. While no common molecule explained colorectal hypersensitivity in these models, hypersensitivity was positively correlated with TGF-β2 mRNA in control, and with IL-1β, inhibin βA and prostaglandin E2 synthase in the dexamethasone-treated post-inflammatory model. In the non-inflammatory model, cyclooxygenase-2 mRNA was negatively correlated with colorectal sensitivity.
Conclusion
These results suggest that persistent functional colorectal hypersensitivity is mediated by condition-specific mediators whose gene expression in the colon is not inevitably sensitive to steroidal anti-inflammatory treatment.
Keywords: colorectal hypersensitivity, inflammation, inflammatory molecules, cytokines
INTRODUCTION
Molecules mediating an inflammatory reaction (e.g., cytokines, prostaglandins, neuropeptides) commonly lead to excitation and/or sensitization of nociceptive sensory neurons, which underlie spontaneous pain arising from and hypersensitivity to stimuli applied to the affected area. In line with this, inflammation in the large intestine has been considered a primary cause of abdominal pain, discomfort and colorectal hypersensitivity associated with inflammatory bowel diseases (IBD), not only in flares but also in remission, and even irritable bowel syndrome (IBS) categorized as ‘functional’ because of the absence of apparent organic abnormalities. Although not consistent across studies, increases in the number of immune cells and the content of inflammatory molecules have been reported in intestinal biopsies or blood from patients with quiescent IBD or IBS(1-3), supporting the hypothesis that an on-going ‘low-grade inflammation’ or low-level inflammatory tissue environment causes abdominal pain and hypersensitivity in these post- and (formerly thought to be) non-inflammatory conditions.
The ‘low-grade inflammation’ hypothesis consequently asks two essential questions: 1) what immune cells/inflammatory molecules mediate abdominal pain/hypersensitivity in quiescent IBD and IBS? and 2) would anti-inflammatory treatments effectively reduce abdominal pain/hypersensitivity in quiescent IBD and IBS? With respect to the first question, an increase in intestinal mast cell mediators in the media of cultured biopsies from IBS patients has been reported(4) which, further, is excitatory when applied to rat mesenteric nerve fibers and cultured sensory neurons(5). Recently, Hughes et al(6) reported increased inflammatory cytokines in the media of cultured peripheral blood mononuclear cells from diarrhea-predominant IBS (D-IBS) patients, some of which (interleukin [IL]-1β, IL-6 and tumor necrosis factor [TNF]-α) sensitized mechanosensitive mouse colorectal afferents. In addition, constipation-predominant IBS patients showed a profile of inflammatory cytokines different from D-IBS patients, suggesting a condition-specific profile and role of inflammatory molecules in different abdominal pain/hypersensitivity conditions.
Several studies have addressed the second question by examining the effect of steroids (prednisolone) or aminosalicylate (mesalamine) on pain/hypersensitivity in IBS patients; prednisolone was found ineffective in post-infectious (PI) IBS patients(7) and mesalamine was inconsistent in its efficacy(8-11). These outcomes are not consistent with the evidence that inflammatory molecules are increased in quiescent IBD and IBS and are able to excite/sensitize visceral sensory nerves. Questioning whether low-grade intestinal inflammation is indeed the underlying mechanism for abdominal pain/hypersensitivity, studies have also reported no obvious correlation between the extent of inflammation and pain/hypersensitivity in either humans or experimental animals(12-15).
Prompted by these conflicting observations regarding the role of inflammation in abdominal pain/hypersensitivity associated with quiescent IBD and IBS, we addressed the above two questions using validated mouse models of functional colorectal hypersensitivity: a post-trinitrobenzenesulfonic acid (TNBS) mouse model that represents post-inflammatory (IBD in remission or PI-IBS) hypersensitivity after mild colitis (16, 17), and an acidified hypertonic saline (AHS) model that represents non-inflammatory (IBS in general) hypersensitivity(18). First, we examined the effect of steroidal anti-inflammatory treatment in the two colorectal hypersensitivity models. Second, we attempted to identify common or condition-specific colonic inflammatory molecules mediating colorectal hypersensitivity and the signature profile characterizing each condition.
MATERIALS AND METHODS
Animal
Adult male C57BL/6J (Jackson Laboratory, Bar Harbor, ME) mice (25–30 g), housed under a 12/12-hr light/dark cycle in an AAALAC accredited facility, were used throughout. Water and food were provided ad libitum. All procedures were approved by the Institutional Animal Care and Use Committee, University of Pittsburgh.
Colorectal sensitivity
A pair of sterile wire electrodes was surgically implanted into the external oblique abdominal musculature with the tips of other ends exposed at the back of the neck for electromyographic recordings of the visceromotor response (VMR) to colorectal distension (CRD) as a measure of colorectal sensitivity. At least five days after electrode implantation, mice were briefly sedated (3% isoflurane) and a 2 cm-long balloon connected to a distension device was inserted trans-anally into the colorectum 1 cm proximal from the anus. The mouse was placed in a plastic cylinder to restrict movement and allowed 30 min to recover from isoflurane sedation. Baseline VMRs to an ascending series of graded CRD (15, 30, 45 and 60 mmHg for 10 sec every 4 min, 3 repetitions at each intensity) were measured two days before day 1 when mice received intracolonic saline, TNBS, or AHS. Colorectal sensitivity was measured on days 10 and 15; VMRs were normalized to the maximum baseline response in each mouse.
Intracolonic treatments
Under 3% isoflurane, 0.1 ml of control saline (in mM: NaCl 140, KCl 5, HEPES 10, MgCl2 1, CaCl2 3 and D-glucose 10, pH 7.4, 300 mosM), TNBS (10 mg ml−1 in 50% ethanol, Sigma-Aldrich) or AHS (pH 6.0, 800 mosM by adding D-mannitol, Sigma-Aldrich) was instilled inside the colorectal lumen trans-anally via a 22 gauge feeding needle. Control saline and AHS were instilled once daily for three consecutive days as reported previously(18).
Dexamethasone treatments
Mice were treated with dexamethasone 21 phosphate disodium salt (Dex, 5 mg kg−1, i.p., Sigma-Aldrich) once daily for four days (days 11-14). Experimental groups with active colon inflammation on day 3 after TNBS received Dex once daily for two days (days 1 and 2). The dose and days of dexamethasone treatment used in this study were previously reported to effectively reduce TNBS-induced colonic inflammation, and post-infectious intestinal dysmotility and visceral hypersensitivity in mice(19, 20).
Gene transcripts assay for colonic inflammatory molecules
One day after final measurements of colorectal sensitivity (day 16), mice were euthanized by CO2 inhalation followed by cervical dislocation, and their full thickness distal colons homogenized in 2 ml of TRIzol® (Invitrogen, Carlsbad, CA) to isolate RNA followed by isopropanol precipitation. Pellets were washed with 70% ethanol, suspended in RNase-free water and the concentration of RNA determined. Five micrograms of RNA was treated with DNase to remove genomic DNA, 1 μg of which was used to generate first strand cDNA by random-hexamer/oligo-d(T)-primed, Superscript II (Invitrogen)-mediated reverse transcription. Gene expression of inflammatory molecules and their receptors were measured by quantitative polymerase chain reaction (qPCR) on a CFX Connect real time cycler (Bio-Rad, Hercules, CA). The targets and sequences of their primer pairs are listed in Table 1. The gene expression of each inflammatory molecule was quantified by linear regression of individual amplification curves using LinReg PCR(21), and normalized to the quantity of internal reference standard gene transcript GAPDH.
Table 1.
