Abstract
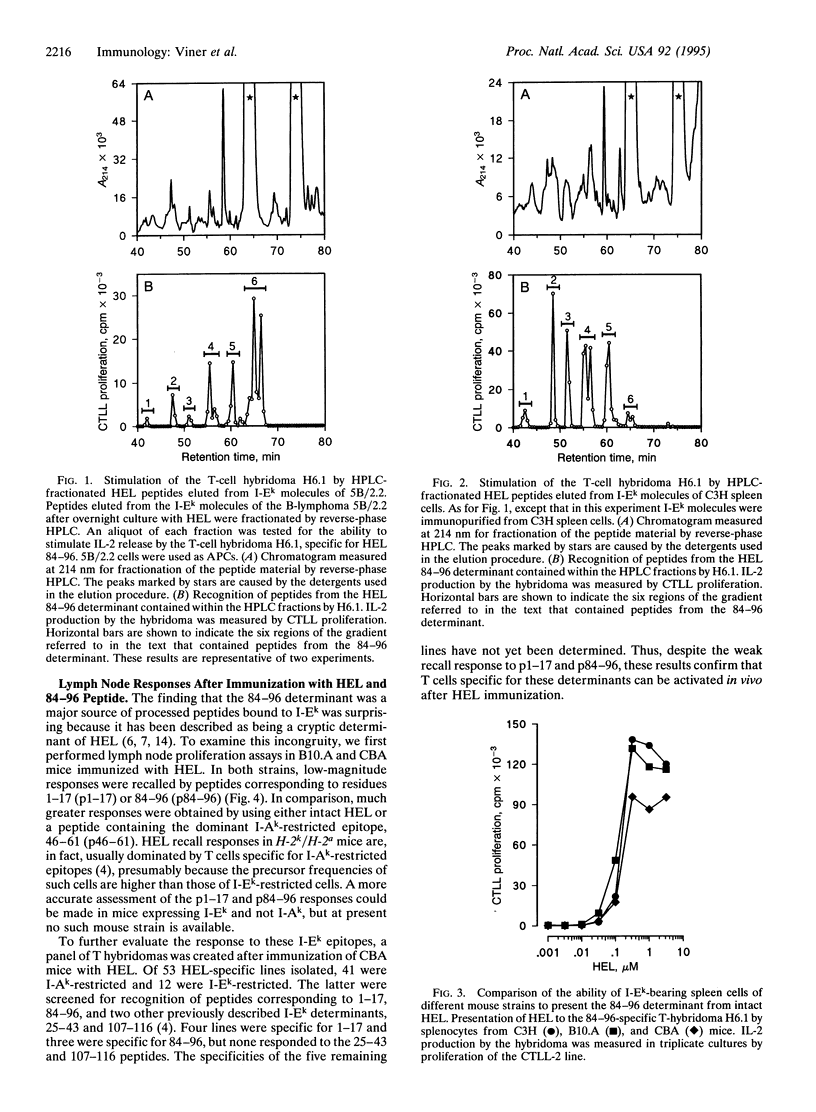
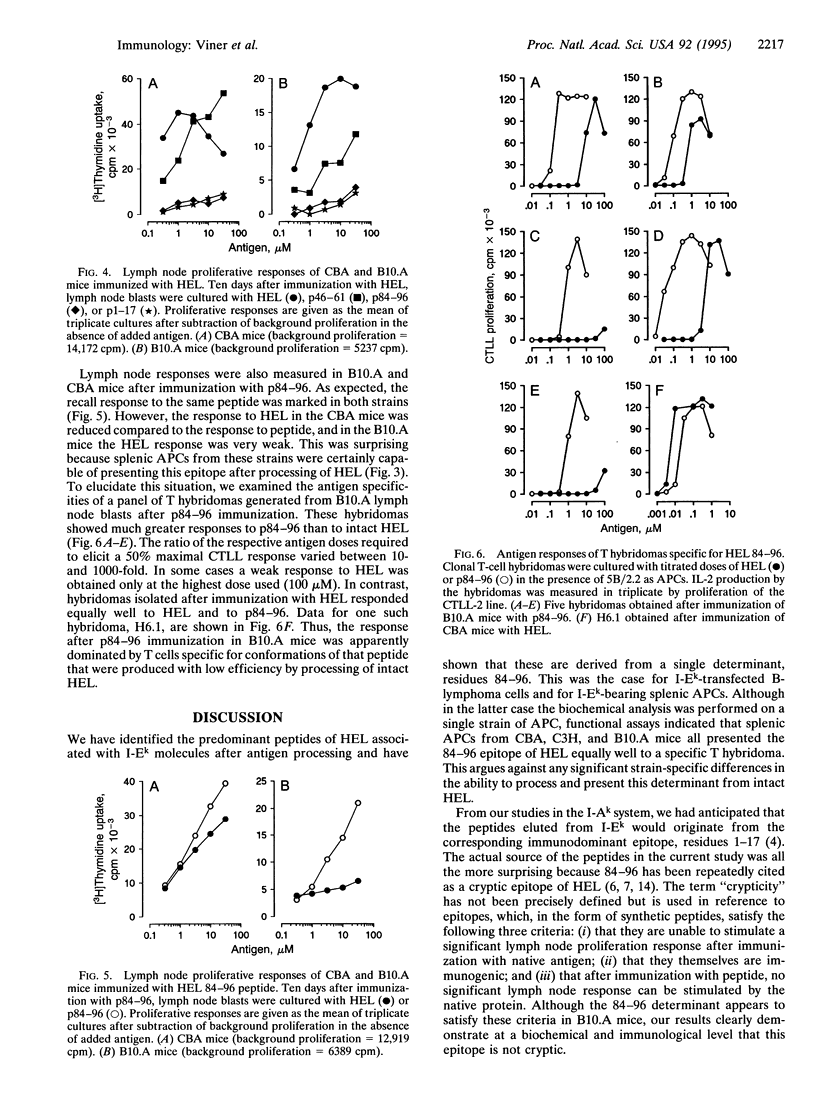
We have chemically analyzed the peptides presented by I-Ek molecules after processing of hen egg lysozyme (HEL) by a murine B-lymphoma line or by splenocytes. In both cases, the identified peptides were derived from a single region of HEL, containing the core residues 85-96 with heterogeneous N and C termini. This was a surprising result because this determinant had previously been described as cryptic--i.e., not presented after processing of intact HEL. Examination of the specificities of T hybridomas isolated after immunization with either HEL or 84-96 peptide (p84-96) provided an explanation for this controversy. Whereas hybridomas induced by immunization with HEL responded equally well to HEL and p84-96, those induced by peptide immunization showed a marked preference for p84-96 over intact HEL. In other words, hybridomas isolated after p84-96 immunization responded poorly to forms of the 84-96 determinant produced by natural processing, leading to the possible erroneous interpretation that 84-96 is a hidden determinant. We conclude that (i) p84-96 is efficiently presented on I-Ek molecules after processing of HEL, (ii) the explanation for the weak lymph node response to this epitope after immunization with HEL lies at the level of the T cell, not the antigen-presenting cell, and (iii) crypticity cannot be defined on the basis of T-cell proliferation studies alone.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adorini L., Appella E., Doria G., Nagy Z. A. Mechanisms influencing the immunodominance of T cell determinants. J Exp Med. 1988 Dec 1;168(6):2091–2104. doi: 10.1084/jem.168.6.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen P. M., Strydom D. J., Unanue E. R. Processing of lysozyme by macrophages: identification of the determinant recognized by two T-cell hybridomas. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2489–2493. doi: 10.1073/pnas.81.8.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ametani A., Apple R., Bhardwaj V., Gammon G., Miller A., Sercarz E. Examining the crypticity of antigenic determinants. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):505–511. doi: 10.1101/sqb.1989.054.01.060. [DOI] [PubMed] [Google Scholar]
