Abstract
Metastatic dissemination to the oral cavity is rare and is usually the evidence of a wide spread disease with an average survival rate of 7 months. In almost a quarter of the cases, oral metastasis was found to be the first indication of an occult malignancy at a distant site. Metastatic lesions can be found anywhere in the oral cavity, however, the jawbones with the molar area is the most frequently involved site. In the oral soft tissues, the gingiva is the most common site, suggesting the possible role of inflammation in the attraction of metastatic deposits. The most common primary malignancies presenting oral metastases were the lung, kidney, liver, and prostate for men, and breast, female genital organs, kidney, and colo-rectum for women. Most patients with jawbone metastasis complain of swelling, pain, and paresthesia. An exophytic lesion is the most common clinical presentation of metastatic lesions in the oral soft tissues. Early lesions, mainly those located in the gingiva, may resemble a hyperplastic or reactive lesion. Once a lesion is recognized as metastasis, the primary tumor site should be identified following clinical, radiological and histopathological investigations. If standardized diagnostic workup fails to detect the site of origin, then the term carcinoma of unknown primary is applied. Personalized medicine tools such as tissue-of-origin assays should be applied, either by immunohistochemical testing or by molecular-profiling methods as these may lead to a more favorable outcome.
Keywords: Oral, Metastasis, Jawbones, Gingiva, Inflammation, Unknown origin
Introduction
Cancer is a complex disease characterized by various biological properties that develop through multistep processes [1, 2]. These properties include sustaining proliferative signaling, evading growth suppressors, resisting cell death, enabling replicative immortality, inducing angiogenesis, and activating invasion and metastasis, in addition to reprogramming of energy metabolism and evading immune destruction [3]. It is, however, the process of metastasis that results in morbidity and eventual mortality in most patients.
The jaws and mouth are uncommon sites for metastatic dissemination with only about 1 % of oral malignancies attributed to metastases [4]. Nevertheless, the incidence of metastatic tumors to the jaws is probably higher than suggested; micrometastatic foci in the jaws were found in 16 % of autopsied carcinoma cases despite the absence of radiologic findings [5]. Because of its rarity and the importance of early detection, the diagnosis is challenging and should be considered in the differential diagnosis of benign common oral inflammatory and reactive lesions [6–10].
The aim of this review is to shortlist several key events in the process of cancer metastasis with special emphasis on jaws and mouth.
The Invasion–Metastasis Cascade
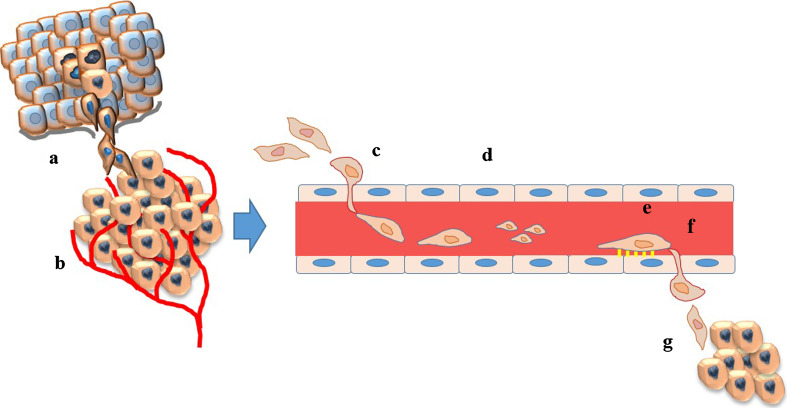
A successful metastatic colony is the result of a process known as the ‘invasion–metastasis cascade’, which involves sequential steps including invasion through the surrounding extracellular matrix (ECM), intravasation into blood vessels, and survival in the circulation (Fig. 1). Circulating tumor cells (CTC) settle in the microvasculature of the target organ and extravasate through the vessel wall. Infiltrated cells may proceed towards overt metastasis with or without an intervening period of latency (dormancy). Cancer cells reinitiate their proliferative programs at the target sites, thereby generating macroscopic, clinically detectable neoplastic growths referred to as ‘metastatic colonization’ [1–3]. Metastasis is a highly inefficient process, in which only a small minority out of thousands of cells released into the circulation each day eventually succeeds in constituting an overt colony at a distant organ [11–14].
Fig. 1.
The metastatic cascade. A successful metastatic colonization represents the end products of a complex sequential events including a invasion through the extracellular matrix (ECM); b proliferation and angiogenesis; c intravasation into the blood vessels; d survival in the circulation. e Circulating cancer cells that survive preferentially adhere to the endothelium in the microvasculature of the target organ and f extravasate through the vessel wall; g tumor cells settle at the target organ, which consists of a permissive microenvironment
The metastatic behavior of malignant cells is regulated by a repertoire of signaling pathways. Mainly those involving oral dissemination will be discussed.
Invasion and dissemination are basic features of cancer cells, achieved mainly by ‘epithelial-to-mesenchymal transition’ (EMT), where cancer cells acquire a mesenchymal phenotype [15–19], and by inducing the formation of new blood vessels (angiogenesis) [20, 21]. Tumor cells invade the underlying ECM using active proteolysis mediated mainly by matrix metalloproteinases (MMPs) to establish a colony. Further tumor growth is angiogenesis dependent and is the result of homeostatic balance between proangiogenic and antiangiogenic [i.e. vascular endothelial growth factor-(VEGF), and thrombospondin respectively], inflammatory, and coagulation factors [20, 21]. Hypoxia is the major stimulus for angiogenesis which leads to the up-regulation of hypoxia-induced transcription factors (HIF), which are the master regulators of proangiogenic signals, mainly the VEGFs [22, 23]. The new blood vessels are largely immature with weak endothelial-cell junctions, allowing tumor cells to intravasate easily into the vasculature [24].
In the circulation, CTC can be passively entrapped in the capillary network of the nearest organ, mainly the lung, which is a highly perfused organ, or as a regulated, site-specific process [25]. CTC actively adhere to the endothelial cells at a specific site, extravasate and adapt to the new microenvironment to establish a metastatic colony. This non-random process was first described by Paget in his ‘seed and soil’ hypothesis; the metastatic ‘seed’ grows preferentially in an organ environment that, in some way, provides a suitable ‘soil’ [26]. It is currently accepted that a successful metastasis requires “pre-metastatic niche” to allow invading cancer cells to survive, colonize, and expand to form a macrometastasis [11, 27].
Chronic inflammation has been linked to numerous steps in the metastatic cascade [28]. The inflammatory microenvironment at the distant site has shown to serve as a favorable pre-metastatic niche for tumor cells to engraft and proliferate [29–31]. Surrounding cells including tumor associated macrophages, infiltrating lymphocytes and cancer associated fibroblasts release a variety of inflammatory mediators such as TNF-α, IL-6, TGF-β, and IL-10 that promote tumor cell proliferation and dissemination [32, 33]. In addition, inflammatory chemokines play a major role in regulating angiogenesis, recruitment of myeloid-derived cells, and facilitating tumor cell extravasation [34].
An increased tendency for malignant transformation in several oral immuno-inflammatory processes (such as oral submucous fibrosis, and although controversial, in some cases of oral lichen planus) can suggest the possible association with chronic inflammatory condition [35]. Some evidence also exists for an association between oral squamous cell carcinoma and the presence of dento-gingival bacterial plaques and chronic periodontitis [36]. However, a direct cause and effect relationship between chronic periodontitis and cancer has not been shown and the role of inflammation as the single cause for cancerous transformation in such cases has not been proven yet [35]. As chronic inflammatory processes in the oral cavity are very common relative to the low incidence of oral cancers, it can be assumed that inflammatory processes on their own only rarely induce cancer development. The contribution of inflammation in the gingiva as a contributing factor in oral metastasis is discussed later.
