Introduction
In this grand rounds, we focus on development, validation and application of neuroimaging biomarkers for Parkinson disease (PD). We cover whether such biomarkers can be used to identify presymptomatic individuals (probably yes), provide a measure of PD severity (in a limited fashion, but frequently done poorly), investigate pathophysiology of parkinsonian disorders (yes, if done carefully), play a role in differential diagnosis of parkinsonism (not well), and investigate pathology underlying cognitive impairment (yes, in conjunction with postmortem data). Along the way, we clarify several issues about definitions of biomarkers and surrogate endpoints. The goal of this lecture is to provide a basis for interpreting current literature and newly proposed clinical tools in PD. In the end, one should be able to critically distinguish fact from fantasy.
Background
PD is a neurodegenerative condition producing motor manifestations including tremor, bradykinesia, rigidity, postural instability, festinating gait and hypophonic speech as well as multiple non-motor symptoms such as orthostasis, constipation, bladder dysfunction, swallowing difficulties, sleep disturbances, mood disorders and cognitive impairment. The underlying pathophysiology includes progressive deposition within brainstem pigmented nuclei of abnormal α-synuclein in intracytoplasmic inclusions (Lewy bodies) and cell processes (Lewy neurites) that also may affect numerous cortical regions. Motor symptoms mostly reflect dysfunction of the dopaminergic nigrostriatal pathway, whereas involvement of other brainstem nuclei and cortical areas likely contributes to non-motor manifestations. Thus the ideal neuroimaging biomarker should be able to quantify regional deposition of abnormal α-synuclein. Development of such a molecular imaging radiotracer has been the focus of much research but is not yet forthcoming.1
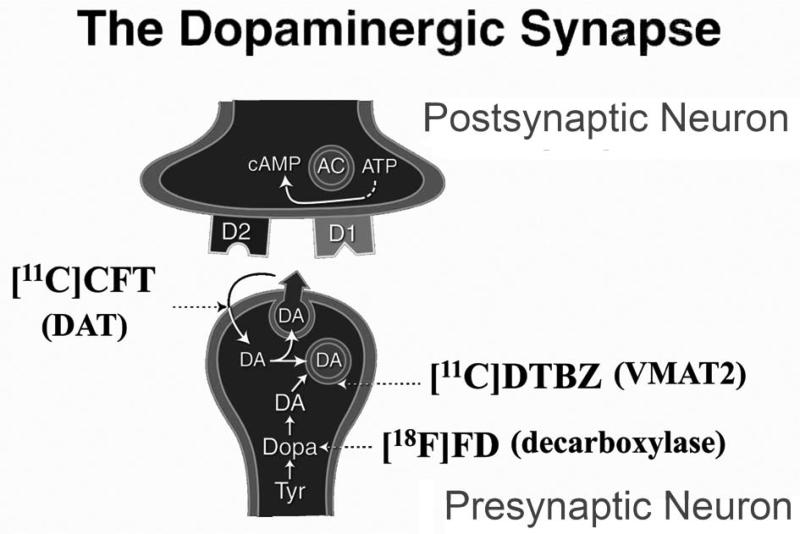
Alternatively, much research effort has been expended to develop measures to quantify nigrostriatal neurons or the effects that loss of these neurons produce. The most direct approach has been to try to quantify presynaptic nigrostriatal dopaminergic neurons. Three classes of radiotracers are commonly used for positron emission tomography (PET) or single photon emission computed tomography (SPECT)-based imaging to assess pre-synaptic dopaminergic nigrostriatal neurons represented by (Fig. 1): 6-[18F]fluorodopa (FD; primarily reflects decarboxylase activity and storage), [11C]dihydrotetrabenazine (DTBZ; reflects vesicular monoamine transporter type 2, VMAT2), and 2-beta-[11C]carbomethoxy-3-beta-4-fluorophenyltropane (CFT; reflects membranous dopamine transporter, DAT)2. Multiple other radiopharmaceuticals have been developed for these molecular targets, including those useful for SPECT.
Figure 1.
Depiction of presynaptic and postsynaptic terminals in a nigrostriatal dopaminergic synapse. Tyr = tyrosine; Dopa = dihydroxyphenylalanine; DA = dopamine; D1 = dopamine D1-like receptor; D2 = dopamine D2-like receptor; [11C]DTBZ = [11C]dihydrotetrabenazine; [18F]FD = 6-[18F]fluorodopa; [11C]CFT = 2-beta-[11C]carbomethoxy-3-beta-4-fluorophenyltropane, DAT = dopamine active transporter; VMAT2 = vesicular monoamine transporter 2; AC = adenylyl cyclase.
Each of these classes of presynaptic radiopharmaceuticals clearly distinguishes those with moderate to severe PD from those without parkinsonism. Such studies have been done in humans with PD as well as in animal models of PD – usually toxin-induced animal models such as 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced nigrostriatal injury in nonhuman primates (Fig. 2).3 On this basis, these types of radiotracers have been applied to a variety of research and potentially clinical relevant uses.
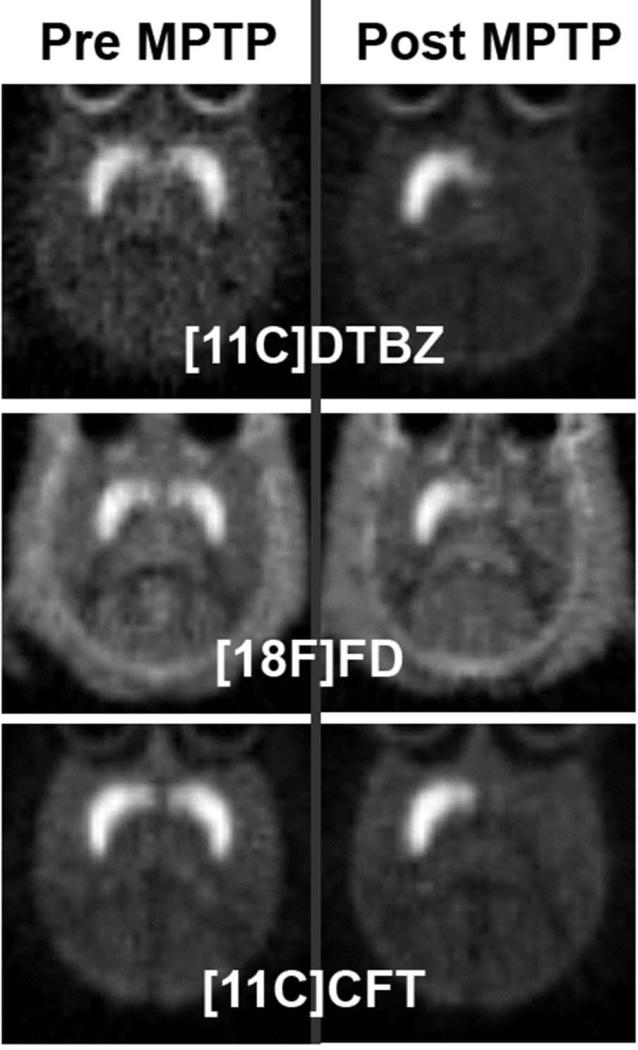
Figure 2.
MPTP induced nigrostriatal injury in non-human primates detected by PET. PET images in the same non-human primate prior to unilateral right intracarotid administration of MPTP (left column) and 8 weeks after MPTP administration (right column). Each row represents PET imaging performed with a different presynaptic radiopharmaceutical: [11C]DTBZ = [11C]dihydrotetrabenazine , [18F]FD = 6-[18F]fluorodopa, and [11C]CFT = 2-beta-[11C]carbomethoxy-3-beta-4-fluorophenyltropane. Note unilateral reduction (right brain) of radioactivity in the caudate and putamen following ipsilateral MPTP injection. Right brain is represented on the right side of the image.
Presymptomatic Diagnosis
One particularly important potential application of these radiotracers could be identification of defects in the nigrostriatal pathway prior to development of motor manifestations of PD. Several studies serendipitously found this may be possible. For example, FD PET scans of monozygotic and dizygotic twins with at least one affected twin revealed low striatal uptake of FD in an unaffected twin who developed clinical manifestations within two years.4 Similarly, a study using sequential doses of MPTP to create progressive lesions of nigrostriatal pathways in a monkey demonstrated reductions in radiolabeled levodopa striatal uptake prior to development of clinically identifiable tremor .5 An earlier PET study demonstrated decreased striatal FD uptake in monkeys given doses of MPTP insufficient to cause motor parkinsonism.6 Although these studies suggest that molecular imaging may be more sensitive than clinical exam or symptomatic history for identification of dysfunction of the nigrostriatal pathway, other studies have raised questions about the potential overlap between normal and abnormal findings in those with either mild symptoms or minimal defects in nigrostriatal dopaminergic pathway.7-9
Nevertheless, molecular neuroimaging, at least in some cases, can be more sensitive than clinical observations. This has potential importance for two scenarios. First, presymptomatic diagnosis could be important once an intervention has been developed to forestall symptom onset in people at risk for development of PD. Of course, other neuroimaging measures quantifying changes in brain networks may be equally or more sensitive than radiotracers targeting presynaptic dopaminergic neurons, but these require additional validation.10 Much research is now focused on developing such biomarkers of early PD which will hopefully have increased relevance once we have a pertinent treatment. Of course, biomarkers to aid with presymptomatic diagnosis are a critical tool to develop an intervention to forestall disease progression. Otherwise, subjects for such a study could not be identified.
Measurement of PD Severity
Can presynaptic radiopharmaceuticals provide a measure of PD severity that is a useful objective measure of disease progression in a clinical trial of a potential disease modifying therapy for PD? Of course, the underlying assumption is that progressive loss of nigrostriatal neurons reflects disease progression.10 In the future, quantification of abnormal deposition of α-synuclein may be a more direct and relevant measure – including cortical as well as brainstem deposition. In the meantime, the relevant question is whether changes in striatal uptake of one of the presynaptic radiopharmaceuticals objectively reflect disease progression?
Before addressing this question, it is important to have a basic understanding of the definition of a primary endpoint of a clinical study, a biomarker and a surrogate endpoint. A primary endpoint of a study is a clinically relevant event that is meaningful to a patient such as death, stroke, time to need a wheelchair or quality of life. These measures can be categorical, like death, or continuous, like quality of life or lifespan after a particular diagnosis.11 A biomarker is an objectively measured indication of a biological process, pathogenic process or response to an intervention.12 A surrogate endpoint is a biomarker that can substitute for a clinically meaningful endpoint. To fulfill this criterion, a biomarker must meet multiple conditions: 1) it must reflect underlying pathophysiology of the disease, 2) the intervention must alter the disease mechanism pathway that the biomarker reflects, 3) the intervention should not directly affect the biomarker without also affecting the underlying disease mechanistic pathway, 4) a statistically significant change in the biomarker must have sufficient magnitude to alter the course of the disease13 and, 5) to be clinically relevant, the biomarker-- if used as a surrogate endpoint -- must reflect toxicity of the intervention.14-16 Thus, the bar for a biomarker to act as a surrogate endpoint of a trial is rather high, but this does not preclude use of a biomarker to assess target engagement or efficacy in a clinical trial.
