Abstract
This study assessed the effects of a resistance exercise training program on the inflammatory response associated with Toll-like receptor (TLR) 2 and TLR4 signaling pathways in senior participants. Twenty-six healthy subjects (age, 69.5 ± 1.3) were randomized to a training (TG; n = 16) or a control (CG; n = 10) group. TG performed an 8-week resistance training program, while CG followed their daily routines. Peripheral blood mononuclear cells were isolated from blood samples obtained before and after the intervention, and levels of proteins involved in the TLR2, TLR4, and myeloid differentiation primary response gene 88 (MyD88)-dependent and MyD88-independent pathways were analyzed. The inflammatory status was evaluated through messenger RNA (mRNA) and protein content of interleukin (IL)-10 and tumor necrosis factor alpha (TNF-α) and plasma levels of C-reactive protein (CRP). After the 8-week resistance training, TLR2 and TLR4 protein expression was reduced in TG. MyD88, p65, phospho-p38, TIR domain-containing adaptor inducing interferon (TRIF), IKKi/IKKε, phospho-interferon regulatory factor (IRF) 3, and phosho-IRF7 were also downregulated in TG after the intervention. The training program induced an increase of phospho-extracellular signal-regulated kinases 1 and 2 (ERK1/2) and Hsp70 and a reduction of Hsp60. While TNF-α mRNA and protein values remained unchanged in both TG and CG, IL-10 mRNA and protein content were upregulated in TG after the intervention. CRP values decreased in TG only. The increase in Hsp70 negatively correlated with TLR2 and TLR4 downregulation. These data suggest that resistance exercise may represent an effective tool to ameliorate the pro-inflammatory status of old participants through an attenuation of MyD88-dependent and MyD88-independent TLR2 and TLR4 signaling pathways.
Keywords: Elderly, Inflammation, Resistance exercise, TLR, MyD88
Introduction
Aging is a natural process characterized by a decline in the normal function of several physiological systems (Partridge 2011). Traditionally, senescence has been also related with changes in the immune system since the increase in life expectancy has promoted that individuals are exposed longer to endogenous and environment antigens. The impairment in immunity in the elderly is known as inmmunosenescence and affects both the innate and the adaptive immune system. The aging process causes a chronic, mostly asymptomatic, low-grade inflammatory state, sometimes called “inflammaging.” This was first suggested by Fagiolo et al. (1993) when they reported greater concentrations of inflammatory cytokines from peripheral blood mononuclear cells of aged subjects compared with young individuals. The increased inflammatory status may be linked to changes in immune cell signaling and/or function, although it has not been clarified yet (Larbi et al. 2004). Independently of the causes, the low-grade chronic inflammation described in aged subjects can lead to a more vulnerable status, increasing the risk of developing chronic illnesses (De la Fuente and Miquel 2009), such as cardiovascular disease, type II diabetes mellitus, or osteoporosis (González-Gallego et al. 2010; Haigis and Yankner 2010; Partridge 2011). Multiple studies have reported an association between low-grade systemic inflammation and physical inactivity (Colbert et al. 2004; Pedersen and Saltin 2006; Kullo et al. 2007), indicating that exercise could be an efficient countermeasure to either prevent or delay the onset of some chronic diseases associated with this low-grade inflammatory status (Pedersen and Saltin 2006; Simpson et al. 2012). However, the mechanisms by which exercise may provide an anti-inflammatory stimulus and enhance the immune response are still not well understood.
Toll-like receptors (TLR), in particular TLR2 and TLR4, may play an important role in the anti-inflammatory effects of a physically active lifestyle (McFarlin et al. 2004). TLRs bind to specific ligands, and the best described for TLR2 and TLR4 are peptidoglycan and lipopolysaccharide (LPS), respectively (Zbinden-Foncea et al. 2012). However, there are other structures, called damage-associated molecular patterns (DAMPs), that are endogenous ligands released from damaged or stressed host tissue which also modulate the activation of both TLRs (Asea et al. 2002). Under normal physiological conditions, these factors are hidden, but after a stress stimulus, they are released into the extracellular environment to be recognized by the host immune system (Neubauer et al. 2013). Numerous studies have showed that apoptotic and necrotic cells release DAMP molecules such as high-mobility group box-1 (HMGB1), S-100 proteins, heat shock proteins, hyaluronan, surfactant protein, interferon-alpha, uric acid, fibronectin, beta defensin, and cardiolipin, which trigger a “sterile inflammatory response” following tissue damage (Martin-Murphy et al. 2010; Neubauer et al. 2013). Among DAMPs, heat shock protein 70 kDa (Hsp70) has an important role in the activation of both TLR2 and TLR4 (Asea et al. 2002). Upon stimulation, these receptors lead to the recruitment of various Toll/interleukin-1 receptor (TIR) domain-containing signaling adaptors such as myeloid differentiation primary response gene 88 (MyD88) and TIR domain-containing adaptor inducing interferon (TRIF). Hence, TLR signaling cascade is divided into a MyD88-dependent and a MyD88-independent pathway (Cristofaro and Opal 2006). Both processes result in the activation of a number of downstream signaling pathways, including nuclear factor kappa B (NF-κB), mitogen-activated protein kinase (MAPK), and interferon regulatory factor (IRF) (Akira and Sato 2003), which control inflammatory and immune responses by inducing the expression of several pro-inflammatory cytokines such as the tumor necrosis factor alpha (TNF-α) and type I interferon (IFN) production (Connolly and O’Neill 2012; Oshiumi et al. 2003).
Studies evaluating the influence of physical activity or exercise training on TLRs have shown that 12 weeks of endurance and resistance training reduced TLR4 expression in both young and old sedentary subjects to the levels found in physically active controls. These changes occurred concomitantly with lower C-reactive protein (CRP) and marginally lower inflammatory cytokine production (Stewart et al. 2005). Therefore, physical activity, but not age, is more important for TLR4 signaling and inflammatory cytokine production (McFarlin et al. 2006). Supporting these data, eccentric-based resistance training prevented exercise-induced pro-inflammatory responses in the elderly (Jimenez-Jimenez et al. 2008), and 6 weeks of eccentric resistance exercise reduced TLR4-mediated activation of the pro-inflammatory response through MyD88-dependent and MyD88-independent pathways in young men (Fernandez-Gonzalo et al. 2012). To this background, it is hypothesized that an 8-week resistance exercise training program would reduce the inflammation in elderly subjects through the TLR2 and TLR4 signaling pathway. Furthermore, Hsp proteins, which are recognized ligands of TLR2 and TLR4, have been involved in a number of remodeling processes associated with exercise training, but their tissue of origin has not been yet determined. A variety of cells or tissues, such as heart, liver, brain, muscle, or even leukocytes, could contribute to the production of Hsp (Fehrenbach et al. 2000). Therefore, we hypothesized that the resistance training-induced adaptations on TLR signaling pathway could be related with an altered expression of Hsp70 and Hsp60 in peripheral blood mononuclear cells from healthy aged individuals.
Methods
Design
The experimental part of the study was completed in 10 weeks. On the first and last week, descriptive measurements, maximal strength tests, and blood samples were collected. The resistance exercise training program was performed during the remaining 8 weeks.
