Abstract
Osteonecrosis of the jaw to a certain extent has been with us for many years. But recently the advent of various medications such as bisphosphonates, VEGF inhibitors, tyrosine kinase inhibitors and humanized antibodies to osteoclastic action have resulted in thousands of cases. While the bisphosphonates continue to be the most common medication associated with osteochemonecrosis antibodies such as denosumab which irreversibly act on osteoclastic action are also being reported. This narrative review will serve as an update with a focus on some of the histopathologic features discussed and reviewed. Perhaps even more uncommonly seen in past reports a discussion of features possibly observed while grossing specimens will be discussed. At the end of this report is hoped that the pathologist will have a better understanding of the historical features, clinical settings, gross examination features as well as histopathologic features associated with osteochemonecrosis.
Keywords: Osteonecrosis of the jaw, Bisphosphonates, Denosumab, Tyrosine kinase inhibitors, Osteochemonecrosis, VEGF inhibitors
Introduction
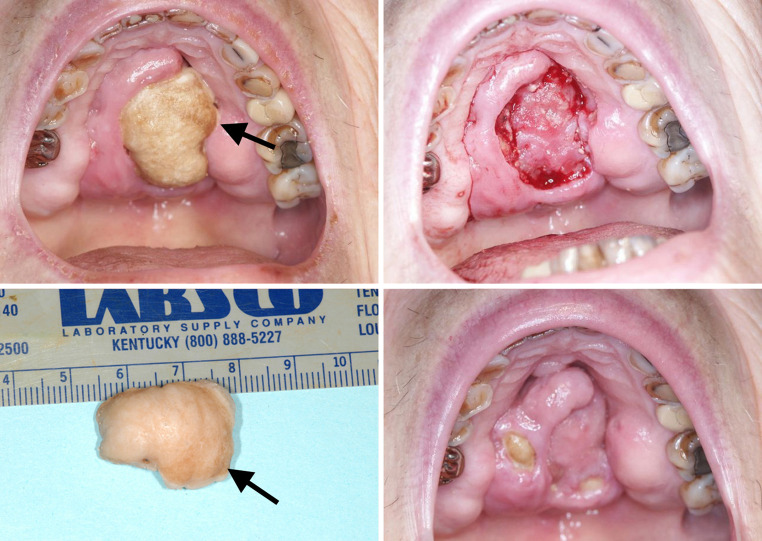
Osteonecrosis in the simplest etymology of the word is: osteo = bone and necrosis = becoming dead. Dorland’s dictionary defines osteonecrosis as “necrosis of bone due to obstruction of its blood supply” [1]. The process of avascular necrosis of the hip follows that definition. But not all osteonecrotic events begin with avascularity. Obstruction of blood supply may be a secondary or tertiary effect of the original cause of osteonecrosis. In osteonecrosis of the jaws (ONJ), traumatic events, bacterial infections, fungal infections, viral infections, immunodeficiency, radiation, and genetic diseases such as osteopetrosis, have all been implicated or shown as causative. The above list is not meant to be exhaustive of all causes of ONJ [2]. This paper will focus primarily on osteonecrosis of the jaw secondary to, or associated with medications (Fig. 1).
Fig. 1.
Bisphosphonate osteochemonecrosis in a low bone density patient. Presentation on palate with underlying granulation tissue, sequestrectomy specimen and healing at about 6 weeks post sequestrectomy. New areas of exposed bone are also noted. Arrows indicate the slight chlorhexidine staining
The current literature continues to expand with acceleration of numbers of articles on the subject each year. A simple computer assisted search on PubMed (US National Library of Medicine Bethesda, MD http://www.ncbi.nlm.nih.gov/pubmed) reveals approximately 2,000 articles as of September 2014. The advent of a series of patients with osteonecrosis associated with bisphosphonate medications was first noted in 2003 in a letter by Marx [3] though some scattered earlier studies suggesting a jaw risk do exist [4–6]. Ruggerio and Lugassy with their colleagues followed with better defined patient studies [7, 8]. In 2006, a narrative review by Woo et al. [9] continued to establish reported trends in the demographics of the growing problem associated with bisphosphonates and jaw necrosis.
Nomenclature
Unfortunately, the nomenclature of the process being discussed has become a confusing amalgam and polyglot of acronyms and words. The simple term osteonecrosis of the jaw (ONJ) without modification, may be the most common term. However, that term is also associated with such things as radiation, trauma, and infection. Terms such as: bisphosphonate osteonecrosis (BON), bisphosphonate associated osteonecrosis of the jaws (BONJ), bisphosphonate related osteonecrosis of the jaws (BRONJ), antiresorptive osteonecrosis of the jaw (ARONJ) and medication related osteonecrosis of the jaw (MRONJ) have all had proponents and all continue to be utilized [10, 11].
However, Migliorati [12–15] first proposed a designation of the disease as osteochemonecrosis. This designation continues to have some cachet due to the increasing numbers of medications associated with the disease, as well as its similarity to the well-recognized designation of osteoradionecrosis. However, none of the terms are totally satisfying, with detractors of the term osteochemonecrosis stating that it implies only oncologic chemotherapy. Medication related osteonecrosis of the jaw (MRONJ) is the latest term proposed by the American Association of Oral and Maxillofacial Surgeons. In their position paper, they emphasize the various medications which have been associated with exposed bone of greater than 8 weeks and not related to radiation therapy [11]. However, in this paper, osteochemonecrosis (OCN) will be preferred regardless of the drug implicated; e.g. bisphosphonates, VEGF inhibitors, tyrosine kinase inhibitors, RANK L antibodies, cathepsin K inhibitors, or immune modulators such as sirolimus.