However, multiple clinical trials of PD comparing presynaptic nigrostriatal molecular imaging biomarkers to clinical measures of disease progression revealed discordant results. The ELLDOPA study prospectively treated 4 groups of otherwise unmedicated PD patients (n=361) with either placebo or three different daily doses of levodopa (150 mg, 300 mg or 600 mg) for 9 months.17 Clinical progression was determined by a clinical rating scale of motor parkinsonism (the Unified Parkinson Disease Rating Scale part 3 – UPDRS 3) that revealed the group treated with 600 mg per day had the least clinical progression despite having stopped levodopa for either one or two weeks; admittedly, the number of participants that could tolerate stopping levodopa for two weeks was relatively small. However, those that did demonstrated no worsening of symptoms from the one week to the two week point after stopping levodopa (suggesting lack of residual symptomatic benefit). The imaging biomarker of disease progression was a DAT SPECT scan done in a subgroup of 116 subjects. After removing 19 participants that did not have a baseline deficit on the SPECT (to reduce the chance of including non-PD subjects in the analysis); the group treated with levodopa 600 mg per day had the greatest additional loss of striatal uptake of the radiopharmaceutical, suggesting greater loss of nigrostriatal dopaminergic neurons. In other words, the clinical measures of the endpoint were discordant with the imaging measures. Several other studies have found similar discordant results.18-22
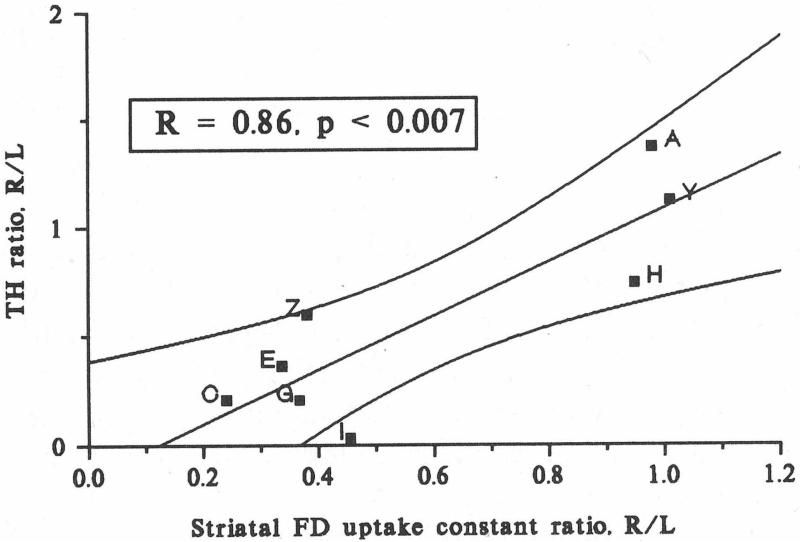
These discrepancies between neuroimaging and clinical measures raise questions about the validity of using such neuroimaging biomarkers as a metric of disease progression. Prior studies to validate that striatal uptake of these widely used neuroimaging biomarkers faithfully reflect reduction of nigrostriatal dopaminergic neurons have been flawed. One early study compared tyrosine hydroxylase (TH) immunostained cells in substantia nigra with PET measures of striatal FD uptake in a small number of monkeys treated with MPTP, a neurotoxin selective for dopaminergic cells.23 TH is a reasonable method to identify dopaminergic cells but that particular study used optical densitometry in representative nigral tissue sections, which is not a reliable means to accurately count cells.
Furthermore, the presumed strong correlation between the PET and in vitro measures (r = 0.86, p < 0.007) was not valid since distribution of the data formed two clusters (Fig. 3) – those strongly affected and those unaffected by MPTP. Rather these data support a categorical difference between the two groups. This study did, however, demonstrate a strong correlation between striatal FD uptake in vivo with ex vivo measures of striatal dopamine. Human studies comparing in vivo FD PET with subsequent post mortem measures had similar problems with inadequate cell count methods and small numbers of subjects.24 A technically impressive study compared high resolution SPECT measures of striatal DAT in rats given either of two neurotoxins to selectively destroy dopaminergic neurons.25 The investigators then compared the SPECT measures with postmortem measures of striatal dopamine or full unbiased stereologic counts of TH nigral neurons. They found strong correlations between striatal dopamine and striatal SPECT measures but examination of their graphs of striatal SPECT DAT measures versus nigral cell counts reveals again two clusters of data – one cluster in the upper right and one in the lower left of the graphs (Fig. 4). The gross bimodal distribution of the data invalidates a correlational analysis. Thus, these findings do not support the notion that the in vivo striatal DAT measures correlate with nigral TH cell counts.
Figure 3.
Linear correlations of the right-to-left ratios (R/L) of striatal FD uptake constants with R/L ratios of TH activity (as measured using optical densitometry) following unilateral MPTP administration. The lines are the best fit and 90% confidence interval. Note, these data are bimodally distributed and invalidate the fit and correlation comparing in vitro cell counts with in vivo SPECT measures. The two clusters may account for those strongly affected and those relatively unaffected by an intervention. Figure adapted from Pate et al, 1993.23
Figure 4.
Regression analysis comparing in vivo DAT SPECT imaging with in vitro nigrostriatal measures. A pin-hole high resolution SPECT scanner was used to image in vivo striatal [123I]FP-CIT uptake in rats treated unilaterally with 6-hydoxydopamine (6-OHDA, which destroys dopaminergic neurons). Regression analysis of A) in vitro measures of striatal DA concentration and striatal [123I]FP-CIT binding ratio, and B) unbiased stereologic in vitro counts of nigral TH+ cell number and striatal [123I]FP-CIT binding ratio. Note, these data are bimodally distributed and invalidate the regression analysis comparing the in vitro cell counts with the in vivo SPECT measures. Values for lesioned and contralateral sham-lesioned hemisphere are shown as black and white spots, respectively (n=26). Figure and legend adapted from Alvarez-Fischer et al, 2007.25
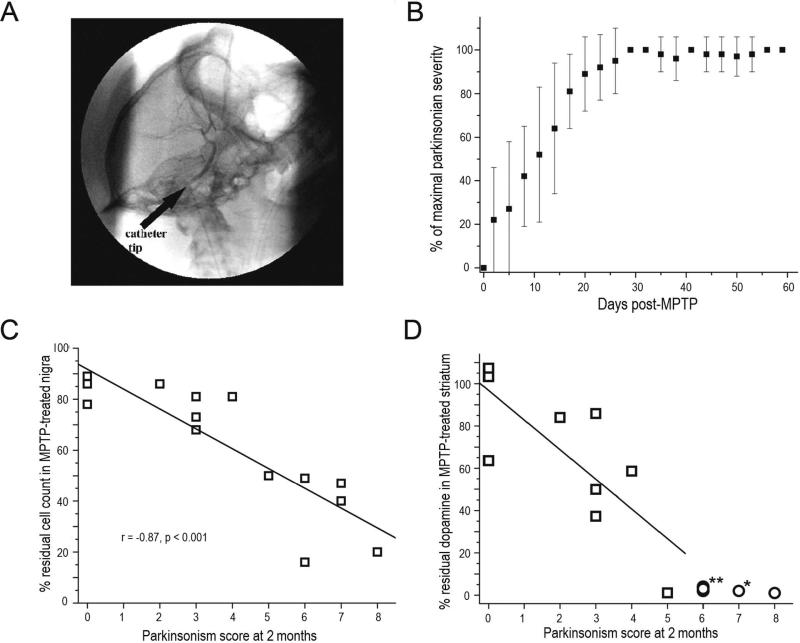
These types of discrepancies led us to determine whether any of the three presynaptic molecular markers faithfully reflect striatal dopamine, nigral dopaminergic neurons or parkinsonian motor behavior. To address these questions, we gave varying doses of MPTP via a unilateral intracarotid infusion to selectively destroy a variable number of nigrostriatal neurons on just one side of the brain in 16 nonhuman primates (Fig. 5A). We had previously demonstrated that once motor parkinsonism reaches a maximum about 3-4 weeks after MPTP (Fig. 5B), it remains unchanged for as long as 2.5 years.3,26 All animals had baseline PET measures using FD (for decarboxylase), DTBZ (for VMAT2) and CFT (for DAT); validated video-based motor ratings of parkinsonism; and subsequent ex vivo measures of quantitative autographic measures of striatal VMAT2 and DAT, striatal dopamine, full unbiased stereologic measures of TH cells in the nigra and stereologic fiber density measures in striatum. All measures and ratings were done blinded to dose of MPTP and other measures.
Figure 5.
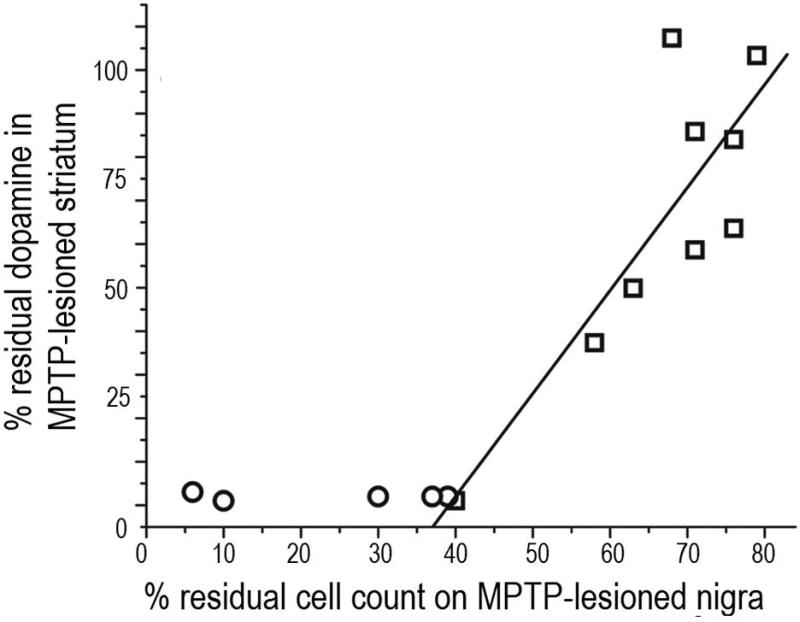
PET correlates with nigrostriatal damage and parkinsonism following unilateral intracarotid MPTP in non-human primates. A) Digital subtraction lateral projection cerebral angiography in a non-human primate demonstrating the location of the unilateral intra-carotid catheter tip just prior to injection of MPTP. B) Plot demonstrating the behavioral response in different animals given varying doses of MPTP (y axis = degree of parkinsonism) over 2 months (x axis = time) following unilateral intra-carotid MPTP administration. Error bars represent standard deviations (N=15). C) Parkinsonism score at 2 months versus percent of residual dopaminergic cell counts and D) dopamine content in the striatum (n=14). Data from monkeys with residual nigral cell counts of less than 50% are depicted by circles (n=5). Squares represent data from monkeys with residual nigral cell counts of 50% or more (n=9). Figure and legend adapted from Tabbal et al., 2012.26
The first key observation of this study was that the degree of nigral cell loss or striatal dopamine loss needed to produce identifiable parkinsonian motor features was much less than commonly thought.8 A reduction of between 14 – 24% of nigral TH neurons or 14 – 34% of striatal dopamine could cause parkinsonian motor manifestations. These values are similar to those reported (about 38% reduction) in low resolution SPECT studies of striatal uptake of a DAT radiotracer in the “normal” side in a group of eight hemiparkinsonian patients27 and recall that striatal DAT measures strongly correlate with striatal dopamine.9 Rather surprisingly, motor parkinsonism correlated well with nigral cell counts but not with striatal dopamine. Once nigral cell counts decreased about 50% striatal dopamine reached a nadir near zero, yet motor parkinsonism continued to increase as nigral cell counts decreased further (Fig. 5C &5D). This rather surprising result does not seem to be limited just to this monkey model of nigrostriatal destruction but also seems to apply to people with PD. A recent study found that postmortem measures in striatum of nigrostriatal neurons appears to reach a nadir when a person had only mild to relatively moderate PD.28 In other words, striatal dopamine measures appear to reach a flooring effect despite progressive worsening of motor parkinsonism as PD progresses. In fact in our monkeys striatal dopamine dropped in a consistent fashion as the reduction of nigral TH cell counts until the nigral cell counts reached 50% (Fig. 6). At that point, the striatal dopamine reached zero and remained there as the nigral cell counts continued to decline with higher doses of MPTP.