Subjects
Twenty-six healthy participants (7 males, 19 females; age range 65–78) volunteered to participate in the study. The inclusion criteria included not to take any medication known to affect the inflammatory response 1 month before or during the study. None of the female participants were taking any hormonal treatment, either before or at the time of the study. Participants did not have any experience in strength training, and they were asked to maintain their physical activity routines during the study period. Before any other activity, a medical screening including anthropometry analysis, the physical activity readiness questionnaire (PAR-Q), a risk factor quiz, blood pressure measurements, and a basal electrocardiogram test were performed in all the participants. Subjects were randomly assigned to a training group (TG; n = 16) or to a control group (CG; n = 10). Age, height, weight, and body mass index were as follows: 69.1 ± 1.1 year, 157.1 ± 1.9 cm, 67.4 ± 2.3 kg, and 27.2 ± 0.6 kg/m2, respectively, for TG and 70.0 ± 0.9 year, 158.9 ± 1.9 cm, 68.1 ± 2.5 kg, and 27.08 ± 0.8 kg/m2, respectively, for CG. Participants from TG followed an 8-week resistance exercise training program, whereas the control group kept their normal daily routines. All participants were informed of the objectives and possible risks of the intervention before written consent for participation was obtained. The study followed the principles of the Declaration of Helsinki, and the local ethics committee approved all procedures.
Maximal strength assessment
Approximately 1 week before and 1 week after the training period, maximal strength was assessed in all the participants. After a standardized 10-min warm-up on a cycle ergometer (Tunturi F35, Tunturi®, Turku, Finland), maximal voluntary isometric contraction (MVIC) test was carried out in a 45° incline leg press device (Gervasport, Madrid, Spain) at 110° knee flexion and in a biceps curl bench device (Gervasport) at 90° elbow flexion. Maximal strength was registered by a strain gauge (Globus Ergometer, Globus, Codogne, Italy). After ∼30 min of rest, one repetition maximum (1RM) test was performed in the same leg press and biceps curl bench previously described and in a seated pec deck machine (BH Fitness Nevada Pro, BH, Vitoria, Spain).
Resistance exercise training
Subjects from the TG completed 16 resistance exercise training sessions over 8 weeks (2 sessions per week), with at least 48 h between sessions. After a 10-min warm-up on a cycle ergometer, participants performed three different exercises, e.g., leg press, biceps curl, and pec deck, in the same exercise devices described above. The number of repetitions per set and load for the three exercises were progressively increased as follows: 3 × 8, 3 × 10, and 3 × 12 at 60 % of 1RM during weeks 1, 2, and 3, respectively; 3 × 8, 3 × 10, and 3 × 12 at 70 % of 1RM during weeks 4, 5, and 6, respectively; and 3 × 8 and 3 × 10 at 80 % of 1RM during weeks 7 and 8, respectively.
Blood sample preparation
Venus blood samples (30 mL) were collected from the brachiocephalic vein using the EDTA anticoagulant Vacutainer™ systems (BD, Franklin Lakes, NJ), 5–6 days before and after the training period. To avoid circadian effects, all samples were collected between 08:00 and 09:00 a.m. Subjects were required to fast for 12 h before the blood test and to avoid any intense exercise during the previous 5–6 days. No caffeine or alcohol was allowed during this time. Food frequency questionnaires (FFQs) were performed all through the study, and participants were asked to maintain their eating habits between both blood sampling. Total blood was centrifuged to isolate plasma and peripheral blood mononuclear cells (PBMCs) with a density gradient centrifugation on Ficoll separation solution (Biochrom AG, Berlin, Germany) (Cuevas et al. 2005).
Reverse transcription and quantitative real-time polymerase chain reaction
Total RNA was isolated from PBMC using a RiboPure™-Blood Kit (Ambion®, Paisley, UK) and quantified by spectrophotometry (NanoDrop 1000, Thermo Scientific, Waltham, MA, USA). DNase I (RNase-free) (Ambion®) was used to removed residual genomic DNA. First-standard complementary DNA (cDNA) was synthesized using High-Capacity cDNA Archive Kit (Applied Biosystems®, Paisley, UK), and then, it was amplified using TaqMan® Universal PCR Master Mix (Applied Biosystems®) on a StepOnePlus™ Real-Time PCR Systems (Applied Biosystems®). TaqMan® primers and probes for interleukin (IL)-10 (GenBank M57627.1 and Hs00961622_m1), TNF-α (GenBank M10988.1 and Hs00174128_m1), and GAPDH as housekeeping gene (GenBank M33197.1 and Hs99999905_m1) were derived from the commercially available TaqMan® Assays-on-Demand Gene (Applied Biosystems®). Relative changes in gene expression levels were determined using the 2−ΔΔCT method as described previously (Veneroso et al. 2009). The cycle number at which the transcripts were detectable (CT) was normalized to the cycle number of GAPDH detection, referred to as ΔCT.
Western blot analysis
For Western blot analysis, PBMCs were suspended on 150 mL of 0.25 mM sucrose, 1 mM EDTA, 10 mM Tris, and a protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO, USA) and physical disrupted using a sonicator. Lysate proteins were fractionated by SDS-PAGE. Polyacrylamide gels (9 % for Hsp60, Hsp70, TLR2, TLR4, TRIF, and IKKi/IKKε; 12 % for MyD88, p65, IRF3, phospho-IRF3, IRF7, phospho-IRF7, extracellular signal-regulated kinases 1 and 2 (ERK1/2), phospho-ERK1/2, p38, phospho-p38 and β-actin; 15 % for TNF-α and IL-10) were running containing 50 μg of the samples and, therefore, transferred to a polyvinylidene fluoride (PVDF) membrane by a Trans-Blot® Turbo™ Transfer System (Bio-Rad®, Hercules, CA, USA). Non-specific binding was blocked by preincubation of the PVDF membranes in PBS containing 2.5 % non-fat milk for 1 h. Then, membranes were incubated overnight at 4 °C with corresponding antibodies. Antibodies against Hsp60 (60 kDa), TLR2 (90–100 kDa), TLR4 (95 kDa), MyD88 (33 kDa), p65 (65 kDa), IRF3 (50 kDa), IRF7 (54 and 18 kDa), phospho-ERK1/2 (42–44 kDa), ERK1/2 (42–44 kDa), TNF-α (26 and 17 kDa), and IL-10 (20 and 37 kDa) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA); antibodies against Hsp70 (70 kDa), TRIF (66 kDa), and IKKi/IKKε; (80 kDa) were purchased from Abcam® (Cambridge, UK, USA); and antibodies against phospho-IRF3 (55 kDa), phospho-IRF7 (65 kDa), p38 (43 kDa), and phospho-p38 (43 kDa) were purchased from Cell Signaling Technology® (Beverly, MA, USA), and β-actin (42 kDa) was purchased from Sigma-Aldrich. Bound primary antibody was detected using an appropriate horseradish peroxidase (HRP)-conjugated secondary antibody (Dako, Glostrup, Denmark) using a chemiluminescent HRP substrate (Luminol Reagent, Santa Cruz Biotechnology). Finally, blots were exposed to autoradiography films and developed. The density of the specific bands was quantified with an imaging densitometer (Image J, Bethesda, MD, USA).
CRP and IL-6 quantification
Plasma CRP and IL-6 were measured by using enzyme-linked immunoabsorbent assay (Quantikine ELISA kit: R&D Systems®, Minneapolis, MN).
Statistical analysis
Data are expressed as relative change from pretraining to posttraining ± standard error of means (SEM). A two-way analysis of variance (ANOVA) with repeated measures for time (pre and post) and group (TG and CG) was performed. Bonferroni post hoc analysis was used where appropriate. Pearson’s correlation analysis was used to assess the relative changes in Hsp70 and Hsp60 in relation to TLR2 and TLR4. Differences were considered significant when p < 0.05. All statistical analyses were performed using SPSS version 18 (SPSS Inc., Chicago, IL, USA).