History and Medication Review of Osteochemonecrosis
There are a number of medication classes associated with OCN. In Table 1, generic names and product names related to patient groups are shown. Protein derived medications are being developed constantly and it is our responsibility to be aware of new drug developments. It was the early days of nitrogen containing bisphosphonates that brought the attention to this discussion of OCN. Nitrogen containing bisphosphonates also continue to be the overwhelmingly most common medication type seen in cases of OCN. Marx is generally given credit for his 2003 letter to the editor [3] to first described bisphosphonate OCN though it was the article by Ruggiero et al. [7] that truly gave substance and attention to the association of jaw necrosis with bisphosphonate use. Ruggiero et al. correctly outlined some of the general demographics which continue to apply today. In general, about 90 % of BRONJ cases are secondary to nitrogen containing bisphosphonates utilized for oncologic reasons and 10 % are secondary to use for low bone density disease. About two-thirds of the cases involve the mandible, and multifocal/multi-arch involvement is relatively common. About three quarters of the cases are in women due to breast cancer as well as women being treated for low bone density. However, some of the specifics have changed since the first reports. One example is the fact that zoledronic acid now has a larger market share than pamidronate and accounts for more overall cases. Another example relates to expanded use of bisphosphonates in addition to malignant disease processes other than multiple myeloma, prostate, and breast cancer. In addition, there are also newer modulators of osteoclastic action that act by different mechanisms.
Table 1.
Medications with osteochemonecrosis of the jaw(s) implications
| Generic | Brand examples | Notes |
|---|---|---|
| Low bone density patients and other non-oncologic uses [10, 11] | ||
| Bisphosphonates | ||
| Clodronate (not available in U.S.) | Bonefos® | Non-nitrogenated |
| Etidronate | Didronel® | Non-nitrogenated |
| Tiludronate | Skelid® | Non-nitrogenated |
| Risedronate | Actonel® | Nitrogenated |
| Atelvia® | ||
| Ibandronate | Boniva®/Bonviva® | Nitrogenated |
| Alendronate | Fosamax® and generic | Nitrogenated |
| Alendronate w cholecalciferol | Fosamax Plus D® | Nitrogenated |
| Ibandronate | Boniva® IV | Nitrogenated |
| Zoledronic acid | Reclast® IV | Nitrogenated |
| Aclasta® IV | ||
| Non-bisphosphonates | ||
| Denosumab | Prolia® | Humanized antibody |
| Odanacatib | Awaiting FDA action | Cathepsin K inhibitor |
|
Oncologic patients Increasing risk with MG dose, number of doses, length of treatment and half-life [10, 11] | ||
| Bisphosphonates | ||
| Clodronate | Bonefos® IV | Non-nitrogenated |
| Ibandronate | Boniva® IV | Nitrogenated |
| Pamidronate | Aredia® IV | Nitrogenated |
| Zoledronic acid | Zometa® IV | Nitrogenated |
| Osteoclast targeted antibody | ||
| Denosumab | XGEVA® SubQ | Humanized antibody |
| Other antibodies/inhibitors | ||
| Sunitinib | Sutent® | Tyrosine kinase inhibitor |
| Sorafenib | Nexavar® | Tyrosine kinase inhibitor |
| Bevacizumab | Avastin® | VEGF inhibitor |
The nitrogenated bisphosphonates, in general, work within the osteoclast only after being absorbed from the hydroxyapatite crystal. The osteoclast is then deactivated thru the isoprenoid pathway intracellularly. The non-nitrogen containing bisphosphonates act differently, are not as potent as the nitrogen containing bisphosphonates, and do not bind to the hydroxyapatite crystal as avidly. The long half-life, especially of the nitrogen containing bisphosphonates, is due to their accumulation within the hydroxyapatite crystal lattice of the bone [16, 17]. The overwhelmingly dominant process to remove the nitrogen containing bisphosphonate from that lattice is through osteoclastic action, but that osteoclastic action necessarily results in intracellular accumulation of the bisphosphonate, which then blocks the osteoclast from further bone remodeling. Thus the overall skeletal turnover is lengthened dramatically and the action of the nitrogen containing bisphosphonate may continue for many years with a half-life of some being estimated at 10 years or more [18–20]. New nitrogen containing bisphosphonates such as neridronate [21] are being developed but cases of osteonecrosis have yet to be reported.
Denosumab is a new humanized antibody with a similar reported frequency/incidence of OCN to nitrogen containing bisphosphonates. Denosumab is a humanized antibody affecting the RANK L system. Denosumab results in a non-reversible deactivation of osteoclasts which persists until osteoclastic cell death. New osteoclasts are formed on a daily basis and thus if an osteoclast is formed after one administration of the drug and before the next administration that osteoclast will be fully functional. The overall action is therefore time limited and related to osteoclast turnover and dosage pattern (though there is some slight reincorporation of some of the antibody upon cell death). In general, the effect of the drug is 2–5 months duration after administration, with a half-life of about 25.4 days [22–30].
Tyrosine kinase inhibitors as well as VEGF inhibitors are FDA approved and currently in use in the United States. The two medications with reported OCN are included in Table 1, though more tyrosine kinase inhibitors are on the market. Tyrosine kinase inhibitors target macrophages which then have an effect on monocytes through colony stimulating factors to inhibit the osteoclast within the osteoclastic developmental feedback loop. VEGF inhibitors work somewhat more directly to inhibit the osteoclasts through their action on monocytes which then are in the osteoclast developmental feedback loop. Further reading on these medications will be left to the reader for exploration [30–40].
Odanacatinib is an inhibitor of cathepsin K. Cathepsin K is a cysteine protease which is instrumental in hydroxyapatite matrix degradation including type I collagen. The current studies on its use center around low bone density patients and FDA approval may occur in the next few years as safety studies continue. A promising feature of the cathepsin K inhibitors is the short and reversible half-life [41, 42].
Medications which act directly on osteoblastic activity seem to have no association with osteochemonecrosis. For instance the parathormone analog teriparatide (Forteo®) has been shown to be helpful during the treatment phase of OCN. Because it also has some benefit in the management of low bone density, it offers the advantage of a therapeutic agent other than a bisphosphonate [43–45]. Its disadvantages are cost and its ability to only be used for a two-year timeframe. Vitamin D also acts directly on the osteoblast and has not been associated with osteochemonecrosis.