- Babbitt B. P., Allen P. M., Matsueda G., Haber E., Unanue E. R. Binding of immunogenic peptides to Ia histocompatibility molecules. 1985 Sep 26-Oct 2Nature. 317(6035):359–361. doi: 10.1038/317359a0. [DOI] [PubMed] [Google Scholar]
- Bhayani H., Paterson Y. Analysis of peptide binding patterns in different major histocompatibility complex/T cell receptor complexes using pigeon cytochrome c-specific T cell hybridomas. Evidence that a single peptide binds major histocompatibility complex in different conformations. J Exp Med. 1989 Nov 1;170(5):1609–1625. doi: 10.1084/jem.170.5.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buus S., Sette A., Colon S. M., Miles C., Grey H. M. The relation between major histocompatibility complex (MHC) restriction and the capacity of Ia to bind immunogenic peptides. Science. 1987 Mar 13;235(4794):1353–1358. doi: 10.1126/science.2435001. [DOI] [PubMed] [Google Scholar]
- Donermeyer D. L., Allen P. M. Binding to Ia protects an immunogenic peptide from proteolytic degradation. J Immunol. 1989 Feb 15;142(4):1063–1068. [PubMed] [Google Scholar]
- Fox B. S., Carbone F. R., Germain R. N., Paterson Y., Schwartz R. H. Processing of a minimal antigenic peptide alters its interaction with MHC molecules. Nature. 1988 Feb 11;331(6156):538–540. doi: 10.1038/331538a0. [DOI] [PubMed] [Google Scholar]
- Gammon G., Shastri N., Cogswell J., Wilbur S., Sadegh-Nasseri S., Krzych U., Miller A., Sercarz E. The choice of T-cell epitopes utilized on a protein antigen depends on multiple factors distant from, as well as at the determinant site. Immunol Rev. 1987 Aug;98:53–73. doi: 10.1111/j.1600-065x.1987.tb00519.x. [DOI] [PubMed] [Google Scholar]
- Kappler J. W., Skidmore B., White J., Marrack P. Antigen-inducible, H-2-restricted, interleukin-2-producing T cell hybridomas. Lack of independent antigen and H-2 recognition. J Exp Med. 1981 May 1;153(5):1198–1214. doi: 10.1084/jem.153.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrack P., Ignatowicz L., Kappler J. W., Boymel J., Freed J. H. Comparison of peptides bound to spleen and thymus class II. J Exp Med. 1993 Dec 1;178(6):2173–2183. doi: 10.1084/jem.178.6.