Results
After the training program, TG showed significant increases in leg press 1RM (p < 0.04) and MVIC (p < 0.03), in biceps curl bench 1RM (p < 0.04) and MVIC (p < 0.05), and in a seated pec deck 1RM (p < 0.05) (Table 1). MVIC and 1RM did not change in CG in any of the exercises analyzed (Table 1).
Table 1.
One repetition maximum (1RM) and maximal voluntary isometric contraction (MVIC), in kilograms (kg), in response to 8 weeks of resistance training for TG and the same period of normal daily routines for CG
| Control group (CG) | Training group (TG) | ||||
|---|---|---|---|---|---|
| Pre | Post | Pre | Post | ||
| Leg press (kg) | 1RM | 160.4 ± 13.5 | 171.3 ± 18.2 | 157.0 ± 13.1 | 198.4 ± 14.3*,** |
| MVIC | 96.3 ± 13.1 | 98.1 ± 15.8 | 109.8 ± 12.2 | 137.4 ± 12.8*,** | |
| Biceps curl bench (kg) | 1RM | 19.0 ± 3.2 | 20.2 ± 4.8 | 19.7 ± 3.1 | 26.0 ± 3.2*,** |
| MVIC | 15.1 ± 2.1 | 16.3 ± 3.3 | 13.2 ± 1.1 | 19.4 ± 2.0*,** | |
| Seated pec deck (kg) | 1RM | 25.7 ± 4.0 | 27.6 ± 5.1 | 24.1 ± 4.3 | 31.5 ± 3.0*,** |
Values are means ± SEM
*p < 0.05 vs CG; **p < 0.05 vs pre within a group
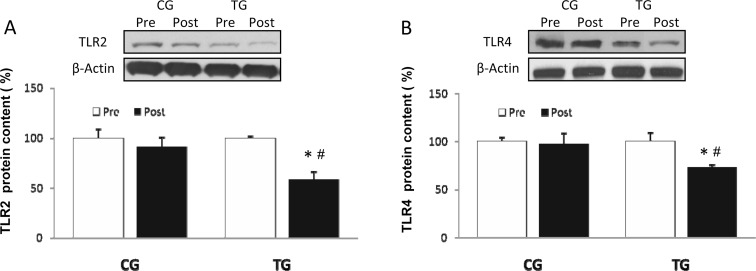
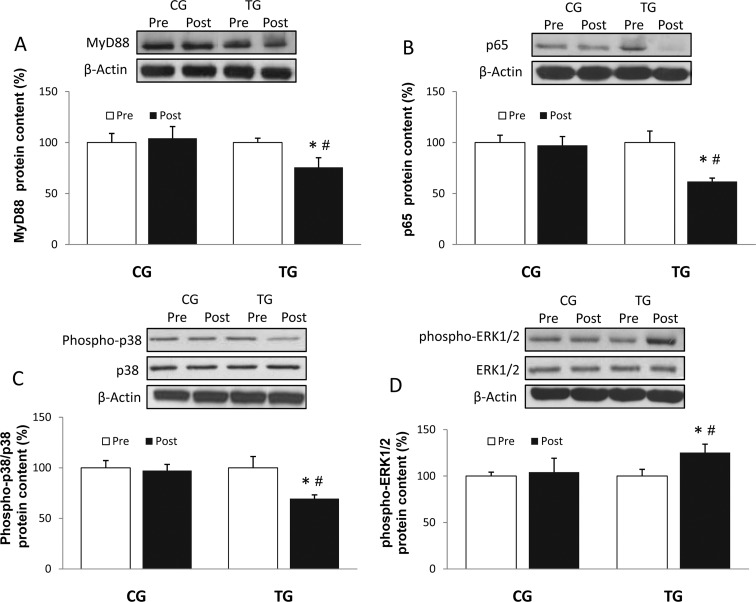
The resistance exercise protocol employed here induced a significant decrease in the protein level of TLR2 (p < 0.04) and TLR4 (p < 0.03) (Fig. 1a, b), whereas CG values did not change. Similar results were observed in the MyD88-dependent pathway, where TG showed a considerable reduction of both MyD88 (p < 0.05) and p65 (p < 0.03) (Fig. 2a, b) protein content after the 8 weeks of training, with no change in CG.
Fig. 1.
Densitometric quantification and representative Western blot of Toll-like receptor 2 (TLR2) (a) and Toll-like receptor 4 (TLR4) (b) in PBMC in response to 8 weeks of resistance training for TG and the same period of normal daily routines for CG. Values are means ± SEM.*p < 0.05 vs CG; #p < 0.05 vs pre within a group
Fig. 2.
Densitometric quantification and representative Western blot of MyD88 (a), p65 (b), phospho-p38/total p38 (c), and phospho-ERK1/2/total ERK1/2 (d) in PBMC in response to 8 weeks of resistance training for TG and the same period of normal daily routines for CG. Values are means ± SEM.*p < 0.05 vs CG; #p < 0.05 vs pre within a group
Phosphorylated p38 content was reduced in response to resistance exercise (Fig. 2c; p < 0.03). This training effect was also present in the protein concentration of cytosolic phospho-ERK 1/2 (Fig. 2d), with significantly higher values (p < 0.02) at posttraining compared to pretraining. Total protein content of both p38 and ERK1/2 remained unchanged in CG and TG.
The training protocol induced an evident downregulation in the most representative MyD88-independent pathway proteins (Fig. 3a, b). Thus, protein expression of TRIF and IKKi/IKKε was reduced after training (p < 0.04 and p < 0.05, respectively). A significant decrease in the phosphorylated state of IRF3 (Fig. 3c; p < 0.02) and IRF7 (Fig. 3d; p < 0.04) was found in TG. None of these proteins changed in CG.
Fig. 3.
Densitometric quantification and representative Western blot of IKKi/IKKε (a) and TRIF (b), phospho-IRF3/total IRF3 (c), and phospho-IRF7/total IRF7 (d) in PBMC in response to 8 weeks of resistance training for TG and the same period of normal daily routines for CG. Values are means ± SEM.*p < 0.05 vs CG; #p < 0.05 vs pre within a group
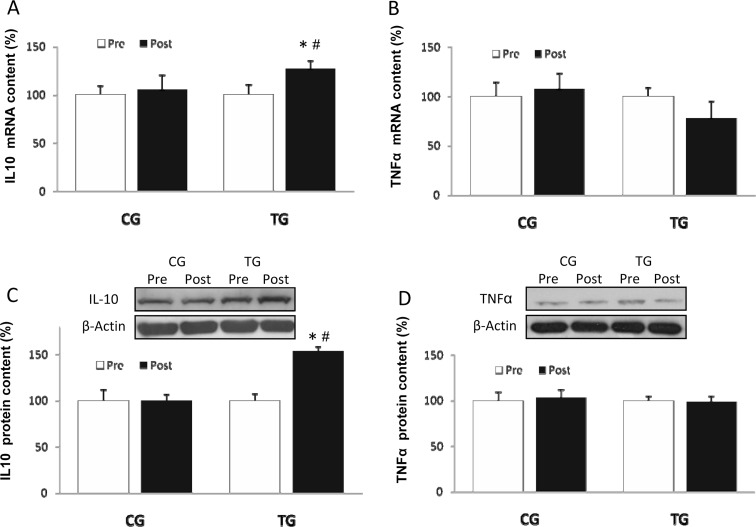
TG showed a significant upregulation (p < 0.04) of PBMC IL-10 messenger RNA (mRNA) levels (Fig. 4a) after the 16 resistance exercise sessions. No significant changes were observed in PBMC TNF-α mRNA levels (Fig. 4b) in any group. IL-10 protein content increased in response to training (Fig. 4c; p < 0.01) whereas TNF-α protein concentration remained constant in both TG and CG (Fig. 4d). The PBMC protein IL-10/TNF-α ratio increased after training in TG (1.05 ± 0.14 vs 1.48 ± 0.17 arbitrary units), indicating greater anti-inflammatory status. This ratio remained unchanged in CG (1.00 ± 0.11 vs 0.97 ± 0.12 arbitrary units).