Figure 6.
Relationship between Striatal dopamine and and substantia nigra TH+ cell counts: Percent of residual dopaminergic cell counts (measured with unbiased stereology) in injected nigra (x-axis) versus percent residual dopamine (measured with HPLC) in the striatum (y-axis, n=14). Data from non-human primates with residual nigral cell counts of less than 50% are depicted in circles (n=5). Squares represent data from primates with residual nigral cell counts of 50% or more (n=9). Figure and legend adapted from Tabbal et al, 2012.26
Substantial additional data support this rather iconoclastic notion that striatal terminal field integrity does not completely explain motor parkinsonism. All three types of in vivo striatal PET measures correlated strongly with ex vivo measures of striatal dopamine (Fig. 7) but not with nigral TH cell counts.9 The in vivo striatal PET measures declined as nigral TH counts declined, but once nigral TH cell counts reached a 50% loss the striatal PET measures reached a nadir close to zero (Fig. 8). Thus, the striatal PET measures behaved exactly like the postmortem striatal dopamine measures. Furthermore, these PET measures of either VMAT2 or DAT do not seem to depend upon the specific PET radioligand, as ex vivo quantitative autographic measures of the maximum number of specific binding sites for VMAT2 and DAT correlated strongly with their respective PET measures and had the same relationship with nigral TH cell counts . Interestingly, we found no preferential change in VMAT2 or DAT with varying loss of nigrostriatal neurons (Fig. 9), and all striatal measures correlated strongly with each other; but note that these postmortem measures were done only 2 months after MPTP.9,29 The consistency of all of the terminal field measures strongly supports that the relationship between terminal fields and nigral cell bodies does not reflect insensitivity of the striatal measures.
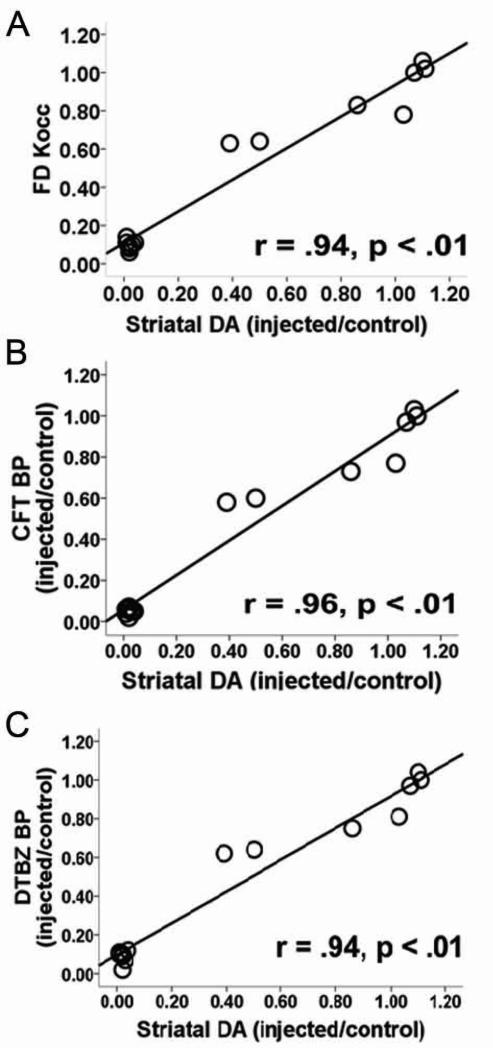
Figure 7.
PET measures correlated with striatal dopamine. Relationship of in vitro measures of striatal dopamine (% control side) compared to in vivo PET measures of striatal A) FD KOCC, B) CFT and C) DTBZ BPND (% control side). Correlation is significant with or without the clustered data points in the lower left corner of each graph. Figure and legend adapted from Karimi et al, 2013.9
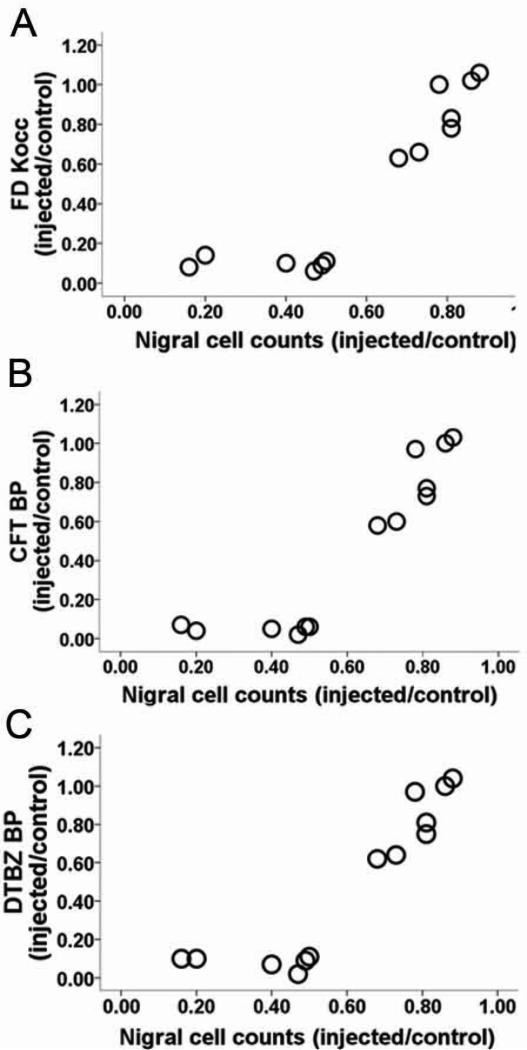
Figure 8.
PET measures have flooring effect in relation to nigral cell counts. Relationship between in vivo PET measures of FD KOCC ratio, CFT and DTBZ BPND ratio with in vitro measures of nigral dopaminergic neurons. All of the striatal PET measures approached zero once nigral cell loss reached 50%. Figure and legend adapted from Karimi et al, 2013.9
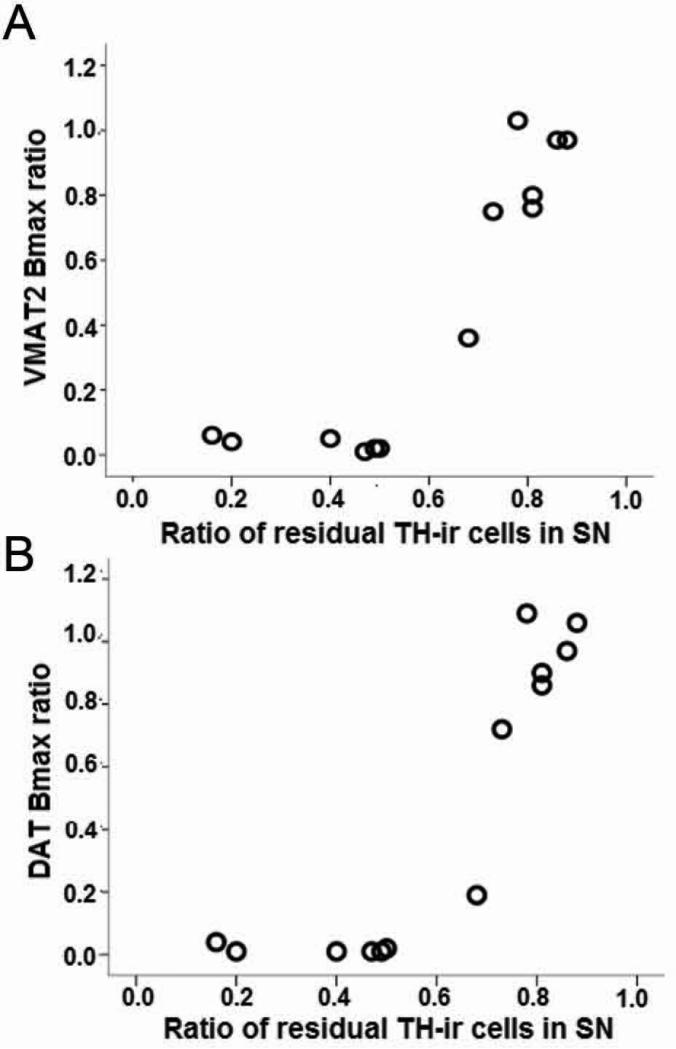
Figure 9.
In vivo PET measures of VMAT2 and DAT correlated with in vitro measures. The relationship between A) VMAT2 or B) DAT Bmax and residual substantia nigra pars compacta tyrosine hydroxylase immunoreactive neurons. The value for each non-human primate was expressed as the ratio of the injected side to the control (n = 13). Thus, these relationships do not depend upon specific molecular imaging radiopharmaceutical but rather reflect underlying pathophysiology. Figure and legend adapted from Tian et al, 2012. 28
These findings may explain in large part the discrepancies between clinical measures of PD motor progression and molecular imaging biomarkers. Many of these clinical studies recruited unmedicated, early-stage PD patients. This could correspond to relatively mild defects in the nigrostriatal pathways. However, as the disease progresses, the striatal measures of these pathways may reach a nadir, a so-called flooring effect, with subsequent changes in the striatal measures reflecting more noise than true progression of PD associated with additional loss of nigrostriatal neurons in substantia nigra and fiber density in the striatum.30
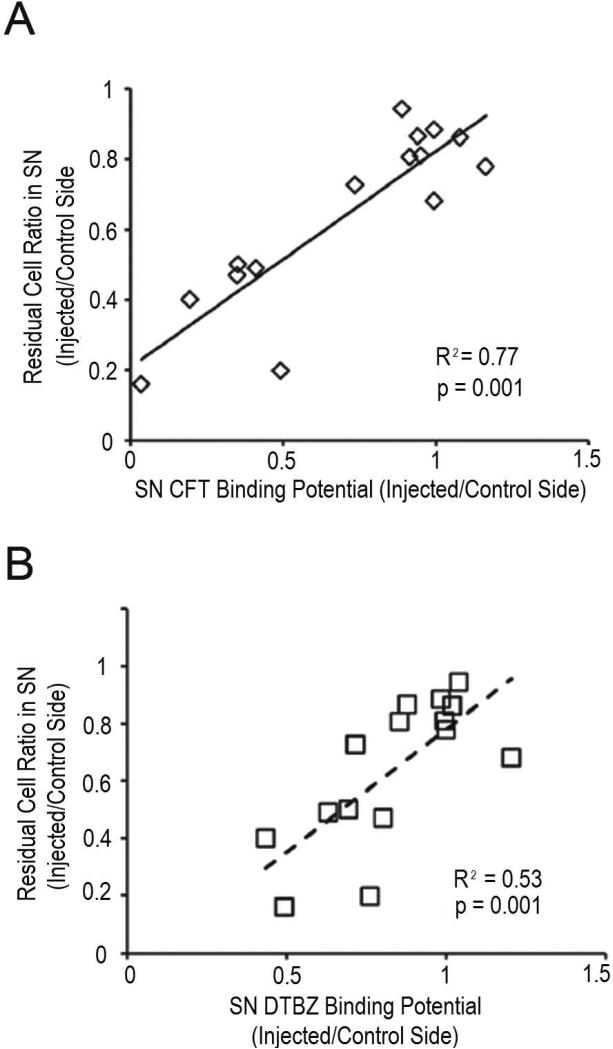
Fortunately, midbrain uptake of either the VMAT2 radioligand (DTBZ) or the DAT (CFT) radioligand does correlate well with stereologic counts of nigral TH neurons whereas FD did not (Fig. 10).31 PET measures of FD in the midbrain were too noisy to provide a reliable measure. Thus, aiming the PET scanner at the midbrain to predominantly reflect substantia nigra uptake may provide a reliable biomarker of loss of nigrostriatal neurons that correlates through the full range of severity of motor parkinsonism.
