Abstract
As our knowledge of disease improves, its classification continually evolves. The last WHO classification of odontogenic tumors was 9 years ago and it is time for revision. We offer the following critique as a constructive, thought provoking challenge to those chosen to provide contemporary insight into the next WHO classification of odontogenic cysts, tumors, and allied conditions.
Keywords: Odontogenic cysts, Odontogenic tumors, Classification of odontogenic tumors
The latest WHO classification of odontogenic tumors was published in Pathology and Genetics. Head and Neck Tumours in 2005 by IARC Press [1]. Chapter 6 dealt with Odontogenic Tumors. Our knowledge and understanding continues to evolve and it is clearly time for a more contemporary classification and discussion of odontogenic tumors. It is not our intention to update our knowledge of each condition; that will be the charge of the new WHO authors and some of the invited experts selected to contribute to this special edition. Rather, our comments will be more conceptual and will represent our opinions and constructive criticism as a guide for improvement. The current version illustrates the challenge of any classification as some tumors contain various combinations of tissues that are incompatible with a classification based on the normal temporal and spatial development of dental hard tissues. The primary value of the WHO classification is now to simply list lesions in a logical order and provide evidence for the validity of each entity. While it does provide guidance for management, its diagnostic utility and value however, are significantly limited by its brevity and lack of accurate terminology. It is also self-evident that a WHO classification must be designed to function at an international level. Some countries have well developed specialties of head and neck or oral/oral and maxillofacial pathology while others have almost none. The classification will become the reference standard for all so that changes can only be made when supported by good evidence. It is very difficult to reverse changes made in a WHO classification, although there is precedence for doing so because the 2005 edition corrected the inaccurate definitions of malignant ameloblastoma and ameloblastic carcinoma from its previous edition. It is interesting that the introduction of the keratocystic odontogenic tumor category has been largely ignored in Europe and North America but adopted elsewhere, perhaps reflecting different perceptions of the classification as definitive, rather than being a proposal that is continually under review.
We recommend that the text begin with definitions of basic terms. As a text on tumors, it would be useful to first define tumor and to set out the intended scope of the classification. Dorland’s Medical Dictionary [2] defines tumor as 1. Swelling, one of the cardinal signs of inflammation, 2. Neoplasm. Stedman’s Medical Dictionary [3] defines tumor as 1. Any swelling or tumefaction, 2. Neoplasm. While many health care providers use tumor today synonymously with neoplasm, others do not and usage varies among different countries. The 1971 WHO classification included “odontogenic tumours, odontogenic cysts and allied lesions.” In 1992, the classification was titled “odontogenic tumours” but still included odontogenic cysts and allied lesions. In 2005 “odontogenic tumours” continued and although some “allied lesions” were still included, unfortunately cysts were eliminated. The WHO classification must follow the uniform structure and terminology used for all other tumors of various tissues and organ systems and so the usage of ‘odontogenic tumour’ will continue. However, we feel the term must be used in its broader context as the classification includes malignant and benign neoplasms and hamartomas.
Confusion has been introduced by reclassifying odontogenic keratocyst as a neoplasm, discussed below, when other cysts seem equally deserving of inclusion in the classification, though not as neoplasms. We strongly recommend including the traditional odontogenic cysts because there are several entities currently excluded that should be considered in the histological differential diagnosis of “odontogenic tumors”, such as glandular odontogenic cyst, botryoid odontogenic cyst, and pure “cystic” variants of the WHOs “calcifying cystic odontogenic tumors.” The terms cyst and neoplasm should be defined and it should be clear, when possible, when a lesion is regarded as developmental or hamartomatous. Because of the conceptual overlap of cysts and cystic neoplasms, both should be discussed, clarified, and classified in the same work. The previous reclassification of odontogenic keratocyst does make this somewhat difficult, but introduction of a category of cysts and cystic neoplasms might provide a suitable structure.
The overall classification is fairly universally accepted; dividing the tumors into benign vs malignant based on their biologic properties and clinical behavior and into epithelial, epithelial with ectomesenchyme (mixed), and ectomesenchymal with or without epithelium based on their histogenetic origin. Since some lesions do not fit readily into this classification, particularly epithelial tumors with “dentinoid” but no ectomesenchymal component, alternative classifications could be considered and there could be advantages to abandoning the developmental framework and simply listing the odontogenic tumors.
Malignant Odontogenic Tumors
The WHO convention to list malignant neoplasms first perhaps places too much emphasis on these rare lesions. There are a few new entities to consider in this group and overall the classification works well; its main failing is over complexity.
There are currently three types of ameloblastic carcinoma recognized, divided into a primary type, a secondary (dedifferentiated) intraosseous type and a secondary (dedifferentiated) peripheral type. Examples of definite high grade transformation of ameloblastoma are few, though clearly the process is possible, but there seems no reason to subdivide the ameloblastic carcinomas themselves as they present similarly and behave similarly. Primary intraosseous squamous cell carcinoma is also subdivided into a solid type, and those derived from keratocystic odontogenic tumor or from other odontogenic cysts. Again, significant reasons for differentiating these on the basis of origin appear lacking and removing the subcategories would considerably simplify the classification.
There is a lack of consensus on criteria for malignancy in odontogenic tumors. Mitotic activity alone is insufficient, but frequent apoptosis may be more indicative. Necrosis and overt invasion at the periphery can be difficult to find or demonstrate and borderline malignant appearances cause significant problems in diagnosis. In one review of 20 malignant odontogenic tumors, only 9 were accepted as truly malignant [4]. The criteria given in the classification for ameloblastic carcinoma are stringent and comprehensive, but do not address the difficulty of borderline lesions where a decision on malignancy can often be facilitated with radiological support.