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J., Germain R. N. Efficient cell surface expression of class II MHC molecules in the absence of associated invariant chain. J Exp Med. 1986 Nov 1;164(5):1478–1489. doi: 10.1084/jem.164.5.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouritsen S., Meldal M., Werdelin O., Hansen A. S., Buus S. MHC molecules protect T cell epitopes against proteolytic destruction. J Immunol. 1992 Sep 15;149(6):1987–1993. [PubMed] [Google Scholar]
- Nelson C. A., Petzold S. J., Unanue E. R. Peptides determine the lifespan of MHC class II molecules in the antigen-presenting cell. Nature. 1994 Sep 15;371(6494):250–252. doi: 10.1038/371250a0. [DOI] [PubMed] [Google Scholar]
- Nelson C. A., Roof R. W., McCourt D. W., Unanue E. R. Identification of the naturally processed form of hen egg white lysozyme bound to the murine major histocompatibility complex class II molecule I-Ak. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7380–7383. doi: 10.1073/pnas.89.16.7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozato K., Mayer N., Sachs D. H. Hybridoma cell lines secreting monoclonal antibodies to mouse H-2 and Ia antigens. J Immunol. 1980 Feb;124(2):533–540. [PubMed] [Google Scholar]
- Schwartz R. H. T-lymphocyte recognition of antigen in association with gene products of the major histocompatibility complex. Annu Rev Immunol. 1985;3:237–261. doi: 10.1146/annurev.iy.03.040185.001321. [DOI] [PubMed] [Google Scholar]
- Sercarz E. E., Lehmann P. V., Ametani A., Benichou G., Miller A., Moudgil K. Dominance and crypticity of T cell antigenic determinants. Annu Rev Immunol. 1993;11:729–766. doi: 10.1146/annurev.iy.11.040193.003501. [DOI] [PubMed] [Google Scholar]
- Sercarz E. E. The architectonics of immune dominance: the aleatory effects of molecular position on the choice of antigenic determinants. Chem Immunol. 1989;46:169–185. [PubMed] [Google Scholar]
- Wade W. F., Chen Z. Z., Maki R., McKercher S., Palmer E., Cambier J. C., Freed J. H. Altered I-A protein-mediated transmembrane signaling in B cells that express truncated I-Ak protein. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6297–6301. doi: 10.1073/pnas.86.16.6297. [DOI] [PMC free article] [PubMed] [Google Scholar]