Many of the metastatic events are clinically silent, appearing years after the establishment of the primary tumor, alluding to metastatic dormancy [37]. The exact mechanism for tumor dormancy and the trigger for reactivation (tumor awakening) is mostly unknown and is proposed to be the result of disruption of the balance between proliferation and cell death [37].
Metastasis to Bone
Bone is a preferred site of metastasis of numerous types of solid neoplasms mainly the breast, prostate, thyroid gland, kidney, and lungs [37, 38]. However, in most cases, bone-only metastasis is infrequent [39].
Bone metastases involve a complex interaction between metastatic tumor cells (MTC) and bone microenvironment which plays a crucial role in homing and growth of tumor cells, and increased expression of growth factors required for tumor survival [40].
In a way similar to the physiological nursing of hematopoietic stem cells, bone marrow stromal cells provide a niche for MTC through various interactions mediated by integrins, chemokines, bone morphogenetic proteins (BMPs), Notch signaling, nestin, and osteopontin [41]. Tumor cells find bone microenvironment favorable for invasion and growth, and recruit resident cells mainly osteoclasts and osteoblasts, to promote the ‘vicious cycle’ of bone metastases (Fig. 2). Receptor activator of nuclear factor-kappa B ligand (RANKL) is essential for the formation, activation, and function of osteoclasts. RANKL functions via its cognate receptor RANK, and it is inhibited by the soluble decoy receptor osteoprotegerin (OPG) [42] (Fig. 2). In skeletal metastases, the ratio of RANKL to OPG is upregulated, which leads to increased osteoclast-mediated bone destruction. Some cancer cells acquire bone cell-like phenotype (osteomimicry) expressing various proteins (BMPs and PTHrP) and transcription factors (Runx2, MSX2) to act directly on bone remodeling [43]. To ensure further growth and progression, MTC not only act on osteoblasts and osteoclasts but modulate the function of other bone marrow residential cells such as platelets, myeloid cells, immune cells, and nerve cells. The balance between bone formation and destruction determines the form of the metastatic lesion whether osteolytic or osteoblastic; in most cases, bone metastases have both osteolytic and osteoblastic elements. Osteoblastic lesions are more typical of prostate cancer, characterized by new bone formation, which is mostly immature woven type of poor quality [44].
Fig. 2.
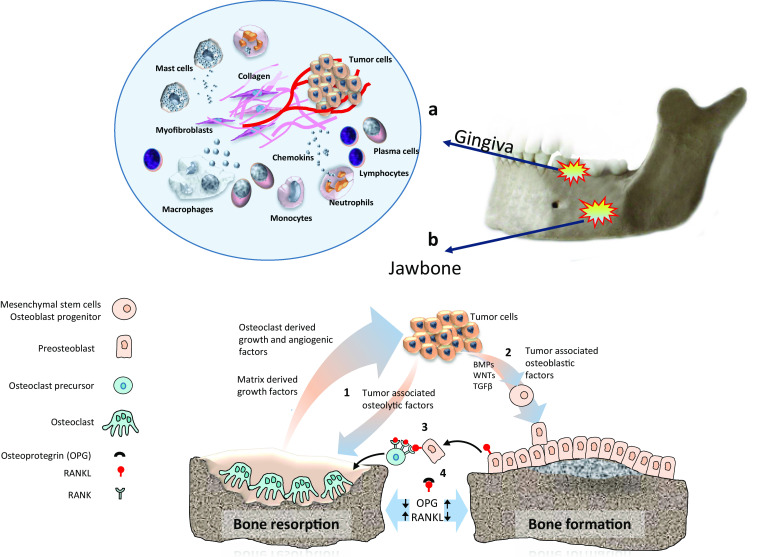
a The microenvironment present in the chronically inflamed gingiva may provide a favorable niche for metastatic cells to colonize and proliferate (circle). Soluble cytokines such as IL-1 and TNF-α are known to facilitate metastatic progression by stimulating angiogenesis and accelerating the generation of extracellular matrix necessary for tumor stroma and also may attract or induce the tumor associated macrophages. b Tumor cells recruit resident cells in the bone marrow mainly osteoclast and osteoblast to promote the ‘vicious cycle’ of bone metastases. (1) Tumor cells stimulate bone resorption which result in release of growth factors [transforming growth factor-β (TGFβ), insulin-like growth factor I (IGF-I)] and ionized calcium from the mineralized bone matrix. In addition, osteoclasts directly produce many protumorigenic factors, including growth factors (such as platelet-derived growth factor, IL-1, TNF), angiogenic factors [vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF)], and matrix metalloproteinases. These factors increase tumor cell proliferation and induce further production of osteolytic and osteoblastic factors creating a positive feedback loop. (2) Metastatic tumor cells express many factors such as transforming growth factor-β (TGFβ), bone morphogenetic proteins (BMPs) and WNT proteins promoting osteoblastic activity. (3) RANKL (receptor activator of NF-κB ligand) functions via its receptor RANK, and is inhibited by the soluble osteoprotegerin (OPG). (4) The ratio of RANKL to OPG is upregulated leading to increased osteoclast-mediated bone destruction
Considering the prognosis of bone metastases, the main goal of therapy is to retard the metastatic progression by impeding the ability of tumor cell invasion and proliferation in the bone microenvironment [45, 46]. Understanding the key pathways regulating homing, colonization and proliferation of MTC in the bone is relevant for the recently development of targeted therapies targeted mainly against OPG/RANK/RANKL pathway, PTHrP, chemokines, and chemokines receptors. The most investigated classes of bone-targeted agents that have an inhibitory effect on osteolysis are the bisphosphonates and the monoclonal antibody against RANKL, denosumab [42]. Even in osteoblastic bone metastases from prostate cancer, there is an increased bone resorption phase providing the rationale for treatment with osteoclast-targeted agents [47, 48].
Many of the factors and signaling pathways involved in the progression and development of bone metastasis may be useful as biomarkers for early detection and monitoring the metastatic disease [40, 49]. Deregulated bone metabolism is associated with the release of biochemical markers amenable to noninvasive measurement in blood or urine. Examples are the amino [N]- and carboxy [C]-terminal cross-linked telopeptides of type I collagen, or NTX and CTX, which reflect osteolysis and the bone-specific alkaline phosphatase (BSAP) levels in serum reflecting the rates of osteogenesis [50]. The levels of NTX, CTX, BSAP and CTCs were found to decrease significantly following bisphosphonates treatment while pathologic levels of NTX and BSAP were significantly associated with mortality [51].
Almost half of the patients with skeletal metastases develop complications collectively termed skeletal-related events (SRE). These include bone pain, hypercalcemia, fracture, and spinal cord compression. SRE cause significant morbidity, reduced performance status, quality of life (QOL) and reduced survival. A reduction in the occurrence of individual SREs with significant decreases in pathological fractures and surgery for bone pain has recently been reported, mainly as the result of bone-targeting treatments [52–54]. Hypercalcaemia, which was highly prevalent in breast cancer patients with bone metastases, is currently rarely seen due to a better understanding of the disease and the frequent use of anti-resorptive therapies [52, 53].
Metastasis to the Jaws and Oral Cavity
Analysis of the literature regarding Jaw and oral metastases is somewhat problematic, as much of the data comes from case reports or small case series with a bias for unusual presentations. Nevertheless, several papers analyzed data reported in the English-language literature since 1916 [6–10].