Primer sequences (5′→3′)
| Forward | Reverse | Accession # | Size (bp) | |
|---|---|---|---|---|
| GAPDH | GTTTGTGATGGGTGTGAACCAC | TGGATGCAGGGATGATGTTCTG | NM_008084 | 263 |
| IL-1β | GGTACATCAGCACCTCACAA | TTAGAAACAGTCCAGCCCATAC | NM_008361.3 | 124 |
| TNF-α | CTACCTTGTTGCCTCCTCTTT | GAGCAGAGGTTCAGTGATGTAG | NM_013693.3 | 116 |
| IL-6 | GATAAGCTGGAGTCACAGAAGG | TTGCCGAGTAGATCTCAAAGTG | NM_031168.1 | 105 |
| TGF-β1 | GTGCGGCAGCTGTACATTGACTTT | TGTACTGTGTGTCCAGGCTCCAAA | NM_011577.1 | 127 |
| TGF-β2 | GGAACCACTGACCATTCTCTATT | CTGGCTTTCCCAAGGACTTTA | NM_009367.3 | 89 |
| TGF-βR1 | ACTACCCTTTGAGGAAGGCAGCTT | AGACCCAACGGACTGACTTTGACA | NM_009370.2 | 202 |
| BAMBI | CACTCCAGCTACTTCTTCATC | GTAGCATCTGATCTCTCCTTTG | NM_026505.2 | 85 |
| Inh βA | AGCCAGGAAGACACTGCACTTTGA | TGGTGACTTTGGTCCTGGTTCTGT | NM_008380.1 | 125 |
| Act R2B | CACAAGAAGATGAGGCCCACGATT | TTTAGGGAGCAGGTCCACATTGGT | NM_007397.2 | 228 |
| FST | TGGATCTTGCAACTCCATCTCGGA | TGCCCAAAGGCTATGTCAACACTG | NM_008046.2 | 137 |
| IL-10 | TGAATTCCCTGGGTGAGAAGCTGA | TGGCCTTGTAGACACCTTGGTCTT | NM_010548.2 | 147 |
| IL-10RA | CACCAAGTAGACAGTGGAATC | GTCCAGAGGGTCAAGTTTATG | NM_008348.2 | 116 |
| COX-1 | AAGATGGGTCCTGGCTTTAC | GGTGATACTGTCGTTCCAGATT | NM_008969.3 | 89 |
| COX-2 | GAAGATTCCCTCCGGTGTTT | CCCTTCTCACTGGCTTATGTAG | NM_011198.3 | 95 |
| PGES | CCACACTCCCTCTTAACCATAAA | GCCAGAATTGTAGGTAGGTCTG | NM_022415.3 | 108 |
| SP | CATGGCCAGATCTCTCACAAA | GCATCGCGCTTCTTTCATAAG | NM_009311.2 | 101 |
| CGRPα | TTTCCTGGTTGTCAGCATCTT | CAGGCGAACTTCTTCTTCACT | NM_007587.2 | 122 |
| CGRPβ | AGTTGAACTCACCATGCCTATTA | ACGGTGGTTTCCCTCATTATC | NM_054084.2 | 108 |
| MCPT-1 | ACCTCAGAAACCCTGAGAGA | CCACACAGACCTGGAAGTTATAG | NM_008570.1 | 98 |
| POMC | CAAGAACGCCATCATCAAGAAC | TCTAAGAGGCTAGAGGTCATCAG | NM_001278581.1 | 115 |
| Occludin | CTGTGATGTGTGTTGAGCTTTG | GGCTGCTGCAAAGATTGATTAG | NM_008756.2 | 91 |
| Claudin 3 | CACCCACCAAGATCCTCTATTC | TTCATCGACTGCTGGTAGTG | NM_009902.4 | 140 |
| TJP 1 | CATCTCCAGTCCCTTACCTTTC | CCTCCAGGCTGACATTAGTTAC | NM_009386.2 | 96 |
| TJP 2 | TGTGAAGCAGATGTGAAGAGG | GGCACTTAGAGAGTCGTGATAAA | NM_001198985.1 | 99 |
| Mucin 1 | TACCCTACCTACCACACTCAC | GAGAGACTGCTACTGCCATTAC | NM_013605.2 | 101 |
| Mucin 2 | CCTCATCATGGACAGCCTATTC | GTACACTGGCACACTCCATATT | NM_023566.2 | 122 |
Data Analyses
Data were expressed as mean±S.E. with n, the number of samples. Proportional data were analyzed by Fisher's exact test (FET). Differences among group means were analyzed by one- (models), two- (before vs. after; Dex vs. Veh) or three-way (models; Veh vs. Dex; normosensitive vs. hypersensitive) ANOVAs with Tukey's multiple comparison tests. When a statistically significant interaction was detected between/among the variables, Bonferroni's post hoc tests were used for pairwise comparisons. Correlation between the magnitude of colorectal sensitivity at 60 mmHg CRD and the amount of gene transcript was analyzed by linear regression. Results were considered statistically significant when p<0.05. In cases where p was greater than 0.05 but less than 0.1, the exact p value is presented.
RESULTS
Effects of dexamethasone (Dex) on colorectal sensitivity
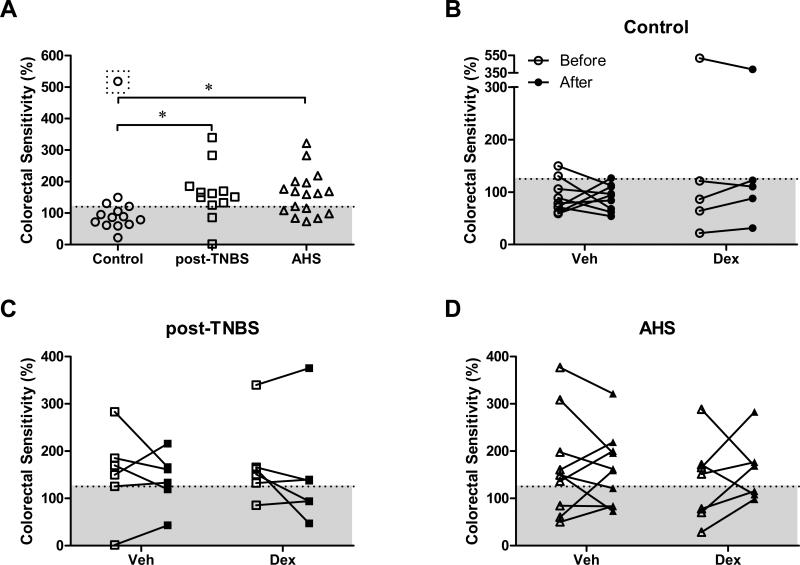
In C57BL/6 mice treated with intracolonic TNBS, inflammation subsides in 10-14 days but colorectal hypersensitivity to CRD persists(16, 17), modeling post-inflammatory colorectal hypersensitivity (post-TNBS). Comparatively, AHS-treated mice show long-lasting hypersensitivity to CRD without any histological/biochemical evidence of inflammation in the colorectum(18), modeling non-inflammatory colorectal hypersensitivity. Replicating these previous findings, we observed significant colorectal sensitivity to 60 mmHg CRD in post-TNBS and AHS groups on day 10 (Fig 1A). Examining each subject individually, however, we noted that some mice were not clearly hypersensitive to CRD after treatments (i.e., colorectal sensitivity was ≤125% of the baseline response, shaded area in Fig 1): 2/12 in post-TNBS and 7/17 in AHS groups. In saline-treated controls, on the other hand, 3 of 14 mice spontaneously developed hypersensitivity, one of which was an outlier; the VMR to 60 mmHg CRD was greater than four times standard deviation (518% of the maximum baseline response, in a dotted box in Fig 1A). The proportion of hypersensitive mice was greater in post-TNBS (p<0.01 by FET) and AHS (p=0.067) groups than in the control group. Excluding the above-mentioned outlier from the control group revealed statistically significant differences in means between control (87.2±9.5%, n=13) and post-TNBS mice (162.6±24.8%, n=12, p<0.05 by 1 way ANOVA with Tukey's test) and between control and AHS-treated mice (161.1±16.9%, n=17, p<0.05). However, we did not exclude this hypersensitive outlier in the control group when comparing gene expression of colonic inflammatory molecules between normosensitive and hypersensitive mice, and correlating it with the magnitude of colorectal sensitivity.