The classification only allows one odontogenic primary intraosseous carcinoma, squamous cell carcinoma. This seems restrictive and other patterns of differentiation are occasionally seen, though squamous is most frequent. It would seem simpler to reduce this to one category of primary intraosseous odontogenic carcinoma. Consideration could be given to include the sclerosing odontogenic carcinoma [5], which may show squamous differentiation, if a review of published cases provides sufficient evidence for its status as an entity. The area of overlap with squamous odontogenic tumor also merits discussion. The squamous odontogenic tumor is poorly defined in terms of behavior and some reported cases have shown aggressive permeative spread that could equally be considered to indicate a well differentiated squamous carcinoma. A further interesting development is the reporting of a series of tumors, in part with clear cells characterized by extensive dentinoid formation. Such an inductive effect is not expected in a tumor composed of cells without requisite odontogenic differentiation and the appearances reported to date are quite variable. Some have been considered benign, but it seems increasingly likely that all such lesions are low grade malignant neoplasms. In this special edition, this entity is reported as odontogenic carcinoma with dentinoid but there will need to be clarification of the histologic overlap with lesions described as adenoid ameloblastoma with dentinoid [6, 7] and adenomatoid dentinoma [8], which seem equally deserving of consideration for inclusion in the classification.
The clear cell odontogenic carcinoma is a well-established entity, but may require some adjustment in the next classification. The description of EWSR1-ATF1 translocations common to several types of clear cell carcinomas may not prove to be a defining molecular change, but it does offer a possible route to subdividing those with a dominant clear cell phenotype from those with minimal cytoplasmic clearing, or provide molecular support to the fact that some clear cell carcinomas appear to develop their clear cell morphology over a period of time in successive recurrences.
The definition of ghost cell odontogenic carcinoma in the current classification highlights a problem with defining lesions by origin in an equivalent benign precursor. As malignant neoplasms may well show a different pattern of differentiation from their benign precursor, this defining characteristic should be removed.
Those responsible for any forthcoming update of the classification should also consider whether a simple division of odontogenic tumors into benign and malignant is adequate. Odontogenic tumors already include a range from high grade malignant neoplasms to minor developmental malformations and several lesions are not easily categorized. Many ameloblastomas show peripheral medullary bone infiltration and since a very small minority “metastasize”; are these intermediate malignant neoplasms? Squamous odontogenic tumor includes aggressive variants. Are all dentinogenic ghost cell tumors aggressive and at risk of recurrence merely because they are solid? The sarcomas and some clear cell lesions are very low grade. The lead taken by the WHO bone and soft tissue tumor authors merits consideration. The lead taken by the WHO Bone and Soft Tissue Tumor classification since 2002 merits consideration. Benign tumors are considered first in each category, followed by those which are intermediate (locally aggressive) and intermediate (rarely metastasizing) and finally those truly malignant [9].
Finally, we appeal to readers to publish cases of possible odontogenic carcinosarcomas that have adequate immunohistochemical or molecular support for the diagnosis. This tumor has been removed from the classification as most published cases do not meet current diagnostic criteria for the sarcoma component. However, occasional cases have been reported with adequate immunohistochemical and/or molecular support [10, 11].
Benign Odontogenic Tumors
Epithelial Tumors
Our criticism of the classification of ameloblastoma is primarily organizational. The authors designate the conventional neoplasm as solid/multicystic. Most conventional ameloblastomas show cystic degeneration, either microscoptically or macroscopically, and because of the concept of unicystic ameloblastoma, we believe the term multicystic adds no value, only confusion. We would recommend that the term “solid/multicystic” is dropped and that “ameloblastoma” is used without qualification for the conventional lesions.
The text states that the conventional ameloblastoma has “virtually no tendency to metastasize” but also states “Metastasizing ameloblastoma is an ameloblastoma that metastasizes in spite of a benign histologic appearance.” These statements are contradictory. They further state that ameloblastoma “occurs exclusively in the jaws, rarely in the sinonasal mucosa.” Since ameloblastomas have been documented rarely in the buccal mucosa [12] as well as the sinonasal mucosa [13], they do not occur “exclusively” in the jaws. We believe that the spectrum of ameloblastoma shows at least six distinct histological patterns; namely, follicular, plexiform, granular cell, acanthomatous, basal cell and desmoplastic. Although the demographic features of desmoplastic ameloblastoma are distinct from the other histologic subtypes, the radiographic features are a function of the desmoplasia, and we do not believe it deserves a separate category. One of the subtypes of plexiform ameloblastoma occurs predominantly in the posterior maxilla [14] and it is not separated as an entity, so we see no purpose to separate desmoplastic ameloblastoma other than to note its distinctive clinical and radiographic features. The histologic subtypes have no clinical significance. It is notable that the odontogenic classification remains very heavily weighted to histological features rather than always fully integrating the clinical, radiological, molecular and behavioral characteristics that define a pathological entity. The authors state the cystogenic ameloblastoma is synonymous with unicystic ameloblastoma. We believe the term cystogenic ameloblastoma has no distinctive meaning and adds only confusion.
Unicystic ameloblastoma remains an area of considerable debate and confusion, particularly to clinicians. The text defines unicystic ameloblastoma using the criteria proposed in the original article by Robinson and Martinez [15] in 1977 and subdivides them into luminal and mural. The text does not acknowledge that subsequent authors have recommended that mural invasion should be viewed as conventional ameloblastoma [16–18]. Li et al. [19] in 2000 reported a recurrence rate of 36 % with mural invasion in unicystic ameloblastomas and a recurrence rate of less than 10 % with the other subtypes. The current WHO authors further state that treatment will depend on depth of invasion. With “limited” mural invasion they recommend follow-up only, but with “deep extension”, further surgery should be considered. This seems somewhat arbitrary and needs to be addressed in the new edition. It should be emphasized that unicystic ameloblastoma cannot be diagnosed definitively on an incisional biopsy.