A new medication in development is also antibody derived and works as a sclerostin inhibitor. Romosozumab [46] development is still very early in its FDA approval process. But it is an intriguing possible management/therapeutic alternative for osteochemonecrosis patients. This is due to its action on the osteoblastic side of bone turnover. But because of its action within the Wnt pathway there are some safety concerns, so studies will undoubtedly continue.
Table 1 is provided so that if a disease is noted in the history, and medications are not up-to-date, the pathologist may check the electronic health record or inquire about medications history. Distant medication use is probably only important for the nitrogen containing bisphosphonates due to their very long half-life. Of additional interest may be the study or review of bisphosphonate use in children with osteogenesis imperfecta. At the current time no cases of osteonecrosis of the jaw in children receiving bisphosphonates have been reported, even after multiple tooth extractions [47–49].
Clinical Diagnosis of Osteochemonecrosis
For this discussion the staging proposed by the American Association of Oral and Maxillofacial Surgeons (AAOMS) will be utilized with comments and minor modifications [11] (Table 2). This staging system requires that patients are taking an antiresorption drug, have no previous H&N radiation therapy or metastatic disease to their jaws, and have signs and/or symptoms of at least 8 weeks duration. For radiographic aspects, due to the long time periods necessary for bone to demonstrate evidence of significant sclerotic or osteolytic change, it is assumed that the duration is of at least 8 weeks. Some other staging systems have been proposed but at this point in time the AAOMS paper is the most widely utilized [11, 50].
Table 2.
Diagnosis and staging of osteochemonecrosis
| Stage 0 | Patients with no clinical evidence of necrotic bone, but present with non-specific symptoms or clinical and radiographic findings |
| Note: In Stage 0 patient must have: | |
| 1. Current or previous treatment with antiresorptive or antiangiogenic agents; | |
| 2. No history of radiation therapy to the jaws | |
| 3. No obvious metastatic disease to the jaws; and | |
| 4. Signs or symptoms of greater than 8 weeks duration with confirmation that signs and/or symptoms that are not of standard odontogenic origin | |
| Stage 1 | Exposed and necrotic bone, or; |
| Fistula(e) that probes to bone, in patients who are asymptomatic and if purulence is evident, it is only pustular and not extensive enough to warrant Stage 3 classification (see Fig. 3) | |
| Stage 2 | Exposed and necrotic bone, or; |
| Fistula(e) that probes to bone, with evidence of infection. Infection is defined as purulence that is more than merely pustular | |
| These patients are typically symptomatic | |
| Stage 3 | Exposed and necrotic bone, or fistulae that probe to bone, with evidence of infection, and one or more of the following: |
| Exposed necrotic bone extending beyond the region of alveolar bone, e.g. inferior border and ramus in the mandible, maxillary sinus and zygoma in the maxilla | |
| Pathologic fracture | |
| Extra-oral fistula | |
| Oral antral/oral nasal communication | |
| Osteolysis extending to the inferior border of the mandible or sinus floor | |
| Note: In Stages 1–3 patient must have: | |
| 1. Current or previous treatment with antiresorptive or antiangiogenic agents; | |
| 2. Intraoral exposed bone or bone that can be probed through a significant intraoral or extraoral fistula(e) in the maxillofacial region that has/have persisted for more than 8 weeks; and | |
| 3. No history of radiation therapy to the jaws; and/or | |
| 4. No obvious metastatic disease to the jaws | |
| Minor editorial changes and notes have been modified by the author. For full explanations and context see original AAOMS article (Ruggiero et al. [11] #1319) | |
One of the deficiencies of the AAOMS system is that it is very difficult to know how to stage patients who have a history of a medication associated with or causing ONJ and also a history of radiotherapy of the jaws and/or obvious metastatic disease of the jaws. Carlson reports several cases that are combined cases of bisphosphonate osteonecrosis the jaw and metastatic disease of the jaw. Though extreme care should be given to diagnose two or more disease processes, the intent of the AAOMS definition should not be interpreted as tautologically eliminating the possibility of diagnosing osteochemonecrosis along with other entities.
Patient Management
Treatment strategies continue to stress prevention. When sequestra present and/or surgical management is necessary, debridement is appropriate and tissue should be forwarded to the pathology laboratory. Sequestra as well as resection specimens are particularly likely to be received in stage 2 or 3 disease [51–53]. It is recommended to surgeons that all sequestra that is removed be submitted for pathologic confirmation. This is particularly important for specimens over 6 mm, because larger sequestra are more frequently associated with malignant disease [54].
Gross Table Considerations
Standard grossing protocols apply with appropriate description of both soft tissue as well as hard tissue components. If soft tissue components are large enough to warrant a separate cassette, they should be separated from the hard tissues to avoid demineralization artifact. The most common specimen received will be a sequestrum. The sequestra are noticeably less dense than what would be considered for normal vital bone and have been described grossly as pumice-like. This decreased density may be due to the almost total lack of soft tissue components within the marrow spaces or probable changes in mineralization.
Demineralization is generally quicker in these less dense specimens with frequent checking of the specimen and solution changes advised to minimize demineralization artifact. A single night is often sufficient for demineralization. Formalin with formic acid is preferred in this author’s laboratory for demineralization (e.g. Calfor® Cancer Diagnostics Inc. Durham, North Carolina). A version more suitable for possible future immunohistochemical reactions is also available. However, standard HCl with EDTA solution (e.g. Cal-Ex™ Thermo Fisher Scientific, Waltham, MA) may also be utilized, especially for more dense specimens. However, the tissue must be properly fixed and closely followed.
The sequestrum/specimen should be examined for the presence of granulation tissue and this will indicate the non-exposed basal bone surface(s). The typical formalin fixed tissue indicators for granulation tissue, such as bloody coagulum or soft tissue fibrillations on the surface are helpful. The base may also be recognized by having a different contour/texture from the exposed bone and is usually self-evident upon exam.
The exposed surfaces of bone are usually indicated by a smoother but finally granular appearance. Brown–black chlorhexidine staining (Fig. 1) may also be evident at the gross table and will indicate exposed bone. If received fresh, the surface microbiome is often a creamy-yellowish-tan surface film and seen once, is easily recognized thereafter. However, the bacterial colonization is often easily appreciated even after fixation. After fixation, the microbiome is usually a granular tan color at the grossing table.