Fig 1.
Effect of dexamethasone (Dex) on post-inflammatory (post-TNBS) and non-inflammatory (AHS) colorectal hypersensitivity in the mouse. (A) Scatter plot showing colorectal sensitivity at 60 mmHg colorectal distension (CRD) measured on day 10 in individual mouse (% of maximum baseline responses). Note the outlier in the control group (in a dotted box). Excluding the outlier revealed significantly greater colorectal sensitivity in post-TNBS and AHS groups than in control (* p<0.05 by 1-way ANOVA). (B-D) Dex (5 mg kg-1), administered once daily for four days (days 11-14) did not alleviate colorectal hypersensitivity in any groups (before, day 10; after, day 15). Veh, saline vehicle. Mice with colorectal sensitivity greater than 125% (above shaded area) were considered hypersensitive to CRD.
After measuring their colorectal sensitivity on day 10, we administered vehicle (Veh) or Dex once daily for four days (days 11-14) and measured the VMR to CRD again on day 15 to study a role of inflammatory molecules in persistent colorectal hypersensitivity in these mice. Dex was ineffective in reducing the magnitude of colorectal hypersensitivity in any group (Fig 1B-D), although it effectively decreased gene expression of some inflammatory molecules (e.g., IL-1β, MCPT-1, and IL-10RA, see below).
Gene expression of inflammatory molecules in the colon
To profile the inflammatory milieu in post- and non-inflammatory hypersensitive conditions, gene transcripts of inflammatory molecules were examined from individual colons one day after the last CRD session, which made it possible to quantify multiple inflammatory molecules from a single sample with high sensitivity, and correlate their gene expression with the magnitude of colorectal sensitivity for identification of molecules underlying persistent functional colorectal hypersensitivity. The following inflammatory molecules were quantified: pro-inflammatory cytokines and mast cell mediators such as IL-1β, IL-6, TNF-α, and mast cell protease-1 (MCPT-1); immune-regulatory cytokines and their receptors such as transforming growth factor (TGF)-β1, TGF-β2, TGF-β receptor 1 (TGF-βR1), BMP and Activin membrane-bound inhibitor homolog (BAMBI, a mock TGF-β receptor), inhibin βA (a subunit of Activin A), Activin receptor 2B (Act R2B), follistatin (FST, endogenous antagonist for Activin), IL-10, IL-10 receptor A (IL-10RA); prostaglandin synthases such as cyclooxygenase (COX)-1, COX-2, prostaglandin E2 synthase (PGES); neuropeptides mediating/modulating inflammation such as substance P (SP), calcitonin gene-related peptide α (CGRPα), CGRPβ, and proopiomelanocortin (POMC, a precursor of β-endorphin); molecules for mucosal barrier function such as occludin, claudin 3, tight junction protein (TJP) 1 and 2, and mucin 1 and 2.
For relative quantification of gene expression, we normalized the amount of each inflammatory molecule gene transcript to that of the internal reference standard GAPDH. Two-way ANOVAs revealed no differences in GAPDH expression either among mouse models (F(2,37)=0.99, p=0.38) or between Veh and Dex treatments (F(1,37)=0.43, p=0.52) without any interaction between the two variables (F(2,37)=1.1, p=0.34), indicating that any change in gene expression of colonic inflammatory molecules was not due to a model- or treatment-dependent change in reference gene expression.
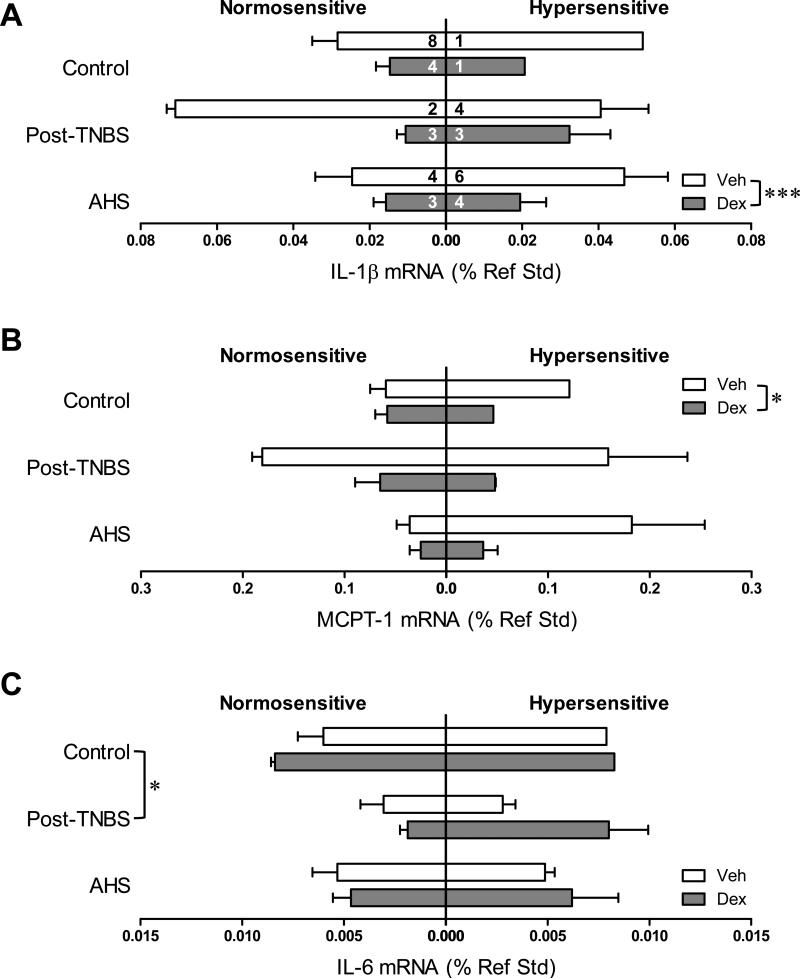
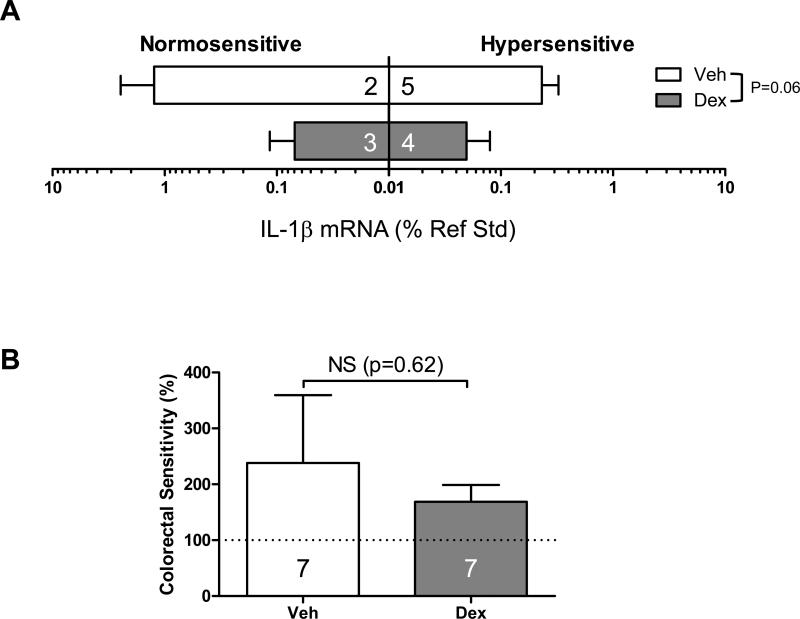
We next examined the colonic gene expression of inflammatory molecules in each model subgrouped by treatment (Veh or Dex) and sensitivity to CRD [normo- (VMR≤125%) or hyper-sensitive (VMR>125%)]. Dex significantly reduced the gene expression of IL-1β (F(1,31)=13.9, p<0.01 by 3 way ANOVA, Fig 2A), MCPT-1 (F(1,31)=4.9, p<0.05, Fig 2B), and IL-10RA (F(1,31)=5.3, p<0.05, Fig 4B) without alleviating colorectal hypersensitivity as described above. The disconnect between the effect of Dex on gene expression of IL-1β, MCPT-1, and IL-10RA and that on colorectal hypersensitivity was further tested in a mouse model of active colon inflammation (TNBS-treated mice on day 3). In the colons from these mice, gene expression of IL-1 β (but neither MCPT-1 nor IL-10RA) was highly up-regulated (18.4-fold increase, n=7, p=0.006 vs. control by Mann-Whitney U-test). Dex effectively inhibited this up-regulation (F(1, 10)=4.5, p=0.06 vs. Veh by 2 way ANOVA, Fig 3A), but still did not prevent colorectal hypersensitivity (Fig 3B), suggesting that targeting colorectal inflammation with steroids may be beneficial to reduce inflammation itself but not sensory symptom.
