Abstract
Albuminuria is both a hallmark and a risk factor for progressive glomerular disease, and results in increased exposure of podocytes to serum albumin with its associated factors. Here in vivo and in vitro models of serum albumin overload were used to test the hypothesis that albumin-induced proteinuria and podocyte injury directly correlate with COX-2 induction. Albumin induced COX-2, MCP-1, CXCL1 and the stress protein HSP25 in both rat glomeruli and cultured podocytes, while B7-1 and HSP70i were also induced in podocytes. Podocyte exposure to albumin induced both mRNA and protein and enhanced the mRNA stability of COX-2, a key regulator of renal hemodynamics and inflammation, which renders podocytes susceptible to injury. Podocyte exposure to albumin also stimulated several kinases (p38 MAPK, MK2, JNK/SAPK and ERK1/2), inhibitors of which (except JNK/SAPK) down-regulated albumin-induced COX-2. Inhibition of AMPK, PKC and NFκB also down-regulated albumin-induced COX-2. Critically, albumin-induced COX-2 was also inhibited by glucocorticoids and thiazolidinediones, both of which directly protect podocytes against injury. Furthermore, specific albumin-associated fatty acids were identified as important contributors to COX-2 induction, podocyte injury and proteinuria. Thus, COX-2 is associated with podocyte injury during albuminuria, as well as with the known podocyte protection imparted by glucocorticoids and thiazolidinediones. Moreover, COX-2 induction, podocyte damage and albuminuria appear mediated largely by serum albumin-associated fatty acids.
Introduction
Proteinuria, manifested predominantly as albuminuria, is not only a marker but also a known risk factor for progressive glomerular disease.1, 2 In this context, albumin-overload in animals is an excellent model to study the structural, pathological and molecular changes in renal diseases.3-6 Although tubulointerstitial injury has been an area of extensive focus in such animal models, there have been very few studies to date of the molecular changes in podocytes, despite the observed structural and pathological changes.3, 4, 6, 7 Moreover, while in vitro studies have shed light on the role of serum albumin (SA) along with its bound factors [i.e. fatty acids (FA) etc.] as mediator of proximal tubule cell (PTC) injury, its molecular effects on podocytes are less well understood.2, 8 Reported responses of podocytes to SA include albumin endocytosis,9 increased TGF-β and p38 MAPK signaling and loss of synaptopodin,10, 11 apoptosis in association with CD2AP down-regulation and endoplasmic stress,12 TRPC6-mediated intracellular Ca2+ increase,13 increased MMP-2 and MMP-914 and modulation of the endothelin-1 gene with actin cytoskeleton reorganization.15
We recently reported increased COX-2 expression in podocytes in response to SA, which was p38 MAPK-dependent.16 COX-2 is a key inducible enzyme of the anabolic cascade of the prostanoid pathway that plays an important role in inflammatory responses, vascular tone, salt/water balance, renin release and in podocyte physiology.17 Moreover, COX-2 expression is transient and regulated at multiple levels, including transcription, mRNA stability, protein synthesis and degradation.18 Abnormally expressed COX-2 has been implicated to play a role in inflammatory disorders, cancer, neurodegenerative diseases and renal injury.17, 19 Increased COX-2 expression in renal cortex and podocytes has been reported in the rat renal ablation model,20 human acute renal allograft rejection,21 in vivo glomerular injury models,22-25 and in vitro by prostaglandin E2 and mechanical stress.26 Additionally, mice with COX-2 overexpressing podocytes demonstrate increased susceptibility to renal injury in adriamycin, puromycin aminonucleoside (PAN) and diabetic nephropathy (DN) models and treatment with COX-2 specific inhibitor ameliorates albuminuria in these renal injury models.23-25
Glucocorticoids (GCs) and thiazolidinediones (TZDs) are the standard therapeutic modalities for nephrotic syndrome (NS) and type II diabetes, respectively.27, 28 Both GCs and TZDs (rosiglitazone, Rosi; and pioglitazone, Pio) have been demonstrated to reduce kidney injury in various experimental models, including PAN-induced nephropathy.29, 30 MAPKs are also known to play crucial roles in the progression of various glomerulopathies, and their inhibition is emerging as a promising therapeutic area for renal diseases.31 We and others have previously shown that GCs, TZDs and MAPK inhibitors all provide direct protective effects against injury in podocytes.16, 32-36 However, the molecular signaling mechanisms responsible for this protection remain elusive, and the possibility that COX-2 may mediate these effects has not previously been explored.
We thus hypothesized that SA-overload induces pro-inflammatory and stress responses which play a role in the pathogenesis of the glomerular/podocyte injury, and that regulation of COX-2 in particular is associated with SA-induced injury and protection by GCs, TZDs and MAPK inhibitors. To test this hypothesis we analyzed the COX-2, pro-inflammatory and stress responses in glomeruli from SA-overload rats and in cultured mouse podocytes, explored the specific signaling pathways involved, and determined the ability of known or potential treatments for podocyte injury to down-regulate COX-2 expression. We also hypothesized that the SA-associated factors such as fatty acids contribute to SA-mediated podocyte injury and tested this hypothesis by identifying specific SA-associated factors contributing to proteinuria, podocyte damage and COX-2 induction upon SA-overload.
Results
SA-Overload in Rats Induces Proteinuria and Glomerular Expression of COX-2, Pro-Inflammatory and Stress Genes
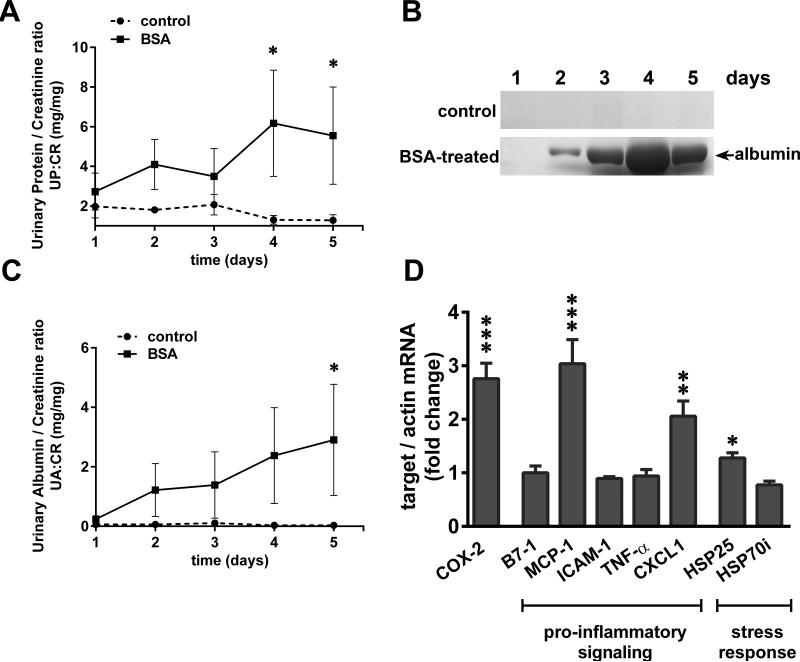
Glomerular injury was induced in the BSA-injected rats, as they developed robust proteinuria and albuminuria, which peaked on days 4-5 (Figure 1A, B, C). Control rats did not show any albumin excretion in the urine. Glomerular gene expression profiles of SA-overload rats showed induction of COX-2, as well as pro-inflammatory signaling and stress response genes (described in Table I) on day 5 after BSA injections (Figure 1D). COX-2 and MCP-1 were induced ~3 fold, CXCL1 was induced ~2 fold and HSP25 ~1.3 fold. Expression of B7-1, ICAM-1, TNFα, and HSP70i was not altered compared to the control group, and the expression of MIP-2 and CXCL5 could not be detected in either group.
Figure 1.
SA-overload in rats induces proteinuria and glomerular expression of COX-2, pro-inflammatory and stress genes. A) Urinary protein/creatinine ratios (UP:CR) of the BSA-treated rats vs control rats (n=3 for each group; *P<0.05 versus control, determined by two way ANOVA Tukey's multiple comparisons test). B) Massive amounts of urinary albumin are excreted from BSA-treated rats but not control rats (representative gels are shown from each group). Urine was collected on five consecutive days and equal volumes (4 μl) analyzed by SDS-PAGE and Coomassie Brilliant Blue staining. C) Urinary albumin/creatinine ratio (UA:CR) of the BSA-treated rats vs control rats (n=3 for each group; *P<0.05 versus control, determined by two way ANOVA Tukey's multiple comparisons test). D) Total RNA was extracted from the isolated glomeruli of control and BSA-treated rats and relative mRNA levels of COX-2, pro-inflammatory and stress genes were measured by qRT-PCR and normalized to β-actin (*P<0.05, **P<0.01 and ***P<0.001 versus control, determined by t test).
Table I Description of genes analyzed in this study
| Gene | Gene Description (Protein) |
| Ptgs2 | Prostaglandin-endoperoxide synthase/Cyclooxygenase-2 (COX-2) |
| Cd80 | Cluster of Differentiation 80 (B7-1) |
| Ccl2 | Chemokine (C-C motif) ligand 2/Monocyte Chemotactic Protein-1 (MCP-1) |
| Icam1 | Intercellular Adhesion Molecule 1 (ICAM-1) |
| Cxcl2 | Chemokine (C-X-C motif) ligand 2/Macrophage Inflammatory protein 2 (MIP-2) |
| Tnf-a | Tumor Necrosis Factor-a (TNF-a) |
| Cxcl1 | Chemokine (C-X-C motif) ligand 1 (CXCL1) |
| Cxcl5 | Chemokine (C-X-C motif) ligand 5 (CXCL5) |
| Tlr4 | Toll-like receptor 4 (TLR4) |
| Hspb1 | Heat Shock Protein 25 (HSP25) |
| Hspa1a | Heat Shock Protein 70 (HSP70i) |
| Actb | β-Actin |
These results indicate that induction of proteinuria in SA-overload rats was associated with induction of COX-2 and several pro-inflammatory and stress response genes.