Metastatic tumors in the jaws and mouth are uncommon. Most cases of metastases to the oral cavity are found in the presence of a widespread disease; therefore, it can be assumed that these develop as a result of secondary spread from other sites, especially from the lungs. However, in almost a quarter of the cases, oral metastasis was found to be the first indication of an occult malignancy at a distant site [9]. CTC may bypass the filtration of the lungs through the valveless vertebral venous plexus; increased intrathoracic pressure can direct blood flow into this system from the caval and azygous venous system accounting for the increased distribution of axial skeleton and head and neck metastasis [55, 56]. In addition, because of their unusual plasticity, CTC may pass through the arteriovenous shunts in the lungs, seeding in distal organs.
Oral Sites
Because of differences in presentation, site of origin and probable pathogenesis, oral metastatic lesions were divided into mucosal and jawbone metastases [9]. The jawbones are twice as common for metastatic colonization than the oral mucosa [9, 57]. Metastatic lesions can be found anywhere in the oral cavity, however, in the jawbones, the mandible was more frequently involved than the maxilla, with the molar area being the most frequent site (> 50 %) followed by the premolar area (38 %) and the angle-ramus (29 %) [7, 9, 57]. In the oral soft tissues the attached gingiva is the most commonly affected site (60 %) followed by the tongue (18 %) [8–10, 57].
The pathogenesis of the metastatic process in the jawbones is not completely understood. In the skeleton, bones with red marrow are the preferred sites for metastatic deposits. Jawbones, especially in elderly, are devoid of active marrow; however, remnants of hematopoietic marrow can exist in the posterior parts of the mandible, especially in cases of focal osteoporotic bone marrow defects. These hematopoietically active sites may serve as a favorable niche attracting MTC.
The gingiva is the most common site for metastatic colonization to oral mucosa [10, 58]. A thorough literature analysis has recently found significant association between gingival metastasis and the presence of teeth, suggesting the possible role of inflammation in the distribution of metastatic deposits to the gingiva [10]. The microenvironment present in the chronically inflamed gingiva may provide a favorable niche for metastatic cells to colonize and proliferate (Fig. 2). Soluble cytokines such as IL-1 and TNF-α which are present in the chronically inflamed gingiva [59, 60] are known to facilitate metastatic progression by stimulating angiogenesis and accelerating the generation of ECM necessary for tumor stroma and also may attract or induce the tumor associated macrophages [61]. Nevertheless, one must consider the relative low incidence of gingival metastases compared with the incidence of gingival inflammation. We can therefore assume that gingival inflammation acts as a co-factor in the attraction of MTC.
Origin of the Primary Tumor
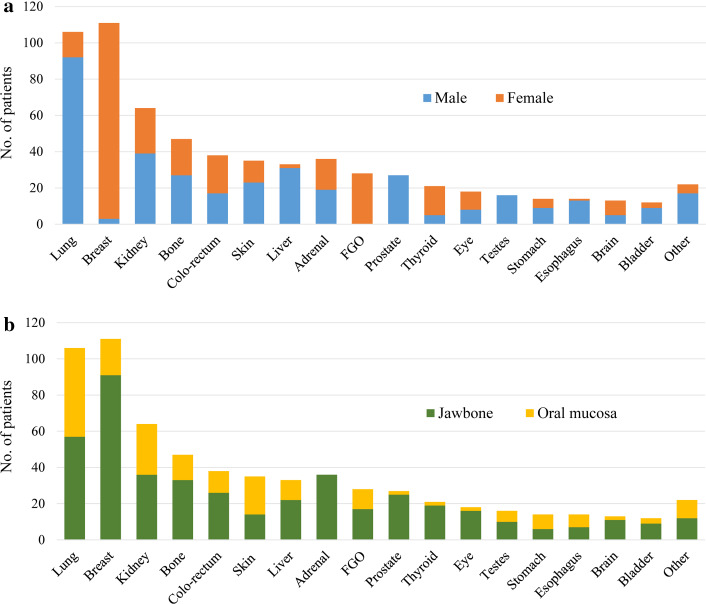
Almost any malignant tumor can metastasize to the oral cavity. Figure 3 presents the global distribution of the most common primary sites. The origin of the metastatic lesions differs between the genders. For men, the most common primary sites are the lung, kidney, liver, and prostate, and for women the breast, female genital organs, kidney, and colo-rectum. Some tumors prefer the jawbone as their metastatic target such as prostatic cancer and breast cancer. In addition, metastatic lesions from the adrenal, thyroid, and eye exclusively preferred the jawbones as their metastatic target [9] (Fig. 4).
Fig. 3.
Distribution of the common primary tumors metastasizing the oral cavity correlated with gender (a) and oral site (b). Based on Hirshberg et al. [8] total 655 cases after omitting the cases of unknown gender. Skin, including malignant melanoma; FGO, female genital organs, including uterus, ovaries, cervix, fallopian tubes; Adrenal, cases of pheochromocytoma and neuroblastoma including those from the retroperitoneum and mediastinum
Fig. 4.
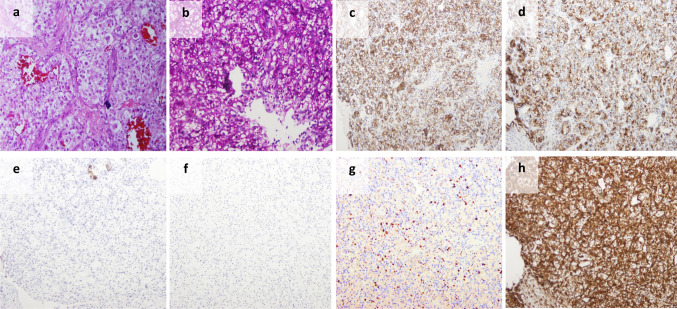
a A metastasis to the mouth exhibited clear cells with moderate atypia, arranged in closely packed groups surrounded by a vascular matrix; b PAS stained the clear cytoplasm. The tumor cells were positive for EMA (c), CD10 (d) and PAX8 (not shown), negative for cytokeratin 7 (e) and cytokeratin 20 (f); g Ki67 is positive in less than 10 % of cells. This pattern of immunohistochemistry is typical for renal cell carcinoma (RCC). h Vimentin was also positive. RCC is one of few carcinomas that stain positive for this general mesenchyme marker. S-100, GFAP and SMA were negative, which ruled-out salivary gland origin. Synaptophysin, calcitonin and chromogranin were negative as well, eliminating the possibility of endocrine carcinoma origin
The most common malignancies are mostly those metastasizing the jaws and mouth. Based on United States reported data obtained from the National Center for Health Statistics [62], prostatic cancer is the most common malignancy affecting males following by the lung, colo-rectum, and urinary bladder (28, 14, 9, and 6 % respectively), while in women the breast is the leading malignancy followed by the lung and colo-rectum (29, 14, and 9 % respectively) [50]. Almost the same incidence was found in Europe, except for women, breast is the leading malignancy followed by the colo-rectum, corpus uteri, and lung [63]. The frequency of oral metastasis is not always consistent with the prevalence of the primary cancers, probably reflecting differences in biologic behavior, aggressiveness and possible differences in affinity toward oral tissues [64]. Some tumors rarely metastasize to the oral cavity, for example pancreatic cancer, which is rarely reported [9]. This is a highly aggressive tumor accounting for 7 % of all cancer mortality and patients probably die before oral metastases are expressed [65].
Differences in the primary site exist between various geographic areas mainly between Eastern and Western countries, which may in part reflect differences in the prevalence of primary malignancies in these countries. A study from Korea found the liver to be the most common primary site for man and thyroid carcinoma for women [64], while in Japanese women the uterus was the most common primary site [65]. However, these studies may be biased mainly due to the small number of cases analyzed, and the combined oral site distribution. For example in the Korean study, no case report of jawbone metastasis from the breast was found, although it is the most common tumor among Korean women [64].