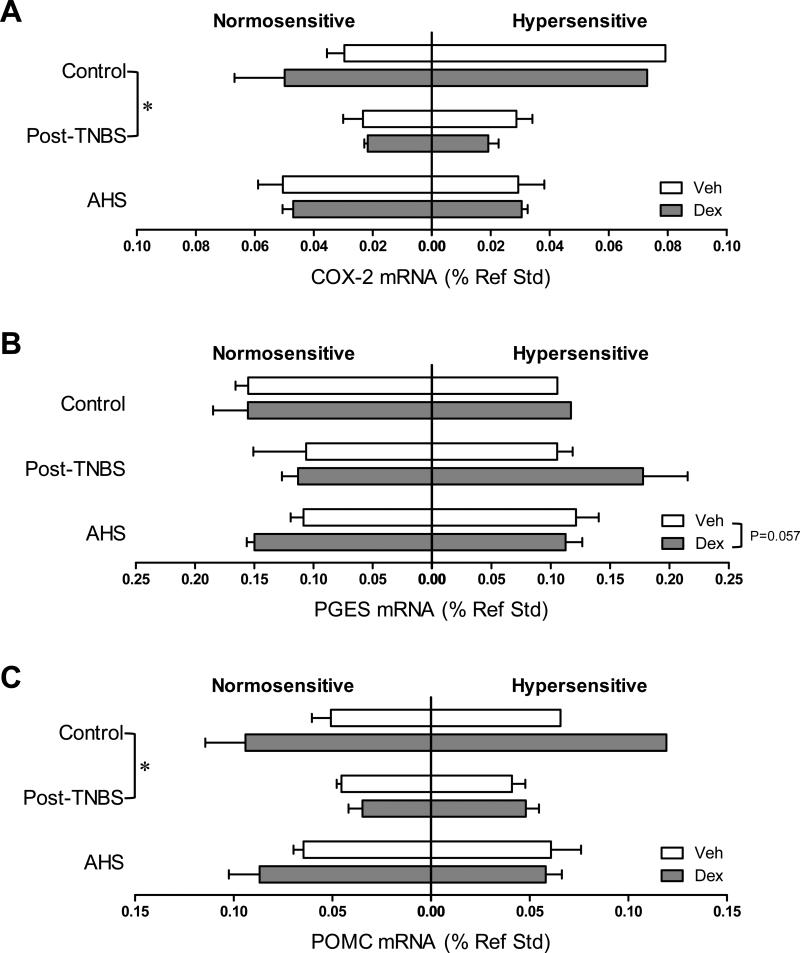
Fig 2.
Colonic gene expression of pro-inflammatory cytokines and mast cell protease. Gene expression data were subgrouped by model (control, post-TNBS, or AHS), treatment (Veh or Dex), and colorectal sensitivity (normo- or hyper-sensitive). Numbers in each horizontal bar in A represent the number of mice in each subgroup throughout. Gene expression of pro-inflammatory cytokines interleukin (IL)-1β (A) and mast cell protease (MCPT)-1 (B) was significantly reduced by Dex (*** p<0.001 and * p<0.05 by 3-way ANOVA). (C) IL-6 gene expression was lower in post-TNBS mice than in control (* p<0.05 by 3-way ANOVA followed by Tukey's multiple comparison tests).
Fig 4.
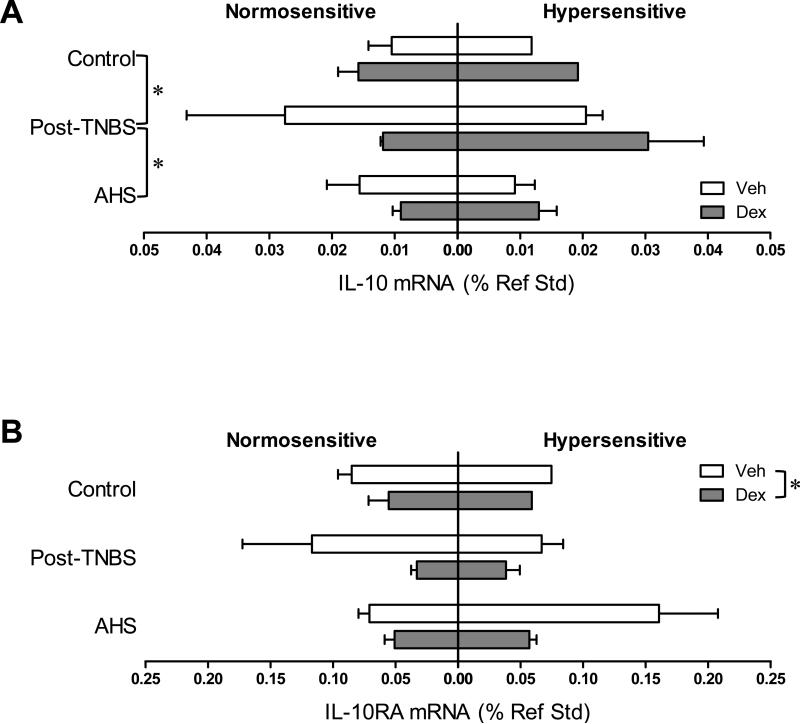
Colonic gene expression of IL-10 and its receptor. (A) IL-10 gene transcript was more abundant in post-TNBS mice than in control and AHS-treated mice (* p<0.05 by 3-way ANOVA followed by Tukey's multiple comparison tests). (B) Gene expression of IL-10 receptor A (RA) was attenuated by Dex (* p<0.05 by 3-way ANOVA).
Fig 3.
Colonic gene expression of IL-1β and colorectal hypersensitivity in mice with active colitis. (A) Dex effectively inhibited up-regulation of IL-1β gene expression in the colons from TNBS-treated mice on day 3. (B) However, Dex did not prevent colorectal hypersensitivity in these mice. Numbers in each bar indicate the number of mice in each group.