Figure 10.
Substantia nigra (SN) PET imaging correlated with nigral cell counts. The relationship between A) CFT and B) DTBZ injected/control side non-displaceable binding potential (or influx constant [Kocc]) in the SN and injected/control side ratio of TH-positive neurons in the SN. Each data point represents 1 non-human primate, and the trend lines are the linear fits of the data. CFT and DTBZ exhibited a tight linear correlation with residual cells in the SN. Midbrain based PET measures correlate with nigral cell counts whereas striatal terminal field measures do not. Figure and legend adapted from Brown et al, 2012.30
Of course, none of these studies have described another potential confound of any of these neuroimaging biomarkers – the potential acute or chronic effects of drugs that could alter the norneuroimaging biomarker without altering the underlying pathophysiology of PD. Symptomatic therapies could alter molecular imaging measurements and their relationships to the underlying pathophysiologic mechanisms in two distinct ways. The drug can directly alter the measure by competing at the specific binding site with the radiotracer (like amphetamine could directly compete with a DAT tracer) or chronic drug administration could alter the underlying biologic relationships of the PET measure with binding sites in presynaptic neurons or terminal fields (eg, if chronic drug exposure altered the number of specific binding sites per neuron). Multiple studies have attempted to address this by comparing the imaging measures either before or after drug administration or comparing long term effects in people. Still results have been quite conflicting19,32-35 although a recent study in MPTP monkeys suggests that levodopa does not alter DAT.34,35 This critical point must also be assessed prior to application of a biomarker to a particular intervention study.
At this point of the Grand Rounds there are several key take home messages. Nigrostriatal reserve is less than commonly thought. Terminal field measures of nigrostriatal neurons are not the same as nigral cell body measures. Surprisingly, severity of motor parkinsonism correlates much better with nigral TH neurons rather than striatal dopamine. Striatal PET measures do not fully correlate with motor parkinsonism but rather PET measures of in vivo midbrain uptake of either a DAT or VMAT2 radiotracer do fully correlate with nigral cell counts and severity of motor parkinsonism in our MPTP monkey model.31 This further supports the notion of the relationship between nigral neurons and motor parkinsonism, regardless of the whether the nigral measures are done in vitro with unbiased stereologic counts of dopaminergic cell bodies or PET-based in vivo measures of DAT or VMAT2. Finally, careful validation is critical prior to application of these types of methods, otherwise interpretation of findings may be misleading and confusing.
Investigations of Pathophysiology
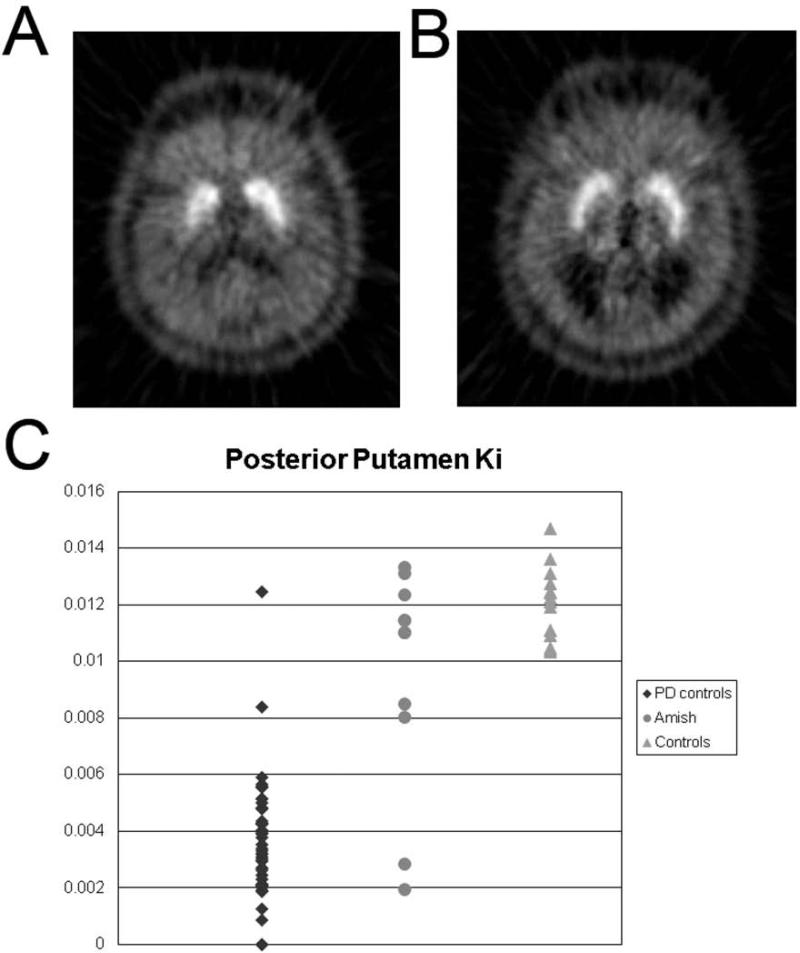
These measures of presynaptic dopaminergic nigrostriatal neurons have been used to investigate the pathophysiology of parkinsonian conditions. Multiple examples demonstrate utility of these tools. Perhaps, the simplest approach has been to use these neuroimaging biomarkers as an endophenotype for genetic studies. Identification of a reduction in striatal uptake of FD using PET in a group of Amish with ambiguous clinical findings helped classify each as either affected or unaffected in a genetic linkage study in a large kindred with high penetrance of parkinsonism (Fig. 11).36 In that study, LOD (logarithm of the odds) score (probability for identification of an abnormal gene locus) increased when including affected participants defined by abnormal imaging that were otherwise excluded from analysis due to ambiguity of clinical diagnosis. Of course, it is important to note that this focused application only identifies a defect in the nigrostriatal pathway that is assumed to reflect PD rather than other parkinsonian conditions due to high prior probability within this group.
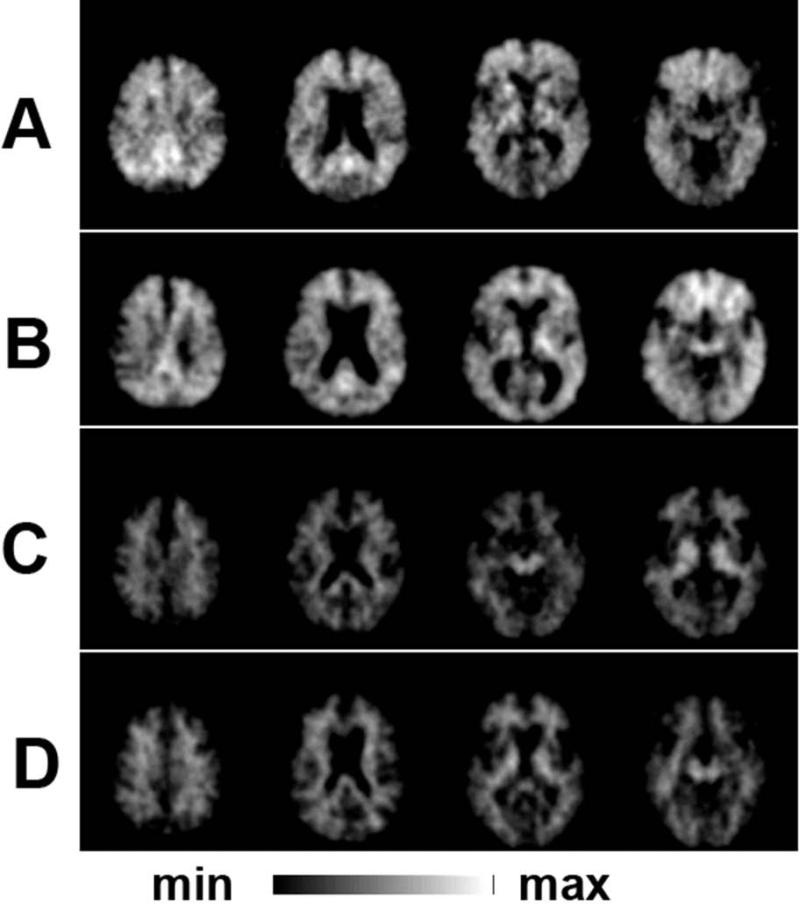
Figure 11.
FD PET reduced striatal uptake endophenotype. A) [18F]FDOPA PET in early PD with reduced uptake in posterior putamen compared to B) an age and gender matched control. C) Ki in control participants (with [n=48] and without [n=24] PD) and Amish (n=11). Forty-seven (98%) of 48 PD controls and four (36%) Amish met PET criteria for PD (Ki for one of the posterior putamen more than three standard deviations below the normal mean) despite clinical features not convincing for or against PD. By categorizing 4 of these as “affected” based upon FD PET phenotype the LOD score improved for the entire linkage analysis. Figure and legend adapted from Racette et al, 2006.31
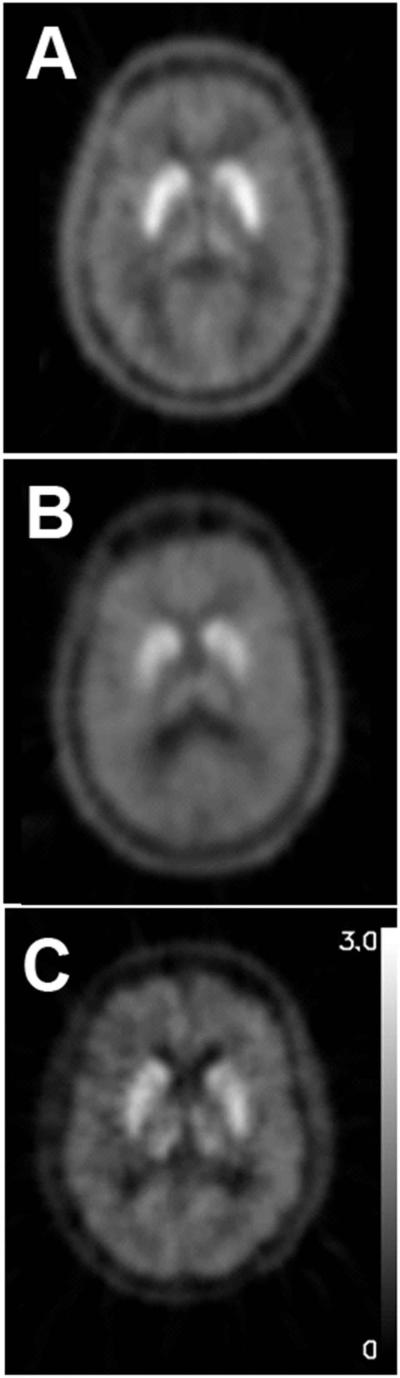
These types of neuroimaging biomarkers also may be applied to investigate involvement of the nigrostriatal pathway in various neurologic conditions. Manganese exposure may cause movement disorders that may or may not be clearly distinguished from PD, but FD PET may demonstrate defects in the nigrostriatal pathway indicating at least some pathophysiologic similarities to PD. Manganese toxicity caused by liver failure can cause parkinsonism and identifiable reductions of striatal uptake of FD in those responsive to levodopa.37,38 The value of FD PET is to indicate a loss of decarboxylase activity rather than another cause of a presynaptic dopaminergic deficit; this limited interpretation applies to those with manganese exposure and levodopa responsiveness. Those not levodopa-responsive may not have an abnormal FD PET39 but it is important to appreciate that a defect in striatal uptake of FD does not necessarily suggest potential dopa responsiveness if coexisting direct striatal damage exits.40 FD PET also revealed defects in the nigrostriatal pathway in asymptomatic welders who presumably have exposure to manganese that emanates from fumes associated with welding (Fig. 12). Interestingly, the pattern of reduced striatal FD uptake revealed greater loss in the caudate compared to posterior putamen; a pattern that contrasts with that typically found in idiopathic PD with greater loss in the posterior putamen.41 These data provide evidence of neurotoxicity in asymptomatic welders. Alternatively one could interpret these data to suggest that those with such striatal defects tend to become welders; yet this explanation seems less likely. In any event, these examples demonstrate the value of using molecular imaging to investigate pathophysiology of parkinsonian conditions or assess the integrity of the nigrostriatal pathway.