In a recent study, Shen et al. [66] extensively reviewed the Chinese literature for metastatic tumors to the oral and maxillofacial region (OMF), with special emphasis to the differences in the constituent ratios of primary cancers between the United States and China. They revealed that metastases to the OMF from the lung, thyroid, liver, esophagus, and stomach were more common in China than in the United States. In addition, they found that the proportion of gingival metastases was by far higher in China than in the United States. The authors attributed these differences to the higher frequency of gingivitis in China as a developing country.
In a small percentage of cases, however, the primary site remains obscure despite thorough clinical and pathological investigation [to be discussed later]. As many of the cases reviewed date back to several decades ago, one can assume that at least in some of these cases, the primary site would have been found today by using advanced imaging technologies and new antibodies available for immunohistochemical analysis.
Clinical Presentation
A rapidly progressing swelling accompanied by pain and paraesthesia are the classic symptoms of a metastatic tumor in the jawbones. Symptoms may vary according to location: for example, trismus in lesions located in the condyle and symptoms related to the sinus and exophthalmos in maxillary lesions. Mental nerve neuropathy or the so-called the “numb chin syndrome” should raise the suspicion of a metastatic disease in the mandible. Similar symptoms can be the result of odontogenic infection, trauma, benign odontogenic tumors, systemic diseases such as amyloidosis, sarcoidosis multiple sclerosis or as neurological manifestation of a non-metastatic malignancy [67, 68]. In most cases, the physical examination reveals a bony swelling with tenderness over the affected area. Radiographic appearance, although non-specific, raise the suspicion of malignancy with most lesions present as a lytic radiolucent lesion with ill-defined margins sometimes with “moth-eaten” appearance simulating osteomyelitis. In some cases, pathologic fracture can be noticed. Occasional osteoblastic appearance can be observed, either as pure or mixed radiopacity. The incidence of sclerotic changes is low, <10 % of the cases, most originating from prostatic carcinomas, but also from the breast and thyroid carcinomas [67]; about 50 % of metastatic prostatic carcinomas appeared as a pure radiopaque or mixed radiopaque-radiolucent lesion and in almost 40 % as a pure radiolucent lesion (8,10). Metastatic disease to the jaws may extend into the overlying soft tissues, appearing to imitate dental or periodontal infection [ 57]. In approximately 5 % of the cases, radiographs do not show any pathological changes [9].
An exophytic, sometimes ulcerated lesion is the most common clinical presentation of metastatic lesions in the oral soft tissues [10]. Early lesions, mainly those located in the gingiva may resemble a hyperplastic or reactive lesion, such as pyogenic granuloma, peripheral giant cell granuloma or fibrous epulis [10]. With the progression of the disease, oral metastatic lesions, especially those located in the soft tissues, may cause progressive discomfort, pain, bleeding, superinfection, dysphagia, interference with mastication, and disfigurement [9, 10]. In some cases, especially those of metastatic hepatocellular carcinoma, severe post-biopsy hemorrhagic episodes had been reported [69].
Metastatic lesions appearing as a soft tissue mass extruding from a recent extraction site have been described [70]. In some cases, the metastatic tumour was probably present before the extraction, causing pain, swelling, and loosening of teeth, which lead the clinician to extract the affected tooth. Tooth extraction can serve as a promoting factor in the metastatic process.
Outcome
The mean time elapsed between the diagnosis of the primary tumor to the discovery of oral metastases has been reported to be 40 months, with over 10 years reported in some cases [9]. The prognosis of a patient with an oral metastasis is grave with an average survival rate of 7 months. Treatment options in most cases include surgical resection, sometimes combined with radiation therapy and/or chemotherapy. As oral metastases are the result of a widespread disease, treatment options are sometimes limited to palliation aimed at preservation of “quality of life”. Newer bisphosphonates and bone-targeted agents may improve the outcome for patients with advanced metastatic cancers; however, studies using these treatment modalities in cases of jaw metastasis have not been published yet.
Introduction of novel imaging modalities such as positron emission tomography with coregistered computed tomography (PET/CT) may enhance early detection of jaw metastasis and will improve survival. In a recently systematic review of metastasis-directed therapy for oligometastatic prostate cancer recurrence, the authors found promising evidence for the use of radiotherapy and surgery [71]. When the oral lesion is found to be the only metastatic lesion, resection seemed to result in improved prognosis [72]. Some cases of metastatic neuroblastoma benefit from chemotherapy even in disseminated disease, which can cause maturation towards ganglioneuroblastoma [73].
Workup of a Suspected Oral Metastasis
The first step in recognizing a lesion as a metastasis is to differentiate primary intraoral malignancies from metastatic tumors. Some primary intraoral malignancies (especially salivary gland malignancies) have histological features resembling tumors from distant organs (e.g.: ductal carcinoma of salivary glands versus metastatic breast carcinoma, intraoral clear cell carcinoma versus metastatic renal cell carcinoma, intraoral squamous cell carcinoma versus metastatic squamous cell carcinoma from lung, or intraoral malignant melanoma versus metastatic malignant melanoma). Malignant soft tissue tumors rarely originate intra-orally, and thus should be worked-up as suspected metastatic rather than primary tumors.
In the presence of a known malignancy, the pathologist’s aim should be to confirm the resemblance between the oral metastasis and the original primary tumor, in both histomorphology and immunohistochemical phenotype. This is important since patients with malignant disease are prone for development of secondary malignancies. A metastatic malignancy without an immediate apparent primary site (before comprehensive investigation) is classified as malignancy of undefined primary origin (MUO) [74, 75]. In many of these patients, a primary tumor site will be identified following clinical, radiological and histopathological investigations. A standardized workup for MUO has been detailed by Amela et al. [76]:
Extensive physical exam (including head and neck, rectal and pelvic examination); Basic blood and biochemistry survey; CT-scan of chest, abdomen, and pelvis; α-fetal-protein and β-HCG in both sexes; Prostate specific antigen in men; Mammography in women.
Oriented workup: Breast MRI for women with inaugural axillary lymph nodes; [18F] fluoro-2-deoxy-d-glucose positron emission tomography; Symptom-oriented endoscopies.
Histopathological evaluation: Immunohistochemical analysis.
The Role of Pathology
The first step for pathological diagnosis of MUO is directed towards establishing a cell lineage, especially in cases in which the histomorphology does not indicate any specific tumor type. For this purpose, a basic panel of immunostains would typically include keratins [AE1/AE3, Cam 5.2, and CK903 (34βE12)] and p63 to rule-out carcinoma, vimentin (sarcoma), S-100 (melanoma), and CD45 (lymphoma) [77, 78]. However, some caution should be applied while interpreting immunohistochemical staining, since for example vimentin, which is positive in most sarcomas, can be expressed by some carcinoma types, and some sarcomas can aberrantly express keratins [77, 78]. Caution is necessary in interpreting immunohistochemical stains, which are subjected to known technical errors with differences between manufacturers, adequate control sample, and handling of the specimen.
Most of the oral metastatic malignancies appear to be of epithelial origin with adenocarcinoma being the most common [6, 7]. Non-epithelial tumors (melanoma, sarcoma, lymphoma, germ cell tumors) should be considered in the differential diagnosis and can be diagnosed using appropriate immunohistochemical testing. These can be satisfactorily managed even without an identifiable primary site.