SA Induces COX-2, Pro-Inflammatory and Stress Genes in Cultured Podocytes
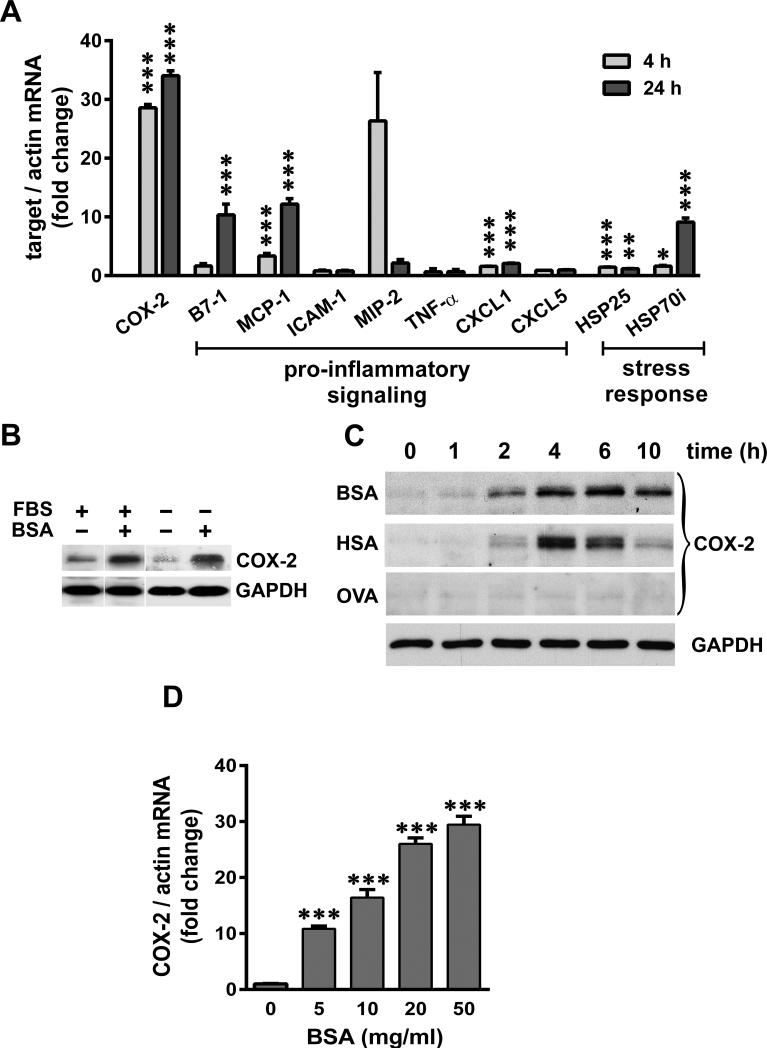
We extended our in vivo findings to an in vitro model of SA-overload using differentiated mouse podocytes and studied the SA response and COX-2 induction in greater detail. We found that BSA induced COX-2, MCP-1, CXCL1 and HSP25 genes in cultured podocytes (Figure 2A), as was also observed in rat glomeruli. COX-2 induction was robust (~30 fold) at both 4 and 24 h in podocytes. We also observed ~10 fold induction of B7-1 and HSP70i at 24h. Consistent with the results in rat glomeruli, no significant induction of ICAM-1, MIP-2, TNFα or CXCL5 was detected in podocytes.
Figure 2.
SA induces COX-2, pro-inflammatory and stress genes in cultured podocytes. A) Serum-starved podocytes were exposed to 40 mg/ml BSA for 4 h and 24 h, and relative mRNA levels of COX-2, pro-inflammatory and stress response genes were measured by qRT-PCR and normalized to β-actin (*P<0.05, **P<0.01 and ***P<0.001 versus time matched control, determined by t test). B) Podocytes were maintained in culture medium containing FBS or serum-starved O/N, and exposed to 40 mg/ml of BSA for 4 h and subjected to immuno-blot analysis for COX-2 and GAPDH. C) Serum-starved podocytes were exposed to 40 mg/ml BSA, human serum albumin (HSA) and Ovalbumin (OVA), and harvested at indicated time points. Protein extracts were analyzed for COX-2 and GAPDH (shown as loading control for a representative blot). D) Serum-starved podocytes were exposed for 4 h to increasing concentrations of BSA as indicated, and mRNA levels of COX-2 were measured by qRT-PCR and normalized to β-actin (***P<0.001 versus control, determined by t test).
When cultured in medium supplemented with 10% FBS (~3.0 mg/ml BSA), podocytes expressed low levels of COX-2 protein, which was further reduced following serum-starvation (Figure 2B). In either culture condition, massive induction of COX-2 was observed in the presence of 40 mg/ml BSA (as well as lower concentrations, as shown below), which corresponds to physiologic concentrations of SA in human plasma (~3.5-5 g/dl). Moreover, COX-2 protein was induced in a time-dependent manner, peaking 4-6 h after exposure to either BSA or human SA (HSA) (Figure 2C). Importantly, exposure to equimolar amounts of ovalbumin (OVA; an unrelated albumin), did not induce COX-2. Moreover, COX-2 was induced by BSA in a concentration-dependent manner (Figure 2D).
Taken together, these data suggest that podocytes respond to SA with a strong induction of COX-2 and moderate induction of several pro-inflammatory and stress genes. Both BSA and HSA induce COX-2 comparably, ruling out species differences, and induction appears to be specific to SA, since OVA did not induce COX-2.
SA and LPS Elicit Disparate COX-2 and Pro-Inflammatory Response in Podocytes
LPS has been shown to induce pro-inflammatory responses via the TLR4 receptor in many cell types, including podocytes.37, 38 To exclude the possibility of SA effects due to LPS contamination, we measured the endotoxin units (EU) in the BSA used, which was found to be very low (0.025 EU/mg of BSA) and comparable to endotoxin levels in the commercial endotoxin-free BSA.
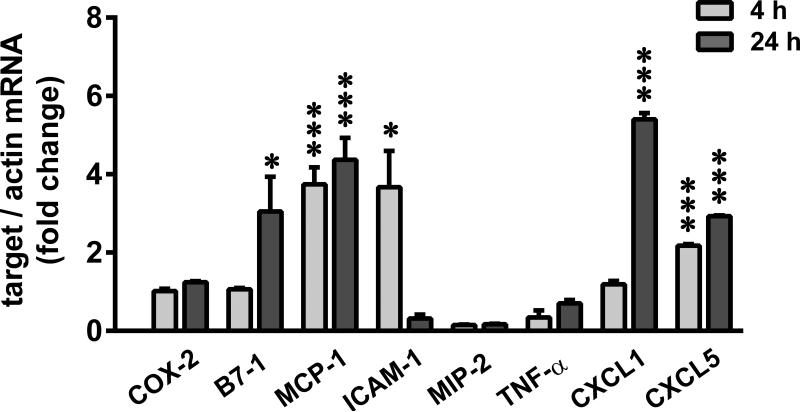
Furthermore, we measured the expression profile of COX-2 and several pro-inflammatory genes following 1 μg/ml LPS treatment (~5000 EU/ml) (Figure 3). At 4 or 24h after the treatment, LPS induced B7-1, MCP-1, ICAM-1, CXCL1, and CXCL5 (2 to 6 fold) but not COX-2, MIP-2 or TNF-α. Even high concentrations of LPS (0.05 ng/ml to 1 mg/ml), failed to induce COX-2 (Supplementary Figure 1). TLR4 expression was confirmed in these podocytes, the levels of which remained unaltered following SA or LPS treatment (Supplementary Figure 2). In addition, using alveolar macrophages as a reference cell line, we demonstrated that COX-2, MCP-1 and TNF-α were greatly induced by the same LPS (026:B6 at 1 μg/ml) that did not produce detectable effects in podocytes (Supplementary Table I). Other LPS serotypes (0127:B8 and 0111:B4) also induced COX-2 in alveolar macrophages. TLR4 expression was found to be ~8 times greater in macrophages than podocytes, which decreased following LPS stimulation (Supplementary Figure 2).
Figure 3.
SA and LPS elicit disparate COX-2 and pro-inflammatory response in podocytes. Serum-starved podocytes were treated with 1 μg/ml LPS (026:B6) for 4 and 24 h and relative mRNA levels of COX-2 and pro-inflammatory signaling genes was measured by qRT-PCR and normalized to β-actin (*P<0.05 and ***P<0.001 versus time matched control, determined by t test).
Thus SA and LPS have disparate effects on the pro-inflammatory response in podocytes, and COX-2 induction is a specific response to SA.
