Abstract
The aim of this work was to investigate the effect of backing films on transdermal delivery of donepezil (DP) from patches. Three backing films, CotranTM 9700, CotranTM 9701, and CotranTM 9726 were chosen as backing layers to prepare transdermal patches containing DP. The transdermal penetration and release amount of DP from each patch were evaluated by rabbit abdominal skin in vitro. The partitioning experiments and attentuated total reflectance Fourier transform infrared (ATR-FTIR) spectroscopy were performed to confirm the existence of interaction between backing films and DP. Results showed that the cumulative release amount of DP from patches with different backing films had the same order of cumulative amount penetrated, i.e. CotranTM 9701 < CotranTM 9700 < CotranTM 9726, which demonstrated that the permeation of DP was mainly limited by release behavior. Partitioning experiments and ATR-FTIR study indicated that CotranTM 9700 and CotranTM 9701 had interaction with DP by H bond formation which decreased the release of drug from the patches. By contrast, CotranTM 9726 could provide the highest flux of skin permeation of DP, because such interaction between them was not found. Moreover, the parameters of backing films were found to have relation to skin hydration, thus affecting the penetration behavior of DP from patches. In conclusion, the effect of backing films on the flux of DP permeation could be attributed to both the interaction of backing films and the changes of skin hydration. Backing films could be a key factor in formulation screening of DP patches.
KEY WORDS: ATR-FTIR, backing films, donepezil, release, transdermal patches
INTRODUCTION
Donepezil (DP, as shown in Fig. 1), a new class of acetylcholinesterase inhibitor, is the second drug approved by FDA for treatment of mild to moderate Alzheimer’s diseases (1,2). It is superior to other cholinesterase inhibitors due to its high potency and selectivity for the enzyme AChE in central nervous system (3). Currently, the dosage forms of DP available on the market are only tablets and capsules. However, oral administration in clinic was often limited because of the gastrointestinal adverse effect and severe patient non-compliance. Therefore, transdermal patch containing DP, which can greatly enhance the treatment compliance, is now in pressing need (4).
Fig. 1.
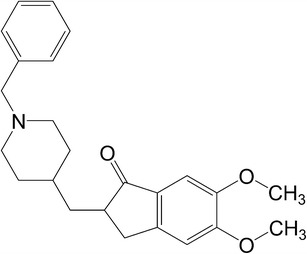
The chemical structure of donepezil
Transdermal patches mainly consist of backing film, adhesive matrix, and release liner. As a component of support in this system and can provide an occlusive environment of skin, backing film is chosen for appearance, flexibility, and need for occlusion. Though important, there are few reports of backing films in formulation screening, and no investigation has ever been made on the interactions between backing films and drugs. Thus, in this work, three different backing films from the comfortable and flexible CotranTM family, CotranTM 9700, CotranTM 9701, and CotranTM 9726, were selected to evaluate their effects on the permeation of DP from patches, and the interactions between backing films and DP were investigated.
MATERIALS AND METHODS
Materials
Donepezil hydrochloride was supplied by Ji’nan Dexinjia Biological Technology Co., Ltd. (Ji’nan, China); methanol of HPLC grade was purchased from Shandong Yuwang Chemicals Co., Ltd. (Shandong, China); PSA DURO-TAK® 87–4098 was obtained from National Starch and Chemical Co., USA (Bridgewater, USA); Release liner ScotchPak® 9744, backing films CotranTM 9700, CotranTM 9701, and CotranTM 9726 were bought from 3M company (3M, USA). All other chemicals were of analytical grade.
Free DP was prepared from DP hydrochloride, according to a process reported by us earlier (5). Then, it was identified using differential scanning calorimetry (DSC).