The diverse and unique expression of CK7 and CK20 in carcinomas has been found to be useful in the differential diagnosis of some carcinomas. Studies have shown that the different expression patterns of CK7 and CK20 are among the most discriminant markers in the differential diagnosis between metastatic colon and ovarian adenocarcinomas, between Merkel cell tumor of skin and small cell carcinomas of other origins and between lung, endometrial, and breast adenocarcinomas and colon adenocarcinoma [79]. Not only the expression, but the lack of expression is indicative for interpretation. For example, the finding of CK7 negativity has greater diagnostic value than its positivity; CK7 negative carcinoma is characteristically in prostate, renal cell, neuroendocrine, hepatocellular, and adrenal carcinomas, carcinoid tumors, germ cell tumors, and squamous cell carcinomas of several origins, excluding cervical. Although individual immunohistochemical stains have only modest specificity and sensitivity, a panel of several markers and recognition of expression patterns are strongly indicative of specific tumors (Table 1). For example a combination of positive TTF-1 and CK7 with negative reactivity for CK20 are highly suggestive for lung, while positive CK20 and CDX-2 and negative CK7 are a typical profile for colorectal adenocarcinoma [78, 80]. Some tumor types are almost always negative for certain markers (e.g., renal for TTF-1, CDX2, p63, PSA, ER), and thus positivity is strong evidence against certain tumor types [78].
Table 1.
Immunohistochemical markers used to diagnose the most common malignancies metastasizing to the jaws and mouth
| Origin | CK7/CK20 | Additional markers |
|---|---|---|
| Breast | 7+/20− | ER/PR, GCDFP-15, Mammaglobin |
| FGO | 7+/20− | PAX8, WT-1 |
| Prostate | 7−/20− | PSA, PSAPa, Prostein, PSMA, NKX3.1, AMACR, Cam 5.2+/CK903- |
| Lung | 7+/20− | TTF-1 (adenocarcinoma), Naspin A, Surfactant |
| Thyroid (papillary and follicular ) | 7+/20− | TTF-1, Thyroglobulin, PAX8 |
| Thyroid medullary | 7+/20− | TTF-1, Calcitonin |
| Kidney | 7−/20− | EMA, PAX2, PAX8, CAIX, RCC, CD10, Vimentin |
| Bladder | 7+/20− | P63, Thrombomodulin (not specific), Uroplakin, GATA3 |
| Colorectal | 7−/20+ | CEA, CDX2,Villin, SATB2 [93] |
| Liver | 7−/20− | Hep-Par1, canalicular CD10, Bile duct specific CEA, αFP, |
| Adrenal | 7−/20− | Inhibin, MelanA, negative AE1 |
| Squamous cell carcinoma | 7b−/20− | P63,CK 5-6 |
Carcinoma of Unknown Primary
The term carcinoma of unknown primary (CUP) which also called “confirmed CUP” should be reserved to cases with histologically confirmed metastatic tumor for which the site of origin is not identified through a standardized diagnostic workup process [80, 81]. In most studies, CUP constitutes 3–5 % of all cancers [66–69] with a decreasing incidence of <2 % during the recent decades. However, variation exists in the definition of CUP between different epidemiological studies making the comparison difficult. Application of a more restrictive definition of CUP results in a considerably lower estimate of incidence under 0.3 % [81–85].
The proportion of CUP in oral metastases varied between the studies. Shen et al. [66] found 1 case of CUP out of 19 (5 %), Seoane et al. [58] found 4 out of 39 (10 %), Van der Waal et al. [86] found CUP in 3 out of 24 cases (12.5 %) and Thiele et al. [87] found CUP in 9 of 52 patients (17 %). These variations probably results from less restricted definition of CUP, and one can assume that at least in some of these cases the primary site would have been found today by sophisticated imaging, new immunohistochemical makers and molecular-profiling tools.
Carcinoma of unknown primary may retain the signature of the primary origin thus molecular technologies may lead to identification of the primary origin [88]. The recent years have produced additional molecular tools for tissue-of-origin profiling of unknown primary cancers. These various tests, employing DNA microarrays, quantitative real-time polymerase-chain-reaction (rtPCR) or tissue-of-origin assays based on messenger RNA (mRNA) or microRNA (miRNA) are based on the premise that in analyzing a large number of genes, the metastatic tumors match their primary tumor. The accuracy of these tests varies between 70 % for mRNA based to 90 % in DNA based tests [89, 90]. These personalized tissue-of-origin assays either by immunohistochemical testing or by molecular-profiling tools in cases of unknown origin, may lead to a more favorable prognosis since recognition of the tumor’s origin serves to guide treatment and allow the implementation of treatment protocols specific for each tumor type [91, 92]. With recent advances in development of various targeted therapy for cancer types, personalized assays could be used to formulate tailored/targeted treatment regimens.
Conclusion
Metastatic tumors of the jaws and mouth are uncommon and are the evidence of a wide spread disease. The diagnosis of a metastatic lesion in the oral region is challenging both in recognition as metastatic lesion and in determination of the site of origin. Recent advancement in imaging technologies, molecular-profiling tools, and immunohistochemical testing improve the identification of the primary site of origin and have an impact on treatment choices.
Acknowledgments
We apologize to the many authors whose work we could not cite because of space constraints.
References
- 1.Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147(2):275–292. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9(4):274–284. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 3.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Servato JP, de Paulo LF, de Faria PR, Cardoso SV, Loyola AM. Metastatic tumours to the head and neck: retrospective analysis from a Brazilian tertiary referral centre. Int J Oral Maxillofac Surg. 2013;42(11):1391–1396. doi: 10.1016/j.ijom.2013.05.020. [DOI] [PubMed] [Google Scholar]
- 5.Hashimoto N, Kurihara K, Yamasaki H, Ohba S, Sakai H, Yoshida S. Pathological characteristics of metastatic carcinoma in the human mandible. J Oral Pathol. 1987;16(7):362–367. doi: 10.1111/j.1600-0714.1987.tb00709.x. [DOI] [PubMed] [Google Scholar]
- 6.Hirshberg A, Leibovich P, Buchner A. Metastases to the oral mucosa: analysis of 157 cases. J Oral Pathol Med. 1993;22(9):385–390. doi: 10.1111/j.1600-0714.1993.tb00128.x. [DOI] [PubMed] [Google Scholar]
- 7.Hirshberg A, Leibovich P, Buchner A. Metastatic tumors to the jaws: analysis of 390 cases. J Oral Pathol Med. 1994;23(8):337–341. doi: 10.1111/j.1600-0714.1994.tb00072.x. [DOI] [PubMed] [Google Scholar]
- 8.Hirshberg A, Buchner A. Metastatic tumours to the oral region. An overview. Oral Oncol. Eur J Cancer. 1995;31B(6):355–360. doi: 10.1016/0964-1955(95)00031-3. [DOI] [PubMed] [Google Scholar]