The adenomatoid odontogenic tumor is an example of the difficulties of classifying odontogenic tumors by their developmental histogenetic origin. While the AOT is primarily an epithelial tumor, it does produce “dentinoid”, clearly not a product of odontogenic epithelium. We agree with the discussion of the remainder of the epithelial tumors, with the exception of the keratocystic odontogenic tumor (KCOT) which will be discussed below.
Mixed Tumors
The most confusing section in the mixed tumor category is the histological spectrum from ameloblastic fibroma, through ameloblastic fibro-dentinoma, ameloblastic fibro-odontoma and ultimately to odontoma, because it includes both neoplasms and hamartomas, and because the lesions are currently inadequately defined. The ameloblastic fibroma appears to be a distinct entity, with a potentially destructive growth pattern. It is conventionally considered a neoplasm of both epithelial and connective tissue components though the evidence for this appears circumstantial. Unfortunately AF may be histologically indistinguishable from early developing odontomas and the lesions currently accepted as ameloblastic fibro-odontoma.
There is evidence that lesions with histologic features identical to ameloblastic fibromas mature into odontomas if untreated [18, 19]. Chau et al. [20] suggested that AF is neoplastic and distinct from AFO and odontoma based on periostin reactivity. Buchner and Vered [21] pointed out that AFs that recur before the age of 22 often recurred as a more mature lesion such as ameloblastic fibro-dentinoma or AFO, and those that recurred after the age of 22, invariably recurred as pure AFs. Accordingly, we and others question the utility of including ameloblastic fibro-dentinoma and ameloblastic fibro-odontoma as entities, as these are not two distinct entities and probably represent developing odontomas [22, 23].
We would recommend revisiting the concept of odontoameloblastoma. We would agree with others that the majority of odontoameloblastomas reported in the literature do not meet the strict diagnostic criteria for that designation [24]. Although ameloblastomas occurring in association with both unerupted teeth and odontomas are well documented, is odontoameloblastoma the most appropriate designation for this entity? Is odontoameloblastoma a unique entity or did the ameloblastoma simply arise in the odontoma? We believe that ameloblastomas can arise from epithelial remnants following odontogenesis and can also arise from those same remnants in odontomas. If odontoameloblatoma was an entity, it would begin as an odontoameloblastoma, evolve as an odontoameloblastoma and recur as an odontoameloblastoma, just as ameloblastic fibromas do. There appears to be no evidence for this, and the name has practical implications for surgeons. If odontoameloblastoma is an entity, do surgeons understand that it behaves exactly as an ameloblastoma? Mosqueda-Taylor et al. [24] concluded that odontoameloblastomas behave as ameloblastomas and should be treated as ameloblastomas. The first person that we are aware of to publically express the concept of ameloblastoma arising in odontomas is Dr. Harvey Kessler of the USA and we believe that “ameloblastoma arising in an odontoma” is a more accurate and clearer designation for this lesion but it probably does not merit a separate category in the classification and odontoameloblastoma should be removed.
There also seems to be merit in adding a specific category for the peripheral odontogenic tumors. These have always been recognized in previous classifications and noted as less aggressive than their intraosseous counterparts. While malignant transformation is possible in peripheral tumors, it appears very rare. Not recognizing that peripheral tumors are innocuous can lead to over treatment. The best example is the inclusion of dentinogenic ghost cell tumor in the last classification with an important defining characteristic being its solid nature. Peripheral calcifying odontogenic cysts had been long recognized as often being solid but probably hamartomatous nodules of no great significance. We have noted cases submitted for publication and presented where a diagnosis of dentinogenic ghost cell tumor has been ascribed incorrectly on the basis of their solid structure and feel that the peripheral tumors need to be described more fully to ensure that patients are correctly treated.
Ectomesenchymal Tumors
The section on mesodermal/ectomesenchymal tumors was relatively well done. The section on odontogenic fibroma (OdF) includes two distinct histologic patterns for this neoplasm; the epithelium poor or “simple type” and the epithelium rich or “WHO type”. The simple type is exceedingly rare and controversial but the epithelium poor tumors often do not contain epithelium at all. Any epithelium present would be coincidental and would be irrelevant to the diagnosis. The WHO definition of OdF is a rare neoplasm characterized by varying amounts of inactive-looking odontogenic epithelium embedded in a mature fibrous stroma. The simple type however, contains little if any epithelium and the stroma is not mature. This definition comes very close to describing hyperplastic dental follicles. We recommend consideration of eliminating the “simple type” and defining the OdF as a neoplasm of mature fibrous connective tissue with variable amounts of epithelium with or without evidence of calcifications. This is another lesion in which the growth pattern and radiological features are important to define it as a neoplasm.
The current classification groups together several entities within the designation of ossifying fibroma, in our opinion incorrectly. This reflects the confusing terminology that has developed in the literature over the last 30 years, during which period a debate raged about the differences between bone and cementum that placed too much emphasis on classification by histological appearances. The literature suggests that the current group contains three distinctive entities and the only one that appears odontogenic is the conventional ossifying fibroma of the jaws. It is challenging to arrive at an appropriate name for a neoplasm that secretes variable amounts and morphologic patterns of matrix of putative odontogenic origin. Ossifying fibroma, cementifying fibroma, cemento-ossifying fibroma, ossifying odontogenic fibroma and periodontoma have all been suggested in the past. Despite the fact that all definitions of cementum include its anatomic association with tooth roots, we prefer cemento-ossifying fibroma as the best name for this entity, because it is a well understood term and because laboratory evidence indicates that periodontal ligament stem cells can produce bone or cementum [25] and that the periodontal ligament and inner lamina dura of the tooth socket probably arise from odontogenic ectomesenchyme. The term cemento-ossifying fibroma also emphasizes that this is exclusively a lesion of the tooth bearing areas of the jaws and avoids confusion with similar long bone tumor terminology. This type should be included with odontogenic tumors.