If both the base and the exposed bone surface of the sequestrum/resection are identified, proper orientation can be achieved. In Fig. 1, the upper right portion of the image shows the underlying granulation tissue immediately after sequestration. Orientation may be challenging because sequestra can have one or more exposed surfaces and one or more bases. An involucrum will have no exposed bone surface. It is preferred to have the demineralized specimen sampled along the entire base with inking at the gross table appropriate to allow for later microscopic identification. Ideally each section will be embedded so as to observe both the nonexposed as well as exposed bone (Fig. 2).
Fig. 2.
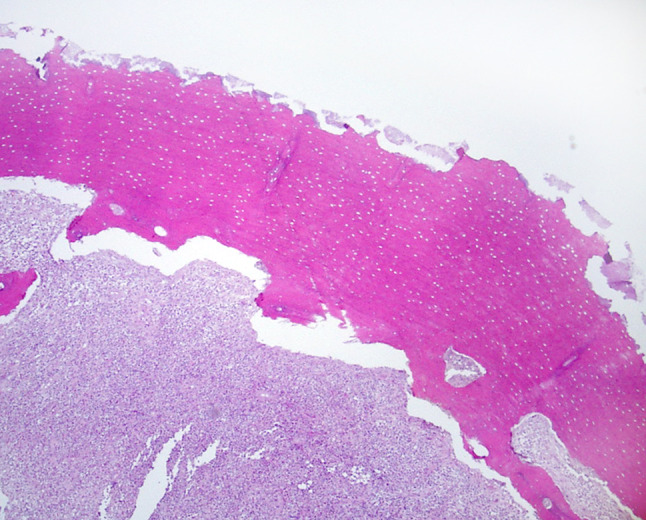
Example of surface bacteria with relatively fine resorptive bone features compared to the underlying nonexposed bone with a coarser resorptive pattern more likely to represent past osteoclastic action
Radiographic Features
Whenever bony samples are obtained, radiographic imaging is often helpful to the pathologist if available. The classic reported anti-resorptive agent changes are sclerotic. Sclerotic changes may be seen in many fashions but a classic feature would be extraction sockets appearing radiopaque [55]. The sequestrum may also be well appreciated on 3-D imaging such as cone beam CTs (Fig. 3). However, a radiolucent appearance is not uncommon, nuclear medicine scans are often unexpectedly hyperintense with admixtures of both radiopaque as well as radiolucent being common. Thus, images are best utilized for assessing features of the individual specimen as well as for appreciating extent of disease and location. Overall interpretation is best left to the radiologist.
Fig. 3.
Radiographic composite image showing axial, sagittal, coronal and “3-D”reconstruction aspects of a sequestrum in the right mandibular alveolus
Microscopic Features
There are some studies related to the microscopic features of osteochemonecrosis, each with strengths and weaknesses [56–65]. Most of the specimens received in a pathology laboratory are the result of sequestrectomies. Because of this, microscopic examinations may show little or no soft tissue component. Microscopically the majority of the bone will be nonvital without evidence of osteocytes within individual lacunae. However, head and neck pathologists are undoubtedly aware of the effect of demineralization on some bone samples. It is not unusual for some osteocytes to “drop out” of individual lacunae but this dropout effect should be splotchy. And if this splotchy pattern is noted it should be considered artifactual. As such, the overall survey of lacunae in vital bone will reveal that many have an osteocyte present. In samples due to osteochemonecrosis, large areas will be nonvital, but basilar areas often display regions of vital lamellar bone. In the areas of the vital lamellar bone, apparently normal osteoclasts may be seen within Howship lacunae.
Some osteoclasts, though normal in size and shape, may be a short distance from the actual bone interface in a detached or so-called “floating” position (Fig. 4). These osteoclasts may also be seen as “giant osteoclasts”. Giant osteoclasts are uncommon but are classically described and should not be overlooked if present. Giant osteoclasts are identified by large numbers of nuclei with cells having more than the expected 3–8 nuclei. Fifteen or more nuclei within the cell are not unusual in giant osteoclasts [66, 67]. It is assumed that any osteoclast that is no longer in close contact with the bone is nonfunctional. In Howship lacunae without an associated osteoclast the lacuna are often relatively shallow. This shallowness may be an indicator of the amount of bone that was able to be resorbed before deactivation of the osteoclast.
Fig. 4.

Additional photomicrograph displaying typical Howship lacunae, detached osteoclasts, neutrophils and granulation tissue
The soft tissue, if seen, will show increased vascularity in the form of granulation tissue with primarily acute inflammatory cells in the form of neutrophils. Neutrophilic debris is often common and some eosinophils may be noted (Fig. 4). Though acute inflammatory cells are most common, this is not meant to imply that some chronic inflammatory cells may also be seen.
Studies on the bacterial microbiome have generally focused on the common presence of actinomycotic colonies (Fig. 5). Because actinomycotic colonies are the only bacterial form easily seen by light microscopic examination, the importance of these colonies is likely overestimated. Figure 5 displays apparent actinomycotic colonies on the soft tissue side with mixed bacterial colonies on the surface. Sedghizadeh has studied these microbial biofilms as well as nanowire properties. His studies undoubtedly show the surface microbiome as being very complex and more complicated than the actinomycotic colonies alone [68, 69].
Fig. 5.