- 9.Hirshberg A, Shnaiderman-Shapiro A, Kaplan I, Berger R. Metastatic tumours to the oral cavity—pathogenesis and analysis of 673 cases. Oral Oncol. 2008;44(8):743–752. doi: 10.1016/j.oraloncology.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 10.Allon I, Pessing A, Kaplan I, Allon DM, Hirshberg A. Metastatic tumors to the gingiva and the presence of teeth as a contributing factor: a literature analysis. J Periodontol. 2014;85(1):132–139. doi: 10.1902/jop.2013.130118. [DOI] [PubMed] [Google Scholar]
- 11.Vanharanta S, Massague J. Origins of metastatic traits. Cancer Cell. 2013;24(4):410–421. doi: 10.1016/j.ccr.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baccelli I, Schneeweiss A, Riethdorf S, Stenzinger A, Schillert A, Vogel V, Klein C, Saini M, Bäuerle T, Wallwiener M, Holland-Letz T, Höfner T, Sprick M, Scharpff M, Marmé F, Sinn HP, Pantel K, Weichert W, Trumpp A. Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nat Biotechnol. 2013;31(6):539–544. doi: 10.1038/nbt.2576. [DOI] [PubMed] [Google Scholar]
- 13.Stott SL, Lee RJ, Nagrath S, Yu M, Miyamoto DT, Ulkus L, Inserra EJ, Ulman M, Springer S, Nakamura Z, Moore AL, Tsukrov DI, Kempner ME, Dahl DM, Wu CL, Iafrate AJ, Smith MR, Tompkins RG, Sequist LV, Toner M, Haber DA, Maheswaran S. Isolation and characterization of circulating tumor cells from patients with localized and metastatic prostate cancer. Sci Transl Med. 2010;2(25):25. doi: 10.1126/scitranslmed.3000403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sleeman JP, Nazarenko I, Thiele W. Do all roads lead to Rome? Routes to metastasis development. Int J Cancer. 2011;128(11):2511–2526. doi: 10.1002/ijc.26027. [DOI] [PubMed] [Google Scholar]
- 15.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15(3):178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsai JH, Yang J. Epithelial-mesenchymal plasticity in carcinoma metastasis. Genes Dev. 2013;27(20):2192–2206. doi: 10.1101/gad.225334.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9(4):239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 19.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141(1):52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29(6 Suppl 16):15–18. doi: 10.1053/sonc.2002.37263. [DOI] [PubMed] [Google Scholar]
- 21.Weis SM, Cheresh DA. Tumor angiogenesis: molecular pathways and therapeutic targets. Nat Med. 2011;17(11):1359–1370. doi: 10.1038/nm.2537. [DOI] [PubMed] [Google Scholar]
- 22.Greer SN, Metcalf JL, Wang Y, Ohh M. The updated biology of hypoxia-inducible factor. EMBO J. 2012;31(11):2448–2460. doi: 10.1038/emboj.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 2003;9(6):677–684. doi: 10.1038/nm0603-677. [DOI] [PubMed] [Google Scholar]
- 24.Reymond N, d’Água BB, Ridley AJ. Crossing the endothelial barrier during metastasis. Nat Rev Cancer. 2013;13(12):858–870. doi: 10.1038/nrc3628. [DOI] [PubMed] [Google Scholar]
- 25.Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9(4):274–284. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 26.Paget S. The distribution of secondary growths in cancer of the breast. Lancet. 1889;1:571–573. doi: 10.1016/S0140-6736(00)49915-0. [DOI] [PubMed] [Google Scholar]
- 27.Psaila B, Lyden D. The metastatic niche: adapting the foreign soil. Nat Rev Cancer. 2009;9(4):285–293. doi: 10.1038/nrc2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aggarwal BB, Shishodia S, Sandur SK, Pandey MK, Sethi G. Inflammation and cancer: how hot is the link? Biochem Pharmacol. 2006;72(11):1605–1621. doi: 10.1016/j.bcp.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 29.Spano D, Zollo M. Tumor microenvironment: a main actor in the metastasis process. Clin Exp Metastasis. 2012;29(4):381–395. doi: 10.1007/s10585-012-9457-5. [DOI] [PubMed] [Google Scholar]
- 30.Borsig L, Wolf MJ, Roblek M, Lorentzen A, Heikenwalder M. Inflammatory chemokines and metastasis–tracing the accessory. Oncogene. 2014;33(25):3217–3224. doi: 10.1038/onc.2013.272. [DOI] [PubMed] [Google Scholar]
- 31.Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. 2013;13(11):759–771. doi: 10.1038/nrc3611. [DOI] [PubMed] [Google Scholar]
- 32.Peinado H, Lavotshkin S, Lyden D. The secreted factors responsible for pre-metastatic niche formation: old sayings and new thoughts. Semin Cancer Biol. 2011;21(2):139–146. doi: 10.1016/j.semcancer.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 33.Landskron G, De la Fuente M, Thuwajit P, Thuwajit C, Hermoso MA. Chronic inflammation and cytokines in the tumor microenvironment. J Immunol Res. 2014;2014:149185. doi: 10.1155/2014/149185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stockmann C, Schadendorf D, Klose R, Helfrich I. The impact of the immune system on tumor: angiogenesis and vascular remodeling. Front Oncol. 2014;4:69. doi: 10.3389/fonc.2014.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feller L, Altini M, Lemmer J. Inflammation in the context of oral cancer. Oral Oncol. 2013;49(9):887–892. doi: 10.1016/j.oraloncology.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 36.Tezal M, Sullivan MA, Hyland A, Marshall JR, Stoler D, Reid ME, Loree TR, Rigual NR, Merzianu M, Hauck L, Lillis C, Wactawski-Wende J, Scannapieco FA. Chronic periodontitis and the incidence of head and neck squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2009;18(9):2406–2412. doi: 10.1158/1055-9965.EPI-09-0334. [DOI] [PubMed] [Google Scholar]
- 37.Wells A, Griffith L, Wells JZ, Taylor DP. The dormancy dilemma: quiescence versus balanced proliferation. Cancer Res. 2013;73(13):3811–3816. doi: 10.1158/0008-5472.CAN-13-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12(20 Pt 2):6243s–6249s. doi: 10.1158/1078-0432.CCR-06-0931. [DOI] [PubMed] [Google Scholar]
- 39.Li S, Peng Y, Weinhandl ED, Blaes AH, Cetin K, Chia VM, Stryker S, Pinzone JJ, Acquavella JF, Arneson TJ. Estimated number of prevalent cases of metastatic bone disease in the US adult population. Clin Epidemiol. 2012;4:87–93. doi: 10.2147/CLEP.S28339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rahim F, Hajizamani S, Mortaz E, Ahmadzadeh A, Shahjahani M, Shahrabi S, Saki N. Molecular regulation of bone marrow metastasis in prostate and breast cancer. Bone Marrow Res. 2014;2014:405920. doi: 10.1155/2014/405920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brown JE, Cook RJ, Major P, Lipton A, Saad F, Smith M, Lee KA, Zheng M, Hei YJ, Coleman RE. Bone turnover markers as predictors of skeletal complications in prostate cancer, lung cancer, and other solid tumors. J Natl Cancer Inst. 2005;97(1):59–69. doi: 10.1093/jnci/dji002. [DOI] [PubMed] [Google Scholar]
- 42.Dougall WC. Molecular pathways: osteoclast-dependent and osteoclast-independent roles of the RANKL/RANK/OPG pathway in tumorigenesis and metastasis. Clin Cancer Res. 2011;18(2):326–335. doi: 10.1158/1078-0432.CCR-10-2507. [DOI] [PubMed] [Google Scholar]
- 43.Rucci N, Teti A. Osteomimicry: how tumor cells try to deceive the bone. Front Biosci (Schol Ed) 2010;1(2):907–915. doi: 10.2741/S110. [DOI] [PubMed] [Google Scholar]