Fig. 4.
mRNA levels of IL-10 (a) and TNF-α (b) and densitometric quantification and representative Western blot of IL-10 (c) and TNF-α (d) in PBMC in response to 8 weeks of resistance training for TG and the same period of normal daily routines for CG. Values are means ± SEM.*p < 0.05 vs CG; #p < 0.05 vs pre within a group
CRP data confirmed the anti-inflammatory effects of the training intervention. CRP levels were significantly lower (p < 0.01) after the training program compared with pretraining values (1.03 ± 0.10 vs 0.64 ± 0.07 mg/L). Plasma IL-6 also was significantly reduced (p < 0.05) as a result of resistance exercise training (2.96 ± 0.09 vs 2.51 ± 0.08 mg/L). No significant differences were observed in the control group for CRP and IL-6 (0.96 ± 0.09 vs 0.90 ± 0.08 mg/L and 2.92 ± 0.11 vs 3.09 ± 0.12, respectively).
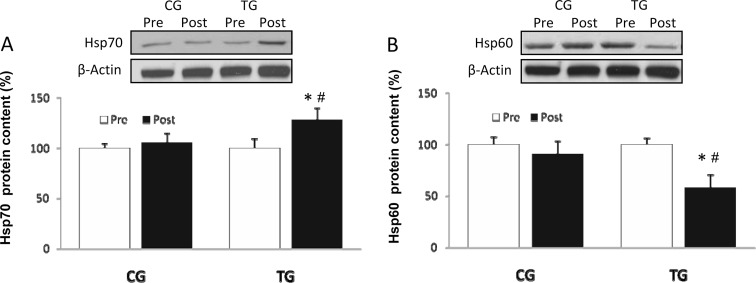
Hsp70 protein concentration (Fig. 5a) increased (p < 0.03) after training. Conversely, Hsp60 (p < 0.01) protein content decreased after the intervention in TG. Both Hsp70 and Hsp60 remained constant in CG (Fig. 5b).
Fig. 5.
Densitometric quantification and representative Western blot of Hsp70 (a) and Hsp60 (b) in PBMC in response to 8 weeks of resistance training for TG and the same period of normal daily routines for CG. Values are means ± SEM.*p < 0.05 vs CG; #p < 0.05 vs pre within a group
There were strong negative correlations between changes in Hsp70 and TLR2 (r = −0.93, p < 0.02), and Hsp70 and TLR4 (r = −0.76, p < 0.05). However, weak correlations were found between the expression of TLR2 and Hsp60 (r = −0.41, p = 0.17), as well as TLR4 and Hsp60 (r = −0.36, p = 0.31), and TLR2 and TLR4 (r = −0.48, p = 0.13).
Discussion
The current study investigated the effects of resistance training on the molecular inflammatory response associated with the TLR2 and TLR4 signaling pathways in PBMCs from healthy old subjects. Data indicate that 8 weeks of resistance training attenuate the TLR2 and TLR4 MyD88-dependent and MyD88-independent pathways, which mediate NF-κB, IRF3, and IRF7 activation, and the related pro-inflammatory response. The TLR2 and TLR4 downregulation was also associated with an increase of Hsp70 protein content. In addition, Hsp60 decreased after resistance training.
Traditionally, aging has been associated with higher levels of pro-inflammatory markers (Bruunsgaard 2002; Ershler and Keller 2005). CRP, IL-6, and TNF-α are the inflammatory markers most consistently associated with age-related chronic diseases and disability (Singh and Newman 2011). However, some studies have reported similar levels of inflammatory biomarkers in young and old subjects (Beharka et al. 2001; Flynn et al. 2003), suggesting that the inflammatory status is more affected by physical activity than age per se (Giannopoulou et al. 2005; Oberbach et al. 2006). Indeed, physical activity seems to induce an anti-inflammatory response through amelioration of inflammatory mediators (Starkie et al. 2003; Keller et al. 2004). Supporting this notion, we here report a decreased CRP plasma levels in old subjects, an adaptation already described (McFarlin et al. 2006). Stewart et al. (2007) supported the use of combined aerobic/resistance training as a modality to reduce the serum concentration of the most commonly utilized marker of inflammation, such as the acute-phase protein CRP, both in young and old physically inactive men. In this line, the research by Donges et al. (2010) also showed that 10 weeks of resistance training resulted in significant attenuation of CRP concentration and no alteration in baseline IL-6. In contrast, the current and other studies (Nicklas et al. 2008) suggest a reduction of both CRP and IL-6 after a period of exercise training, supporting the role of IL-6 as a key factor controlling chronic elevation of CRP in older adults (Singh and Newman 2011). The resistance exercise program employed in the current investigation reduced the protein concentration of TLR2 and TLR4 in PBMC of ∼70-year-old subjects. It is believed that TLR2 and TLR4 have synergistic effects over the immune cells (Zbinden-Foncea et al. 2012) controlling the inflammatory response via a regulatory loop between TLRs and cytokines. Indeed, TLRs induce cytokine expression via NF-κB pathway, which is involved in the modulation of TLR expression (Miettinen et al. 2001; Seibl et al. 2003). Our results are supported by previous studies suggesting that physically active subjects present significantly lower cell surface TLR4 expression and inflammatory cytokine production than physically inactive subjects (Flynn et al. 2003; Stewart et al. 2005; McFarlin et al. 2006). Therefore, it is plausible that low TLR2 and TLR4 expression inhibits pro-inflammatory cytokine production, which may explain, at least partially, the anti-inflammatory effect attributed to regular exercise (McFarlin et al. 2004).
TLR2 and TLR4 meditate the early inflammatory response by the main downstream adaptor MyD88 (Jiang et al. 2010). This protein is essential to stimulate pro-inflammatory cytokine production (Kawai and Akira 2006). Previous results from our group indicate that the MyD88-dependent pathway plays an important role in the inflammatory response induced by eccentric-based resistance exercise (Fernandez-Gonzalo et al. 2012, 2014). In support, data from the current investigation suggest that the lower TLR2 and TLR4 expression induced by resistance training was accompanied by a decrease in MyD88 protein concentration in PBMC of old subjects. In contrast, MyD88 protein expression was not altered in PBMC of obese subjects after 10 weeks of endurance training (Nickel et al. 2011). The different exercise stimulus (high-intensity low-volume vs low-intensity high-volume) may affect the MyD88 pathway differently, explaining the discrepancies between these studies.