Another entity known in the US as juvenile active ossifying fibroma and in Europe as psammomatoid ossifying fibroma and other complex names is entirely different and is not odontogenic, affecting both the extragnathic craniofacial bones as well as the jaws. The term cementum or “cementicles” is inappropriate to describe the calcifications in this lesion, despite the histological appearances. This lesion is less easily enucleated and has greater growth potential with likelihood of recurrence, perhaps because of its less well defined periphery. This condition is well placed in the section on bone related lesions.
The third member of the group, often termed trabecular ossifying fibroma, perhaps has a better claim to the description ‘juvenile’ and needs to be distinguished primarily because of its worrying histological appearances that can mimic osteosarcoma. However, on the basis of existing evidence, this variant is also well documented in extragnathic craniofacial sites, as well as the non-tooth bearing areas of the jaws and also does not appear to be an odontogenic tumor.
Both “juvenile” variants should be mentioned in the new classification because of their relationship with other ossifying fibromas, if in name only. Some have argued that juvenile should be dropped because both diagnoses are rendered on unique histologic features and both lesions can occur later than adolescence. However, the term juvenile has been associated with these lesions for at least 30 years, is well recognized, and both variants do have a strong predilection for children and teens.
The Cyst/Neoplasm Interface
Odontogenic Keratocyst (OKC)/Keratocystic Odontogenic Tumor (KCOT)
The 2005 WHO classification was the first to exclude a classification of the odontogenic cysts, especially since one of the most controversial features of the classification was the discussion and distinction of the cyst/neoplasm interface, and the reclassification of lesions originally considered simple or developmental cysts to lesions that some authors now consider neoplasms. There is much overlap and controversy between cysts and tumors, both in terms of histologic features and clinical presentation (Figs. 1, 2, 3, 4). Additionally, the pathogenesis of a number of these entities remains unknown and hotly debated. Many authors avoid this dilemma by referring to “tumors” without defining whether they mean “neoplasm” or use the term cyst loosely, or synonymously with “non-neoplastic” or “developmental.” This debate would be improved once again with clear definitions of cyst, neoplasm and cystic neoplasm. Four conditions require discussion; odontogenic keratocyst/keratocystic odontogenic tumor, calcifying odontogenic cyst/calcifying cystic odontogenic tumor, glandular odontogenic cyst/mucoepidermoid carcinoma and ameloblastoma/multicystic ameloblastoma/unicystic ameloblastoma.
Fig. 1.

Calcifying odontogenic cyst/calcifying cystic odontogenic tumor: Cyst or neoplasm?
Fig. 2.

Odontogenic keratocyst/keratocystic odontogenic tumor: Cyst or neoplasm?
Fig. 3.

Unicystic ameloblastoma: Cyst or neoplasm?
Fig. 4.
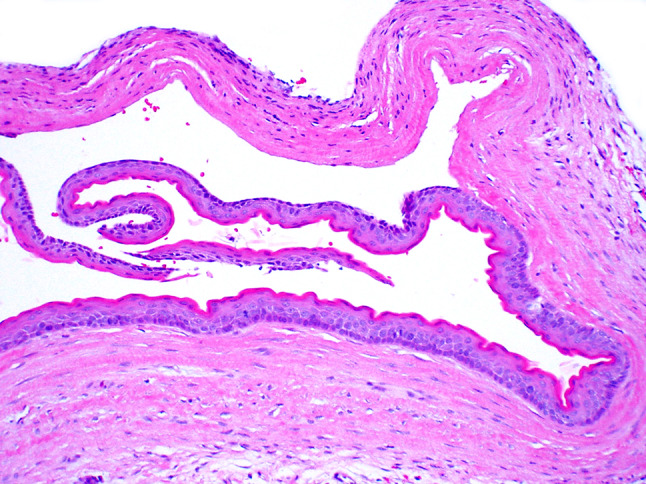
Cutaneous keratocyst in a syndromic patient: Cyst or neoplasm?
In 2005 the WHO reclassified odontogenic keratocyst (OKC) as a neoplasm and recommended keratocystic odontogenic tumor (KCOT) as the appropriate designation. In justifying the reclassification, the authors stressed “aggressive” behavior, recurrence, the occasional occurrence of a “solid” variant, and mutations in the PTCH gene. However, the original references on mutations were all on syndromic patients and the 6 WHO references on non-syndromic cases included syndromic patients. There were numerous subsequent papers dealing with PTCH mutations in syndromic and nonsyndromic OKCs, which have shown mutations in approximately 85 % of syndromic cases and 30 % of non-syndromic cases [26–32]. The mutations, however, are not clonal or limited to PTCH. Mutations of p16, P53, MCC, TSLC1, LTAS2, and FHIT have also been reported in OKCs [33–35]. In 1999 the Karolinska Institute designed and made publically available a PTCH mutation database. As of June of 2010, there were 291 different mutations. We personally do not find sufficient evidence to justify reclassifying OKCs as neoplastic. While neoplasms are characterized by genetic aberrations, there is currently no single genetic alteration that defines neoplasia. For example, fibrous dysplasia is due to a mutation but it has not been reclassified as a neoplasm. It is possible that the molecular/genetic alteration that affects some OKCs might affects their biologic behavior without defining the cyst as a neoplasm and it was likely intentional that the recent review of OKCs by Gomes et al. was titled “Review of the molecular pathogenesis of the odontogenic keratocyts” [36]. Currently the definition of neoplasia in virtually every general pathology textbook is based on phenotype and clinical behavior, not molecular aberrations. The major general pathology texts all define a neoplasm as a new growth of tissue which is not regulated by the normal growth regulatory factors and one in which the neoplasm continues to grow, even if the stimulus which produced it is removed. Neoplasms are characterized by growth autonomy and they should not resolve spontaneously. OKCs have been well documented to resolve with decompression [37], which may result in the loss of the characteristic histologic features that revert to a lining more like the mucosa of the oral cavity. It has also been shown that most OKCs produce PTCH exon 1b, but expression may be lost by decompression [38].