SA Stabilizes COX-2 mRNA
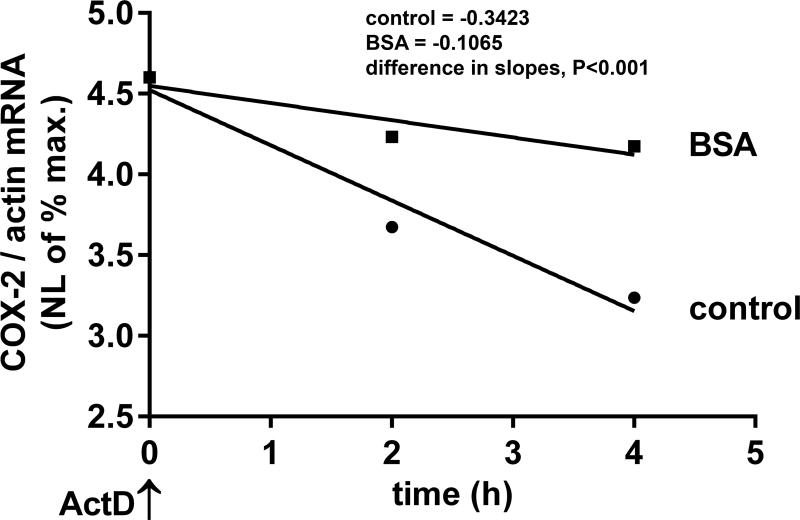
In addition to transcription, COX-2 expression is known to be regulated by its mRNA stability via the AU-rich elements (ARE) in the 3’UTR.18, 39 To determine the ability of SA to regulate COX-2 mRNA stability, cells were treated with actinomycin D to inhibit nascent mRNA synthesis after exposure to BSA or media alone. BSA exposure resulted in increased stability of COX-2 mRNA, with a significantly longer half-life (t1/2 = 6 h) compared to media control (t1/2 = 1.8h) (Figure 4). Thus, our data suggest that physiologic concentrations of SA have the ability to regulate COX-2 mRNA stability, as well as transcription.
Figure 4.
SA stabilizes COX-2 mRNA. Podocytes were exposed to 20 mg/ml BSA or medium alone (control) for 2 h, transcription was inhibited with actinomycin D (ActD) and RNA was extracted after 2 and 4 h. COX-2 mRNA levels were quantified by qRT-PCR and normalized to β–actin. Natural logarithmic (NL) values of the maximum mRNA amount were plotted against time and half-lives calculated (control: t1/2=1.8h, BSA: t1/2=6h) from the slopes of linear regression analysis which were significantly different (P<0.001) between control and BSA groups (control: y = 0.3423*x + 4.523, BSA: y = 0.1065*x + 4.549).
Signaling Pathways Involving Kinases Mediate COX-2 Induction by SA
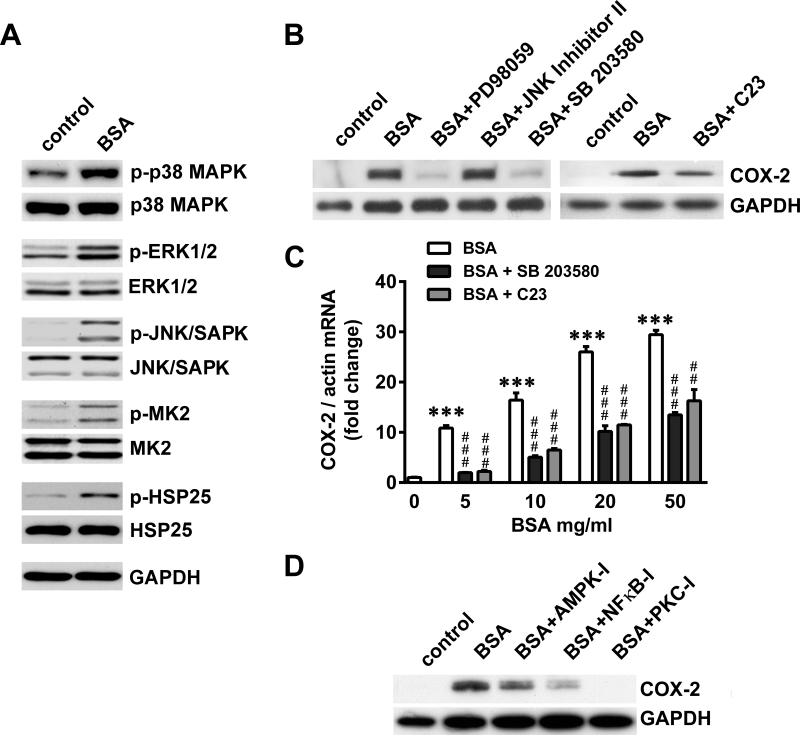
To identify the signaling pathways involved in regulating SA-induced COX-2, we first demonstrated the activation of various MAPK pathways in response to BSA, as seen by increased phosphorylation of p38 MAPK, ERK1/2 and JNK/SAPK (Figure 5A). p38 MAPK activation led to phosphorylation of its downstream kinase, MK2, and its major substrate, HSP25. Subsequently, the contribution of these MAPK pathways to SA-induced COX-2 regulation was determined using specific inhibitors of these kinases. Inhibition of ERK1/- and p38 MAPK, but not JNK/SAPK, resulted in down-regulation of BSA-induced COX-2 (Figure 5B). MK2 inhibition also resulted in partial down-regulation of BSA-induced COX-2. Moreover, we confirmed the effectiveness of these inhibitors by analyzing the phosphorylation status of ERK1/2 and the downstream substrates MK2 (for p38 MAPK), HSP25 (for p38MAPK and MK2) and c-Jun (for JNK/SAPK) in the presence of the inhibitors (data not shown).
Figure 5.
Signaling pathways involving kinases mediate COX-2 induction by SA. A) Serum- starved podocytes were treated with 20 mg/ml BSA for 1 h and subjected to immuno-blot analysis for phosphorylated (p) and total forms of p38 MAPK, ERK1/2, JNK/SAPK, MK2, HSP25 and GAPDH. B) Serum-starved podocytes were treated with 20 mg/ml BSA for 4 h following 1 h pre-treatment with inhibitors at 20μM for ERK1/2 (PD98059), JNK/SAPK (JNK Inhibitor II), p38 MAPK (SB 203580) and MK2 (C23). Cells were subjected to immuno-blot analysis for COX-2 and GAPDH. C) Serum-starved podocytes were treated with increasing concentrations of BSA as indicated for 4 h following 24 h pre-treatment with 30 ZM SB203580 (p38 MAPK inhibitor) or 10 μM C23 (MK2 inhibitor), COX-2 mRNA levels were quantified by qRT-PCR and normalized to β-actin (***P<0.001 versus control; **P<0.01 and ***P<0.001 versus BSA, determined by one way ANOVA). D) Serum-starved podocytes were treated with 20 mg/ml BSA for 4 h following 1 h pre-treatment with inhibitors at 5μM for AMPK (Compound C), NFκB (NFκB-I) and PKC (PKC-I). Cells were subjected to immuno-blot analysis for COX-2 and GAPDH.
Due to the well-defined abilities of p38 MAPK and MK2 inhibitors to provide podocyte protection,16 we studied their abilities to down-regulate COX-2 mRNA in the presence of SA in greater detail. Both p38 MAPK and MK2 inhibitors reduced BSA-induced COX-2, which was dependent on the BSA concentration (Figure 5C). Additionally, the roles of AMPK, NFκB and PKC in SA-induced COX-2 were demonstrated by the abilities of their inhibitors to down- regulate BSA-induced COX-2, albeit to different degrees (Figure 5D).
In summary, a number of signaling pathways contribute to the regulation of SA-induced COX-2, including the ERK1/2, p38 MAPK, MK2, AMPK, PKC and NFκB pathways.
Glucocorticoids and Thiazolidinediones Inhibit SA-Induced COX-2
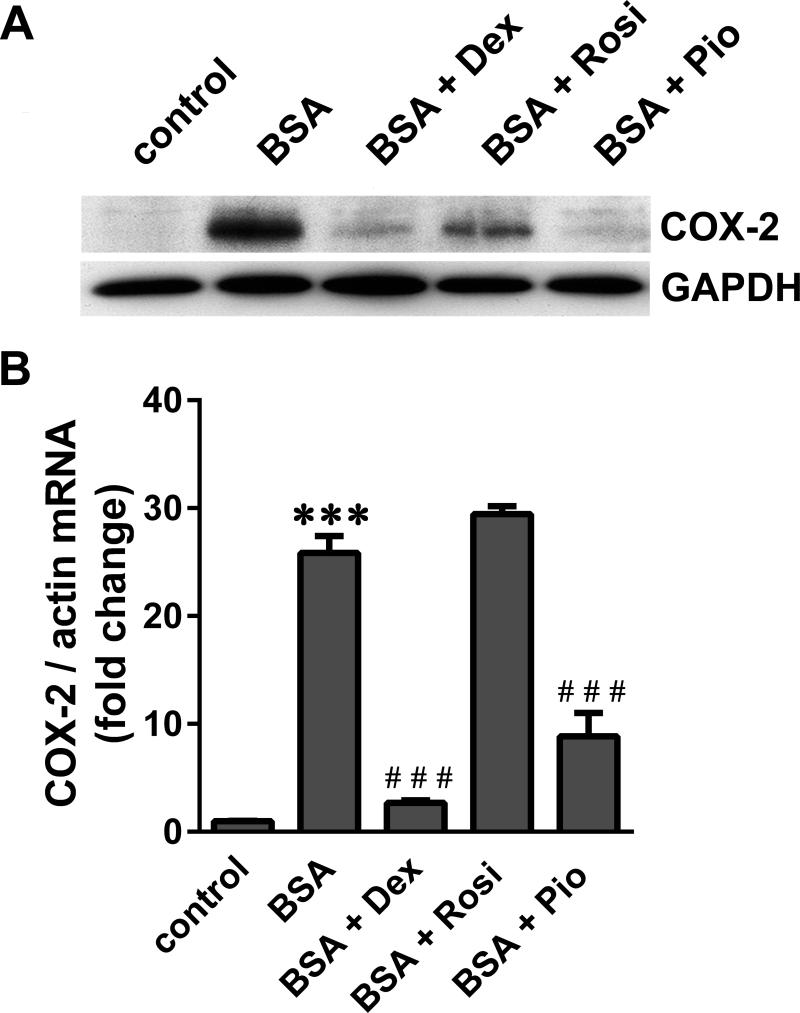
We and others have previously demonstrated the direct protective effects of both GCs and TZDs on podocytes.32-36 To determine if these protective agents can inhibit SA-induced COX-2, podocytes were pre-treated with dexamethasone (Dex), and the PPARγ agonists Rosi and Pio for 24 h prior to BSA exposure. While Dex and Pio inhibited BSA-induced COX-2 nearly completely, Rosi only partially inhibited COX-2 protein (Figure 6A). Dex and Pio also inhibited BSA-induced COX-2 mRNA, while Rosi had no effect (Figure 6B).