Figure 12.
FD PET reduced uptake in nigrostriatal pathway in asymptomatic welders differs from PD. FDOPA PET composite images of decay-corrected counts from 24 to 94 minutes from a representative A) control. B) welder, and C) subject with idiopathic Parkinson disease (IPD) normalized to the reference region. FDOPA uptake is reduced in the caudate region of the welder in comparison to the control subject while the posterior putamen is the most affected region in the subject with IPD. Data previously presented in Criswell et al, 2011.33
Differential Diagnosis
Many have proposed using molecular imaging methods to help diagnose those with PD. In this review we will focus on presynaptic radiopharmaceuticals. There are basically two levels of diagnosis that have either clinical or research applications. The most straightforward is whether these presynaptic markers of decarboxylase activity, VMAT2 or DAT, distinguish PD from normal. Multiple studies have demonstrated this.42-50 The Food and Drug Administration (FDA) recently approved a SPECT-based scanning radiopharmaceutical for DAT, and this is widely advertised to help with diagnosis. The clearest utility of this type of approach is to use an abnormal scan as part of the inclusion criteria for research studies in an attempt to avoid including non-PD participants. While this approach may make the participant group more homogenous, it does reduce the generalizability of study findings to those that have been screened by such a scan.
Clinical application requires these types of scans to be useful for differential diagnosis. That is, can an abnormal scan differentiate PD from a host of other conditions that may mimic PD, and does it do so sufficiently reliably to provide clinical benefit? Several studies have focused on trying to differentiate people with essential tremor from those with PD. Many such studies suggest that normal striatal uptake of these radiotracers would predict a low probability of developing PD in the next two years.51 Others have followed those with normal scans for longer than three years and found that between 15-20% developed a neurodegenerative parkinsonian condition, including dopa-responsive idiopathic PD.7 In fact, longer follow up may reveal that a greater relative frequency of “normal” scans occur in those with neurodegenerative parkinsonian syndromes including potentially treatable idiopathic PD.52 Thus, if a clinician relies only on this type of molecular imaging to determine whether an individual does or does not have PD, then that clinician may miss a treatable condition.
One of the best studies to assess whether the SPECT DAT scan can distinguish among a total of 35 people with essential tremor, dystonic tremor, psychogenic parkinsonism, drug-induced parkinsonism and neurodegenerative parkinsonism obtained participants from community physician referral.53 They had two movement disorders experts evaluate each participant, obtained a DAT SPECT scan for each and one of the movement disorders experts and re-examined the participants six months later still blinded to the SPECT results. The final diagnosis of the movement disorders expert was considered the gold standard, and the SPECT data (when analyzed by a quantitative rather than visual based method) performed well with respect to the clinical exam. The reasonable interpretation for these findings was that the SPECT scan predicted the 6-month follow-up exam – it was almost as good as an expert clinician. Appropriately, the authors noted that reduced striatal uptake of the DAT radioligand indicated a defect in the nigrostriatal pathway that can occur in multiple neurodegenerative conditions in addition to PD; thus it does not differentiate among idiopathic PD, multiple systems atrophy, corticobasal syndrome, vascular parkinsonism or progressive supranuclear palsy. Of course, the number of participants in this study was relatively small.
Thus, the clinical benefit of using this type of scan remains to be proven. In fact, a reanalysis of the data submitted to the FDA in support of clinical implementation of a SPECT radiopharmaceutical to quantify striatal DAT demonstrated that the SPECT DAT scan provided no additional benefit beyond clinical exam to distinguish neurodegenerative parkinsonism.54 One may further argue that if there is a clinical question of idiopathic PD, then the important clinical question is whether symptoms respond to levodopa. Giving a trial of levodopa is far less expensive than obtaining a SPECT DAT or PET scan of a presynaptic radiopharmaceutical although some have argued this point. The charge for a SPECT DAT scan can range up to $4000, whereas a 1-2 month trial of an escalating dose of levodopa may cost up to $200 and does not include any radiation exposure. Either approach should include an initial and follow up visit with the clinician.52,55-57
Nevertheless, multiple clinical applications could potentially be useful, if evidence supports them.58 Presymptomatic diagnosis in at-risk populations could be critically valuable if we had a low risk intervention to forestall the onset of symptoms in PD. However, we currently do not have such an intervention. Can we use this type of scanning to distinguish idiopathic PD from psychogenic parkinsonism? That seems reasonable, but one must be careful about those with a dopa-responsive parkinsonian syndrome due to GTP-cyclohydrolase deficiency. These patients will have normal striatal uptake of any of the presynaptic tracers yet have parkinsonism.59 Thus, a normal scan would support a diagnosis of psychogenic parkinsonism, but could also occur with GTP-cyclohydrolase deficiency or occasionally with idiopathic PD at an early stage. Of course, one must be careful as a normal radiotracer imaging scan can be found in those with idiopathic Parkinson disease or other neurodegenerative condition, and as noted this may occur relatively commonly.7,52 Another potential clinical application could be helping to select a target for implantation of a deep brain stimulation electrode for treatment of people with action/postural tremor as well as some parkinsonian features.58 If that person only has an essential tremor, then targeting the ventral intermediate nucleus (VIM) of the thalamus would be commonly done. On the other hand, if that person also had underlying PD, then targeting either the internal segment of the pallidum or the subthalamic nucleus (STN) would be indicated – although the efficacy of either STN or pallidal stimulation for essential tremor remains unknown. Nevertheless, this application seems entirely rational but has not yet been subjected to a clinical trial to prove that it would be a valuable means to make a clinical decision. For example, we do not even know whether in such a scenario that STN stimulation would be inferior to VIM stimulation for essential tremor.
Some have suggested that these presynaptic neuroimaging markers may help distinguish dementia associated with synucleinopathy since these patients have nigrostriatal defects as part of their parkinsonism. SPECT studies have demonstrated reduced striatal dopamine transporter uptake in those with diffuse cortical synuclienopathy but not in those only with AD.60 However, reduced striatal DAT also occurs in other parkinsonian syndromes such as multiple systems atrophy, corticobasal degeneration, and progressive supranuclear palsy. Furthermore, striatal reduction of DAT does not exclude co-existing AD. Furthermore, the presumed clinical benefit is to help avoid typical neuroleptics in people with dementia if they have underlying nigrostriatal defects – since that could exacerbate parkinsonism. The simpler clinical approach is to avoid typical neuroleptics in all demented people and particularly if they have any evidence of clinical parkinsonism. This clinical approach saves valuable resources and reduces perhaps unnecessary radiation exposure. Thus, these neuroimaging tests do not provide an important clinical benefit, although some would disagree.61
The take home messages these last two sections include that molecular imaging of presynaptic neurons can play an important role for investigation of the pathophysiology of parkinsonian disorders but clinical application for differential diagnosis may be risky. Differential diagnosis and clinical care still depend upon a careful clinical assessment and follow up. Otherwise, patient management may suffer.
Molecular Imaging of Cognitive Impairment in PD
The next part of this Grand Rounds describes a series of studies related to validation and interpretation of a different sort of molecular imaging tool to investigate the underlying pathophysiology of cognitive impairment that commonly occurs in PD. As many as 80% of people with PD develop dementia within 20 years after the onset of motor symptoms, decreasing quality of life and reducing survival.62,63 Not so many years ago, we taught that the underlying pathophysiology of cognitive impairment associated with PD was due to co-existing Alzheimer disease (AD) in half of the patients and due to cortical synucleinopathy in the other half. The development of the PET amyloid imaging radiopharmaceutical Pittsburgh compound B (PiB) permitted in vivo measurement of fibrillar Aβ amyloid in the brain.64 The underlying pathological changes found in the brain of people with AD include abnormal Aβ amyloid that occurs many years prior to onset of dementia, and then abnormal deposition of tau.65-67 Thus, we began a study more than eight years ago to determine whether PET PiB imaging could distinguish those people with PD that develop cognitive impairment have co-existing AD or only abnormal cortical α-synucleinopathy. This story is relevant since this provides another demonstration of the importance of validating neuroimaging biomarkers for each specific application.
Several PET studies demonstrated increased uptake of brain PiB in early dementia due to AD68-71 or other pathologic condition like amyloid angiopathy.72,73 Thus, we started a series of prospective studies to determine whether we could use PET PiB to identify cognitive impairment due to coexisting AD in those with PD. We recruited people with PD with and without cognitive impairment as well as age matched healthy adults. Measures included clinical assessments, cognitive testing, high resolution MRI, genetic analyses, CSF collection and PET PiB scans. We then followed participants and collected brain tissue from those that died during the course of the study.
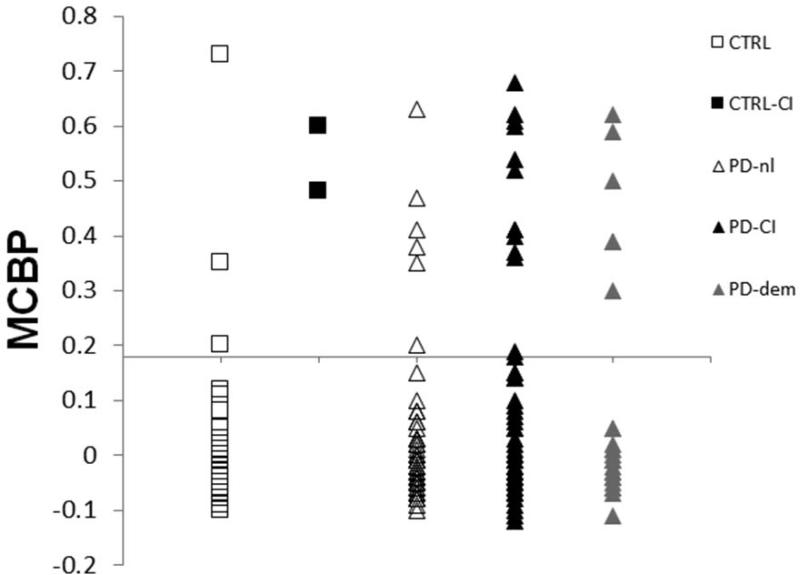
The first observation was that the chance of having a positive PiB PET scan was about the same in those with early onset cognitive impairment compared to those with later onset cognitive impairment with respect to the onset of motor manifestations. Although the numbers were small in the original report71 we are in the process of extending this analysis to more than 200 participants but at this point only have separated the groups by cognitive impairment (Fig. 13). The percent of PiB positivity did not significantly vary across groups based upon cognitive status.