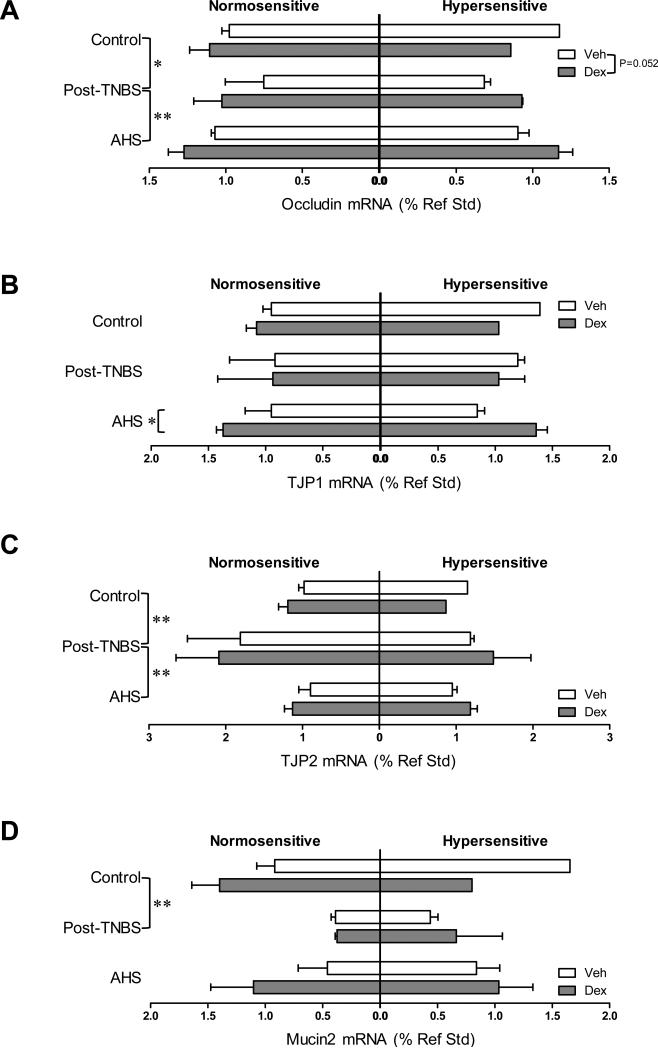
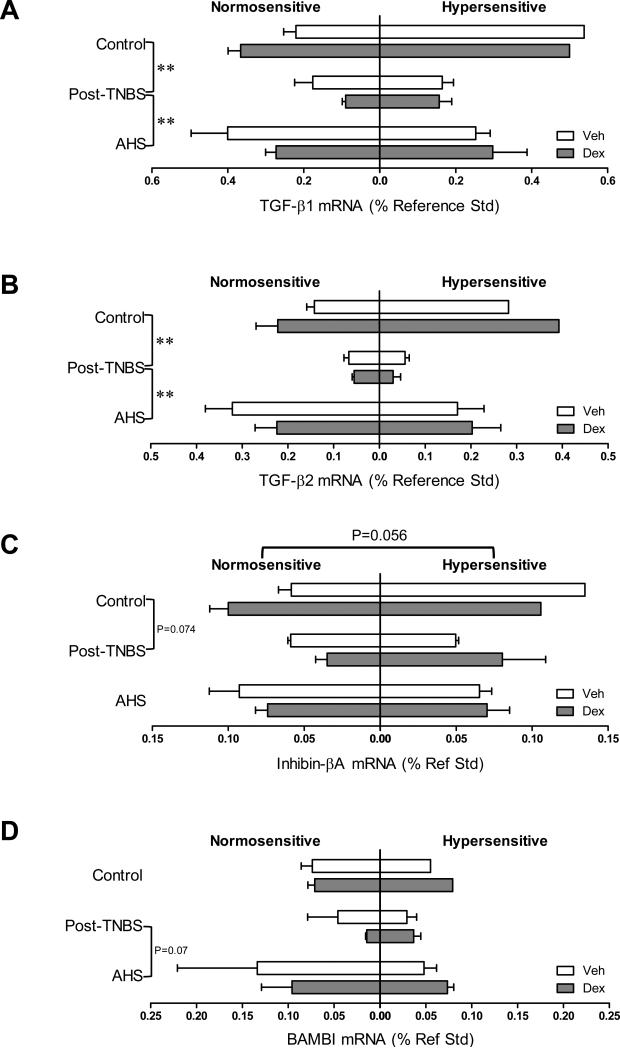
Dex tended to increase the gene expression of PGES and occludin (one of the main components of tight junctions) [F(1,31)=3.9, p=0.057 for PGES (Fig 5B); F(1,31)=4.1, p=0.052 for occludin (Fig 7A)], and significantly increased the gene expression of TJP1 only in AHS/non-inflammatory model (Fmodel × treatment (2,31)=3.1, p=0.061 by 3 way ANOVA and p<0.05 by Bonferroni's t-test, Fig 7B). Colons from post-TNBS/post-inflammatory mice exhibited lower gene expression of IL-6 (p<0.05 by 3 way ANOVA followed by Tukey's test, Fig 2C), COX-2 (p<0.05, Fig 5A), POMC (p<0.05, Fig 5C), and mucin 2 (p<0.01, Fig 7D) than colons from control mice. Post-TNBS/post-inflammatory colons also showed significantly lower gene expression of TGF-β1 (p<0.01, Fig 6A), TGF-β2 (p<0.01, Fig 6B), and occludin (p<0.01, Fig 7A), but higher gene expression of IL-10 (p<0.05, Fig 4A) and TJP2 (p<0.01, Fig 7C) than colons from either control or the AHS/non-inflammatory model. In addition, tendencies to lower gene expression of inhibin βA than in control mice (p=0.074, Fig 6C) and lower gene expression of BAMBI than in the AHS/non-inflammatory mice (p=0.07, Fig 6D) were observed in the post-TNBS/post-inflammatory mice. Gene expression of TNF-α, FST, COX-1, TGF-βR1, Act R2B, SP, CGRPα, CGRPβ, claudin 3, and mucin 1 in the colon did not differ among models, between Veh- and Dex-treated mice, or between normo- and hyper-sensitive mice (data not shown).
Fig 5.
Colonic gene expression of prostaglandin synthases and β-endorphin precursor, proopiomelanocortin (POMC). (A) Gene expression of cyclooxygenase (COX)-2 was lower in post-TNBS mice than in control (* p<0.05 by 3-way ANOVA followed by Tukey's multiple comparison tests). (B) Dex tended to increase the gene expression of prostaglandin E synthase (PGES, p=0.057 by 3 way ANOVA). (C) Gene expression of POMC was lower in post-TNBS mice than in control (p<0.05 by 3-way ANOVA followed by Tukey's multiple comparison tests).
Fig 7.
Colonic gene expression of tight junction proteins and mucins. (A) Gene expression of occludin was decreased in post-TNBS mice, and increased by Dex (p=0.052 by 3-way ANOVA). (B) Gene expression of TJP1 was increased by Dex in AHS-treated mice (p<0.05 by Bonferroni's t-test). (C) Post-TNBS mice showed an increase in the gene expression of TJP2. (D) Gene expression of mucin 2 was lower in post-TNBS mice than in control. ** p<0.01 and * p<0.05 by 3-way ANOVA.
Fig 6.
Colonic gene expression of transforming growth factor (TGF)-β cytokine family and their receptors. Gene expression of TGF-β1 (A) and TGF-β2 (B) was significantly lower in post-TNBS mice than in control or AHS-treated mice (** p<0.01 by 3-way ANOVA followed by Tukey's multiple comparison tests). (C) Gene expression of inhibin βA showed a tendency to a decrease in post-TNBS mice (p=0.074 vs. control). Its gene expression also tended to be higher in hypersensitive mice (p=0.056). (D) Gene expression of the mock receptor for TGF-β, BAMBI, tended to be higher in AHS-treated mice than in post-TNBS mice (p=0.07).