Figure 6.
Glucocorticoids and thiazolidinediones inhibit SA-induced COX-2. Serum–starved podocytes were treated with 20 mg/ml BSA for 4 h following pre-treatment with 1 μM dexamethasone (Dex), 10 μM rosiglitazone (Rosi) and 0.1 μM pioglitazone (Pio) for 24 h. A) COX-2 and GAPDH protein were visualized by immuno-blot analysis, and B) COX-2 mRNA levels were quantified by qRT-PCR and normalized to β-actin (***P<0.001 versus control; ***P<0.001 versus BSA, determined by one way ANOVA).
These results suggest that the known protective effects of GCs and TZDs may be mediated, at least in part, via COX-2 inhibition. Additionally, the observed drug-specific differences support the possibility of COX-2 regulation both at the transcriptional and post-transcriptional levels.
SA-Associated Factors Contribute To COX-2 Induction in Podocytes
To determine the contribution of SA per se or SA-associated factors (SAAFs) in COX-2 induction, podocytes were exposed to various forms of SA, and SA disassociated from selective factors (Figure 7A, B).
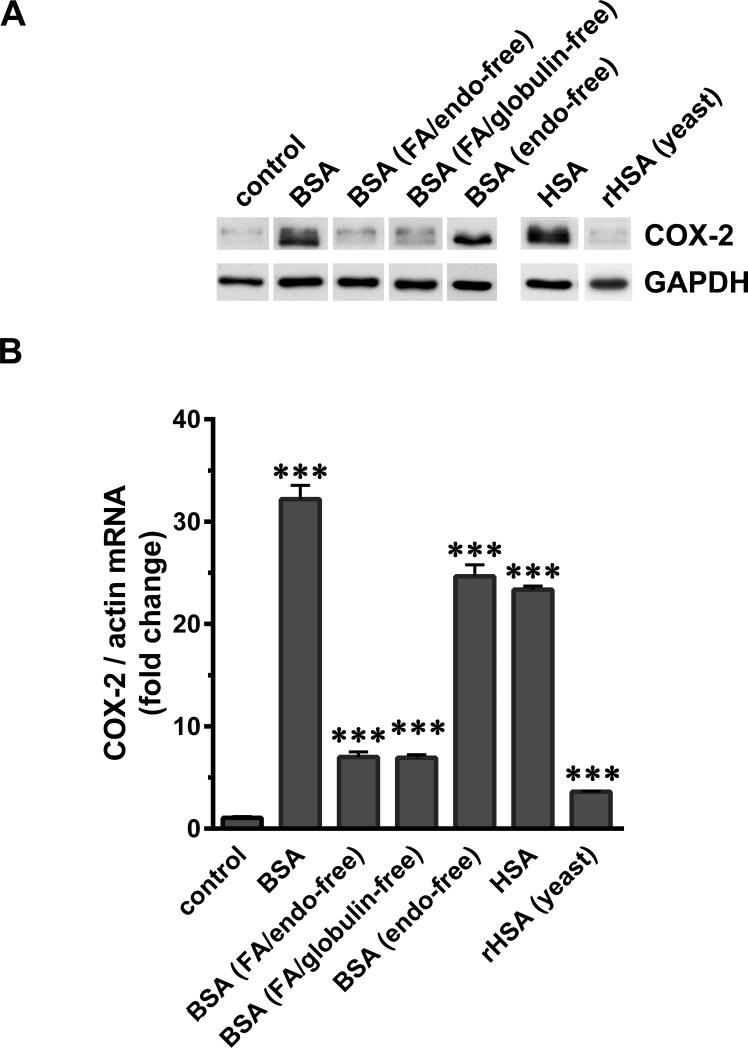
Figure 7.
SA-associated factors contribute to COX-2 induction in podocytes. Serum-starved podocytes were exposed to 40 mg/ml of BSA, charcoal-treated FA/endotoxin-free BSA, FA/globulin-free BSA, endotoxin-free BSA, HSA and recombinant HSA made in yeast (rHSA). (A) Cells were harvested after 4 h, processed for SDS-PAGE and western blotting and analyzed for COX-2 and GAPDH. (B) Total RNA was extracted and COX-2 mRNA was measured by RT-PCR and normalized to the β-actin mRNA (***P<0.001 versus control, determined by t test).
Charcoal-treated BSA (FA and endotoxin-free) and BSA depleted of FA (together with globulins) resulted in moderate induction of COX-2 mRNA, although COX-2 protein was not detectable (Figure 7A, B). Similar results were also obtained with charcoal-treatment of HSA and BSA according to the previously described protocol (data not shown).40 Use of commercial endotoxin-free BSA did not diminish the COX-2 inducing activity. Recombinant HSA made in yeast (rHSA) resulted in no detectable COX-2 protein induction, while mRNA was induced at low but significant levels. Specific depletion of globulins, glycoproteins and low-molecular- mass filtrate fraction (<20kDa) from BSA preparations did not have any diminishing effect on COX-2 induction by BSA and free FA in the absence of albumin did not induce COX-2 (data not shown).
Collectively, these data are informative with regard to the nature of SAAFs responsible for COX-2 induction: i) Globulins, endotoxins, glycoproteins and low molecular weight molecules (<20kDa) in BSA preparation are unlikely to contribute to COX-2 induction in podocytes, and ii) FA/lipds may be one of the candidate SAAFs partially contributing to COX-2 induction.
Fatty Acids Complexed with SA Contribute to COX-2 Induction in Podocytes
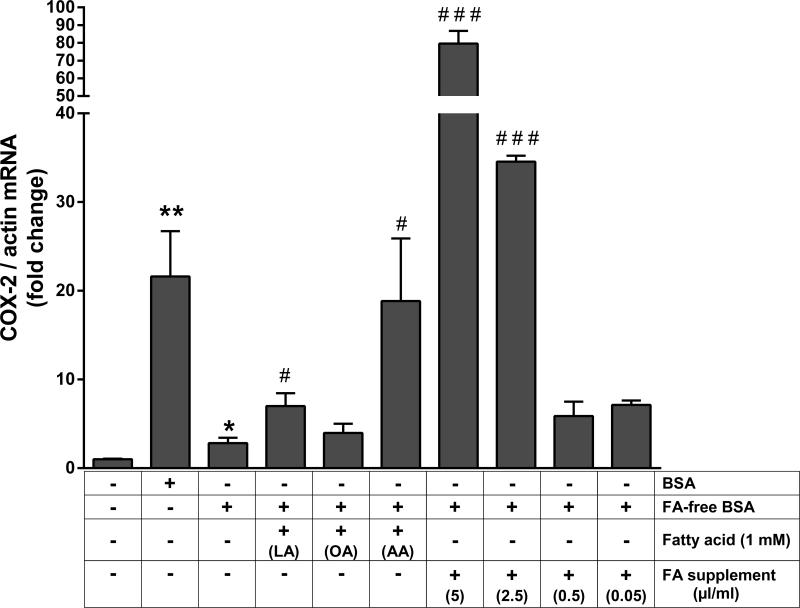
To identify the role and nature of fatty acids (FA) complexed with SA in contributing to COX-2 induction, podocytes were exposed to several specific FAs in the presence of FA-free BSA. FA-free BSA complexed with arachidonic acid greatly enhanced the modest COX-2 induction seen with FA-free BSA, while lauric acid had lesser effect and oleic acid did not show any effect at 1mM concentration (Figure 8). At higher concentration (10mM) oleic acid (+ FA-free BSA) did enhance COX-2 induction, but both lauric acid and arachidonic acid induced podocyte viability loss (data now shown). FA supplement complexed with FA-free BSA enhanced COX-2 induction greatly and in a dose-dependent manner (Figure 8).
Figure 8.
Fatty acids bound to SA contribute to COX-2 induction in podocytes. Serum-starved podocytes were exposed to 40 mg/ml of BSA, FA-free BSA, and FA-free BSA in combination with 1 mM Lauric acid (LA), 1 mM Oleic acid (OA), 1 mM Arachidonic acid (AA) and a combined fatty acid supplement at 5, 2.5, 0.5 and 0.05 μl/ml cell culture media. Cells were harvested after 4 h, total RNA was extracted and COX-2 mRNA was measured by RT-PCR and normalized to the β-actin mRNA (*P<0.05 and **P<0.01 versus control; *P<0.05 and ***P<0.001 versus FA-free BSA, determined by t test).