Furthermore, orthokeratinized odontogenic cysts and dentigerous cysts have been shown to contain PTCH mutations [38]. Cutaneous cysts in syndromic as well as non-syndromic patients have been shown to be histologically identical to OKCs but they are classified as cysts, not neoplasms [39, 40]. It is clear therefore, that this debate is not resolved and the question remains, are these latter cysts neoplasms also? And what about the OKCs that do not show mutations, are they neoplastic? And if they are not, how do we tell histologically which are cysts and which are neoplasms? One criticism of the current WHO text is the elimination of the category of odontogenic cysts. This leaves it unclear whether the authors recommended using KCOT for all OKCs or only those that show the mutation. We may reach a point where neoplasms can be defined at the molecular level and we are open to redefining the concept of neoplasia, but we are not there yet and we believe reclassifying OKCs as neoplastic was premature and a solution to reclassifying them needs to be found. Therefore we recommend that we revert to the previous terminology of odontogenic keratocyst.
Calcifying Odontogenic Cyst (COC)/Calcifying Cystic Odontogenic Tumor (CCOT), Dentinogenic Ghost Cell Tumor (DGCT), Ghost Cell Odontogenic Carcinoma (GCOC)
In 2005 the WHO authors also chose to classify all ghost cell lesions as neoplasms, with little evidence besides the fact that ghost cell lesions occupy a spectrum from completely cystic to solid growths of cells. We do not take exception to the concept that the solid lesions are neoplasms, but there appears no evidence that the purely cystic lesions are neoplastic. In 1981, Praetorius et al. [41] were one of the first to suggest that ghost cell lesions comprise a spectrum between true (developmental) cysts and solid neoplasms. They described three basic variants of the cystic lesions;
A cyst with a moderate amount of mural proliferation of epithelium with limited amounts of dentinoid.
A cyst in association with an odontoma
A cyst with extensive “ameloblastoma-like” proliferation in the wall or lumen of the cyst
For the solid, neoplastic variant, they suggested the term dentinogenic ghost cell tumor (DGCT). With very little evidence or justification, the WHO subsequently classified all ghost cell lesions as neoplasms and suggested calcifying cystic odontogenic tumor (CCOT) for the cystic lesions and dentinogenic ghost cell tumor for the solid variant. In 2008, an international collaborative group [42] reviewed the WHO classification of ghost cell neoplasms and suggested that further work was needed to define more precisely their biologic behavior. They divided their tumors into CCOT, DGCT and ghost cell odontogenic carcinoma (GCOC). They further subdivided the CCOT in four distinct types;
Type 1: simple cystic
Type 2: CCOT associated with an odontoma
Type 3: with ameloblastomatous-like proliferation
Type 4: associated with other benign odontogenic tumors other than odontoma.
Interestingly, 87 % of their cases were classified as Type 1 or 2, lesions for which there is minimal if any evidence of neoplastic potential, and lesions that are biologically nonaggressive with a recurrence rate of around 5 % [42]. Most authorities recognize a variant of CCOT with “ameloblastomatous-like proliferation” in its wall or lumen and also recognize ameloblastic COC or ameloblastoma ex COC. The distinction between true ameloblastoma and ameloblastoma-like is not objectively defined and none of the ameloblastic COCs reported to date have recurred as ameloblastoma [42]. The entire spectrum of ghost cell lesions needs to be revisited with the great majority of lesions in this category returned to the nonaggressive cystic classification and the terminology simplified.
Glandular Odontogenic cyst (GOC)/Central Mucoepidermoid Carcinoma (CMEC)
GOC and CMEC are both relevant to a classification of odontogenic cysts and tumors and should be included. GOCs are well documented as odontogenic cysts and while there is some evidence for an origin of CMEC from intraosseous salivary glands, there is also significant evidence demonstrating origin from odontogenic cysts. It is likely therefore that at least some CMECs are of odontogenic origin and consideration needs to be given to their place in the overall classification of odontogenic cysts and tumors. However, recent molecular studies have suggested that GOC and CMEC are distinct and unrelated entities [43].
Ameloblastoma/Unicystic Ameloblastoma
There is minimal to no debate about the appropriate classification of ameloblastoma as a neoplasm, regardless of whether it is unicystic, multicystic or solid. In the original description of unicystic ameloblastoma, the authors suggested three groups;
a simple cyst in which the lumen is lined by variable epithelium but with areas showing ameloblastic differentiation limited to the luminal epithelium
a cyst with intraluminal proliferation of ameloblastoma, often in a plexiform pattern, but without mural involvement
a cyst with mural infiltration of ameloblastoma into the cyst wall but not completely through the entire cyst wall
The 2005 WHO classification concurs with this grouping. However, as pointed out earlier, other authors have recommend eliminating lesions with mural invasion as a “unicystic” variant and there is limited evidence that mural invasion does result in a more biologically aggressive neoplasm with a higher recurrence rate following conservative treatment. Additional verification is needed but we recommend that mural invasion should preclude a classification of “unicystic.” We would therefore recommend that the category of unicystic ameloblastoma only contain two variants; the simple neoplastic cyst and the luminal variant. Lesions with mural infiltration should be regarded as conventional ameloblastoma until further studies clarify this dilemma.