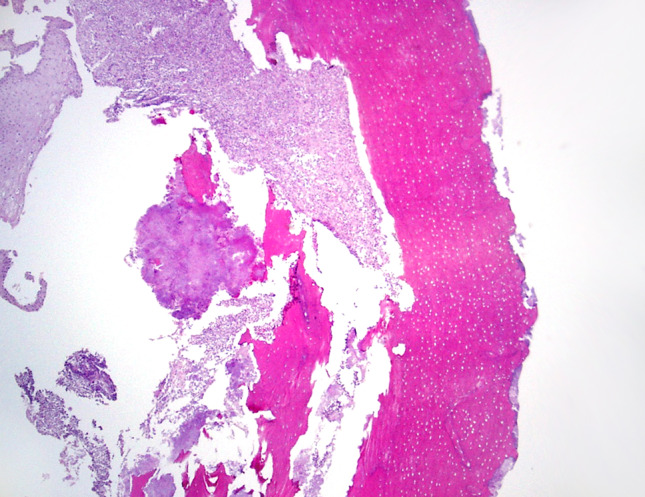
Actinomycotic colonies seen on the nonexposed side near soft tissue, with a thin layer of bacterial colonization on the exposed bone surface
Common along the bone surface at the base is evidence of resorption without evidence of associated osteoclasts. The pattern of resorption is often different than that resulting from osteoclasts and the Howship lacunae though bacteria can often backfill some of these as well. The pattern is more like that of the scalloping and advancing border associated with dental caries, especially that of root caries. It would appear that the possibility of the bacterial microbiome acting as a resorption agent separate from osteoclastic action may be at play in some specimens. This bacterial action is also implied in the Sedghizadeh article though not directly stated as such. I suspect that one component of some sequestration events may be aided through the action of bacterial acidic action and the resulting resorption. Reversal lines, resorption pattern, bacterial colonization, distribution of vital bone elements as compared to nonvital areas and other mentioned features are all helpful in assessing osteochemonecrosis. Though no single or combined microscopic feature is pathognomonic for osteochemonecrosis although significant numbers of detached osteoclasts may be most helpful.
Marx and Turson mention the detached osteoclasts, but overall their paper is more optimistic than practical in definitively diagnosing osteochemonecrosis by microscopic features alone [64]. A rebuttal to the paper is well discussed by Kalmar in his Letter to the Editor [63, 65].
Conclusion
Devitalized bone is often removed from the jaws and submitted to a pathology lab. It is important for practicing pathologists to be aware that bone necrosis from the jaws can result from infection (bacterial, viral, and fungal), trauma, radiation, “spontaneous sequestration” or secondary to a variety of antiresorptive agents.
Medications associated with osteochemonecrosis of the jaw will likely continue to expand with the advent of new medications. These medications may prove effective for low bone density as well as in skeletally related events secondary to primary malignancy or metastatic disease. It is hoped that the shorter half-life of newer medications will prove to be beneficial in at least resolving osteochemonecrosis more quickly than in the current era where nitrogen containing bisphosphonates predominate. However, it must be remembered that these nitrogen bisphosphonates have been extraordinarily helpful in reducing hip fractures by approximately 43 % and extending both quality of life and lifespan in patients with various skeletally related malignancies, and they will undoubtedly continue to be used [70–76].
References
- 1.Dorland’s Illustrated Medical Dictionary. 30th ed. Philadelphia: W.B. Saunders and Co.; 2000.
- 2.Almazrooa SA, Woo SB. Bisphosphonate and nonbisphosphonate-associated osteonecrosis of the jaw: a review. J Am Dent Assoc. 2009;140(7):864–875. doi: 10.14219/jada.archive.2009.0280. [DOI] [PubMed] [Google Scholar]
- 3.Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg. 2003;61(9):1115–1117. doi: 10.1016/S0278-2391(03)00720-1. [DOI] [PubMed] [Google Scholar]
- 4.Handick K. The effect of etidronate on alveolar bone remodeling in dog mandible, University of Indiana; 1998.
- 5.Gotcher JE, Jee WS. The progress of the periodontal syndrome in the rice rat. II. The effects of a diphosphonate on the periodontium. J Periodontal Res. 1981;16(4):441–455. doi: 10.1111/j.1600-0765.1981.tb00995.x. [DOI] [PubMed] [Google Scholar]
- 6.Gotcher JE, Jee WS. The progress of the periodontal syndrome in the rice rat. I. Morphometric and autoradiographic studies. J Periodontal Res. 1981;16(3):275–291. doi: 10.1111/j.1600-0765.1981.tb00976.x. [DOI] [PubMed] [Google Scholar]
- 7.Ruggiero SL, Mehrotra B, Rosenberg TJ, et al. Osteonecrosis of the jaws associated with the use of bisphosphonates: a review of 63 cases. J Oral Maxillofac Surg. 2004;62(5):527–534. doi: 10.1016/j.joms.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Lugassy G, Shaham R, Nemets A, et al. Severe osteomyelitis of the jaw in long-term survivors of multiple myeloma: a new clinical entity. AmJMed. 2004;117(6):440–441. doi: 10.1016/j.amjmed.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 9.Woo SB, Hellstein JW, Kalmar JR. Narrative [corrected] review: bisphosphonates and osteonecrosis of the jaws. Ann Intern Med. 2006;144(10):753–761. doi: 10.7326/0003-4819-144-10-200605160-00009. [DOI] [PubMed] [Google Scholar]
- 10.Hellstein JW, Adler RA, Edwards B, et al. Managing the care of patients receiving antiresorptive therapy for prevention and treatment of osteoporosis: executive summary of recommendations from the American Dental Association Council on Scientific Affairs. J Am Dent Assoc. 2011;142(11):1243–1251. doi: 10.14219/jada.archive.2011.0108. [DOI] [PubMed] [Google Scholar]
- 11.Ruggiero SL, Dodson TB, Fantasia J, et al. American association of oral and maxillofacial surgeons position paper on medication-related osteonecrosis of the jaw—2014 update. J Oral Maxillofac Surg. 2014;72(10):1938–1956. doi: 10.1016/j.joms.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 12.Migliorati CA. Bisphosphanates and oral cavity avascular bone necrosis. J Clin Oncol. 2003;21(22):4253–4254. doi: 10.1200/JCO.2003.99.132. [DOI] [PubMed] [Google Scholar]