Following recruitment of MyD88, the next step in the early phase activation of NF-κB though TLR2 and TLR4 signaling is mediated by tumor necrosis factor receptor-associated factor 6 (TRAF6) and transforming growth factor-β-activated protein kinase 1 (TAK1) among other proteins (Coll and O’Neill 2010), which lead to the recruitment of p50 in the cytoplasm and the translocation of p65 to the nucleus (Kawai and Akira 2006). Both p50 and p65 form the most frequent heterodimer of NF-κB (McFarlin et al. 2006). In the current study, the resistance training program induced a significant decrease of p65 protein content in TG, asserting the anti-inflammatory effect attributed of resistance exercise.
MyD88 is also involved in the activation of MAPK signaling. Literature supports a differential response of these kinases after exercise, although most authors report transient activations following either resistance or endurance exercise (van Ginneken et al. 2006; Kramer and Goodyear 2007). Our data support this differential activation state between p38 and ERK1/2. Thus, while phospho-p38, the activated form, was significantly reduced after the training period, the phosphorylation state of ERK1/2 was significantly upregulated in TG. The discrepancy between the response of these proteins may be explained by the highly exercise specific and contraction form dependent of MAPK signaling (Lee et al. 2002). Thus, while some MAPK downstream molecules show the same transitional repression identified in other components of the NF-κB pathway, the overexpression of ERK1/2 is consistent with other studies which show an increase after resistance exercise (Taylor et al. 2012).
Additionally to the MyD88-dependent pathway, NF-κB, and, therefore, cytokine production can also be activated in TLR signaling by the TRIF-dependent pathway (Kawai and Akira 2006). Moreover, TRIF interacts with the heterodimer TANK-binding kinase 1 (TBK1)-IKKi/IKKε which mediates the phosphorylation of IRF3. This transcriptional factor is dimerized and, then, translocated to the nucleus to bind DNA and stimulate the expression of type I IFN (Kawai and Akira 2006). Our results indicate that the MyD88-independent pathway, measured through TRIF, IKKi/IKKε, and the phosphorylation of IRF3 and IRF7, is also significantly downregulated after 8 weeks of resistance exercise in old subjects. Therefore, these data show that the anti-inflammatory effects of resistance exercise are mediated by both MyD88-dependent and MyD88-independent pathways in the elderly.
The current study suggests that the main pathways activated by TLR2 and TLR4, as well as both receptors, are downregulated after 8 weeks of resistance training in older adults. However, the mechanisms by which TLR2 and TLR4 expression is reduced after exercise are far from understood. A relationship between endogenous ligands such as cytokines or Hsps and TLR expression has been suggested (Beg 2002). Indeed, exercise is able to increase the plasma concentration of several cytokines that induce a decrease in TLR expression (Gleeson et al. 2006). This idea is supported by the immunoregulatory effect of chronic exercise, which induces an increase of IL-10 and an amelioration of TNF-α in plasma (Moldoveanu et al. 2001; Steensberg et al. 2003; Vieira et al. 2007). Our data show that resistance exercise induced a marked upregulation of IL-10 protein expression, yet TNF-α protein level was unchanged. It is worth noting that IL-10 acts as a natural antagonist of TNF-α, being able to inhibit NF-κβ signaling (Schottelius et al. 1999). Furthermore, ERK1/2 is involved in the phosphorylation of the IL-10 proximal promoter in macrophages (Hofmann et al. 2012). In support, the increased expression of phospho-ERK1/2 reported in the current study was coincident with higher protein level of IL-10 in mononuclear cells. In addition, analyzing the IL-10/TNF-α ratio, which is often used as an indicator of the inflammatory status (Petersen and Pedersen 2005; Lira et al. 2009), our results indicate that the resistance exercise training triggered molecular changes toward an anti-inflammatory status.
Exercise is also associated with transient elevations of Hsp expression, which may reduce inflammatory mediators (Noble and Shen 2012), suggesting that these proteins act as mediators of the effects of exercise on the immune function (Walsh et al. 2001). Hsp70 is one of many DAMPs recognized by both TLR2 and TLR4 (Asea et al. 2002). An increase of Hsp70 concentration after exercise has been reported in both animals (Locke et al. 1990; Samelman 2000) and humans (Walsh et al. 2001; Weber et al. 2012), but it should be noted that the production of Hsp70 after exercise is intensity- and frequency-dependent (Harris and Starnes 2001; Milne and Noble 2002). Although Walsh et al. (2001) clearly showed that exercise results in an increase in circulating Hsp72, the inducible form of the Hsp70, they could not determine the tissue of origin. The appearance of Hsp72 in the serum preceded any increase in Hsp72 gene expression in skeletal muscle, so it is likely that the origin of the circulating Hsp72 was not from the contracting muscle. Furthermore, Weber et al. showed that the lymphocyte HSP70 content was higher at the middle and end than at the beginning of the season in women handball players (Weber et al. 2012). On the other hand, it has been also demonstrated that the expression of HSP70 in leukocytes is increased after a half marathon (Fehrenbach et al. 2000). However, 1-h walking sessions, 3 days a week on a running track for 8 weeks, had no impact on leukocyte Hsp72 expression in elderly people (Simar et al. 2012). Our exercise protocol seems to be intense enough to promote an increase of Hsp70 in PBMC, which could be the origin of the circulating Hsps. Finally, although some studies support the hypothesis that exercise increases Hsp72 production within monocytes (Fehrenbach et al. 2000), we cannot rule out that other cells or tissues contribute to increase Hsp70 expression in response to exercise.
Hsp70 can directly stimulate anti-inflammatory cytokines due to an inhibition of the NF-κB pathway (Schell et al. 2005; Weiss et al. 2007). This inhibition reduces the expression of pro-inflammatory cytokines regulated by NF-κB, such as TNF-α, IL-6, or IL-1β (De et al. 2000; Pockley et al. 2009). In this line, our data show that the exercise-induced decrease of TLR2 and TLR4 was inversely correlated with the increase of Hsp70 in PBMC. In addition, circulating Hsp60 activates TLR2 and TLR4 (Ohashi et al. 2000; Asea et al. 2002; Kilmartin and Reen 2004) resulting in the production of inflammatory mediators (Hao et al. 2010). However, although the correlation analysis between Hsp60 and TLRs did not reach statistical signification, there is a weak positive association which could support that the amelioration of Hsp60 protein content is also related with the anti-inflammatory status induced by the resistance training protocol employed. Apart from the Hsp, additional sterile inflammation signals released into the circulation by stressed or/and injured cells not assessed in the current investigation may also influence TLR signaling pathways in PBMCs. In this line, Neubauer et al. (2013) suggest that DAMPs, hypothetically originating from damaged skeletal muscle tissue, are associated with the activation of TLRs in neutrophils. Moreover, it is important to highlight that the function of these endogenous “danger signals” might be also altered with aging, as a result of increased oxidative stress and inflammation in old subjects (Murlasits et al. 2006).
In summary, this study shows that an 8-week resistance exercise training program downregulates TLR2 and TLR4 basal expression, inducing an anti-inflammatory status in elderly subjects. In addition and as previously reported in young men, resistance training impacted both MyD88-dependent and MyD88-independent pathways. The anti-inflammatory effect induced by resistance training on the TLR pathways seems to be associated with changes in the expression of Hsp70, and possibly in Hsp60, which may also confer further protection against other age-related disorders. Altogether, the current study suggests that resistance exercise represents a useful tool to induce positive anti-inflammatory adaptations in the elderly.
Acknowledgments
This study was supported by Plan Nacional I+D+I DEP2010-17574, Spain.