The WHO chapter on “odontogenic tumours” concludes with a discussion of a variety of allied conditions that are not neoplastic or odontogenic. We concur with this approach and prefer the original inclusion of odontogenic cysts with the tumors, and further recommend consideration of expanding the classification to include “Odontogenic cysts, neoplasms, and allied condition” as this would significantly improve the utility of the classification by including lesions that need to be considered in the differential diagnosis of odontogenic tumors or resemble them histologically and provide a more complete overview of lesions that affect the jaws predominantly or exclusively.
Summary and Conclusions
Lastly, since the latest WHO edition, much has been published on odontogenic tumors. The new edition will obviously address new entities and genetic/molecular alterations in odontogenic tumors, and it was not the intent of this review to provide updates of all of the tumors but tumors such as primordial odontogenic tumor [44] and odontogenic carcinoma with dentinoid (in this special edition) deserve consideration. Lastly, as we approach the next iteration of the classification of odontogenic tumors, we would emphasize that classifications should be simple, reproducible, have clinical utility, and be easy for non-specialist pathologists to understand and use. We present a suggested classification of odontogenic cysts, tumors, and allied conditions (Table 1).
Table 1.
Classification of odontogenic cysts, tumours, and allied conditions
| A classification of odontogenic cysts |
| Cysts of inflammatory origin |
| Radicular cysts |
| Inflammatory collateral (paradental) cysts |
| Cysts of developmental (or unknown) origin |
| Dentigerous cyst |
| Odontogenic keratocyst |
| Lateral periodontal cysts |
| Gingival cysts |
| Glandular odontogenic cyst |
| Calcifying odontogenic cyst |
| Orthokeratinised odontogenic cyst |
| A classification of odontogenic tumours and allied lesions |
| Malignant tumours |
| Odontogenic carcinomas |
| Metastasizing (malignant) ameloblastoma |
| Ameloblastic carcinoma |
| Primary intraosseous carcinoma NOS |
| Clear cell odontogenic carcinoma |
| Ghost cell odontogenic carcinoma |
| Odontogenic sarcomas |
| Ameloblastic fibrosarcoma |
| Odontogenic sarcomas, NOS |
| Benign tumours |
| Epithelial odontogenic tumours |
| Ameloblastoma |
| Ameloblastoma, unicystic type |
| Squamous odontogenic tumour |
| Calcifying epithelial odontogenic tumour |
| Adenomatoid odontogenic tumor |
| Mixed odontogenic tumors (epithelial and mesenchymal) |
| Ameloblastic fibroma |
| Odontoma and developing odontoma |
| Mesenchymal odontogenic tumours (with limited or without odontogenic epithelium) |
| Odontogenic fibroma |
| Granular cell odontogenic tumour |
| Odontogenic myxoma/myxofibroma |
| Cementoblastoma |
| Cemento-ossifying fibroma |
| Peripheral odontogenic tumours |
| Allied lesions |
| Psammomatoid ossifying fibroma |
| Trabecular ossifying fibroma |
| Fibrous dysplasia |
| Cemento-osseous dysplasias |
| Central giant cell granuloma |
| Cherubism |
| Aneurysmal bone cyst |
| Simple bone cyst |
| Melanotic neuroectodermal tumour of infancy |
In writing this opinion piece, the authors have often struggled to agree on nomenclature, what constitutes an entity, and at what point the evidence is sufficient to make a change to the classification. Indeed, there are several contentious areas where we have not been able to agree and a majority view has been adopted. These difficulties between only four authors experienced with odontogenic tumors reflect the challenge of this process.
References
- 1.Barnes L, Eveson JW, Reichart P, Sidransky D. World Health Organization classification of tumours: pathology and genetics, head and neck tumours. Lyon: IARC Press; 2005. [Google Scholar]
- 2.Dorlands Illustrated Medical Dictionary. 32rd ed. Saunders: Elsevier; 2012, p. 1985.
- 3.Stedmans Medical Dictionary. 27th ed. Philadelphia: Lippincott Williams Wilkins; 2000. p. 1894.