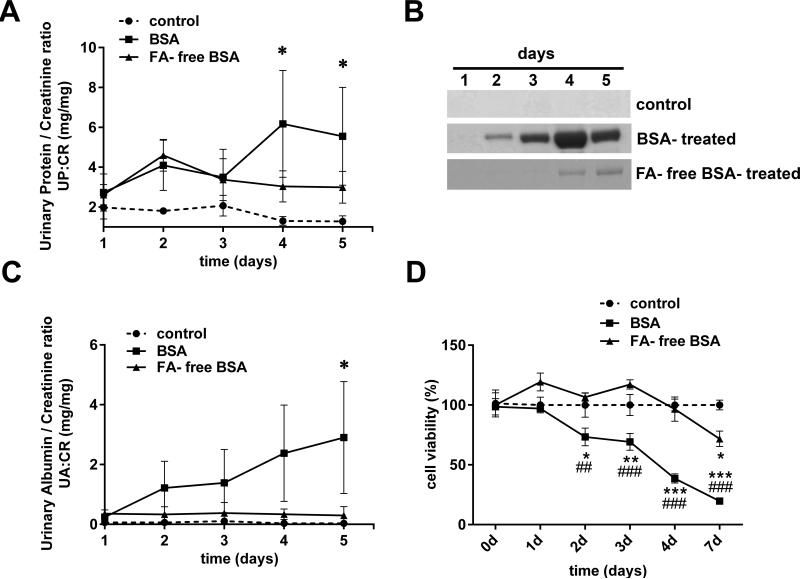
Delipidation of SA Attenuates Proteinuria and Podocyte Damage
To determine the role of FA complexed with SA in inducing proteinuria in vivo, we compared proteinuria in control rats and those treated with BSA and FA-free BSA. BSA-treated rats developed massive albuminuria and significant proteinuria as compared to control rats (Figure 9). In contrast, FA-free BSA treated rats did not develop significant proteinuria or albuminuria as compared to control rats (Figure 9A, B. C). Furthermore, to compare podocyte injury induced by SA to that of delipidated-SA, podocytes were exposed to BSA and FA-free BSA and cell viability analyzed. Exposure to BSA resulted in significantly greater loss of podocyte viability in a time-dependent manner compared to either FA-free BSA or control (Figure 9D).
Figure 9.
Delipidation of BSA attenuates proteinuria and podocyte damage. A) Urinary protein/creatinine ratio (UP:CR) of the control, BSA-treated and FA-free BSA-treated rats (n=3 for each group; *P<0.05 versus control, determined by two way ANOVA Tukey's multiple comparisons test). B) Representative gels from each group show that only small amounts of albumin are excreted from FA-free BSA-treated rats, while BSA treated rats excreted massive amounts of albumin in their urine compared to control rats. Urine was collected on five consecutive days and equal volumes (2.5 μl) analyzed by SDS PAGE and Coomassie Brilliant Blue staining. C) Urinary albumin/creatinine ratios (UA:CR) from control, BSA-treated and FA-free BSA-treated rats (n=3 for each group; *P<0.05 versus control, determined by two way ANOVA Tukey's multiple comparisons test). D) Cultured podocytes were exposed to media (control) or 40 mg/ml BSA and FA-free BSA for 0, 1, 2, 3, 4 and 7 days and assayed for cell viability using MTT (*P<0.05, **P<0.01 and ***P<0.001 versus control; ***P<0.01 and ***P<0.001 versus FA-free BSA, determined by two way ANOVA Tukey's multiple comparisons test).
Discussion
In the present study, we used both in vivo and in vitro models of SA overload to test the hypothesis that exposure to SA, along with its associated factors, induces podocyte expression of COX-2, podocyte injury, and proteinuria. We found that in both rat glomeruli and cultured mouse podocytes, SA-overload induced expression of COX-2, the pro-inflammatory genes MCP-1 and CXCL1 and the stress gene HSP25 and further delineated the signaling pathways involved. Notably, SA-induced COX-2 was down-regulated by glucocorticoids and thiazolidinediones, both of which are known to directly protect podocytes against injury. Furthermore, specific SA-associated fatty acids were identified as important contributors to COX-2 induction, proteinuria and podocyte injury. Together, these findings associate COX-2 with podocyte injury during albuminuria, as well as with the known podocyte protection imparted by glucocorticoids and thiazolidinediones. Moreover, podocyte injury, COX-2 induction, and albuminuria appear mediated in part by SA–associated fatty acids.
There are several reports indicating a strong relationship between SA-overload and tubulointerstitial injury in rats and mice, characterized by a pro-inflammatory response, infiltration by macrophages and lymphocytes, tubular atrophy and interstitial fibrosis.5, 6, 41, 42 Such infiltration by immune cells is not evident in glomeruli, but there is an increase in IgG, IgM and C3 and C5 antigens in the glomeruli, which become enlarged, while podocytes undergo effacement and detachment from the basement membrane. 3, 4, 6, 7, 43 Despite the observed glomerular structural and pathological changes, the molecular changes in glomeruli following SA-overload have not been studied in detail. A few relevant studies report increased osteopontin expression in the renal cortex and TNF-α and IL-1β induction in the glomeruli of BSA-injected rats and mice, respectively.41, 44 Since the glomerular lesions following SA-overload in mice are mild compared to rats, we specifically studied the molecular responses in the glomeruli of SA-overload rats,6, 43 and found induction of COX-2, pro-inflammatory genes, MCP-1 and CXCL1, and stress gene HSP25. We speculate that these responses may play a role in the pathogenesis of the ensuing glomerular lesions. Glomerular COX-2 induction in the SA-overload model is consistent with prior findings of COX-2 induction in the glomeruli during renal injury in both humans and in experimental models.20-25 Moreover, COX-2 over-expression in podocytes is known to make them susceptible to further injury.23-25 Glomerular expression of MCP-1 correlates with renal damage in both inflammatory and non-inflammatory models of glomerular injury,45 and its up-regulation in podocytes leads to increased cell motility and albumin permeability.46
We also utilized the in vitro SA-overload model in podocytes to analyze the expression of pro-inflammatory genes and to study the molecular mechanism of COX-2 regulation in podocytes in greater detail. Although the effects of SA or SAAFs on PTCs have been extensively studied, there are relatively few reports in podocytes. It has also been suggested that the intrinsic pro-inflammatory signaling in podocytes might contribute to the progression of albuminuria.47. Proximal tubule cells have been reported to reabsorb physiologic amounts of albumin in the primary urine,48 and albumin-overload in PTCs results in the activation of various molecular signaling pathways and production of chemo-attractants, pro-fibrotic agents and matrix proteins which ultimately result in interstitial inflammation and fibrosis.2 The role of TGF-β, endothelin-1, p38 MAPK, MMP-2, MMP-9, CD2AP, GRP78 and TRP6 signaling in podocytes upon albumin-exposure has been well-documented.10-15 Additionally, exposure to SA results in direct podocyte injury, involving reorganization of the actin cytoskeleton, apoptosis and loss of cell viability.10, 11, 15 In the current study we observed a massive induction of COX-2 and significant induction of B7-1, MCP-1, CXCL1, HSP25 and HSP70i in podocytes exposed to SA. We also observed SA concentration-dependent COX-2 induction, suggesting that even subtle increases in SA concentrations in the urinary space (such as during the initial phases of albuminuria or during chronic progressive glomerular disease) can result in increased COX-2 expression in podocytes. Furthermore, COX-2 induction could be a part of a stress response, as its expression has recently been shown to be regulated by heat-shock transcription factors which typically act on the promoters of stress genes.49 Consistent with this, we found induction of both HSP25 and HSP70i in podocytes. Thus, in addition to COX-2, induction of these pro-inflammatory and stress molecules suggest additional potential molecular mechanisms by which SA may have detrimental effects on podocytes.
Albumin is the most prevalent protein in blood, and it binds and transports many different kinds of molecules, including FAs, hormones, vitamins, bile acids, cations, bilirubin etc. In light of this, we further studied the induction of COX-2 using various forms of SA, and SA treated in various ways to selectively dissociate it from specific factors. We excluded the role of endotoxins, glycoproteins, globulins, low molecular weight compounds (<20kDa) and free FAs alone in inducing COX-2 in podocytes, in contrast to the known effects of some of these substances in other cells.50, 51 Recombinant, FA-free and charcoal-treated SA reduced the COX-2 mRNA induction to low levels and protein induction to undetectable levels, suggesting that FA may be the key SAAF contributing to COX-2 induction by SA. Furthermore, we found that the addition of a FA supplement containing a mixture of FA and arachidonic acid complexed with SA, restored the ability of SA to strongly induce COX-2 expression. Of even greater potential interest, we also found that FA-free BSA induced significantly less podocyte injury in cultured cells, and less proteinuria and albuminuria in rats, than standard BSA. This observation provides direct confirmation of the previously reported theory that the renal toxicity of filtered SA depends on its lipid moiety.52, 53 In vivo, podocytes exposed to SA is likely associated with many factors rather than free albumin molecule, since SA associates with 99% of the FA in plasma. Also consistent with our findings, it has been reported that FA associated with SA and not the SA molecule per se, lead to podocyte apoptosis and necrosis,8 tubulointerstitial injury in vivo53 and detrimental effects in PTCs.2 Overall, our findings suggest that fatty acids, primarily arachidonic acid, contribute significantly to the SA-induced injury to podocytes.
Both SA and LPS stimulation resulted in induction of B7-1, which has been shown to be critically associated with NS and induced by LPS and PAN in podocytes.37 However, we found that the COX-2 induction was a specific response to SA, but not to LPS, in podocytes. LPS induced CXCL1, CXCL5, B7-1 and ICAM-1 while SA did not induce CXCL5 and ICAM-1. Increased CXCL-1 and CXCL-5 expression has also been reported to be induced by LPS in podocytes.54 In summary, the gene expression profile of COX-2 and other pro-inflammatory molecules in podocytes was found to be disparate between LPS and SA treatments. In support of this, a recent study has also identified disparate pro-inflammatory effects of LPS and PAN in podocytes, suggesting the role of complementary pathways in disease induction.38
The nonsteroidal anti-inflammatory drugs inhibit COX-2 directly, but they have been associated with renal abnormalities in ~ 1-3% of cases.55 The use of COX-2 selective inhibitor, celecoxib is however, controversial. Despite its known renal side effects,56, 57 it has also been shown to be protective in NS in some cases. 58, 59 Selective COX-2 inhibitors have been shown to reduce proteinuria and foot process effacement in Adriamycin-induced, PAN-induced and diabetic nephropathy animal models, 60, 24, 23, 25 and to attenuate podocyte death in vitro.61 In this context, our data also suggests that the COX-2 inhibitor, celecoxib, may have beneficial effects in reducing SA-induced injury to the podocyte actin cytoskeleton (Supplementary Figure 3).