- 13.Migliorati CA. Bisphosphonate-associated oral osteonecrosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99(2):135. doi: 10.1016/j.tripleo.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Migliorati CA, Casiglia J, Epstein J, et al. Managing the care of patients with bisphosphonate-associated osteonecrosis: an American Academy of Oral Medicine position paper. J Am Dent Assoc. 2005;136(12):1658–1668. doi: 10.14219/jada.archive.2005.0108. [DOI] [PubMed] [Google Scholar]
- 15.Migliorati CA, Schubert MM, Peterson DE, et al. Bisphosphonate-associated osteonecrosis of mandibular and maxillary bone: an emerging oral complication of supportive cancer therapy. Cancer. 2005;104(1):83–93. doi: 10.1002/cncr.21130. [DOI] [PubMed] [Google Scholar]
- 16.Russell RG, Rogers MJ, Frith JC, et al. The pharmacology of bisphosphonates and new insights into their mechanisms of action. J Bone Miner Res. 1999;14(Suppl 2):53–65. doi: 10.1002/jbmr.5650140212. [DOI] [PubMed] [Google Scholar]
- 17.Fleisch H. Introduction to bisphosphonates. History and functional mechanisms. Orthopade. 2007;36(2):103. doi: 10.1007/s00132-006-1040-9. [DOI] [PubMed] [Google Scholar]
- 18.Wood J, Bonjean K, Ruetz S, et al. Novel antiangiogenic effects of the bisphosphonate compound zoledronic acid. J Pharmacol Exp Ther. 2002;302(3):1055–1061. doi: 10.1124/jpet.102.035295. [DOI] [PubMed] [Google Scholar]
- 19.Santini D, Vespasiani GU, Vincenzi B, et al. The antineoplastic role of bisphosphonates: from basic research to clinical evidence. AnnOncol. 2003;14(10):1468–1476. doi: 10.1093/annonc/mdg401. [DOI] [PubMed] [Google Scholar]
- 20.Vitte C, Fleisch H, Guenther HL. Bisphosphonates induce osteoblasts to secrete an inhibitor of osteoclast-mediated resorption. Endocrinology. 1996;137(6):2324–2333. doi: 10.1210/endo.137.6.8641182. [DOI] [PubMed] [Google Scholar]
- 21.Maines E, Monti E, Doro F, et al. Children and adolescents treated with neridronate for osteogenesis imperfecta show no evidence of any osteonecrosis of the jaw. J Bone Miner Metab. 2012;30(4):434–438. doi: 10.1007/s00774-011-0331-3. [DOI] [PubMed] [Google Scholar]
- 22.Aghaloo TL, Felsenfeld AL, Tetradis S. Osteonecrosis of the jaw in a patient on Denosumab. J Oral Maxillofac Surg. 2010;68(5):959–963. doi: 10.1016/j.joms.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cummings SR, San MJ, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361(8):756–765. doi: 10.1056/NEJMoa0809493. [DOI] [PubMed] [Google Scholar]
- 24.Fusco V, Galassi C, Berruti A, et al. Osteonecrosis of the jaw after zoledronic acid and denosumab treatment. J Clin Oncol. 2011;29(17):e521–e522. doi: 10.1200/JCO.2011.35.1551. [DOI] [PubMed] [Google Scholar]
- 25.Henry DH, Costa L, Goldwasser F, et al. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol. 2011;29(9):1125–1132. doi: 10.1200/JCO.2010.31.3304. [DOI] [PubMed] [Google Scholar]
- 26.Lipton A, Fizazi K, Stopeck AT, et al. Superiority of denosumab to zoledronic acid for prevention of skeletal-related events: a combined analysis of 3 pivotal, randomised, phase 3 trials. Eur J Cancer. 2012;48(16):3082–3092. doi: 10.1016/j.ejca.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 27.Miller PD, Bolognese MA, Lewiecki EM, et al. Effect of denosumab on bone density and turnover in postmenopausal women with low bone mass after long-term continued, discontinued, and restarting of therapy: a randomized blinded phase 2 clinical trial. Bone. 2008;43(2):222–229. doi: 10.1016/j.bone.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 28.Papapoulos S, Chapurlat R, Libanati C, et al. Five years of denosumab exposure in women with postmenopausal osteoporosis: results from the first two years of the FREEDOM extension. J Bone Miner Res. 2012;27(3):694–701. doi: 10.1002/jbmr.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stopeck AT, Lipton A, Body JJ, et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol. 2010;28(35):5132–5139. doi: 10.1200/JCO.2010.29.7101. [DOI] [PubMed] [Google Scholar]
- 30.Troeltzsch M, Woodlock T, Kriegelstein S, et al. Physiology and pharmacology of nonbisphosphonate drugs implicated in osteonecrosis of the jaw. J Can Dent Assoc. 2012;78:c85. [PubMed] [Google Scholar]
- 31.Bozas G, Roy A, Ramasamy V, et al. Osteonecrosis of the jaw after a single bisphosphonate infusion in a patient with metastatic renal cancer treated with sunitinib. Onkologie. 2010;33(6):321–323. doi: 10.1159/000313680. [DOI] [PubMed] [Google Scholar]
- 32.Brunello A, Saia G, Bedogni A, et al. Worsening of osteonecrosis of the jaw during treatment with sunitinib in a patient with metastatic renal cell carcinoma. Bone. 2009;44(1):173–175. doi: 10.1016/j.bone.2008.08.132. [DOI] [PubMed] [Google Scholar]
- 33.Hoefert S, Eufinger H. Sunitinib may raise the risk of bisphosphonate-related osteonecrosis of the jaw: presentation of three cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;110(4):463–469. doi: 10.1016/j.tripleo.2010.04.049. [DOI] [PubMed] [Google Scholar]
- 34.Disel U, Besen AA, Ozyilkan O, et al. A case report of bevacizumab-related osteonecrosis of the jaw: old problem, new culprit. Oral Oncol. 2012;48(2):e2–e3. doi: 10.1016/j.oraloncology.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 35.Estilo CL, Fornier M, Farooki A, et al. Osteonecrosis of the jaw related to bevacizumab. J Clin Oncol. 2008;26(24):4037–4038. doi: 10.1200/JCO.2007.15.5424. [DOI] [PubMed] [Google Scholar]