Gene expression of inflammatory molecules correlated with colorectal hypersensitivity
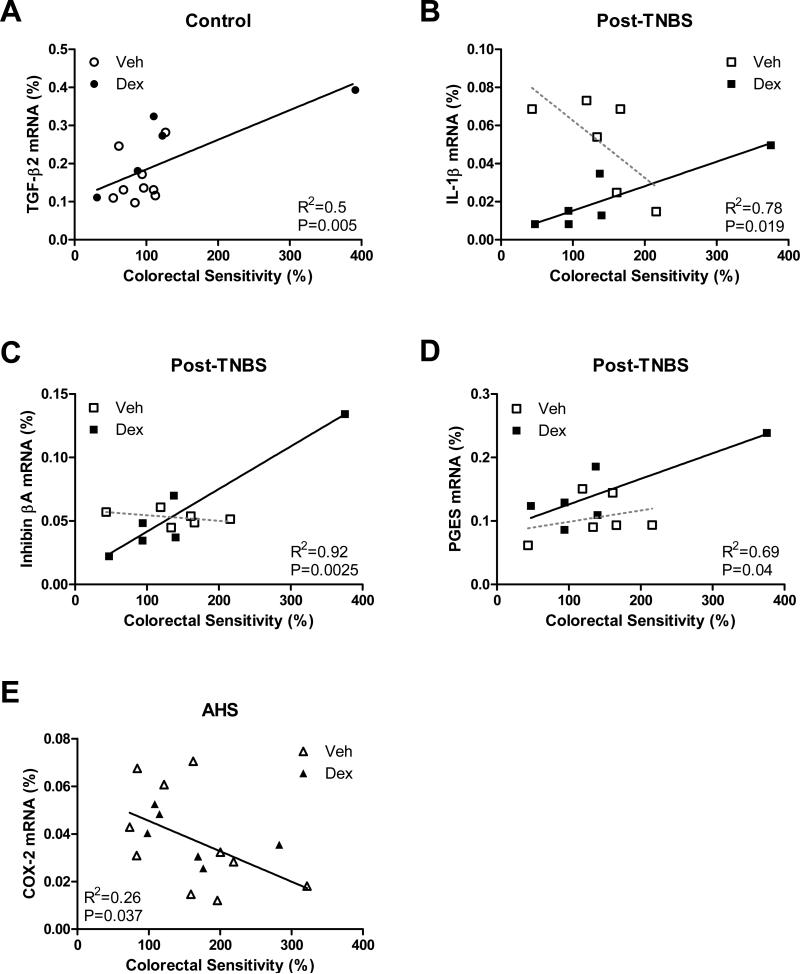
In the gene expression of IL-1β, TGF-β1, TGF-β2, inhibin βA, PGES, and COX-2, we observed a strong interaction between variables (i.e., model, treatment and sensitivity): F model × treatment × sensitivity (2,31)=3.2, p=0.056 for IL-1β; Fmodel × sensitivity (2,31)=9.2, p<0.001 for TGF-β1; Fmodel × sensitivity (2,31)=5.9, p<0.01 for TGF-β2; F model × treatment × sensitivity (2,31)=3.0, p=0.064 for inhibin βA; F model × treatment × sensitivity (2,31)=3.2, p=0.054 for PGES; F model × sensitivity (2,31)=7.3, p<0.01 for COX-2), suggesting that gene expression of these molecules is correlated with the magnitude of colorectal sensitivity in a model- and treatment (condition)-specific fashion. Subsequent pairwise comparisons using Bonferroni's t-tests and regression analyses revealed that colorectal sensitivity was positively correlated with TGF-β2 (R2=0.5, p=0.005, Fig 8A) in control mice, with IL-1β (R2=0.78, p<0.02, Fig 8B), inhibin βA (R2=0.92, p<0.003, Fig 8C) and PGES (R2=0.69, p<0.05, Fig 8D) in Dex-treated post-TNBS/post-inflammatory mice, and negatively with COX-2 gene expression (R2=0.26, p<0.04, Fig 8E) in the AHS /non-inflammatory mice.
Fig 8.
Condition-specific correlation between gene expression of inflammatory molecules and colorectal sensitivity. Colorectal sensitivity was positively correlated with TGF-β2 (A) in control, and with IL-1β (B), inhibin βA (C) and PGES (D) in Dex-treated post-TNBS mice. Broken lines in B-D indicate statistically insignificant linear regressions in Veh-treated post-TNBS mice. (E) Gene expression of COX-2 was negatively correlated with colorectal sensitivity in AHS-treated mice.
When gene expression data from all mice were used for correlation analysis, no common colonic inflammatory molecule was found to significantly account for colorectal hypersensitivity in any group/model; gene expression of inhibin βA showed a tendency to a positive correlation with colorectal sensitivity (p=0.072), but with a poor coefficient of determination (R2=0.077).
DISCUSSION
This study tested the hypothesis that an on-going ‘low-grade’ intestinal inflammation is an underlying mechanism for abdominal pain and hypersensitivity associated with quiescent IBD and IBS by examining the effect of steroidal anti-inflammatory treatment on, and correlating the gene expression pattern of inflammatory molecules in the colon with, post- and non-inflammatory colorectal hypersensitivity using mouse models that represent the two conditions. We found: 1) no inhibitory effect of dexamethasone on colorectal hypersensitivity in any model, 2) model-specific gene expression of inflammatory molecules in the colon, and 3) condition-specific correlation between the magnitude of colorectal sensitivity and gene expression of TGF-β2 (in control), IL-1β, inhibin βA and PGES (in Dex-treated post-TNBS/post-inflammatory condition) and COX-2 (in AHS/non-inflammatory condition). The inability of dexamethasone to affect persistent colorectal hypersensitivity appears to be due to its ineffectiveness on colonic gene expression of important inflammatory molecules in each corresponding condition despite its effective down-regulation of IL-1β, MCPT-1, and IL-10RA gene expression. It is unclear what confers this ‘condition-specificity’ on these inflammatory molecules, but considering ‘context’-dependent differential actions of cytokines in immune regulation(22, 23), one could speculate that the effects of inflammatory molecules on colorectal afferents is determined by conditions of or at their sites of action (properties of both local tissue environment and the afferents themselves). The condition-specific gene expression profiles of inflammatory molecules in the colon support this speculation to some extent in that colons from mice in control, post- and non-inflammatory models with/without Dex treatment have different quality ‘inflammatory milieus’ and hence TGF-β2, IL-1β, Activin A, and prostaglandins may result in different outcomes in each condition. It needs further investigation to understand how these molecules (TGF-β2, IL-1β, Activin A, and prostaglandins) might regulate visceral sensation. Studies suggest that they could directly act on colorectal afferents to increase visceral sensitivity to CRD. Cytokines belonging to the multifunctional TGF-β cytokine superfamily (e.g., TGF-β1 and Activin A) have been shown to up-regulate TRPV1(24-26) in primary afferent neurons to exert nociceptive actions. IL-1β and prostaglandins, specifically PGE2, activate/sensitize primary afferent neurons(27-32). However, the effect of IL-1β on primary afferents is dose-dependent in a bell shape(32) and subpopulation-dependent(33), supporting the notion of its ‘condition-specific’ role in colorectal hypersensitivity. Similarly, PGE2 can also exert inhibitory/analgesic actions in an inflammation state-dependent manner through EP3 receptors that are abundantly expressed in primary afferent neurons(34), and a prostaglandin metabolite can persistently inhibit TRPA1 to cause analgesia(35), both of which might explain the negative correlation between the gene expression of COX-2 and colorectal sensitivity in the AHS/non-inflammatory model.
In addition to potential ‘low-grade inflammation’ in the intestines of patients with quiescent IBD and IBS, compromised intestinal mucosal barriers have been observed in such patients(36), suggesting that increased mucosal permeability renders the tissue underneath more susceptible to pathogen invasion and subsequent local inflammation(37). In the present study, we also found that colons from mice in the post-TNBS/post-inflammatory group had decreased gene expression of occludin, one of the main components of tight junctions, together with mucin 2, a protectant for the mucosal surface, suggesting diminished mucosal barrier function in this mouse model. However, none of these changes accounted for visceral hypersensitivity in this post-inflammatory model. Furthermore, our findings that dexamethasone did not substantially alleviate visceral hypersensitivity despite its beneficial effect on the gene expression of occludin and TJP1 (in the AHS/non-inflammatory model) question a direct cause-effect correlation between increased mucosal permeability and visceral hypersensitivity. It would be interesting though to study whether such changes in mucosal barrier integrity alter other intestinal functions (e.g., colonic motility) in these mouse models and whether dexamethasone could restore the alterations.