Figure 13.
PiB PET does not distinguish subtypes of cognitive impairment in PD. Mean cortical binding potentials (MCBPs) for participant groups that include CTRL = healthy control with a Clinical Demetia Rating (CDR =0; n=48; 6% PiB+), CTRL-CI = control with cognitive impairment (CDR = 0.5; n=2; 100% PiB+), PD-nl = Parkinson disease, no cognitive impairment (CDR = 0; n=66; 9% PiB+), PD-CI = Parkinson disease, cognitive impairment (CDR = 0.5; n = 67; 15% PiB+), PD-dem = Parkinson disease with dementia (CDR = 1 or more; n = 24; 21% PiB+). Each point represents an individual participant (N = 207). The horizontal scale crossing the Y-axis at 0.18 is the recommended cut-off for PIB+. Figure and legend updated from data presented by Foster et al, 2010.59
But does a positive PiB PET scan indicate AD pathology? Postmortem examination of the first three people that died during the course of this study was quite instructive.74 All three of these people had PD associated with severe dementia. Two of them (case 1 and 2) had a positive PiB scan and their brain examinations revealed abnormal Aβ deposition as well as cortical synucleinopathy, but no abnormal tau deposition. Accordingly, these two would not satisfy NIA Reagan criteria for pathological evidence of AD that accounted for dementia. The third participant (case 3) had a negative PiB PET scan and brain autopsy revealed only cortical synucleinopathy (Fig. 14); this confirmed that PiB did not target abnormal α-synuclein, which also has an amyloid conformation like Aβ. Thus, this study suggested that PiB PET was specific for abnormal Aβ but not sufficient to indicate co-existing AD. Some might argue that these two patients were just early in the course of AD since we now know that Aβ deposition precedes tau deposition. However, these two people were old, demented and no longer living. Thus, given the severity of their dementia, this seemed different from the typical course of AD.
Figure 14.
PiB scans target abnormal Aβ deposition, but not α-synuclein. [11C]-PIB PET images for A) Case 1 and B) Case 2 demonstrates increased signal in multiple cortical areas, including orbitofrontal and prefrontal cortex, precuneus, and temporal lobes. C) Case 3 and D) a control participant have minimal PIB signal in cortical areas. PIB retention in white matter areas is likely due to nonspecific binding. Data previously presented in Burack et al, 2010.60
An autopsy series of 32 people with PD and dementia from our Movement Disorders Center confirmed these findings.75 In that group, all had abnormal cortical synucleinopathy; 60% also had abnormal Aβ. Only a single individual had sufficient abnormal tau to meet NIA Reagan criteria for coexisting AD. While immunohistology of autopsy brain tissue revealed that 60% had abnormal Aβ, only about 15-20% of the dementia or cognitively impaired participants in our studies have had positive PiB PET scans. The difference in time between the PET and autopsy could explain this discrepancy – of course autopsies are done later in the course, but we doubt this is the key factor. Much more likely is that PET PiB is sensitive to detection of fibrillar Aβ amyloid and does not as avidly bind to diffuse Aβ amyloid.76 Careful comparisons of the post mortem distribution with the PET PiB findings will help address this question.
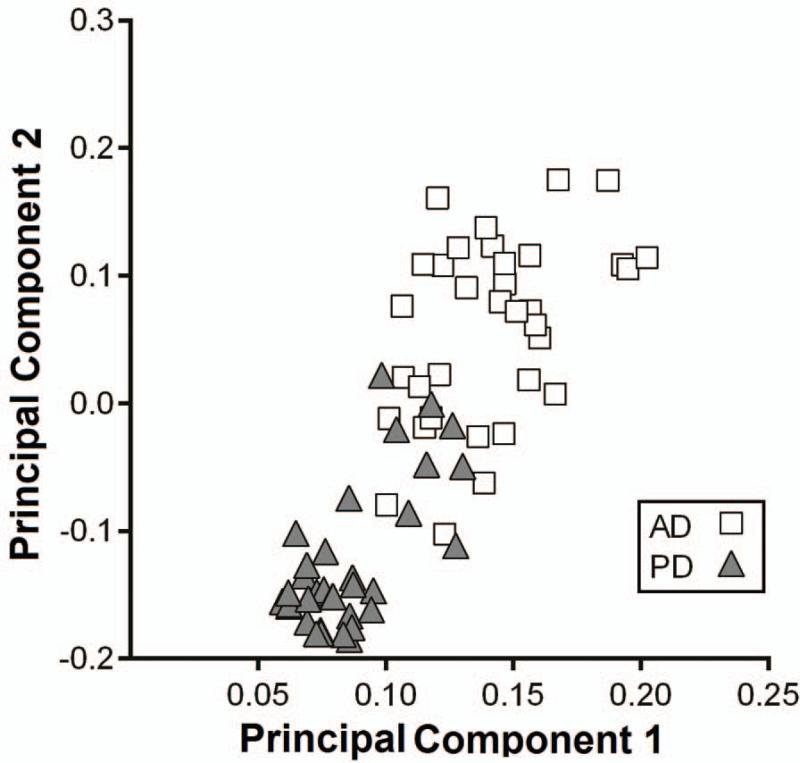
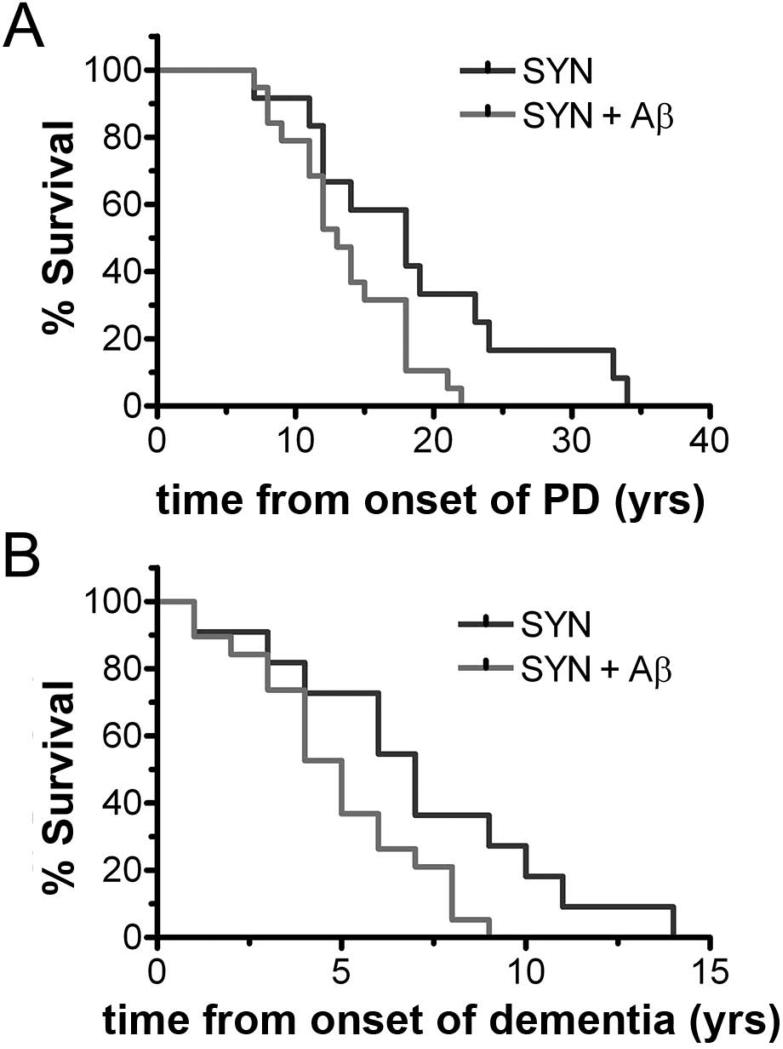
Principal components analysis of the distribution of PiB in people with PD differs from that found in those with AD (Fig. 15).77 This type of statistical analysis effectively determines patterns of voxel-based distribution that helps distinguish the different groups of patients. This difference holds even when limiting the groups to those PD and Alzheimer participants with comparable severity of cognitive impairment. This suggests that the Aβ deposition in PD may have a different pathologic mechanism from AD and may be related to abnormal α-synuclein deposition, but this remains to be determined. The other question is whether Aβ deposition contributes to the cognitive impairment or dysfunction in those with PD. Although the numbers were relatively small in the original report, those with Aβ deposition as well as cortical synucleinopathy in a postmortem study had shorter lifespan whether determined from onset of motor parkinsonism or onset of dementia (Fig. 16).75 Furthermore, a higher PiB uptake contributed to the risk of progressive cognitive decline in PD subjects with and without mild cognitive decline at baseline.78 PD patients with low CSF Aβ levels, a measure inversely correlated with Aβ plaque deposition, experience faster cognitive decline over two years.79 However, previous analyses of a smaller (earlier) subset (n=47) from our cohort indicated that PiB binding does not differ between PD with early versus late onset of cognitive impairment.71,77 This supports the notion that the frequency of PiB positivity is independent of the relative timing of cognitive impairment relative to motor impairment in PD.
Figure 15.
Distribution of principal component analysis component weights differ between PD and Alzheimer patients. Scatterplot represents the component weights of the primary principal components (PC1 and PC2) for the direct comparison of study participants with PD and Alzheimer disease. Note, that this distinction exists when limiting this analysis to those PD and AD patients with comparable degrees of cognitive impairment. Data previously presented in Campbell et al, 2011.63
Figure 16.
Comparison of survival in demented PD patients with postmortem findings of only synucleinopathy versus synucleinopathy plus abnormal Aβ deposition. Kaplan-Meier survival curves show A) the percentage of survival with respect to time from the onset of PD and B) time from the PD onset to dementia. Data previously presented in Kotzbauer et al, 2012.61
Interestingly, in some PD patients, CSF α-synuclein and Aβ1-42 were reduced compared to subjects with normal cognition, but CSF tau was not increased in those with PD as occurs in AD.80 CSF Aβ1-42 inversely correlated with PiB mean cortical binding potential, with PiB positive PD participants having lower CSF Aβ1-42 compared to PiB negative PD participants. Furthermore, CSF α-synuclein positively correlated with Aβ1-42 in PD participants but not in controls, suggesting a pathophysiologic connection between the metabolisms of these proteins in PD.
The key messages from this part of the Grand Rounds are that a positive PET PiB scan in a person with PD indicates abnormal Aβ deposition but does not necessarily indicate co-existing AD, at least in patients recruited from a Movement Disorders Center. Postmortem examination of brain tissue was critical for proper interpretation and understanding of application of this existing neuroimaging biomarker to a disorder different from its initial application. PiB may provide a sensitive neuroimaging biomarker for fibrillar Aβ which occurs in early AD but abnormal uptake of PiB is not specific for AD since other conditions including PD may have accompanying abnormal Aβ deposition in the brain. Thus, application of a neuroimaging biomarker requires proper validation for each application. Look before you leap, otherwise fantasy may supersede fact.