- 4.Goldenberg D, Sciubba J, Koch W, Tufano RP. Malignant odontogenic tumors: a 22-year experience. Laryngoscope. 2004;114:1770–1774. doi: 10.1097/00005537-200410000-00018. [DOI] [PubMed] [Google Scholar]
- 5.Koutlas IG, Allen CM, Warnock GR, Manivel JC. Sclerosing odontogenic carcinoma. A previously unreported variant of a locally aggressive odontogenic neoplasm eithout apparent metastatic potential. Am J Surg Pathol. 2008;32:1613–1619. doi: 10.1097/PAS.0b013e31817a8a58. [DOI] [PubMed] [Google Scholar]
- 6.Matsumoto Y, MIzoue K, Seto K. Atypical plexiform ameloblastoma with dentinoid: adenoid ameloblastoma with dentinoid. J Oral Pathol Med. 2001;30:251–254. doi: 10.1034/j.1600-0714.2001.300410.x. [DOI] [PubMed] [Google Scholar]
- 7.Evans BL, Carr RF, Phillipe LJ. Adenoid ameloblastoma with dentinoid: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;98:583–588. doi: 10.1016/j.tripleo.2004.02.077. [DOI] [PubMed] [Google Scholar]
- 8.Allen CM, Neville BW, Hammond HL. Adenomatoid dentinoma. Report of four cases of an unusual odontogenic lesion. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;86:313–317. doi: 10.1016/S1079-2104(98)90178-0. [DOI] [PubMed] [Google Scholar]
- 9.Fletcher CD, Hogendoorn P, Mertens F, Bridge J. WHO Classification of Tumours of Soft Tissue and Bone. 4. Lyon, France: IARC Press; 2013. [Google Scholar]
- 10.Chikosi R, Segall N, Augusto P, Freedman P. Odontogenic carcinosarcoma: case, report and literature review. J Oral Maxillofac Surg. 2011;69:1501–1507. doi: 10.1016/j.joms.2010.05.071. [DOI] [PubMed] [Google Scholar]
- 11.DeLair D, Bejarano PA, Peleg M, El-Mofty SK. Ameloblastic carcinosarcoma arising in ameloblastic fibroma of the mandible: a case report and review of literature. Oral Surg Oral Med Oral Radiol Endod. 2007;103:516–520. doi: 10.1016/j.tripleo.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 12.Yamanishi T, Ando S, Aikawa T, Kishino M, Nakano Y, Sasai K, Isomura Tanaka E, Tsuji T, Koizumi H, Iida S, Kogo M. A case of extragingival peripheral ameloblastoma in the buccal mucosa. J Oral Pathol Med. 2007;36:184–186. doi: 10.1111/j.1600-0714.2006.00480.x. [DOI] [PubMed] [Google Scholar]
- 13.Schafer DR, Thompson LDR, Smith BC, Wenig BM. Primary ameloblastoma of the sinonasal tract: a clinicopathologic study of 24 cases. Cancer. 1998;82:667–674. doi: 10.1002/(SICI)1097-0142(19980215)82:4<667::AID-CNCR8>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 14.Tsaknis PJ, Nelson JF. The maxillary ameloblastoma; an analysis of 24 cases. J Oral Surg. 1980;38:336–342. [PubMed] [Google Scholar]
- 15.Robinson L, Martinez MG. Unicystic ameloblastoma. A prognostically distinct entity. Cancer. 1977;40:2278–2285. doi: 10.1002/1097-0142(197711)40:5<2278::AID-CNCR2820400539>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 16.Gardner DG. A pathologists approach to the treatment of ameloblastoma. J Oral Maxillofac Surg. 1984;42:161–166. doi: 10.1016/S0278-2391(84)80026-9. [DOI] [PubMed] [Google Scholar]
- 17.Ackerman GL, Altini M, Shear M. The unicystic ameloblastoma: a clinicopathologic study of 57 cases. J Oral Pathol. 1988;17:541–546. doi: 10.1111/j.1600-0714.1988.tb01331.x. [DOI] [PubMed] [Google Scholar]
- 18.Philipsen HP, Reichart PA. Unicystic ameloblastoma. A review of 193 cases from the literature. Oral Oncol. 1998;34:317–325. doi: 10.1016/S1368-8375(98)00012-8. [DOI] [PubMed] [Google Scholar]
- 19.Li T-J, Wu Y-T, Yu S-F, Yu G-Y. Unicystic ameloblastoma. Clinicopathologic study of 33 Chinese patients. Am J Surg Pathol. 2000;24:1385–1392. doi: 10.1097/00000478-200010000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Chau E, Daley T, Darling MR, Hamilton D. The expression and immunohistochemical localization of periostin in odontogenic tumors of mixed epithelial/mesenchymal origin. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116:214–220. doi: 10.1016/j.oooo.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 21.Buchner A, Vered M. Ameloblastic fibroma: a stage in the development of a hamartomatous odontoma or a true neoplasm? Critical analysis of 162 previously reported cases plus 10 new cases. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116:598–606. doi: 10.1016/j.oooo.2013.06.039. [DOI] [PubMed] [Google Scholar]
- 22.Slootweg PJ. An analysis of the interrelationship of the mixed odontogenic tumors-ameloblastic fibroma, ameloblastic fibro-odontoma, and the odontomas. Oral Surg Oral Med Oral Pathol. 1981;51:266–277. doi: 10.1016/0030-4220(81)90056-6. [DOI] [PubMed] [Google Scholar]
- 23.Philipsen HP, Reichart PA, Praetorius F. Mixed odontogenic tumours and odontomas. Considerations on iInterrelationship. Review of the literature and presentation of 134 new cases of odontomas. Oral Oncol. 1997;33:86–99. doi: 10.1016/S0964-1955(96)00067-X. [DOI] [PubMed] [Google Scholar]
- 24.Mosqueda-Taylor A, Carlos-Bregni R, Ramírez-Amador V, et al. Odontoameloblastoma: clinico-pathologic study of three cases and critical review of the literature. Oral Oncol. 2002;38:800–805. doi: 10.1016/S1368-8375(02)00046-5. [DOI] [PubMed] [Google Scholar]