Among the various inflammatory molecules quantified in this study (pro- and anti-inflammatory cytokines, mast cell mediators, prostaglandin synthases, and neuropetides for neurogenic inflammation, immune regulation and anti-nociception), we found none commonly correlated with colorectal hypersensitivity in any of the conditions tested. We acknowledge possibilities that 1) other molecules not quantified in the present study could be correlated with colorectal hypersensitivity in these models/conditions, 2) sampling full-thickness colon could obscure a potentially significant focal change in limited areas of the colon wall, to which colorectal hypersensitivity in these models could be attributed, and 3) our assessment of gene transcript quantities might not linearly reflect the amounts of proteins in the colon. However, it is noteworthy that in a study that attempted to correlate visceral sensitivity in IBS patients with immunological changes detected in their colonic mucosa, about 50% of recruited IBS patients (all IBS subtypes pooled) were found to be normally sensitive to rectal distension, resulting in no significant correlation between the two(12). Likewise, patients with quiescent IBD also do not always exhibit IBS-like symptoms. In fact, some patients with mildly active or quiescent IBD are found to be less sensitive to distension(13, 38). To explain this ‘no correlation’ between the visceral inflammatory environment and visceral pain/hypersensitivity, activation of endogenous anti-nociceptive mechanisms has been proposed, including hydrogen sulfide(39), local immune cell-driven β-endorphin(40, 41), and descending inhibitory systems(38, 42). In this study, we quantified the colonic gene expression of POMC, the precursor of β-endorphin, expecting that mice that developed hypersensitivity might have decreased gene expression of this endogenous opioid. We did observe a decrease in the gene expression of POMC in the post-TNBS/post-inflammatory model, but it was not correlated with colorectal sensitivity, suggesting again that peripheral mediators for functional colorectal hypersensitivity could be condition/context-specific, and behavioral functional visceral pain/hypersensitivity is likely to be a net outcome of multiple counteracting/cross-talking factors.
To clearly understand the role of peripheral mediators in visceral pain/hypersensitivity, it seems inevitable to look at not only the periphery (as in this study) but also the visceral sensory system itself that ultimately detects, responds to and transmits intestinal events to the central nervous system for perception. It is not unrealistic that the visceral sensory system undergoes adaptive changes to cope with an intestinal inflammatory environment. In previous studies, we detected increased responses to stretch in muscular class colorectal afferents in post-TNBS and AHS-treated mice(16, 18), which we proposed as contributory to behavioral hypersensitivity to CRD. Although technically challenging, it would be interesting in future studies to correlate neuronal response to inflammatory molecules and gene expression pattern of involved signaling components in colorectal afferent neurons with behavioral hypersensitivity, which might reveal significance of inflammatory molecules whose gene expression ‘in the colon’ was not found to be clearly correlated with behavioral colorectal hypersensitivity in the present study, and suggest potential therapeutic targets ‘in sensory neurons’ for managing abdominal pain/hypersensitivity in quiescent IBD and IBS.
In conclusion, this study demonstrates the presence of condition-specific, steroid-insensitive inflammatory molecules in the colon for mediating persistent colorectal hypersensitivity in post- and non-inflammatory conditions, suggesting that steroid treatments effective in reducing inflammation may not guarantee attenuation of sensory symptoms in quiescent IBD and IBS. Future studies focusing on responses of sensory neurons to local inflammatory molecules and correlating the response characteristics with persistent hypersensitivity in these conditions deserve further attention.
ACKNOWLEDGEMENT
We thank Sonali Joyce for technical assistance in molecular biological experiments.
GRANT
NIH award DK093525
Footnotes
Author Contributions:
La JH: concept and design, acquisition, analysis and interpretation of data; drafting of the manuscript
Gebhart GF: obtaining funding; study supervision; interpretation of data; review of the manuscript.
DISCLOSURE
The authors have nothing to disclose.
REFERENCES
- 1.Bjerrum JT, Hansen M, Olsen J, Nielsen OH. Genome-wide gene expression analysis of mucosal colonic biopsies and isolated colonocytes suggests a continuous inflammatory state in the lamina propria of patients with quiescent ulcerative colitis. Inflammatory bowel diseases. 2010;16:999–1007. doi: 10.1002/ibd.21142. [DOI] [PubMed] [Google Scholar]
- 2.Matricon J, Meleine M, Gelot A, et al. Review article: Associations between immune activation, intestinal permeability and the irritable bowel syndrome. Alimentary pharmacology & therapeutics. 2012;36:1009–1031. doi: 10.1111/apt.12080. [DOI] [PubMed] [Google Scholar]
- 3.Vivinus-Nebot M, Frin-Mathy G, Bzioueche H, et al. Functional bowel symptoms in quiescent inflammatory bowel diseases: role of epithelial barrier disruption and low-grade inflammation. Gut. 2014;63:744–752. doi: 10.1136/gutjnl-2012-304066. [DOI] [PubMed] [Google Scholar]
- 4.Barbara G, Stanghellini V, De Giorgio R, et al. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126:693–702. doi: 10.1053/j.gastro.2003.11.055. [DOI] [PubMed] [Google Scholar]
- 5.Barbara G, Wang B, Stanghellini V, et al. Mast cell-dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology. 2007;132:26–37. doi: 10.1053/j.gastro.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 6.Hughes PA, Harrington AM, Castro J, et al. Sensory neuro-immune interactions differ between irritable bowel syndrome subtypes. Gut. 2013;62:1456–1465. doi: 10.1136/gutjnl-2011-301856. [DOI] [PubMed] [Google Scholar]
- 7.Dunlop SP, Jenkins D, Neal KR, et al. Randomized, double-blind, placebo-controlled trial of prednisolone in post-infectious irritable bowel syndrome. Alimentary pharmacology & therapeutics. 2003;18:77–84. doi: 10.1046/j.1365-2036.2003.01640.x. [DOI] [PubMed] [Google Scholar]
- 8.Andrews CN, Griffiths TA, Kaufman J, Vergnolle N, Surette MG, Rioux KP. Mesalazine (5-aminosalicylic acid) alters faecal bacterial profiles, but not mucosal proteolytic activity in diarrhoea-predominant irritable bowel syndrome. Alimentary pharmacology & therapeutics. 2011;34:374–383. doi: 10.1111/j.1365-2036.2011.04732.x. [DOI] [PubMed] [Google Scholar]
- 9.Corinaldesi R, Stanghellini V, Cremon C, et al. Effect of mesalazine on mucosal immune biomarkers in irritable bowel syndrome: a randomized controlled proof-of-concept study. Alimentary pharmacology & therapeutics. 2009;30:245–252. doi: 10.1111/j.1365-2036.2009.04041.x. [DOI] [PubMed] [Google Scholar]
- 10.Dorofeyev AE, Kiriyan EA, Vasilenko IV, Rassokhina OA, Elin AF. Clinical, endoscopical and morphological efficacy of mesalazine in patients with irritable bowel syndrome. Clinical and experimental gastroenterology. 2011;4:141–153. doi: 10.2147/CEG.S18381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tuteja AK, Fang JC, Al-Suqi M, Stoddard GJ, Hale DC. Double-blind placebo-controlled study of mesalamine in post-infective irritable bowel syndrome--a pilot study. Scandinavian journal of gastroenterology. 2012;47:1159–1164. doi: 10.3109/00365521.2012.694903. [DOI] [PubMed] [Google Scholar]
- 12.Braak B, Klooker TK, Wouters MM, et al. Mucosal immune cell numbers and visceral sensitivity in patients with irritable bowel syndrome: is there any relationship? The American journal of gastroenterology. 2012;107:715–726. doi: 10.1038/ajg.2012.54. [DOI] [PubMed] [Google Scholar]