Acknowledgments
This study was funded by NIH (NS050425, NS058714, NS41509, and NS075321); Michael J Fox Foundation; Murphy Fund; American Parkinson Disease Association (APDA) Center for Advanced PD Research at Washington University; Greater St. Louis Chapter of the APDA; McDonnell Center for Higher Brain Function; Barnes-Jewish Hospital Foundation (Elliot Stein Family Fund for PD Research & the Parkinson Disease Research Fund).
We also thank colleagues that have collaborated on these research studies including Drs. Stephen Moerlein, Morvarid Karimi, LinLin Tian, Meghan Campbell, Brad Racette, Susan Criswell, Jonathan Mink, Paul Kotzbauer, Nigel Cairns, Johanna Hartlein and William Powers. We also thank Amanda Norris and Hugh Flores for assistance with figure preparation.
References
- 1.Bagchi DP, Yu L, Perlmutter JS, et al. Binding of the radioligand SIL23 to alpha-synuclein fibrils in Parkinson disease brain tissue establishes feasibility and screening approaches for developing a Parkinson disease imaging agent. PLoS One. 2013;8:e55031. doi: 10.1371/journal.pone.0055031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brooks DJ, Frey KA, Marek KL, et al. Assessment of neuroimaging techniques as biomarkers of the progression of Parkinson's disease. Exp Neurol. 2003;184(Suppl 1):S68–S79. doi: 10.1016/j.expneurol.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 3.Perlmutter JS, Tempel LW, Black KJ, et al. MPTP induces dystonia and parkinsonism: Clues to the pathophysiology of dystonia. Neurology. 1997;49:1432–1438. doi: 10.1212/wnl.49.5.1432. [DOI] [PubMed] [Google Scholar]
- 4.Burn DJ, Mark MH, Playford ED, et al. Parkinson's disease in twins studied with 18F-dopa and positron emission tomography. Neurology. 1992;42:1894–1900. doi: 10.1212/wnl.42.10.1894. [DOI] [PubMed] [Google Scholar]
- 5.Nagai Y, Obayashi S, Ando K, et al. Progressive changes of pre- and post-synaptic dopaminergic biomarkers in conscious MPTP-treated cynomolgus monkeys measured by positron emission tomography. Synapse. 2007;61:809–819. doi: 10.1002/syn.20431. [DOI] [PubMed] [Google Scholar]
- 6.Guttman M, Yong VW, Kim SU, et al. Asymptomatic striatal dopamine depletion: PET scans in unilateral MPTP monkeys. Synapse. 1988;2:469–473. doi: 10.1002/syn.890020502. [DOI] [PubMed] [Google Scholar]
- 7.Serrano VJ, Garcia BL, Duran BC, et al. [Negative predictive value of SPECT with I123 ioflupane in movement disorders (reply)]. Rev Esp Med Nucl. 2009;28:267–268. doi: 10.1016/j.remn.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Tabbal SD, Tian L, Karimi M, et al. Low nigrostriatal reserve for motor parkinsonism in nonhuman primates. Exp Neurol. 2012;237:355–362. doi: 10.1016/j.expneurol.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karimi M, Tian L, Brown CA, et al. Validation of nigrostriatal positron emission tomography measures: critical limits. Ann Neurol. 2013;73:390–396. doi: 10.1002/ana.23798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang CC, Poston KL, Dhawan V, et al. Abnormalities in metabolic network activity precede the onset of motor symptoms in Parkinson's disease. J Neurosci. 2010;30:1049–1056. doi: 10.1523/JNEUROSCI.4188-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bucher HC, Guyatt GH, Cook DJ, et al. Users’ guides to the medical literature: XIX. Applying clinical trial results. A. How to use an article measuring the effect of an intervention on surrogate end points. Evidence-Based Medicine Working Group. J Am Med Assoc. 1999;282:771–778. doi: 10.1001/jama.282.8.771. [DOI] [PubMed] [Google Scholar]
- 12.Fleming TR, DeMets DL. Surrogate end points in clinical trials: are we being misled? Ann Intern Med. 1996;125:605–613. doi: 10.7326/0003-4819-125-7-199610010-00011. [DOI] [PubMed] [Google Scholar]
- 13.Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT-LLT). J Am Med Assoc. 2002;288:2998–3007. doi: 10.1001/jama.288.23.2998. [DOI] [PubMed] [Google Scholar]
- 14.Echt DS, Liebson PR, Mitchell LB, et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med. 1991;324:781–788. doi: 10.1056/NEJM199103213241201. [DOI] [PubMed] [Google Scholar]
- 15.Effects of encainide, flecainide, imipramine and moricizine on ventricular arrhythmias during the year after acute myocardial infarction: the CAPS. Am J Cardiol. 1988;61:501–509. doi: 10.1016/0002-9149(88)90754-0. [DOI] [PubMed] [Google Scholar]
- 16.Markus HS, Droste DW, Kaps M, et al. Dual antiplatelet therapy with clopidogrel and aspirin in symptomatic carotid stenosis evaluated using doppler embolic signal detection: the Clopidogrel and Aspirin for Reduction of Emboli in Symptomatic Carotid Stenosis (CARESS) trial. Circulation. 2005;111:2233–2240. doi: 10.1161/01.CIR.0000163561.90680.1C. [DOI] [PubMed] [Google Scholar]
- 17.Fahn S, Oakes D, Shoulson I, et al. Levodopa and the progression of Parkinson's disease. N Engl J Med. 2004;351:2498–2508. doi: 10.1056/NEJMoa033447. [DOI] [PubMed] [Google Scholar]
- 18.Whone AL, Watts RL, Stoessl AJ, et al. Slower progression of Parkinson's disease with ropinirole versus levodopa: The REAL-PET study. Ann Neurol. 2003;54:93–101. doi: 10.1002/ana.10609. [DOI] [PubMed] [Google Scholar]
- 19.Ravina B, Eidelberg D, Ahlskog JE, et al. The role of radiotracer imaging in Parkinson disease. Neurology. 2005;64:208–215. doi: 10.1212/01.WNL.0000149403.14458.7F. [DOI] [PubMed] [Google Scholar]
- 20.Lang AE, Gill S, Patel NK, et al. Randomized controlled trial of intraputamenal glial cell line-derived neurotrophic factor infusion in Parkinson disease. Ann Neurol. 2006;59:459–466. doi: 10.1002/ana.20737. [DOI] [PubMed] [Google Scholar]
- 21.Dorsey ER, Holloway RG, Ravina BM. Biomarkers in Parkinson's disease. Expert Rev Neurother. 2006;6:823–831. doi: 10.1586/14737175.6.6.823. [DOI] [PubMed] [Google Scholar]
- 22.Freed CR, Greene PE, Breeze RE, et al. Transplantation of embryonic dopamine neurons for severe Parkinson's disease. New Engl J Med. 2001;344:710–718. doi: 10.1056/NEJM200103083441002. [DOI] [PubMed] [Google Scholar]
- 23.Pate BD, Kawamata T, Yamada T, et al. Correlation of striatal fluorodopa uptake in the MPTP monkey with dopaminergic indices. Ann Neurol. 1993;34:331–338. doi: 10.1002/ana.410340306. [DOI] [PubMed] [Google Scholar]
- 24.Snow BJ, Tooyama I, McGeer EG, et al. Human positron emission tomographic [18F]fluorodopa studies correlate with dopamine cell counts and levels. Ann Neurol. 1993;34:324–330. doi: 10.1002/ana.410340304. [DOI] [PubMed] [Google Scholar]
- 25.Alvarez-Fischer D, Blessmann G, Trosowski C, et al. Quantitative [(123)I]FP-CIT pinhole SPECT imaging predicts striatal dopamine levels, but not number of nigral neurons in different mouse models of Parkinson's disease. Neuroimage. 2007;38:5–12. doi: 10.1016/j.neuroimage.2007.05.056. [DOI] [PubMed] [Google Scholar]
- 26.Tabbal SD, Mink JW, Antenor JA, et al. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced acute transient dystonia in monkeys associated with low striatal dopamine. Neuroscience. 2006;141:1281–1287. doi: 10.1016/j.neuroscience.2006.04.072. [DOI] [PubMed] [Google Scholar]
- 27.Marek KL, Seibyl JP, Zoghbi SS, et al. [123I] beta-CIT/SPECT imaging demonstrates bilateral loss of dopamine transporters in hemi-Parkinson's disease. Neurology. 1996;46:231–237. doi: 10.1212/wnl.46.1.231. [DOI] [PubMed] [Google Scholar]
- 28.Kordower JH, Olanow CW, Dodiya HB, et al. Disease duration and the integrity of the nigrostriatal system in Parkinson's disease. Brain. 2013;136:2419–2431. doi: 10.1093/brain/awt192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tian L, Karimi M, Loftin SK, et al. No differential regulation of dopamine transporter (DAT) and vesicular monoamine transporter 2 (VMAT2) binding in a primate model of Parkinson disease. PLoS One. 2012;7:e31439. doi: 10.1371/journal.pone.0031439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tian L, Karimi M, Brown CA, et al. In vivo measures of nigrostriatal neuronal response to unilateral MPTP treatment. Brain Res. 2014 doi: 10.1016/j.brainres.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown CA, Karimi MK, Tian L, et al. Validation of midbrain positron emission tomography measures for nigrostriatal neurons in macaques. Ann Neurol. 2013;74:602–610. doi: 10.1002/ana.23939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Au WL, Adams JR, Troiano AR, et al. Parkinson's disease: in vivo assessment of disease progression using positron emission tomography. Brain Res Mol Brain Res. 2005;134:24–33. doi: 10.1016/j.molbrainres.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 33.Guttman M, Stewart D, Hussey D, et al. Influence of L-dopa and pramipexole on striatal dopamine transporter in early PD. Neurology. 2001;56:1559–1564. doi: 10.1212/wnl.56.11.1559. [DOI] [PubMed] [Google Scholar]
- 34.Fernagut PO, Li Q, Dovero S, et al. Dopamine transporter binding is unaffected by L-DOPA administration in normal and MPTP-treated monkeys. PLoS One. 2010;5:e14053. doi: 10.1371/journal.pone.0014053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Innis RB, Marek KL, Sheff K, et al. Effect of treatment with L-dopa/carbidopa or L-selegiline on striatal dopamine transporter SPECT imaging with [123I]beta-CIT. Mov Disord. 1999;14:436–442. doi: 10.1002/1531-8257(199905)14:3<436::aid-mds1008>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 36.Racette BA, Good L, Antenor JA, et al. [18F]FDOPA PET as an endophenotype for Parkinson's Disease linkage studies. Am J Med Genet B Neuropsychiatr Genet. 2006;141:245–249. doi: 10.1002/ajmg.b.30293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Racette BA, Antenor JA, McGee-Minnich L, et al. [18F]FDOPA PET and clinical features in parkinsonism due to manganism. Mov Disord. 2005;20:492–496. doi: 10.1002/mds.20381. [DOI] [PubMed] [Google Scholar]
- 38.Criswell SR, Perlmutter JS, Crippin JS, et al. Reduced uptake of FDOPA PET in end-stage liver disease with elevated manganese levels. Arch Neurol. 2012;69:394–397. doi: 10.1001/archneurol.2011.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wolters EC, Huang CC, Clark C, et al. Positron emission tomography in manganese intoxication. Ann Neurol. 1989;26:647–651. doi: 10.1002/ana.410260510. [DOI] [PubMed] [Google Scholar]