References
- Akira S, Sato S. Toll-like receptors and their signaling mechanisms. Scand J Infect Dis. 2003;35:555–562. doi: 10.1080/00365540310015683. [DOI] [PubMed] [Google Scholar]
- Asea A, Rehli M, Kabingu E, Boch JA, Bare O, Auron PE, Stevenson MA, Calderwood SK. Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J Biol Chem. 2002;277:15028–15034. doi: 10.1074/jbc.M200497200. [DOI] [PubMed] [Google Scholar]
- Beg AA. Endogenous ligands of toll-like receptors: implications for regulating inflammatory and immune responses. Trends Immunol. 2002;23:509–512. doi: 10.1016/S1471-4906(02)02317-7. [DOI] [PubMed] [Google Scholar]
- Beharka AA, Meydani M, Wu D, Leka LS, Meydani A, Meydani SN. Interleukin-6 production does not increase with age. J Gerontol A Biol Sci Med Sci. 2001;56:B81–B88. doi: 10.1093/gerona/56.2.B81. [DOI] [PubMed] [Google Scholar]
- Bruunsgaard H. Effects of tumor necrosis factor-alpha and interleukin-6 in elderly populations. Eur Cytokine Netw. 2002;13:389–391. [PubMed] [Google Scholar]
- Colbert LH, Visser M, Simonsick EM, Tracy RP, Newman AB, Kritchevsky SB, Pahor M, Taaffe DR, Brach J, Rubin S, Harris TB. Physical activity, exercise, and inflammatory markers in older adults: findings from the health, aging and body composition study. J Am Geriatr Soc. 2004;52:1098–1104. doi: 10.1111/j.1532-5415.2004.52307.x. [DOI] [PubMed] [Google Scholar]
- Coll RC, O’Neill LA. New insights into the regulation of signalling by toll-like receptors and nod-like receptors. J Innate Immun. 2010;2:406–421. doi: 10.1159/000315469. [DOI] [PubMed] [Google Scholar]
- Connolly DJ, O’Neill LA. New developments in toll-like receptor targeted therapeutics. Curr Opin Pharmacol. 2012;12:510–518. doi: 10.1016/j.coph.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Cristofaro P, Opal SM. Role of toll-like receptors in infection and immunity: clinical implications. Drugs. 2006;66:15–29. doi: 10.2165/00003495-200666010-00002. [DOI] [PubMed] [Google Scholar]
- Cuevas MJ, Almar M, García-Glez JC, García-López D, De Paz JA, Alvear-Ordenes I, González-Gallego J. Changes in oxidative stress markers and NF-kappaB activation induced by sprint exercise. Free Radic Res. 2005;39:431–439. doi: 10.1080/10715760500072149. [DOI] [PubMed] [Google Scholar]
- De la Fuente M, Miquel J. An update of the oxidation-inflammation theory of aging: the involvement of the immune system in oxi-inflamm-aging. Curr Pharm Des. 2009;15:3003–3026. doi: 10.2174/138161209789058110. [DOI] [PubMed] [Google Scholar]
- De AK, Kodys KM, Yeh BS, Miller-Graziano C. Exaggerated human monocyte IL-10 concomitant to minimal TNF-alpha induction by heat-shock protein 27 (Hsp27) suggests Hsp27 is primarily an antiinflammatory stimulus. J Immunol. 2000;165:3951–3958. doi: 10.4049/jimmunol.165.7.3951. [DOI] [PubMed] [Google Scholar]
- Donges CE1, Duffield R, Drinkwater EJ. Effects of resistance or aerobic exercise training on interleukin-6, C-reactive protein, and body composition. Med Sci Sports Exerc. 2010;42:304–312. doi: 10.1249/MSS.0b013e3181b117ca. [DOI] [PubMed] [Google Scholar]
- Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu Rev Med. 2005;51:245–270. doi: 10.1146/annurev.med.51.1.245. [DOI] [PubMed] [Google Scholar]
- Fagiolo U, Cossarizza A, Scala E, Fanalesbelasio E, Ortolani C, Cozzi E, Monti D, Franceschi C, Paganelli R. Increased cytokine production in mononuclear-cells of healthy elderly people. Eur J Immunol. 1993;23:2375–2378. doi: 10.1002/eji.1830230950. [DOI] [PubMed] [Google Scholar]
- Fehrenbach E, Niess AM, Schlotz E, Passek F, Dickhuth HH, Northoff H. Transcriptional and translational regulation of heat shock proteins in leukocytes of endurance runners. J Appl Physiol. 2000;89(1985):704–710. doi: 10.1152/jappl.2000.89.2.704. [DOI] [PubMed] [Google Scholar]
- Fernandez-Gonzalo R, De Paz JA, Rodriguez-Miguelez P, Cuevas MJ, González-Gallego J. Effects of eccentric exercise on toll-like receptor 4 signaling pathway in peripheral blood mononuclear cells. J Appl Physiol. 2012;112:2011–2018. doi: 10.1152/japplphysiol.01499.2011. [DOI] [PubMed] [Google Scholar]
- Fernandez-Gonzalo R, De Paz JA, Rodriguez-Miguelez P, Cuevas MJ, González-Gallego J (2014) TLR4-mediated blunting of inflammatory responses to eccentric exercise in young women. Mediators Inflamm doi:10.1155/2014/479395 [DOI] [PMC free article] [PubMed]
- Flynn MG, McFarlin BK, Phillips MD, Stewart LK, Timmerman KL. Toll-like receptor 4 and CD14 mRNA expression are lower in resistive exercise-trained elderly women. J Appl Physiol. 2003;95:1833–1842. doi: 10.1152/japplphysiol.00359.2003. [DOI] [PubMed] [Google Scholar]
- Giannopoulou I, Fernhall B, Carhart R, Weinstock RS, Baynard T, Figueroa A, Kanaley JA. Effects of diet and/or exercise on the adipocytokine and inflammatory cytokine levels of postmenopausal women with type 2 diabetes. Metabolism. 2005;54:866–875. doi: 10.1016/j.metabol.2005.01.033. [DOI] [PubMed] [Google Scholar]
- Gleeson M, McFarlin B, Flynn M. Exercise and toll-like receptors. Exerc Immunol Rev. 2006;12:34–53. [PubMed] [Google Scholar]
- González-Gallego J, García-Mediavilla MV, Sánchez-Campos S, Tuñón MJ. Fruit polyphenols, immunity and inflammation. Br J Nutr. 2010;104(Suppl 3):S15–S27. doi: 10.1017/S0007114510003910. [DOI] [PubMed] [Google Scholar]
- Haigis MC, Yankner BA. The aging stress response. Mol Cell. 2010;40:333–344. doi: 10.1016/j.molcel.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao HN, Zheng B, Nasser S, Ren W, Latteier M, Wooley P, Morawa L. The roles of monocytic heat shock protein 60 and toll-like receptors in the regional inflammation response to wear debris particles. J Biomed Mater Res A. 2010;92:1373–1381. doi: 10.1002/jbm.a.32474. [DOI] [PubMed] [Google Scholar]
- Harris MB, Starnes JW. Effects of body temperature during exercise training on myocardial adaptations. Am J Physiol Heart Circ Physiol. 2001;280:H2271–H2280. doi: 10.1152/ajpheart.2001.280.5.H2271. [DOI] [PubMed] [Google Scholar]
- Hofmann SR, Morbach H, Schwarz T, Rosen-Wolff A, Girschick HJ, Hedrich CM. Attenuated TLR4/MAPK signaling in monocytes from patients with CRMO results in impaired IL-10 expression. Clin Immunol. 2012;145:69–76. doi: 10.1016/j.clim.2012.07.012. [DOI] [PubMed] [Google Scholar]