- 44.Clezardin P, Teti A. Bone metastasis: pathogenesis and therapeutic implications. Clin Exp Metastasis. 2007;24(8):599–608. doi: 10.1007/s10585-007-9112-8. [DOI] [PubMed] [Google Scholar]
- 45.Weilbaecher KN, Guise TA, McCauley LK. Cancer to bone: a fatal attraction. Nat Rev Cancer. 2011;11(6):411–425. doi: 10.1038/nrc3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oyewumi MO, Alazizi A, Wehrung D, Manochakian R, Safadi FF. Emerging lung cancer therapeutic targets based on the pathogenesis of bone metastases. Int J Cell Biol. 2014;2014:236246. doi: 10.1155/2014/236246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gartrell BA, Saad F. Managing bone metastases and reducing skeletal related events in prostate cancer. Nat Rev Clin Oncol. 2014;11(6):335–345. doi: 10.1038/nrclinonc.2014.70. [DOI] [PubMed] [Google Scholar]
- 48.Yonou H, Ochiai A, Goya M, Kanomata N, Hokama S, Morozumi M, Sugaya K, Hatano T, Ogawa Y. Intraosseous growth of human prostate cancer in implanted adult human bone: relationship of prostate cancer cells to osteoclasts in osteoblastic metastatic lesions. Prostate. 2004;58(4):406–413. doi: 10.1002/pros.10349. [DOI] [PubMed] [Google Scholar]
- 49.Jung K, Lein M. Bone turnover markers in serum and urine as diagnostic, prognostic and monitoring biomarkers of bone metastasis. Biochim Biophys Acta. 2014;1846(2):425–438. doi: 10.1016/j.bbcan.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 50.Coleman R, Costa L, Saad F, Cook R, Hadji P, Terpos E, Garnero P, Brown J, Body JJ, Smith M, Lee KA, Major P, Dimopoulos M, Lipton A. Consensus on the utility of bone markers in the malignant bone disease setting. Crit Rev Oncol Hematol. 2011;80(3):411–432. doi: 10.1016/j.critrevonc.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 51.Barnadas A, Manso L, de la Piedra C, Meseguer C, Crespo C, Gómez P, Calvo L, Martinez P, Ruiz-Borrego M, Perelló A, Antón A, Codes M, Margelí M, Murias A, Salvador J, Seguí MÁ, de Juan A, Gavilá J, Luque M, Pérez D, Zamora P, Arizcuma A, Chacón JI, Heras L, Martin-Fernández M, Mahillo-Fernández I, Tusquets I. Bone turnover markers as predictive indicators of outcome in patients with breast cancer and bone metastases treated with bisphosphonates: results from a 2-year multicentre observational study (ZOMAR study) Bone. 2014;68:32–40. doi: 10.1016/j.bone.2014.07.036. [DOI] [PubMed] [Google Scholar]
- 52.Poon M, Zeng L, Zhang L, Lam H, Emmenegger U, Wong E, Bedard G, Lao N, Chow R, Chow E. Incidence of skeletal-related events over time from solid tumour bone metastases reported in randomised trials using bone-modifying agents. Clin Oncol (R Coll Radiol) 2013;25(7):435–444. doi: 10.1016/j.clon.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 53.Peddi P, Lopez-Olivo MA, Pratt GF, Suarez-Almazor ME. Denosumab in patients with cancer and skeletal metastases: a systematic review and meta-analysis. Cancer Treat Rev. 2013;39(1):97–104. doi: 10.1016/j.ctrv.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Deng X, He G, Liu J, Luo F, Peng X, Tang S, Gao Z, Lin Q, Keller JM, Yang T, Keller ET. Recent advances in bone-targeted therapies of metastatic prostate cancer. Cancer Treat Rev. 2014;40(6):730–738. doi: 10.1016/j.ctrv.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Batson OV. The function of the vertebral veins and their role in the spread of metastases. Ann Surg. 1940;112(1):138–149. doi: 10.1097/00000658-194007000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cummings J, Hacking N, Fairhurst J, Ackery D, Jenkins JD. Distribution of bony metastases in prostatic carcinoma. Br J Urol. 1990;66(4):411–414. doi: 10.1111/j.1464-410X.1990.tb14964.x. [DOI] [PubMed] [Google Scholar]
- 57.D’silva N, Summerline DJ, Cordell KG, Abdelsayed RA, Tomish CE, Hanks CT, Fear D, Meyrowitz S. Metastatic tumors in the jaws. A retrospective study of 114 cases. JADA. 2006;137(12):1667–1672. doi: 10.14219/jada.archive.2006.0112. [DOI] [PubMed] [Google Scholar]
- 58.Seoane J, Van der Waal I, Van der Waal RI, Cameselle-Teijeiro J, Antón I, Tardio A, Alcázar-Otero JJ, Varela-Centelles P, Diz P, Antón I, Tardio A, Alcázar-Otero JJ. Metastatic tumours to the oral cavity: a survival study with a special focus on gingival metastases. J Clin Periodontol. 2009;36(6):488–492. doi: 10.1111/j.1600-051X.2009.01407.x. [DOI] [PubMed] [Google Scholar]
- 59.Otenio CC, Fonseca I, Martins MF, Ribeiro LC, Assis NM, Ferreira AP, Ribeiro RA. Expression of IL-1β, IL-6, TNF-α, and iNOS in pregnant women with periodontal disease. Genet Mol Res. 2012;11(4):4468–4478. doi: 10.4238/2012.September.20.3. [DOI] [PubMed] [Google Scholar]
- 60.Bickel M, Axtelius B, Solioz C, Attstrom R. Cytokine gene expression in chronic periodontitis. J Clin Periodontol. 2001;28(9):840–847. doi: 10.1034/j.1600-051x.2001.028009840.x. [DOI] [PubMed] [Google Scholar]
- 61.Allavena P, Sica A, Solinas G, Porta C, Mantovani A. The inflammatory microenvironment in tumor progression: the role of tumor-associated macrophages. Crit Rev Oncol Hematol. 2008;66(1):1–9. doi: 10.1016/j.critrevonc.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 62.Siegel R, Naishadham D, Jemal A. Cancer Statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 63.Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D, Bray F. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49(6):1374–1403. doi: 10.1016/j.ejca.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 64.Lim SY, Kim SA, Ahn SG, Kim HK, Kim SG, Hwang HK, Kim BO, Lee SH, Kim JD, Yoon JH. Metastatic tumours to the jaws and oral soft tissues: a retrospective analysis of 41 Korean patients. Int J Oral Maxillofac Surg. 2006;35(5):412–415. doi: 10.1016/j.ijom.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 65.Nishimura Y, Yakata H, Kawasaki T, Nakajima T. Metastatic tumours of the mouth and jaws. A review of the Japanese literature. J Maxillofac Surg. 1982;10(4):253–258. doi: 10.1016/S0301-0503(82)80050-7. [DOI] [PubMed] [Google Scholar]
- 66.Shen ML, Kang J, Wen YL, Ying WM, Yi J, Hua CG, Tang XF, Wen YM. Metastatic tumors to the oral and maxillofacial region: a retrospective study of 19 cases in West China and review of the Chinese and English literature. J Oral Maxillofac Surg. 2009;67(4):718–737. doi: 10.1016/j.joms.2008.06.032. [DOI] [PubMed] [Google Scholar]
- 67.Laurencet FM, Anchisi S, Tullen E, Dietrich PY. Mental neuropathy: report of five cases and review of the literature. Crit Rev Oncol Hematol. 2000;34(1):71–79. doi: 10.1016/S1040-8428(00)00050-0. [DOI] [PubMed] [Google Scholar]
- 68.Smith SF, Blackman G, Hopper C. Numb chin syndrome: a nonmetastatic neurological manifestation of malignancy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105(3):e53–e56. doi: 10.1016/j.tripleo.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 69.Ahsar A, Khateery SM, Kovacs A. Mandibular metastatic hepatocellular carcinoma: a case involving severe post biopsy hemorrhage. J Oral Maxillofac Surg. 1997;55(6):547–552. doi: 10.1016/S0278-2391(97)90480-8. [DOI] [PubMed] [Google Scholar]