- 36.Greuter S, Schmid F, Ruhstaller T, et al. Bevacizumab-associated osteonecrosis of the jaw. Ann Oncol. 2008;19(12):2091–2092. doi: 10.1093/annonc/mdn653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guarneri V, Miles D, Robert N, et al. Bevacizumab and osteonecrosis of the jaw: incidence and association with bisphosphonate therapy in three large prospective trials in advanced breast cancer. Breast Cancer Res Treat. 2010;122(1):181–188. doi: 10.1007/s10549-010-0866-3. [DOI] [PubMed] [Google Scholar]
- 38.Hopp RN, Pucci J, Santos-Silva AR, et al. Osteonecrosis after administration of intravitreous bevacizumab. J Oral Maxillofac Surg. 2012;70(3):632–635. doi: 10.1016/j.joms.2011.02.104. [DOI] [PubMed] [Google Scholar]
- 39.Koch FP, Walter C, Hansen T, et al. Osteonecrosis of the jaw related to sunitinib. Oral and maxillofacial surgery. 2011;15(1):63–66. doi: 10.1007/s10006-010-0224-y. [DOI] [PubMed] [Google Scholar]
- 40.Smidt-Hansen T, Folkmar TB, Fode K, et al. Combination of zoledronic Acid and targeted therapy is active but may induce osteonecrosis of the jaw in patients with metastatic renal cell carcinoma. J Oral Maxillofac Surg. 2013;71(9):1532–1540. doi: 10.1016/j.joms.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 41.Nakamura T, Shiraki M, Fukunaga M, et al. Effect of the cathepsin K inhibitor odanacatib administered once weekly on bone mineral density in Japanese patients with osteoporosis–a double-blind, randomized, dose-finding study. Osteoporos Int. 2014;25(1):367–376. doi: 10.1007/s00198-013-2398-2. [DOI] [PubMed] [Google Scholar]
- 42.Zhuo Y, Gauthier JY, Black WC, et al. Inhibition of bone resorption by the cathepsin K inhibitor odanacatib is fully reversible. Bone. 2014;67:269–280. doi: 10.1016/j.bone.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 43.Lee JJ, Cheng SJ, Jeng JH, et al. Successful treatment of advanced bisphosphonate-related osteonecrosis of the mandible with adjunctive teriparatide therapy. Head Neck. 2011;33(9):1366–1371. doi: 10.1002/hed.21380. [DOI] [PubMed] [Google Scholar]
- 44.Subramanian G, Cohen HV, Quek SY. A model for the pathogenesis of bisphosphonate-associated osteonecrosis of the jaw and teriparatide’s potential role in its resolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112(6):744–753. doi: 10.1016/j.tripleo.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 45.Subramanian G, Quek SY. Teriparatide’s role in the management of bisphosphonate-associated osteonecrosis of the jaw. Osteoporos Int. 2012;23(11):2727–2728. doi: 10.1007/s00198-012-1951-8. [DOI] [PubMed] [Google Scholar]
- 46.McClung MR, Grauer A, Boonen S, et al. Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med. 2014;370(5):412–420. doi: 10.1056/NEJMoa1305224. [DOI] [PubMed] [Google Scholar]
- 47.Castillo H, Samson-Fang L, Academy American, for Cerebral P, et al. Effects of bisphosphonates in children with osteogenesis imperfecta: an AACPDM systematic review. Dev Med Child Neurol. 2009;51(1):17–29. doi: 10.1111/j.1469-8749.2008.03222.x. [DOI] [PubMed] [Google Scholar]
- 48.Hennedige AA, Jayasinghe J, Khajeh J, et al. Systematic review on the incidence of bisphosphonate related osteonecrosis of the jaw in children diagnosed with osteogenesis imperfecta. J Oral Maxillofac Res. 2013;4(4):e1. doi: 10.5037/jomr.2013.4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Malmgren B, Astrom E, Soderhall S. No osteonecrosis in jaws of young patients with osteogenesis imperfecta treated with bisphosphonates. J Oral Pathol Med. 2008;37(4):196–200. doi: 10.1111/j.1600-0714.2007.00607.x. [DOI] [PubMed] [Google Scholar]
- 50.Bagan JV, Hens-Aumente E, Leopoldo-Rodado M, et al. Bisphosphonate-related osteonecrosis of the jaws: study of the staging system in a series of clinical cases. Oral Oncol. 2012;48(8):753–757. doi: 10.1016/j.oraloncology.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 51.Carlson ER, Basile JD. The role of surgical resection in the management of bisphosphonate-related osteonecrosis of the jaws. J Oral Maxillofac Surg. 2009;67(5 Suppl):85–95. doi: 10.1016/j.joms.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 52.Ruggiero SL, Carlson ER, Assael LA. Comprehensive review of bisphosphonate therapy: implications for the oral and maxillofacial surgery patient. J Oral Maxillofac Surg. 2009;67(5 Suppl):1. doi: 10.1016/j.joms.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 53.Carlson ER. Management of antiresorptive osteonecrosis of the jaws with primary surgical resection. J Oral Maxillofac Surg. 2014;72(4):655–657. doi: 10.1016/j.joms.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 54.Carlson ER, Fleisher KE, Ruggiero SL. Metastatic cancer identified in osteonecrosis specimens of the jaws in patients receiving intravenous bisphosphonate medications. J Oral Maxillofac Surg. 2013;71(12):2077–2086. doi: 10.1016/j.joms.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 55.Fleisher KE, Welch G, Kottal S, et al. Predicting risk for bisphosphonate-related osteonecrosis of the jaws: CTX versus radiographic markers. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;110(4):509–16. [DOI] [PubMed]