- 40.Otsuka M, Kuwabara Y, Ichiya Y, et al. Differentiating between multiple system atrophy and Parkinson's disease by positron emission tomography with 18F-dopa and 18F-FDG. Ann Nucl Med. 1997;11:251–257. doi: 10.1007/BF03164771. [DOI] [PubMed] [Google Scholar]
- 41.Criswell SR, Perlmutter JS, Videen TO, et al. Reduced uptake of [(1)(8)F]FDOPA PET in asymptomatic welders with occupational manganese exposure. Neurology. 2011;76:1296–1301. doi: 10.1212/WNL.0b013e3182152830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leenders KL, Salmon EP, Tyrrell P, et al. The nigrostriatal dopaminergic system assessed in vivo by positron emission tomography in healthy volunteer subjects and patients with Parkinson's disease. Arch Neurol. 1990;47:1290–1298. doi: 10.1001/archneur.1990.00530120034007. [DOI] [PubMed] [Google Scholar]
- 43.Frost JJ, Rosier AJ, Reich SG, et al. Positron emission tomographic imaging of the dopamine transporter with 11C-WIN 35,428 reveals marked declines in mild Parkinson's disease. Ann Neurol. 1993;34:423–431. doi: 10.1002/ana.410340331. [DOI] [PubMed] [Google Scholar]
- 44.Eidelberg D, Moeller JR, Dhawan V, et al. The metabolic anatomy of Parkinson's disease: Complementary [18F]fluorodeoxyglucose and [18F]fluorodopa positron emission tomographic studies. Mov Disord. 1990;5:203–213. doi: 10.1002/mds.870050304. [DOI] [PubMed] [Google Scholar]
- 45.Antonini A, Vontobel P, Psylla M, et al. Complementary positron emission tomographic studies of the striatal dopaminergic system in Parkinson's disease. Arch Neurol. 1995;52:1183–1190. doi: 10.1001/archneur.1995.00540360061017. [DOI] [PubMed] [Google Scholar]
- 46.Ishikawa T, Dhawan V, Chaly T, et al. Clinical significance of striatal DOPA decarboxylase activity in Parkinson's disease. J Nucl Med. 1996;37:216–222. [PubMed] [Google Scholar]
- 47.Morrish PK, Sawle GV, Brooks DJ. An [18F]dopa-PET and clinical study of the rate of the progresssion in Parkinson's disease. Brain. 1996;119:585–591. doi: 10.1093/brain/119.2.585. [DOI] [PubMed] [Google Scholar]
- 48.Bohnen NI, Albin RL, Koeppe RA, et al. Positron emission tomography of monoaminergic vesicular binding in aging and Parkinson disease. J Cereb Blood Flow Metab. 2006 doi: 10.1038/sj.jcbfm.9600276. [DOI] [PubMed] [Google Scholar]
- 49.Seibyl JP, Marek KL, Quinlan D, et al. Decreased single-photon emission computed tomographic [123I]beta-CIT striatal uptake correlates with symptom severity in Parkinson's disease. Ann Neurol. 1995;38:589–598. doi: 10.1002/ana.410380407. [DOI] [PubMed] [Google Scholar]
- 50.Sawle GV, Playford ED, Burn DJ, et al. Separating Parkinson's disease from normality. Discriminant function analysis of fluorodopa F 18 positron emission tomography data. Arch Neurol. 1994;51:237–243. doi: 10.1001/archneur.1994.00540150027011. [DOI] [PubMed] [Google Scholar]
- 51.Ceravolo R, Antonini A, Volterrani D, et al. Predictive value of nigrostriatal dysfunction in isolated tremor: a clinical and SPECT study. Mov Disord. 2008;23:2049–2054. doi: 10.1002/mds.22259. [DOI] [PubMed] [Google Scholar]
- 52.Menendez-Gonzalez M, Tavares F, Zeidan N, et al. Diagnoses behind patients with hard-to-classify tremor and normal DaT-SPECT: a clinical follow up study. Front Aging Neurosci. 2014;6:56. doi: 10.3389/fnagi.2014.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jennings DL, Seibyl JP, Oakes D, et al. (123I) beta-CIT and single-photon emission computed tomographic imaging vs clinical evaluation in Parkinsonian syndrome: unmasking an early diagnosis. Arch Neurol. 2004;61:1224–1229. doi: 10.1001/archneur.61.8.1224. [DOI] [PubMed] [Google Scholar]
- 54.Fuente-Fernandez R. Role of DaTSCAN and clinical diagnosis in Parkinson disease. Neurology. 2012;78:696–701. doi: 10.1212/WNL.0b013e318248e520. [DOI] [PubMed] [Google Scholar]
- 55.Van Laere K, Everaert L, Annemans L, et al. The cost effectiveness of 123I-FP-CIT SPECT imaging in patients with an uncertain clinical diagnosis of parkinsonism. Eur J Nucl Med Mol Imaging. 2008;35:1367–1376. doi: 10.1007/s00259-008-0777-2. [DOI] [PubMed] [Google Scholar]
- 56.Dodel RC, Hoffken H, Moller JC, et al. Dopamine transporter imaging and SPECT in diagnostic work-up of Parkinson's disease: a decision-analytic approach. Mov Disord. 2003;18(Suppl 7):S52–S62. doi: 10.1002/mds.10580. [DOI] [PubMed] [Google Scholar]
- 57.Stoessl AJ. Nature Clinical Practice Neurology. Vol. 5. MacMillan; 2009. Radionuclide scanning to diagnose Parkinson disease: is it cost-effective? pp. 10–11. [DOI] [PubMed] [Google Scholar]
- 58.Perlmutter JS, Eidelberg D. To scan or not to scan: DaT is the question. Neurology. 2012;78:688–689. doi: 10.1212/WNL.0b013e3182494c72. [DOI] [PubMed] [Google Scholar]
- 59.Takahashi H, Levine RA, Galloway MP, et al. Biochemical and fluorodopa positron emission tomographic findings in an asymptomatic carrier of the gene for dopa-responsive dystonia. Ann Neurol. 1994;35:354–356. doi: 10.1002/ana.410350317. [DOI] [PubMed] [Google Scholar]
- 60.O'Brien JT, Colloby S, Fenwick J, et al. Dopamine transporter loss visualized with FP-CIT SPECT in the differential diagnosis of dementia with Lewy bodies. Arch Neurol. 2004;61:919–925. doi: 10.1001/archneur.61.6.919. [DOI] [PubMed] [Google Scholar]
- 61.McKeith I, O'Brien J, Walker Z, et al. Sensitivity and specificity of dopamine transporter imaging with 123I-FP-CIT SPECT in dementia with Lewy bodies: a phase III, multicentre study. Lancet Neurol. 2007;6:305–313. doi: 10.1016/S1474-4422(07)70057-1. [DOI] [PubMed] [Google Scholar]
- 62.Aarsland D, Kurz MW. The epidemiology of dementia associated with Parkinson disease. J Neurol Sci. 2010;289:18–22. doi: 10.1016/j.jns.2009.08.034. [DOI] [PubMed] [Google Scholar]
- 63.Hely MA, Reid WG, Adena MA, et al. The Sydney multicenter study of Parkinson's disease: the inevitability of dementia at 20 years. Mov Disord. 2008;23:837–844. doi: 10.1002/mds.21956. [DOI] [PubMed] [Google Scholar]
- 64.Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 65.Mintun MA, LaRossa GN, Sheline YI, et al. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology. 2006;67:446–452. doi: 10.1212/01.wnl.0000228230.26044.a4. [DOI] [PubMed] [Google Scholar]
- 66.Morris JC, Roe CM, Grant EA, et al. Pittsburgh compound B imaging and prediction of progression from cognitive normality to symptomatic Alzheimer disease. Arch Neurol. 2009;66:1469–1475. doi: 10.1001/archneurol.2009.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bateman RJ, Xiong C, Benzinger TL, et al. Clinical and biomarker changes in dominantly inherited Alzheimer's disease. N Engl J Med. 2012;367:795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Edison P, Rowe CC, Rinne JO, et al. Amyloid load in Parkinson's disease dementia and Lewy body dementia measured with [11C]PIB positron emission tomography. J Neurol Neurosurg Psychiatry. 2008;79:1331–1338. doi: 10.1136/jnnp.2007.127878. [DOI] [PubMed] [Google Scholar]
- 69.Maetzler W, Liepelt I, Reimold M, et al. Cortical PIB binding in Lewy body disease is associated with Alzheimer-like characteristics. Neurobiol Dis. 2009;34:107–112. doi: 10.1016/j.nbd.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 70.Gomperts SN, Rentz DM, Moran E, et al. Imaging amyloid deposition in Lewy body diseases. Neurology. 2008;71:903–910. doi: 10.1212/01.wnl.0000326146.60732.d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Foster ER, Campbell MC, Burack MA, et al. Amyloid imaging of Lewy body-associated disorders. Mov Disord. 2010;15:2516–2523. doi: 10.1002/mds.23393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Johnson KA, Gregas M, Becker JA, et al. Imaging of amyloid burden and distribution in cerebral amyloid angiopathy. Ann Neurol. 2007;62:229–234. doi: 10.1002/ana.21164. [DOI] [PubMed] [Google Scholar]
- 73.Baron JC, Farid K, Dolan E, et al. Diagnostic utility of amyloid PET in cerebral amyloid angiopathy-related symptomatic intracerebral hemorrhage. J Cereb Blood Flow Metab. 2014;34:753–758. doi: 10.1038/jcbfm.2014.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Burack MA, Hartlein J, Flores HP, et al. In vivo amyloid imaging in autopsy-confirmed Parkinson disease with dementia. Neurology. 2010;74:77–84. doi: 10.1212/WNL.0b013e3181c7da8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kotzbauer PT, Cairns NJ, Campbell MC, et al. Pathologic accumulation of alpha-synuclein and Abeta in Parkinson disease patients with dementia. Arch Neurol. 2012;69:1326–1331. doi: 10.1001/archneurol.2012.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cairns NJ, Ikonomovic MD, Benzinger T, et al. Absence of Pittsburgh compound B detection of cerebral amyloid beta in a patient with clinical, cognitive, and cerebrospinal fluid markers of Alzheimer disease: a case report. Arch Neurol. 2009;66:1557–1562. doi: 10.1001/archneurol.2009.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Campbell MC, Markham J, Flores H, et al. Principal component analysis of PiB distribution in Parkinson and Alzheimer diseases. Neurology. 2013;81:520–527. doi: 10.1212/WNL.0b013e31829e6f94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gomperts SN, Locascio JJ, Rentz D, et al. Amyloid is linked to cognitive decline in patients with Parkinson disease without dementia. Neurology. 2013;80:85–91. doi: 10.1212/WNL.0b013e31827b1a07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Siderowf A, Xie SX, Hurtig H, et al. CSF amyloid {beta} 1-42 predicts cognitive decline in Parkinson disease. Neurology. 2010 doi: 10.1212/WNL.0b013e3181f39a78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Buddhala C, Campbell MC, Perlmutter JS, et al. Correlation Between Decreased CSF á-Synuclein and Aâ1-42 in Parkinson Disease. Neurobiology of Aging. 2014 doi: 10.1016/j.neurobiolaging.2014.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]