- 13.Chang L, Munakata J, Mayer EA, et al. Perceptual responses in patients with inflammatory and functional bowel disease. Gut. 2000;47:497–505. doi: 10.1136/gut.47.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larsson MH, Rapp L, Lindstrom E. Effect of DSS-induced colitis on visceral sensitivity to colorectal distension in mice. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2006;18:144–152. doi: 10.1111/j.1365-2982.2005.00736.x. [DOI] [PubMed] [Google Scholar]
- 15.Sharkey KA. Visceral sensation and colitis: inflammation and hypersensitivity do not always go hand in hand. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2006;18:87–90. doi: 10.1111/j.1365-2982.2005.00743.x. [DOI] [PubMed] [Google Scholar]
- 16.Feng B, La JH, Tanaka T, Schwartz ES, McMurray TP, Gebhart GF. Altered colorectal afferent function associated with TNBS-induced visceral hypersensitivity in mice. American journal of physiology Gastrointestinal and liver physiology. 2012;303:G817–824. doi: 10.1152/ajpgi.00257.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanaka T, Shinoda M, Feng B, Albers KM, Gebhart GF. Modulation of visceral hypersensitivity by glial cell line-derived neurotrophic factor family receptor α-3 in colorectal afferents. American journal of physiology Gastrointestinal and liver physiology. 2011;300:G418–424. doi: 10.1152/ajpgi.00456.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.La JH, Feng B, Schwartz ES, Brumovsky PR, Gebhart GF. Luminal hypertonicity and acidity modulate colorectal afferents and induce persistent visceral hypersensitivity. American journal of physiology Gastrointestinal and liver physiology. 2012;303:G802–809. doi: 10.1152/ajpgi.00259.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bercik P, Wang L, Verdu EF, et al. Visceral hyperalgesia and intestinal dysmotility in a mouse model of postinfective gut dysfunction. Gastroenterology. 2004;127:179–187. doi: 10.1053/j.gastro.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 20.Reuter KC, Grunwitz CR, Kaminski BM, Steinhilber D, Radeke HH, Stein J. Selective glucocorticoid receptor agonists for the treatment of inflammatory bowel disease: studies in mice with acute trinitrobenzene sulfonic acid colitis. The Journal of pharmacology and experimental therapeutics. 2012;341:68–80. doi: 10.1124/jpet.111.183947. [DOI] [PubMed] [Google Scholar]
- 21.Ruijter JM, Ramakers C, Hoogaars WM, et al. Amplification efficiency: linking baseline and bias in the analysis of quantitative PCR data. Nucleic acids research. 2009;37:e45. doi: 10.1093/nar/gkp045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Touzot M, Grandclaudon M, Cappuccio A, et al. Combinatorial flexibility of cytokine function during human T helper cell differentiation. Nature communications. 2014;5:3987. doi: 10.1038/ncomms4987. [DOI] [PubMed] [Google Scholar]
- 23.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 24.Utreras E, Keller J, Terse A, Prochazkova M, Iadarola MJ, Kulkarni AB. Transforming growth factor-beta1 regulates Cdk5 activity in primary sensory neurons. The Journal of biological chemistry. 2012;287:16917–16929. doi: 10.1074/jbc.M111.329979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu Q, Zhang XM, Duan KZ, et al. Peripheral TGF-beta1 signaling is a critical event in bone cancer-induced hyperalgesia in rodents. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:19099–19111. doi: 10.1523/JNEUROSCI.4852-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu W, Xu P, Cuascut FX, Hall AK, Oxford GS. Activin acutely sensitizes dorsal root ganglion neurons and induces hyperalgesia via PKC-mediated potentiation of transient receptor potential vanilloid I. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:13770–13780. doi: 10.1523/JNEUROSCI.3822-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moriyama T, Higashi T, Togashi K, et al. Sensitization of TRPV1 by EP1 and IP reveals peripheral nociceptive mechanism of prostaglandins. Molecular pain. 2005;1:3. doi: 10.1186/1744-8069-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith JA, Amagasu SM, Eglen RM, Hunter JC, Bley KR. Characterization of prostanoid receptor-evoked responses in rat sensory neurones. British journal of pharmacology. 1998;124:513–523. doi: 10.1038/sj.bjp.0701853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith JA, Davis CL, Burgess GM. Prostaglandin E2-induced sensitization of bradykinin-evoked responses in rat dorsal root ganglion neurons is mediated by cAMP-dependent protein kinase A. The European journal of neuroscience. 2000;12:3250–3258. doi: 10.1046/j.1460-9568.2000.00218.x. [DOI] [PubMed] [Google Scholar]
- 30.St-Jacques B, Ma W. Peripheral prostaglandin E2 prolongs the sensitization of nociceptive dorsal root ganglion neurons possibly by facilitating the synthesis and anterograde axonal trafficking of EP4 receptors. Experimental neurology. 2014 doi: 10.1016/j.expneurol.2014.05.028. [DOI] [PubMed] [Google Scholar]
- 31.Ozaktay AC, Kallakuri S, Takebayashi T, et al. Effects of interleukin-1 beta, interleukin-6, and tumor necrosis factor on sensitivity of dorsal root ganglion and peripheral receptive fields in rats. European spine journal : official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2006;15:1529–1537. doi: 10.1007/s00586-005-0058-8. [DOI] [PubMed] [Google Scholar]
- 32.Zelenka M, Schafers M, Sommer C. Intraneural injection of interleukin-1beta and tumor necrosis factor-alpha into rat sciatic nerve at physiological doses induces signs of neuropathic pain. Pain. 2005;116:257–263. doi: 10.1016/j.pain.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 33.Stemkowski PL, Smith PA. Long-term IL-1beta exposure causes subpopulation-dependent alterations in rat dorsal root ganglion neuron excitability. Journal of neurophysiology. 2012;107:1586–1597. doi: 10.1152/jn.00587.2011. [DOI] [PubMed] [Google Scholar]
- 34.Natura G, Bar KJ, Eitner A, et al. Neuronal prostaglandin E2 receptor subtype EP3 mediates antinociception during inflammation. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:13648–13653. doi: 10.1073/pnas.1300820110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weng Y, Batista-Schepman PA, Barabas ME, et al. Prostaglandin metabolite induces inhibition of TRPA1 and channel-dependent nociception. Molecular pain. 2012;8:75. doi: 10.1186/1744-8069-8-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Camilleri M, Madsen K, Spiller R, Greenwood-Van Meerveld B, Verne GN. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2012;24:503–512. doi: 10.1111/j.1365-2982.2012.01921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Porter CK, Cash BD, Pimentel M, Akinseye A, Riddle MS. Risk of inflammatory bowel disease following a diagnosis of irritable bowel syndrome. BMC gastroenterology. 2012;12:55. doi: 10.1186/1471-230X-12-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bernstein CN, Niazi N, Robert M, et al. Rectal afferent function in patients with inflammatory and functional intestinal disorders. Pain. 1996;66:151–161. doi: 10.1016/0304-3959(96)03062-x. [DOI] [PubMed] [Google Scholar]
- 39.Distrutti E, Sediari L, Mencarelli A, et al. Evidence that hydrogen sulfide exerts antinociceptive effects in the gastrointestinal tract by activating KATP channels. The Journal of pharmacology and experimental therapeutics. 2006;316:325–335. doi: 10.1124/jpet.105.091595. [DOI] [PubMed] [Google Scholar]
- 40.Verma-Gandhu M, Bercik P, Motomura Y, et al. CD4+ T-cell modulation of visceral nociception in mice. Gastroenterology. 2006;130:1721–1728. doi: 10.1053/j.gastro.2006.01.045. [DOI] [PubMed] [Google Scholar]
- 41.Verma-Gandhu M, Verdu EF, Bercik P, et al. Visceral pain perception is determined by the duration of colitis and associated neuropeptide expression in the mouse. Gut. 2007;56:358–364. doi: 10.1136/gut.2006.100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mayer EA, Berman S, Suyenobu B, et al. Differences in brain responses to visceral pain between patients with irritable bowel syndrome and ulcerative colitis. Pain. 2005;115:398–409. doi: 10.1016/j.pain.2005.03.023. [DOI] [PubMed] [Google Scholar]