- 56.Hansen T, Kunkel M, Kirkpatrick CJ, et al. Actinomyces in infected osteoradionecrosis–underestimated? Hum Pathol. 2006;37(1):61–67. doi: 10.1016/j.humpath.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 57.Hall V. Actinomyces–gathering evidence of human colonization and infection. Anaerobe. 2008;14(1):1–7. doi: 10.1016/j.anaerobe.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 58.Hansen T, Kunkel M, Springer E, et al. Actinomycosis of the jaws–histopathological study of 45 patients shows significant involvement in bisphosphonate-associated osteonecrosis and infected osteoradionecrosis. Virchows Archiv. 2007;451(6):1009–1017. doi: 10.1007/s00428-007-0516-2. [DOI] [PubMed] [Google Scholar]
- 59.Naik NH, Russo TA. Bisphosphonate-related osteonecrosis of the jaw: the role of actinomyces. Clin Infect Dis. 2009;49(11):1729–1732. doi: 10.1086/648075. [DOI] [PubMed] [Google Scholar]
- 60.De Ceulaer J, Tacconelli E, Vandecasteele SJ. Actinomyces osteomyelitis in bisphosphonate-related osteonecrosis of the jaw (BRONJ): the missing link? Eur J Clin Microbiol Infect Dis. 2014;33(11):1873–80. [DOI] [PubMed]
- 61.Favia G, Pilolli GP, Maiorano E. Histologic and histomorphometric features of bisphosphonate-related osteonecrosis of the jaws: an analysis of 31 cases with confocal laser scanning microscopy. Bone. 2009;45(3):406–413. doi: 10.1016/j.bone.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 62.Favia G, Piattelli A, Sportelli P, et al. Osteonecrosis of the posterior mandible after implant insertion: a clinical and histological case report. Clin Implant Dent Relat Res. 2011;13(1):58–63. doi: 10.1111/j.1708-8208.2009.00181.x. [DOI] [PubMed] [Google Scholar]
- 63.Marx RE, Tursun R. Response to—a commentary on “Suppurative osteomyelitis, bisphosphonate induced 1 osteonecrosis, osteoradionecrosis: a blinded histopathologic comparison and its 2 implications for the mechanism of each disease” by R.E. Marx and R. Tursun [Int. J. Oral. Maxillofac. Surg. 41 (3) (2012) 283–289] Int J Oral Maxillofac Surg. 2013;42(1):148–149. doi: 10.1016/j.ijom.2012.06.027. [DOI] [PubMed] [Google Scholar]
- 64.Marx RE, Tursun R. Suppurative osteomyelitis, bisphosphonate induced osteonecrosis, osteoradionecrosis: a blinded histopathologic comparison and its implications for the mechanism of each disease. Int J Oral Maxillofac Surg. 2012;41(3):283–289. doi: 10.1016/j.ijom.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 65.Kalmar JR. A commentary on “Suppurative osteomyelitis, bisphosphonate induced osteonecrosis, osteoradionecrosis: a blinded histopathologic comparison and its implications for the mechanism of each disease” by R.E. Marx and R. Tursun [Int. J. Oral Maxillofac. Surg. 41 (2012) 283–289] Int J Oral Maxillofac Surg. 2013;42(1):147–148. doi: 10.1016/j.ijom.2012.06.026. [DOI] [PubMed] [Google Scholar]
- 66.Weinstein RS, Roberson PK, Manolagas SC. Giant osteoclast formation and long-term oral bisphosphonate therapy. N Engl J Med. 2009;360(1):53–62. doi: 10.1056/NEJMoa0802633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jain N, Weinstein RS. Giant osteoclasts after long-term bisphosphonate therapy: diagnostic challenges. Nat Rev Rheumatol. 2009;5(6):341–346. doi: 10.1038/nrrheum.2009.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sedghizadeh PP, Kumar SK, Gorur A, et al. Identification of microbial biofilms in osteonecrosis of the jaws secondary to bisphosphonate therapy. J Oral Maxillofac Surg. 2008;66(4):767–775. doi: 10.1016/j.joms.2007.11.035. [DOI] [PubMed] [Google Scholar]
- 69.Sedghizadeh PP, Kumar SK, Gorur A, et al. Microbial biofilms in osteomyelitis of the jaw and osteonecrosis of the jaw secondary to bisphosphonate therapy. J Am Dent Assoc. 2009;140(10):1259–1265. doi: 10.14219/jada.archive.2009.0049. [DOI] [PubMed] [Google Scholar]
- 70.Mauri D, Valachis A, Polyzos IP, et al. Osteonecrosis of the jaw and use of bisphosphonates in adjuvant breast cancer treatment: a meta-analysis. Breast Cancer Res Treat. 2009;116(3):433–439. doi: 10.1007/s10549-009-0432-z. [DOI] [PubMed] [Google Scholar]
- 71.Van PC. The phenomenon of osteonecrosis of the jaw in patients with metastatic breast cancer. Cancer Invest. 2006;24(1):110–112. doi: 10.1080/07357900500449652. [DOI] [PubMed] [Google Scholar]
- 72.Khosla S, Burr D, Cauley J, et al. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2007;22(10):1479–1491. doi: 10.1359/jbmr.0707onj. [DOI] [PubMed] [Google Scholar]
- 73.Estilo CL, Van Poznak CH, Wiliams T, et al. Osteonecrosis of the maxilla and mandible in patients with advanced cancer treated with bisphosphonate therapy. Oncologist. 2008;13(8):911–920. doi: 10.1634/theoncologist.2008-0091. [DOI] [PubMed] [Google Scholar]
- 74.Van Poznak C. Making a case for defining osteonecrosis of the jaw. J Dent Res. 2011;90(4):399–401. doi: 10.1177/0022034510396884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Van Poznak CH, Temin S, Yee GC, et al. American Society of Clinical Oncology executive summary of the clinical practice guideline update on the role of bone-modifying agents in metastatic breast cancer. J Clin Oncol. 2011;29(9):1221–1227. doi: 10.1200/JCO.2010.32.5209. [DOI] [PubMed] [Google Scholar]
- 76.Saad F, Brown JE, Van Poznak C, et al. Incidence, risk factors, and outcomes of osteonecrosis of the jaw: integrated analysis from three blinded active-controlled phase III trials in cancer patients with bone metastases. Ann Oncol. 2012;23(5):1341–1347. doi: 10.1093/annonc/mdr435. [DOI] [PubMed] [Google Scholar]