- Jiang W, Hu M, Rao J, Xu X, Wang X, Kong L. Over-expression of toll-like receptors and their ligands in small-for-size graft. Hepatol Res. 2010;40:318–329. doi: 10.1111/j.1872-034X.2009.00603.x. [DOI] [PubMed] [Google Scholar]
- Jimenez-Jimenez R, Cuevas MJ, Almar M, Lima E, García-López D, De Paz JA, González-Gallego J. Eccentric training impairs NF-kappaB activation and over-expression of inflammation-related genes induced by acute eccentric exercise in the elderly. Mech Ageing Dev. 2008;129:313–321. doi: 10.1016/j.mad.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. TLR signaling. Cell Death Differ. 2006;13:816–825. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- Keller C, Keller P, Giralt M, Hidalgo J, Pedersen BK. Exercise normalises overexpression of TNF-alpha in knockout mice. Biochem Biophys Res Commun. 2004;321:179–182. doi: 10.1016/j.bbrc.2004.06.129. [DOI] [PubMed] [Google Scholar]
- Kilmartin B, Reen DJ. HSP60 induces self-tolerance to repeated HSP60 stimulation and cross-tolerance to other pro-inflammatory stimuli. Eur J Immunol. 2004;34:2041–2051. doi: 10.1002/eji.200425108. [DOI] [PubMed] [Google Scholar]
- Kramer HF, Goodyear LJ. Exercise, MAPK, and NF-kappaB signaling in skeletal muscle. J Appl Physiol. 2007;103:388–395. doi: 10.1152/japplphysiol.00085.2007. [DOI] [PubMed] [Google Scholar]
- Kullo IJ, Khaleghi M, Hensrud DD. Markers of inflammation are inversely associated with VO2 max in asymptomatic men. J Appl Physiol. 2007;102:1374–1379. doi: 10.1152/japplphysiol.01028.2006. [DOI] [PubMed] [Google Scholar]
- Larbi A, Dupuis G, Douziech N, Khalil A, Fulop T. Low-grade inflammation with aging has consequences for T-lymphocyte signaling. signal transduction pathways, chromatin structure, and gene expression mechanisms as therapeutic targets. Ann N Y Acad Sci. 2004;1030:125–133. doi: 10.1196/annals.1329.016. [DOI] [PubMed] [Google Scholar]
- Lee JS, Bruce CR, Spurrell BE, Hawley JA. Effect of training on activation of extracellular signal-regulated kinase 1/2 and p38 mitogen-activated protein kinase pathways in rat soleus muscle. Clin Exp Pharmacol Physiol. 2002;29:655–660. doi: 10.1046/j.1440-1681.2002.03713.x. [DOI] [PubMed] [Google Scholar]
- Lira FS, Rosa JC, Zanchi NE, Yamashita AS, Lopes RD, Lopes AC, Batista ML, Jr, Seelaender M. Regulation of inflammation in the adipose tissue in cancer cachexia: effect of exercise. Cell Biochem Funct. 2009;27:71–75. doi: 10.1002/cbf.1540. [DOI] [PubMed] [Google Scholar]
- Locke M, Noble EG, Atkinson BG. Exercising mammals synthesize stress proteins. Am J Physiol. 1990;258:C723–C729. doi: 10.1152/ajpcell.1990.258.4.C723. [DOI] [PubMed] [Google Scholar]
- Martin-Murphy BV, Holt MP, Ju C. The role of damage associated molecular pattern molecules in acetaminophen-induced liver injury in mice. Toxicol Lett. 2010;192:387–394. doi: 10.1016/j.toxlet.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlin BK, Flynn MG, Campbell WW, Stewart LK, Timmerman KL. TLR4 is lower in resistance-trained older women and related to inflammatory cytokines. Med Sci Sports Exerc. 2004;36:1876–1883. doi: 10.1249/01.MSS.0000145465.71269.10. [DOI] [PubMed] [Google Scholar]
- McFarlin BK, Flynn MG, Campbell WW, Craig BA, Robinson JP, Stewart LK, Timmerman KL, Coen PM. Physical activity status, but not age, influences inflammatory biomarkers and toll-like receptor 4. J Gerontol A Biol Sci Med Sci. 2006;61:388–393. doi: 10.1093/gerona/61.4.388. [DOI] [PubMed] [Google Scholar]
- Miettinen M, Sareneva T, Julkunen I, Matikainen S. IFNs activate toll-like receptor gene expression in viral infections. Genes Immun. 2001;2:349–355. doi: 10.1038/sj.gene.6363791. [DOI] [PubMed] [Google Scholar]
- Milne KJ, Noble EG. Exercise-induced elevation of HSP70 is intensity dependent. J Appl Physiol. 2002;93:561–568. doi: 10.1152/japplphysiol.00528.2001. [DOI] [PubMed] [Google Scholar]
- Moldoveanu AI, Shephard RJ, Shek PN. The cytokine response to physical activity and training. Sports Med. 2001;31:115–144. doi: 10.2165/00007256-200131020-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murlasits Z, Cutlip RG, Geronilla KB, Rao KM, Wonderlin WF, Alway SE. Resistance training increases heat shock protein levels in skeletal muscle of young and old rats. Exp Gerontol. 2006;41:398–406. doi: 10.1016/j.exger.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Neubauer O, Sabapathy S, Lazarus R, Jowett JB, Desbrow B, Peake JM, Cameron-Smith D, Haseler LJ, Wagner KH, Bulmer AC. Transcriptome analysis of neutrophils after endurance exercise reveals novel signaling mechanisms in the immune response to physiological stress. J Appl Physiol. 2013;114:1677–1688. doi: 10.1152/japplphysiol.00143.2013. [DOI] [PubMed] [Google Scholar]
- Nickel T, Hanssen H, Emslander I, Drexel V, Hertel G, Schmidt-Trucksäss A, Summo C, Sisic Z, Lambert M, Hoster E, Halle M, Weis M. Immunomodulatory effects of aerobic training in obesity. Mediators Inflamm. 2011 doi: 10.1155/2011/308965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicklas BJ, Hsu FC, Brinkley TJ, Church T, Goodpaster BH, Kritchevsky SB, Pahor M. Exercise training and plasma C-reactive protein and interleukin-6 in elderly people. J Am Geriatr Soc. 2008;56:2045–2052. doi: 10.1111/j.1532-5415.2008.01994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble EG, Shen GX. Impact of exercise and metabolic disorders on heat shock proteins and vascular inflammation. Autoimmune Dis. 2012 doi: 10.1155/2012/836519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberbach A, Tonjes A, Kloting N, Fasshauer M, Kratzsch J, Busse MW, Paschke R, Stumvoll M, Blüher M. Effect of a 4 week physical training program on plasma concentrations of inflammatory markers in patients with abnormal glucose tolerance. Eur J Endocrinol. 2006;154:577–585. doi: 10.1530/eje.1.02127. [DOI] [PubMed] [Google Scholar]
- Ohashi K, Burkart V, Flohe S, Kolb H. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J Immunol. 2000;164:558–561. doi: 10.4049/jimmunol.164.2.558. [DOI] [PubMed] [Google Scholar]