- 70.Hirshberg A, Leibovich P, Horowitz I, Buchner A. metastatic tumors to post-extraction site. J Oral Maxillofac Surg. 1993;51(12):1334–1337. doi: 10.1016/S0278-2391(10)80138-7. [DOI] [PubMed] [Google Scholar]
- 71.Ost P, Bossi A, Decaestecker K, De Meerleer G, Giannarini G, Karnes RJ, Roach M 3rd, Briganti A. Metastasis-directed therapy of regional and distant recurrences after curative treatment of prostate cancer: a systematic review of the literature. Eur Urol. 2014. doi:10.1016/j.eururo.2014.09.004. [DOI] [PubMed]
- 72.Nakamura T, Ishimaru JI, Mizui T, Kobayashi A, Iwata H, Shimokawa K. Osteosarcoma metastatic to the mandible: a case report. Oral Pathol Oral Radiol Endod. 2001;91(4):452–454. doi: 10.1067/moe.2001.113107. [DOI] [PubMed] [Google Scholar]
- 73.Bhattacharyya I, Williamson A, Cohen DM, Bever JL. Metastatic neuroblastoma with ganglionuromtous differentiation and mandibular involvement. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88(5):582–586. doi: 10.1016/S1079-2104(99)70090-9. [DOI] [PubMed] [Google Scholar]
- 74.Taylor MB, Bronham NR, Arnold SE. Carcinoma of unknown primary: key radiological issues from the recent National Institute for Health and Clinical Excellence guidelines. Br J Radiol. 1014;2012(85):661–671. doi: 10.1259/bjr/75018360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.National Institute for Health and Clinical Excellence . Diagnosis and management of metastatic disease of unknown primary origin (clinical guideline 104) London UK: National Institute for Health and Clinical Excellence; 2010. [PubMed] [Google Scholar]
- 76.Amela EY, Lauridant-Philippin G, Cousin S, Ryckewaert T, Adenis A, Penel A. Management of “unfavourable” carcinoma of unknown primary site: synthesis of recent literarture. Crit Rev Oncol Hematol. 2012;84(2):213–223. doi: 10.1016/j.critrevonc.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 77.Rekhtman N, Bishop JA. Quick reference handbook for surgical pathologists. Berlin: Springer; 2011. [Google Scholar]
- 78.Pavilidis N, Pentheroudokis G. Cancer of unknown primary site. Lancet. 2012;379:1428–1435. doi: 10.1016/S0140-6736(11)61178-1. [DOI] [PubMed] [Google Scholar]
- 79.Pepper C, Pai I, Hay A, Deery A, Wilson P, Williamson P, Pitkin L. Investigatin strategy in the management of metastatic adenocarcinoma of unknown primary presenting as cervical lymphadenopathy. Acta Otolaryngol. 2014;134(8):838–842. doi: 10.3109/00016489.2014.884726. [DOI] [PubMed] [Google Scholar]
- 80.Pavlidis N, Fizazi K. Cancer of unknown primary (CUP) Crit Rev Oncol Hematol. 2005;54(3):243–250. doi: 10.1016/j.critrevonc.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 81.Luke C, Koczwara B, Karapetis C, Pittman K, Price T, Kotasek D, Beckmann K, Brown MP, Roder D. Exploring the epidemiological characteristics of cancers of unknown primary site in an Australian population: implications for research and clinical care. Aust N Z J Public Health. 2008;32(4):383–389. doi: 10.1111/j.1753-6405.2008.00260.x. [DOI] [PubMed] [Google Scholar]
- 82.Shu X, Sundquist K, Sundquist J, Hemminki K. Time trends in incidence, causes of death, and survival of cancer of unknown primary in Sweden. Eur J Cancer Prev. 2012;21(3):281–288. doi: 10.1097/CEJ.0b013e32834c9ceb. [DOI] [PubMed] [Google Scholar]
- 83.Brewster DH, Lang J, Bhatti LA, Thomson CS, Oien KA. Descriptive epidemiology of cancer of unknown primary site in Scotland, 1961–2010. Cancer Epidemiol. 2014;38(3):227–234. doi: 10.1016/j.canep.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 84.Urban D, Rao A, Bressel M, Lawrence YR, Mileshkin L. Cancer of unknown primary: a population-based analysis of temporal change and socioeconomic disparities. Br J Cancer. 2013;109(5):1318–1324. doi: 10.1038/bjc.2013.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Brustugun OT, Helland A. Rapid reduction in the incidence of cancer of unknown primary: a population-based study. Acta Oncol. 2014;53(1):134–137. doi: 10.3109/0284186X.2013.783230. [DOI] [PubMed] [Google Scholar]
- 86.van der Waal RI, Buter J, van der Waal I. Oral metastases: report of 24 cases. Br J Oral Maxillofac Surg. 2003;41(1):3–6. doi: 10.1016/S0266-4356(02)00301-7. [DOI] [PubMed] [Google Scholar]
- 87.Thiele OC, Freier K, Bacon C, Flechtenmacher C, Scherfler S, Seeberger R. Craniofacial metastases: a 20-year survey. J Craniomaxillofac Surg. 2011;39(2):135–137. doi: 10.1016/j.jcms.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 88.Varadhachary GR, Raber MN. Cancer of unknown primary site. N Engl J Med. 2014;371(8):757–765. doi: 10.1056/NEJMra1303917. [DOI] [PubMed] [Google Scholar]
- 89.Monzon FA, Koen TJ. Diagnosis of metastatic neoplasms: molecular approaches for identification of tissue of origin. Arch Pathol Lab Med. 2010;134(2):216–224. doi: 10.5858/134.2.216. [DOI] [PubMed] [Google Scholar]
- 90.Rosenfeld N, Aharonov R, Meiri E, Rosenwald S, Spector Y, Zepeniuk M, Benjamin H, Shabes N, Tabak S, Levy A, Lebanony D, Goren Y, Silberschein E, Targan N, Ben-Ari A, Gilad S, Sion-Vardy N, Tobar A, Feinmesser M, Kharenko O, Nativ O, Nass D, Perelman M, Yosepovich A, Shalmon B, Polak-Charcon S, Fridman E, Avniel A, Bentwich I, Bentwich Z, Cohen D, Chajut A, Barshack I. MicroRNAs accurately identify cancer tissue origin. Nat Biotechnol. 2008;26(4):462–469. doi: 10.1038/nbt1392. [DOI] [PubMed] [Google Scholar]
- 91.Epstein JI, Egevad L, Humphrey PA, Montironi R. Members of the ISUP Immunohistochemistry in Diagnostic Urologic Pathology Group. Best practices recommendations in the application of immunohistochemistry in the prostate: report from the International Society of Urologic Pathology consensus conference. Am J Surg Pathol. 2014;38(8):e6–e19. doi: 10.1097/PAS.0000000000000238. [DOI] [PubMed] [Google Scholar]
- 92.Reuter VE, Argani P, Zhou M, Delahunt B. Members of the ISUP Immunohistochemistry in Diagnostic Urologic Pathology Group. Best practices recommendations in the application of immunohistochemistry in the kidney tumors: report from the International Society of Urologic Pathology consensus conference. Am J Surg Pathol. 2014;38(8):e35–e49. doi: 10.1097/PAS.0000000000000258. [DOI] [PubMed] [Google Scholar]
- 93.Dragomir A, de Wit M, Johansson C, Uhlen M, Pontén F. The role of SATB2 as a diagnostic marker for tumors of colorectal origin: results of a pathology-based clinical prospective study. Am J Clin Pathol. 2014;141(5):630–638. doi: 10.1309/AJCPWW2URZ9JKQJU. [DOI] [PubMed] [Google Scholar]
- 94.Chu P, Wu E, Weiss LM. Cytokeratin 7 and cytokeratin 20 expression in epithelial neoplasms: a survey of 435 cases. Mod Pathol. 2000;13(9):962–972. doi: 10.1038/modpathol.3880175. [DOI] [PubMed] [Google Scholar]