Our data suggest that COX-2 is regulated by a complex signaling network in podocytes which can be activated by SA with its (i.e. SAAFs) inhibited by GCs and TZDs (Figure 10). SA-induced COX-2 is also down-regulated by inhibitors to several kinases (p38 MAPK, ERK1/2 and MK2) which have also been shown to protect podocytes and reduce proteinuria in various models of glomerular disease.16, 31 Inhibition of ERK1/2 in colorectal tumor cells62 and of both p38 MAPK and ERK1/2, but not JNK/SAPK in intestinal epithelial cells63 has been reported to down-regulate COX-2 expression. Similarly, a JNK/SAPK inhibitor did not block SA-induced COX-2 in podocytes in our study, although inhibition of JNK/SAPK has been shown to reduce COX-2 expression in macrophages.64 Furthermore, upon activation of the p38 MAPK signaling cascade, phosphorylated HSP25 has been shown to bind ARE in the 3’UTR of COX-2 and alter its stability.39 Consistent with this, we also observed an increase in the COX-2 mRNA stability with SA exposure, along with simultaneous phosphorylation of p38 MAPK, MK2 and HSP25. Additionally, we and others have previously shown the protective effects of GCs and TZDs in podocytes and their ability to attenuate MAPK signaling.16, 32-36, 65 GCs and TZDs have also been reported to inhibit COX-2 in certain cell types,65, 66 but to up-regulate COX-2 in other cell types via the mediation of CCAAT/enhancer binding protein-β with GCs and by the action of TZDs on the PPRE element in the COX-2 promoter region.67, 68 In the present study, we found that GCs and TZDs inhibit SA-induced COX-2 in podocytes. Moreover, we found increased expression of the p65 subunit of NFκB in SA-exposed podocytes (data not shown). Activated receptors for GCs and TZDs bind to NFκB and block the expression of pro- inflammatory molecules, including COX-2, via their trans-repression activities.69 Involvement of AMPK, p38 MAPK and PKC in SA-induced COX-2 also indicates the possibility of stimulation of G-protein coupled receptor pathways by SA in podocytes.26, 61 Furthermore, reduction of SA-induced COX-2 by GCs and TZDs in podocytes may also be mediated via AMPK and PKC pathways, respectively, as reported in other cell types.70, 71
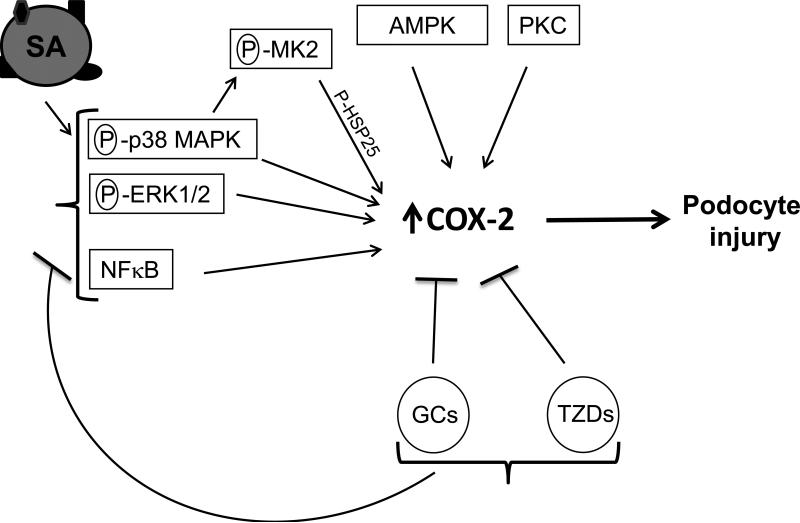
Figure 10.
Proposed schematic of COX-2 as a mediator of podocyte injury by SA and protection by GCs, TZDs and MAPK inhibitors. Serum Albumin (SA) exists as a complex entity and along with its associated factors (i.e. fatty acids, hormones, vitamins and bilirubin etc., depicted as ▲/ ■ / ● on SA molecule on left), it induces COX-2 in podocytes. This is coupled with an increase in phosphorylation (P) of kinases p38 MAPK, ERK1/2, MK2 and its downstream substrate HSP25. Inhibitors to p38 MAPK, MK2, ERK1/2, AMPK and PKC (depicted in rectangles) down-regulate SA-induced COX-2. Glucocorticoids (GCs) and thiazolidinediones (TZDs), which are known to protect podocytes against injury, also inhibit SA- induced COX-2. They have been previously shown to attenuate MAPK signaling and also impart their trans-repression activities via coupling of their respective ligand-bound receptors (GR and PPARγ) with NFκB to block COX-2 expression via the NFκB response element on COX-2. NFκB (p65 subunit) is over-expressed following SA-exposure and NFκB inhibition down-regulates SA-induced COX-2.
In summary, our study suggests that podocyte exposure to SA, as occurs during glomerular disease, induces COX-2 as well as other pro-inflammatory and stress molecules in both glomeruli in vivo and in podocytes in vitro. Moreover, SA-induced COX-2 was inhibited by GCs and TZDs, both of which directly protect podocytes against injury. Together, these findings associate COX-2 with podocyte injury during albuminuria, as well as with the known podocyte protection imparted by GCs and TZDs. Moreover, podocyte injury, COX-2 induction, and albuminuria appear mediated in large part by specific SA–associated fatty acids.
Concise Methods
Albumin overload model in rats
We followed the established protocol for the SA-overload model in rats with minor modifications,4, 72 and the study was performed in accordance with the animal protocols approved by the Institutional Animal Care and Use Committee. After sacrificing the rats on day 5, the kidneys were harvested, glomeruli isolated and total mRNA was extracted. Detailed protocols of animal study are shown in Supplementary Materials online.
Podocyte culture and treatments
The conditionally immortalized mouse podocyte cell line (MPC-5) was cultured as previously described.32 For exposure to SA, differentiated podocytes were incubated with different kinds of purified bovine or human serum albumin (BSA, HSA) following which the cells were harvested and processed for total mRNA and protein extraction. BSA and HSA used in this study were all from Sigma (St Louis, MO); BSA (A7906), charcoal-treated FA/endotoxin-free BSA (A8806), FA/globulin-free BSA (A7030), endotoxin-free BSA (A9430), globulin-free BSA (A2058), HSA (A1653), FA- free HSA (A1887) and recombinant HSA made in yeast (A7736). The individual FAs (Nu-check Prep, Elysian, MN) and FA supplement (Sigma; F7050) were complexed with FA-free BSA (A8806) before treating podocytes. Detailed protocols for cell culture experiments and reagents used are described in Supplementary Materials online.
RNA extraction and real time polymerase chain reaction
Total RNA was extracted from podocytes or glomeruli and used for quantitative reverse transcription-polymerase chain reaction (qRT-PCR) as described previously.32 COX-2, B7-1, MCP-1, ICAM-1, MIP-2, TNF-α, CXCL1, CXCL5, TLR4, HSP25, HSP70i and β-actin mRNAs were quantified by qRT-PCR using the SYBR green method on an iQ5 thermal cycler (Bio-Rad Labs, Hercules, CA), and using primers listed in Supplementary Table II. Detailed protocols for RNA extraction and qRT-PCR are shown in Supplementary Materials online.
Transcript stability and mRNA half life
Stability of COX-2 mRNA under control and SA-exposed conditions in differentiated podocytes was assayed and is described in detail in Supplementary Materials online.
Protein extraction, SDS PAGE, and western blotting
Podocyte extracts were prepared and processed for SDS-PAGE followed by western blotting as previously described.32 Detailed protocols for protein extraction, SDS-PAGE, western blotting and antibodies used are described in Supplementary Materials online.
Statistical analyses
Statistical significance was determined by the unpaired Student's t test for single comparisons and one way ANOVA or two way ANOVA for multiple group comparisons, using the GraphPad Prism software version 6.00 for Windows. P values are indicated in the respective figure legends (*P<0.05, **P<0.01 and ***P<0.001; *P<0.05, **P<0.01 and ***P<0.001). Experiments were performed at least three times, and either the representative blots are shown or the data are presented as mean ± SEM.
Acknowledgements
We thank Rainer Benndorf and Bryce A Kerlin for critical reading of the manuscript. We also thank Amanda Waller for assistance with albuminuria determination, Ruma Pengal for assistance with some MAPK inhibitor experiments, Xiaojing Nie for assistance with microscopic images, and Tad Eichler for assistance with glomerular preparations. This work was supported in part by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (Grant RO1 DK077283) and internal funds from Nationwide Children’s Hospital to WES. Part of this work was presented as a poster (abstract) at the American Society of Nephrology (ASN) Kidney Week, 1–4 November 2012, San Diego, CA.
Footnotes
Part of this work was presented as a poster (abstract) at the American Society of Nephrology (ASN) Kidney Week, Nov 1-4, 2012, San Diego, CA.