- Oshiumi H, Matsumoto M, Funami K, Akazawa T, Seya T. TICAM-1, an adaptor molecule that participates in toll-like receptor 3-mediated interferon-beta induction. Nat Immunol. 2003;4:161–167. doi: 10.1038/ni886. [DOI] [PubMed] [Google Scholar]
- Partridge L. Some highlights of research on aging with invertebrates, 2010. Aging Cell. 2011;10:5–9. doi: 10.1111/j.1474-9726.2010.00649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen BK, Saltin B. Evidence for prescribing exercise as therapy in chronic disease. Scand J Med Sci Sports. 2006;16(Suppl 1):3–63. doi: 10.1111/j.1600-0838.2006.00520.x. [DOI] [PubMed] [Google Scholar]
- Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol. 2005;98:1154–1162. doi: 10.1152/japplphysiol.00164.2004. [DOI] [PubMed] [Google Scholar]
- Pockley AG, Calderwood SK, Multhoff G. The atheroprotective properties of Hsp70: a role for Hsp70-endothelial interactions? Cell Stress Chaperones. 2009;14:545–553. doi: 10.1007/s12192-009-0113-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samelman TR. Heat shock protein expression is increased in cardiac and skeletal muscles of Fischer 344 rats after endurance training. Exp Physiol. 2000;85:92–102. doi: 10.1111/j.1469-445X.2000.01894.x. [DOI] [PubMed] [Google Scholar]
- Schell MT, Spitzer AL, Johnson JA, Lee D, Harris HW. Heat shock inhibits NF-kB activation in a dose- and time-dependent manner. J Surg Res. 2005;129:90–93. doi: 10.1016/j.jss.2005.05.025. [DOI] [PubMed] [Google Scholar]
- Schottelius AJ, Mayo MW, Sartor RB, Baldwin AS., Jr Interleukin-10 signaling blocks inhibitor of kappaB kinase activity and nuclear factor kappaB DNA binding. J Biol Chem. 1999;274:31868–31874. doi: 10.1074/jbc.274.45.31868. [DOI] [PubMed] [Google Scholar]
- Seibl R, Birchler T, Loeliger S, Hossle JP, Gay RE, Saurenmann T, Michel BA, Seger RA, Gay S, Lauener RP. Expression and regulation of toll-like receptor 2 in rheumatoid arthritis synovium. Am J Pathol. 2003;162:1221–1227. doi: 10.1016/S0002-9440(10)63918-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simar D, Malatesta D, Mas E, Delage M, Caillaud C. Effect of an 8-weeks aerobic training program in elderly on oxidative stress and HSP72 expression in leukocytes during antioxidant supplementation. J Nutr Health Aging. 2012;16:155–161. doi: 10.1007/s12603-011-0106-5. [DOI] [PubMed] [Google Scholar]
- Simpson RJ, Lowder TW, Spielmann G, Bigley AB, LaVoy EC, Kunz H. Exercise and the aging immune system. Ageing Res Rev. 2012;11:404–420. doi: 10.1016/j.arr.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Singh T, Newman AB. Inflammatory markers in population studies of aging. Ageing Res Rev. 2011;10:319–329. doi: 10.1016/j.arr.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkie R, Ostrowski SR, Jauffred S, Febbraio M, Pedersen BK. Exercise and IL-6 infusion inhibit endotoxin-induced TNF-alpha production in humans. FASEB J. 2003;17:884–886. doi: 10.1096/fj.02-0670fje. [DOI] [PubMed] [Google Scholar]
- Steensberg A, Fischer CP, Keller C, Moller K, Pedersen BK. IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans. Am J Physiol Endocrinol Metab. 2003;285:E433–E437. doi: 10.1152/ajpendo.00074.2003. [DOI] [PubMed] [Google Scholar]
- Stewart LK, Flynn MG, Campbell WW, Craig BA, Robinson JP, McFarlin BK, Timmerman KL, Coen PM, Felker J, Talbert E. Influence of exercise training and age on CD14+ cell-surface expression of toll-like receptor 2 and 4. Brain Behav Immun. 2005;19:389–397. doi: 10.1016/j.bbi.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Stewart LK, Flynn MG, Campbell WW, Craig BA, Robinson JP, Timmerman KL, McFarlin BK, Coen PM, Talbert E. The influence of exercise training on inflammatory cytokines and C-reactive protein. Med Sci Sports Exerc. 2007;39:389–397. doi: 10.1249/mss.0b013e31811ece1c. [DOI] [PubMed] [Google Scholar]
- Taylor LW, Wilborn CD, Kreider RB, Willoughby DS. Effects of resistance exercise intensity on extracellular signal-regulated kinase 1/2 mitogen-activated protein kinase activation in men. J Strength Cond Res. 2012;26:599–607. doi: 10.1519/JSC.0b013e318242f92d. [DOI] [PubMed] [Google Scholar]
- van Ginneken MM, de Graaf-Roelfsema E, Keizer HA, van Dam KG, Wijnberg ID, van der Kolk JH, van Breda E. Effect of exercise on activation of the p38 mitogen-activated protein kinase pathway, c-Jun NH2 terminal kinase, and heat shock protein 27 in equine skeletal muscle. Am J Vet Res. 2006;67:837–844. doi: 10.2460/ajvr.67.5.837. [DOI] [PubMed] [Google Scholar]
- Veneroso C, Tuñón MJ, González-Gallego J, Collado PS. Melatonin reduces cardiac inflammatory injury induced by acute exercise. J Pineal Res. 2009;47:184–191. doi: 10.1111/j.1600-079X.2009.00699.x. [DOI] [PubMed] [Google Scholar]
- Vieira RP, Claudino RC, Duarte AC, Santos AB, Perini A, Faria Neto HC, Mauad T, Martins MA, Dolhnikoff M, Carvalho CR. Aerobic exercise decreases chronic allergic lung inflammation and airway remodeling in mice. Am J Respir Crit Care Med. 2007;176:871–877. doi: 10.1164/rccm.200610-1567OC. [DOI] [PubMed] [Google Scholar]
- Walsh RC, Koukoulas I, Garnham A, Moseley PL, Hargreaves M, Febbraio MA. Exercise increases serum Hsp72 in humans. Cell Stress Chaperones. 2001;6:386–393. doi: 10.1379/1466-1268(2001)006<0386:EISHIH>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber MH, da Rocha RF, Schnorr CE, Schröder R, Moreira JC. Changes in lymphocyte HSP70 levels in women handball players throughout 1 year of training: the role of estrogen levels. J Physiol Biochem. 2012;68:365–375. doi: 10.1007/s13105-012-0148-0. [DOI] [PubMed] [Google Scholar]
- Weiss YG, Bromberg Z, Raj N, Raphael J, Goloubinoff P, Ben-Neriah Y, Deutschman CS. Enhanced heat shock protein 70 expression alters proteasomal degradation of IkappaB kinase in experimental acute respiratory distress syndrome. Crit Care Med. 2007;35:2128–2138. doi: 10.1097/01.CCM.0000278915.78030.74. [DOI] [PubMed] [Google Scholar]
- Zbinden-Foncea H, Raymackers JM, Deldicque L, Renard P, Francaux M. TLR2 and TLR4 activate p38 MAPK and JNK during endurance exercise in skeletal muscle. Med Sci Sports Exerc. 2012;44:1463–1472. doi: 10.1249/MSS.0b013e31824e0d5d. [DOI] [PubMed] [Google Scholar]