- 25.Roguljic H, Matthews BG, Yang W, Cvija H, Mina M, Kalajzic I. In vivo identification of periodontal progenitor cells. J Dent Res. 2013;92:709–715. doi: 10.1177/0022034513493434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gu XM, Zhao HS, Sun LS, Li TJ. PTCH mutations in sporadic and Gorlin-syndrome-related odontogenic keratocysts. J Dent Res. 2006;85:859–863. doi: 10.1177/154405910608500916. [DOI] [PubMed] [Google Scholar]
- 27.Li TJ, Yuan JW, Gu XM, Zhao HS. PTCH germline mutations in Chinese nevoid basal cell carcinoma syndrome patients. Oral Dis. 2008;25:174–179. doi: 10.1111/j.1601-0825.2007.01369.x. [DOI] [PubMed] [Google Scholar]
- 28.Sun LS, Li XF, Li TJ. PTCH1 and SMO gene alterations in keratocystic odontogenic tumors. J Dent Res. 2008;87:575–579. doi: 10.1177/154405910808700616. [DOI] [PubMed] [Google Scholar]
- 29.Pan S, Li TJ. Mechanisms of inactivation of PTCH1 gene in keratocystic odontogenic tumors: modifivation of the two-hit hypothesis. Clin Cancer Res. 2010;16:442–450. doi: 10.1158/1078-0432.CCR-09-2574. [DOI] [PubMed] [Google Scholar]
- 30.Barreto DC, Gomez RS, Bale AE, Boson WL, De Marco L. PTCH gene mutations in odontogenic keratocysts J Dent Res. 2000;79:1418–1422. doi: 10.1177/00220345000790061101. [DOI] [PubMed] [Google Scholar]
- 31.Ohki K, Kumamoto H, Ichinohasama R, Sato T, et al. PTC gene mutations and expression of SHH, PTC, SMO, and GLI-1 in odontogenic keratocysts. Int J Oral Maxillofac Surg. 2009;33:584–592. doi: 10.1016/j.ijom.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 32.Song YL, Zhang WF, Peng B, Wang CN, et al. Germline mutations of the pTCH ggene in families with odontogenic keratocysts and nevoid basal cell carcinoma syndrome. Tumor Biol. 2006;27:175–180. doi: 10.1159/000093054. [DOI] [PubMed] [Google Scholar]
- 33.Agaram NP, Collins B, Barnes L, Lomago D, et al. Molecular analysis to demonstrate that odontogenic keratocysts are neoplastic. Arch Pathol Lab Med. 2004;128:313–317. doi: 10.5858/2004-128-313-MATDTO. [DOI] [PubMed] [Google Scholar]
- 34.Henley J, Summerlin D-J, Tomich C, Zhang S, Cheng L. Molecular evidence supporting the neoplastic nature of odontogenic keratocyst: a laser capture microdissection study of 15 cases. Histopath. 2005;47:582–586. doi: 10.1111/j.1365-2559.2005.02267.x. [DOI] [PubMed] [Google Scholar]
- 35.Malcić A, Jukić S, Anić I, Pavelić B, et al. Alterations of FHIT and p53 genes in keratocystic odontogenic tumours, dentigerous cyst and radicular cyst. J Oral Pathol Med. 2008;37:294–301. doi: 10.1111/j.1600-0714.2007.00622.x. [DOI] [PubMed] [Google Scholar]
- 36.Gomes CC, Diniz MG, Gomez RS. Review of the pathogenesis of the odontogenic keratocyst. Oral Oncol. 2009;45:1011–1014. doi: 10.1016/j.oraloncology.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 37.Pogrel MA, Jordan RC. Marsupialization as a definitive treatment for the odontogenic keratocyst. J Oral Maxillofac Surg. 2004;62:651–655. doi: 10.1016/j.joms.2003.08.029. [DOI] [PubMed] [Google Scholar]
- 38.Diniz MG, Galvao CF, Macedo PS, Gomes CC, Gomez RS. Evidence of loss of heterozygosity of the PTCH gene in orthokeratinized odontogenic cyst. J Oral Pathol Med. 2011;40:277–280. doi: 10.1111/j.1600-0714.2010.00977.x. [DOI] [PubMed] [Google Scholar]
- 39.Cassarino DS, Linden KG, Barr RJ. Cutaneous keratocyst arising independently of the nevoid basal cell carincoma syndrome. Am J Dermatopathol. 2005;27:177–178. doi: 10.1097/01.dad.0000154434.61000.31. [DOI] [PubMed] [Google Scholar]
- 40.Fernandez-Flores A. Cutaneous keratocyst: a renaming as isthmic-anagenic cyst protocol. Am J Dermatopathol. 2008;30:87–88. doi: 10.1097/DAD.0b013e31815e8b2f. [DOI] [PubMed] [Google Scholar]
- 41.Pretorius F, Hjorting-Hansen E, Gorlin RJ, Vickers RA. Calcifying odontogenic cyst: range, variations and neoplastic potential. Acta Odontol Scand. 1981;39:221–240. doi: 10.3109/00016358109162284. [DOI] [PubMed] [Google Scholar]
- 42.Ledesma-Montes C, Gorlin RJ, Shear M, Praetorius F, et al. International collaborative study on ghost cell odontogenic tumours: calcifying cystic odontogenic tumour, dentinogenic ghost cell tumour and ghost cell odontogenic carcinoma. J Oral Pathol Med. 2008;37:302–308. doi: 10.1111/j.1600-0714.2007.00623.x. [DOI] [PubMed] [Google Scholar]
- 43.Bishop JA, Yonescu R, Batista D, Warnock GR, Westra WH. Glandular odontogenic cysts (GOCs) lack MAML2 rearrangements: a finding to discredit the putative nature of GOC as a precursor to central mucoepidermoid carcinoma. Head Neck Pathol. 2014 doi: 10.1007/s12105-014-0534-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mosqueda-Taylor A, Pires FR, Aguirre-Urızar JM, et al. Primordial odontogenic tumour: clinicopathological analysis of six cases of a previously undescribed entity. Histopathology. 2014 doi: 10.1111/his.12451. [DOI] [PubMed] [Google Scholar]