Disclosures
None
References
- 1.Iseki K, Ikemiya Y, Iseki C, et al. Proteinuria and the risk of developing end-stage renal disease. Kidney international. 2003;63:1468–1474. doi: 10.1046/j.1523-1755.2003.00868.x. [DOI] [PubMed] [Google Scholar]
- 2.Brunskill NJ. Albumin signals the coming of age of proteinuric nephropathy. J Am Soc Nephrol. 2004;15:504–505. doi: 10.1097/01.asn.0000112912.40303.81. [DOI] [PubMed] [Google Scholar]
- 3.Ashworth CT, James JA. Glomerular excretion of macromolecular substances. Electron microscopic study of rat kidney after administration of human serum albumin. Am J Pathol. 1961;39:307–316. [PMC free article] [PubMed] [Google Scholar]
- 4.Lawrence GM, Brewer DB. Effect of strain and sex on the induction of hyperalbuminaemic proteinuria in the rat. Clinical science. 1981;61:751–756. doi: 10.1042/cs0610751. [DOI] [PubMed] [Google Scholar]
- 5.Eddy AA, Kim H, Lopez-Guisa J, et al. Interstitial fibrosis in mice with overload proteinuria: deficiency of TIMP-1 is not protective. Kidney international. 2000;58:618–628. doi: 10.1046/j.1523-1755.2000.00208.x. [DOI] [PubMed] [Google Scholar]
- 6.Eddy AA. Interstitial nephritis induced by protein-overload proteinuria. Am J Pathol. 1989;135:719–733. [PMC free article] [PubMed] [Google Scholar]
- 7.Weening JJ, Van Guldener C, Daha MR, et al. The pathophysiology of protein-overload proteinuria. Am J Pathol. 1987;129:64–73. [PMC free article] [PubMed] [Google Scholar]
- 8.Sieber J, Lindenmeyer MT, Kampe K, et al. Regulation of podocyte survival and endoplasmic reticulum stress by fatty acids. Am J Physiol Renal Physiol. 2010;299:F821–829. doi: 10.1152/ajprenal.00196.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eyre J, Ioannou K, Grubb BD, et al. Statin-sensitive endocytosis of albumin by glomerular podocytes. Am J Physiol Renal Physiol. 2007;292:F674–681. doi: 10.1152/ajprenal.00272.2006. [DOI] [PubMed] [Google Scholar]
- 10.Abbate M, Zoja C, Morigi M, et al. Transforming growth factor-beta1 is up-regulated by podocytes in response to excess intraglomerular passage of proteins: a central pathway in progressive glomerulosclerosis. Am J Pathol. 2002;161:2179–2193. doi: 10.1016/s0002-9440(10)64495-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoshida S, Nagase M, Shibata S, et al. Podocyte injury induced by albumin overload in vivo and in vitro: involvement of TGF-beta and p38 MAPK. Nephron Exp Nephrol. 2008;108:e57–68. doi: 10.1159/000124236. [DOI] [PubMed] [Google Scholar]
- 12.He F, Chen S, Wang H, et al. Regulation of CD2-associated protein influences podocyte endoplasmic reticulum stress-mediated apoptosis induced by albumin overload. Gene. 2011;484:18–25. doi: 10.1016/j.gene.2011.05.025. [DOI] [PubMed] [Google Scholar]
- 13.Chen S, He FF, Wang H, et al. Calcium entry via TRPC6 mediates albumin overload-induced endoplasmic reticulum stress and apoptosis in podocytes. Cell calcium. 2011;50:523–529. doi: 10.1016/j.ceca.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 14.Fang Z, He F, Chen S, et al. Albumin modulates the production of matrix metalloproteinases-2 and -9 in podocytes. Journal of Huazhong University of Science and Technology Medical sciences = Hua zhong ke ji da xue xue bao Yi xue Ying De wen ban = Huazhong keji daxue xuebao Yixue Yingdewen ban. 2009;29:710–714. doi: 10.1007/s11596-009-0607-1. [DOI] [PubMed] [Google Scholar]
- 15.Morigi M, Buelli S, Angioletti S, et al. In response to protein load podocytes reorganize cytoskeleton and modulate endothelin-1 gene: implication for permselective dysfunction of chronic nephropathies. Am J Pathol. 2005;166:1309–1320. doi: 10.1016/S0002-9440(10)62350-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pengal R, Guess AJ, Agrawal S, et al. Inhibition of the protein kinase MK-2 protects podocytes from nephrotic syndrome-related injury. Am J Physiol Renal Physiol. 2011;301:F509–519. doi: 10.1152/ajprenal.00661.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris RC. COX-2 and the kidney. J Cardiovasc Pharmacol. 2006;47(Suppl 1):S37–42. doi: 10.1097/00005344-200605001-00007. [DOI] [PubMed] [Google Scholar]
- 18.Kang YJ, Mbonye UR, DeLong CJ, et al. Regulation of intracellular cyclooxygenase levels by gene transcription and protein degradation. Prog Lipid Res. 2007;46:108–125. doi: 10.1016/j.plipres.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Vries EF. Imaging of cyclooxygenase-2 (COX-2) expression: potential use in diagnosis and drug evaluation. Curr Pharm Des. 2006;12:3847–3856. doi: 10.2174/138161206778559650. [DOI] [PubMed] [Google Scholar]
- 20.Wang JL, Cheng HF, Zhang MZ, et al. Selective increase of cyclooxygenase-2 expression in a model of renal ablation. Am J Physiol. 1998;275:F613–622. doi: 10.1152/ajprenal.1998.275.4.F613. [DOI] [PubMed] [Google Scholar]
- 21.Rangel EB, Moura LA, Franco MF, et al. Up-regulation of cyclooxygenase-2 during acute human renal allograft rejection. Clin Transplant. 2005;19:543–550. doi: 10.1111/j.1399-0012.2005.00386.x. [DOI] [PubMed] [Google Scholar]
- 22.Komers R, Lindsley JN, Oyama TT, et al. Immunohistochemical and functional correlations of renal cyclooxygenase-2 in experimental diabetes. The Journal of clinical investigation. 2001;107:889–898. doi: 10.1172/JCI10228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng H, Wang S, Jo YI, et al. Overexpression of cyclooxygenase-2 predisposes to podocyte injury. J Am Soc Nephrol. 2007;18:551–559. doi: 10.1681/ASN.2006090990. [DOI] [PubMed] [Google Scholar]
- 24.Jo YI, Cheng H, Wang S, et al. Puromycin induces reversible proteinuric injury in transgenic mice expressing cyclooxygenase-2 in podocytes. Nephron Exp Nephrol. 2007;107:e87–94. doi: 10.1159/000108653. [DOI] [PubMed] [Google Scholar]
- 25.Cheng H, Fan X, Moeckel GW, et al. Podocyte COX-2 exacerbates diabetic nephropathy by increasing podocyte (pro)renin receptor expression. J Am Soc Nephrol. 2011;22:1240–1251. doi: 10.1681/ASN.2010111149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Faour WH, Gomi K, Kennedy CR. PGE(2) induces COX-2 expression in podocytes via the EP(4) receptor through a PKA-independent mechanism. Cell Signal. 2008;20:2156–2164. doi: 10.1016/j.cellsig.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 27.Greenbaum LA, Benndorf R, Smoyer WE. Childhood nephrotic syndrome--current and future therapies. Nature reviews Nephrology. 2012;8:445–458. doi: 10.1038/nrneph.2012.115. [DOI] [PubMed] [Google Scholar]
- 28.Sarafidis PA, Stafylas PC, Georgianos PI, et al. Effect of thiazolidinediones on albuminuria and proteinuria in diabetes: a meta-analysis. Am J Kidney Dis. 2010;55:835–847. doi: 10.1053/j.ajkd.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 29.Kawamura T, Yoshioka T, Bills T, et al. Glucocorticoid activates glomerular antioxidant enzymes and protects glomeruli from oxidant injuries. Kidney international. 1991;40:291–301. doi: 10.1038/ki.1991.213. [DOI] [PubMed] [Google Scholar]
- 30.Zuo Y, Yang HC, Potthoff SA, et al. Protective effects of PPARgamma agonist in acute nephrotic syndrome. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2012;27:174–181. doi: 10.1093/ndt/gfr240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grande MT, Lopez-Novoa JM. Therapeutical relevance of MAP-kinase inhibitors in renal diseases: current knowledge and future clinical perspectives. Curr Med Chem. 2008;15:2054–2070. doi: 10.2174/092986708785132889. [DOI] [PubMed] [Google Scholar]
- 32.Agrawal S, Guess AJ, Benndorf R, et al. Comparison of direct action of thiazolidinediones and glucocorticoids on renal podocytes: protection from injury and molecular effects. Mol Pharmacol. 2011;80:389–399. doi: 10.1124/mol.111.071654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ransom RF, Lam NG, Hallett MA, et al. Glucocorticoids protect and enhance recovery of cultured murine podocytes via actin filament stabilization. Kidney international. 2005;68:2473–2483. doi: 10.1111/j.1523-1755.2005.00723.x. [DOI] [PubMed] [Google Scholar]
- 34.Wada T, Pippin JW, Marshall CB, et al. Dexamethasone prevents podocyte apoptosis induced by puromycin aminonucleoside: role of p53 and Bcl-2-related family proteins. J Am Soc Nephrol. 2005;16:2615–2625. doi: 10.1681/ASN.2005020142. [DOI] [PubMed] [Google Scholar]
- 35.Miglio G, Rosa AC, Rattazzi L, et al. Protective effects of peroxisome proliferator-activated receptor agonists on human podocytes: proposed mechanisms of action. British journal of pharmacology. 2012;167:641–653. doi: 10.1111/j.1476-5381.2012.02026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kanjanabuch T, Ma LJ, Chen J, et al. PPAR-gamma agonist protects podocytes from injury. Kidney international. 2007;71:1232–1239. doi: 10.1038/sj.ki.5002248. [DOI] [PubMed] [Google Scholar]
- 37.Reiser J, von Gersdorff G, Loos M, et al. Induction of B7-1 in podocytes is associated with nephrotic syndrome. The Journal of clinical investigation. 2004;113:1390–1397. doi: 10.1172/JCI20402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Srivastava T, Sharma M, Yew KH, et al. LPS and PAN-induced podocyte injury in an in vitro model of minimal change disease: changes in TLR profile. Journal of cell communication and signaling. 2013;7:49–60. doi: 10.1007/s12079-012-0184-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lasa M, Mahtani KR, Finch A, et al. Regulation of cyclooxygenase 2 mRNA stability by the mitogen-activated protein kinase p38 signaling cascade. Mol Cell Biol. 2000;20:4265–4274. doi: 10.1128/mcb.20.12.4265-4274.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen RF. Removal of fatty acids from serum albumin by charcoal treatment. J Biol Chem. 1967;242:173–181. [PubMed] [Google Scholar]
- 41.Eddy AA, Giachelli CM. Renal expression of genes that promote interstitial inflammation and fibrosis in rats with protein-overload proteinuria. Kidney international. 1995;47:1546–1557. doi: 10.1038/ki.1995.218. [DOI] [PubMed] [Google Scholar]
- 42.Anderson MS, Recant L. Fine structural alterations in the rat kidney following intraperitoneal bovine albumin. Am J Pathol. 1962;40:555–569. [PMC free article] [PubMed] [Google Scholar]
- 43.Ishola DA, Jr., van der Giezen DM, Hahnel B, et al. In mice, proteinuria and renal inflammatory responses to albumin overload are strain-dependent. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2006;21:591–597. doi: 10.1093/ndt/gfi303. [DOI] [PubMed] [Google Scholar]
- 44.Okamura K, Dummer P, Kopp J, et al. Endocytosis of albumin by podocytes elicits an inflammatory response and induces apoptotic cell death. PloS one. 2013;8:e54817. doi: 10.1371/journal.pone.0054817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Viedt C, Orth SR. Monocyte chemoattractant protein-1 (MCP-1) in the kidney: does it more than simply attract monocytes? Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2002;17:2043–2047. doi: 10.1093/ndt/17.12.2043. [DOI] [PubMed] [Google Scholar]
- 46.Lee EY, Chung CH, Khoury CC, et al. The monocyte chemoattractant protein-1/CCR2 loop, inducible by TGF-beta, increases podocyte motility and albumin permeability. Am J Physiol Renal Physiol. 2009;297:F85–94. doi: 10.1152/ajprenal.90642.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brinkkoetter PT, Ising C, Benzing T. The role of the podocyte in albumin filtration. Nature reviews Nephrology. 2013;9:328–336. doi: 10.1038/nrneph.2013.78. [DOI] [PubMed] [Google Scholar]
- 48.Tenten V, Menzel S, Kunter U, et al. Albumin Is Recycled from the Primary Urine by Tubular Transcytosis. J Am Soc Nephrol. 2013 doi: 10.1681/ASN.2013010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rossi A, Coccia M, Trotta E, et al. Regulation of cyclooxygenase-2 expression by heat: a novel aspect of heat shock factor 1 function in human cells. PloS one. 2012;7:e31304. doi: 10.1371/journal.pone.0031304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vega A, Chacon P, Alba G, et al. Modulation of IgE-dependent COX-2 gene expression by reactive oxygen species in human neutrophils. Journal of leukocyte biology. 2006;80:152–163. doi: 10.1189/jlb.0705411. [DOI] [PubMed] [Google Scholar]
- 51.Alvarez S, Serramia MJ, Fresno M, et al. Human immunodeficiency virus type 1 envelope glycoprotein 120 induces cyclooxygenase-2 expression in neuroblastoma cells through a nuclear factor-kappaB and activating protein-1 mediated mechanism. Journal of neurochemistry. 2005;94:850–861. doi: 10.1111/j.1471-4159.2005.03267.x. [DOI] [PubMed] [Google Scholar]
- 52.Kamijo A, Kimura K, Sugaya T, et al. Urinary free fatty acids bound to albumin aggravate tubulointerstitial damage. Kidney international. 2002;62:1628–1637. doi: 10.1046/j.1523-1755.2002.00618.x. [DOI] [PubMed] [Google Scholar]
- 53.Thomas ME, Harris KP, Walls J, et al. Fatty acids exacerbate tubulointerstitial injury in protein-overload proteinuria. Am J Physiol Renal Physiol. 2002;283:F640–647. doi: 10.1152/ajprenal.00001.2002. [DOI] [PubMed] [Google Scholar]
- 54.Banas MC, Banas B, Hudkins KL, et al. TLR4 links podocytes with the innate immune system to mediate glomerular injury. J Am Soc Nephrol. 2008;19:704–713. doi: 10.1681/ASN.2007040395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.DeBroe ME, Porter GA, Bennett WM, et al. In: Nonsteroidal anti-inflammatory drugs: effects on kidney function. 9th edition. Whelton A, Watson AJ, editors. Kluwer Academic Publishers Dordrecht; The Netherlands: 1998. [Google Scholar]
- 56.Markowitz GS, Falkowitz DC, Isom R, et al. Membranous glomerulopathy and acute interstitial nephritis following treatment with celecoxib. Clinical nephrology. 2003;59:137–142. doi: 10.5414/cnp59137. [DOI] [PubMed] [Google Scholar]
- 57.Alper AB, Jr., Meleg-Smith S, Krane NK. Nephrotic syndrome and interstitial nephritis associated with celecoxib. Am J Kidney Dis. 2002;40:1086–1090. doi: 10.1053/ajkd.2002.36349. [DOI] [PubMed] [Google Scholar]
- 58.Vogt L, de Zeeuw D, Woittiez AJ, et al. Selective cyclooxygenase-2 (COX-2) inhibition reduces proteinuria in renal patients. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2009;24:1182–1189. doi: 10.1093/ndt/gfn644. [DOI] [PubMed] [Google Scholar]
- 59.Mihovilovic K, Ljubanovic D, Knotek M. Safe administration of celecoxib to a patient with repeated episodes of nephrotic syndrome induced by NSAIDs. Clinical drug investigation. 2011;31:351–355. doi: 10.1007/BF03256934. [DOI] [PubMed] [Google Scholar]
- 60.Lee DW, Kwak IS, Lee SB, et al. Effects of celecoxib and nordihydroguaiaretic acid on puromycin aminonucleoside-induced nephrosis in the rat. Journal of Korean medical science. 2009;24(Suppl):S183–188. doi: 10.3346/jkms.2009.24.S1.S183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang L, Flannery PJ, Rosenberg PB, et al. Gq-dependent signaling upregulates COX2 in glomerular podocytes. J Am Soc Nephrol. 2008;19:2108–2118. doi: 10.1681/ASN.2008010113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Elder DJ, Halton DE, Playle LC, et al. The MEK/ERK pathway mediates COX-2-selective NSAID-induced apoptosis and induced COX-2 protein expression in colorectal carcinoma cells. Int J Cancer. 2002;99:323–327. doi: 10.1002/ijc.10330. [DOI] [PubMed] [Google Scholar]
- 63.Shafer LM, Slice LW. Anisomycin induces COX-2 mRNA expression through p38(MAPK) and CREB independent of small GTPases in intestinal epithelial cells. Biochim Biophys Acta. 2005;1745:393–400. doi: 10.1016/j.bbamcr.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 64.Nieminen R, Lahti A, Jalonen U, et al. JNK inhibitor SP600125 reduces COX-2 expression by attenuating mRNA in activated murine J774 macrophages. Int Immunopharmacol. 2006;6:987–996. doi: 10.1016/j.intimp.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 65.Lasa M, Brook M, Saklatvala J, et al. Dexamethasone destabilizes cyclooxygenase 2 mRNA by inhibiting mitogen-activated protein kinase p38. Mol Cell Biol. 2001;21:771–780. doi: 10.1128/MCB.21.3.771-780.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Han S, Inoue H, Flowers LC, et al. Control of COX-2 gene expression through peroxisome proliferator-activated receptor gamma in human cervical cancer cells. Clin Cancer Res. 2003;9:4627–4635. [PubMed] [Google Scholar]
- 67.Meade EA, McIntyre TM, Zimmerman GA, et al. Peroxisome proliferators enhance cyclooxygenase-2 expression in epithelial cells. J Biol Chem. 1999;274:8328–8334. doi: 10.1074/jbc.274.12.8328. [DOI] [PubMed] [Google Scholar]
- 68.Sun H, Sheveleva E, Xu B, et al. Corticosteroids induce COX-2 expression in cardiomyocytes: role of glucocorticoid receptor and C/EBP-beta. Am J Physiol Cell Physiol. 2008;295:C915–922. doi: 10.1152/ajpcell.90646.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li MD, Yang X. A Retrospective on Nuclear Receptor Regulation of Inflammation: Lessons from GR and PPARs. PPAR research. 2011;2011:742785. doi: 10.1155/2011/742785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Christ-Crain M, Kola B, Lolli F, et al. AMP-activated protein kinase mediates glucocorticoid-induced metabolic changes: a novel mechanism in Cushing's syndrome. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2008;22:1672–1683. doi: 10.1096/fj.07-094144. [DOI] [PubMed] [Google Scholar]
- 71.Scoditti E, Massaro M, Carluccio MA, et al. PPARgamma agonists inhibit angiogenesis by suppressing PKCalpha- and CREB-mediated COX-2 expression in the human endothelium. Cardiovasc Res. 2010;86:302–310. doi: 10.1093/cvr/cvp400. [DOI] [PubMed] [Google Scholar]
- 72.Pippin JW, Brinkkoetter PT, Cormack-Aboud FC, et al. Inducible rodent models of acquired podocyte diseases. Am J Physiol Renal Physiol. 2009;296:F213–229. doi: 10.1152/ajprenal.90421.2008. [DOI] [PubMed] [Google Scholar]