In Vitro Permeation Experiments
All animal experiments were carried out in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals as well as the guidelines for animal use published by Life Science Research Center of Shenyang Pharmaceutical University. DP patches and rabbit abdominal skin were prepared as described in a previous study (5). In vitro permeation experiments were performed using a two-chamber side-by-side glass diffusion cell in accordance with the method of Sun et al. (5), except that pH 7.4 phosphate buffer solution (PBS) and PEG400 in ratio of 5:1 were used as the receptor solution. Two-milliliter samples were taken at pre-determined time intervals from receptor compartment for analysis and replaced with the same volume of fresh medium to maintain sink conditions.
Release of DP from Patches
The experiments of drug release study were carried out in accordance with that in the permeation study mentioned above, except that dialysis membrane was mounted in place of the excised rabbit skin.
Partition of DP Between Backing Film and Medium
The partitioning studies were carried out with weighed pieces (1 cm2) of CotranTM 9700, CotranTM 9701, and CotranTM 9726, and each of these was placed in a 4 mL solution of DP (5 μg/mL) in a sealed glass vial, with 1:49 (v/v) of methanol and water as solvent. All vials were shaken for 24 h at 32°C to reach equilibrium. After centrifugation, the supernatant solution was analyzed by HPLC for drug content. The amount of the drug bound to three backing films were calculated by subtracting the amount of the drug present in the supernatant from the initial drug concentration. Partition coefficients were calculated from DP in the backing films and that in donor solution by the following equation: K = (C0 − C)/C
where C0 and C represent drug concentration in the DP solution before and after partitioning, respectively.
ATR-FTIR Measurements
Backing films CotranTM 9700, CotranTM 9701, and CotranTM 9726 were cut into 1 × 1 cm square pieces, and every type of backing film was divided into two groups. In one group, these backing films were treated following the process in section “Partition of DP between Backing Film and Medium”. In the other group, the weighed pieces (1 cm2) of backing film were incubated for 24 h in 4 mL of the blank solution (methanol and water, 1:49, v/v) as control. Thereafter, the backing films were oven dried at 40°C for 2 h. After drying, spectral measurements were made with a Nicolet NEXUS 470 Fourier Transform Infrared Spectrometer (Thermo Nicolet, USA) equipped with an attentuated total reflectance (ATR) attachment. All spectra were obtained as an average of 32 scans recorded between 4,000 and 400 at 4 cm−1 resolution.
Quantitative Analysis
DP was quantitatively analyzed by HPLC method. The HPLC system consists of an L-2130 pump (Hitachi Ltd, Japan) and an L-2420 variable wavelength ultraviolet absorption detector (Hitachi Ltd., Japan). A Diamonsil® C18 column (200 mm × 4.6 mm × 5 μm) was used, and its temperature was maintained at 40°C. Elution was performed with methanol, 0.05 mol/L potassium dihydrogen phosphate solution and triethylamine by a volume ratio of 55:45:0.5, and the pH was adjusted to 2.7 with phosphoric acid. The flow rate was 1 mL/min, and detection wavelength was set at 271 nm.
Data Analysis
Each experiment value was an average of minimum four parallel measurements, and the data were expressed as mean ± SD, except where indicated. Student’s t test was carried out to evaluate the statistical significance of data from different groups. P < 0.05 was considered to indicate significant differences.
RESULTS AND DISCUSSION
DSC Characterization of Free DP
As was seen from the DSC curves (Fig. 2), the free DP had a sharp endothermic peak with a melting point of 88.6°C, which was lower than that of donepezil hydrochloride. This suggested that free DP might be prepared.
Fig. 2.
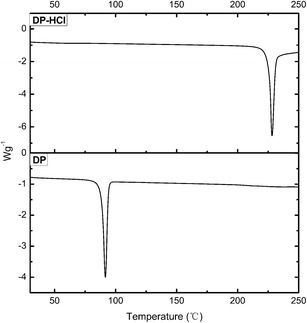
DSC curves of donepezil hydrochloride and donepezil at a heating rate of 10°C min−1
In Vitro Permeation Experiments
The effect of backing films on the permeation of DP from patches was examined by in vitro permeation experiments. As shown in Fig. 3, the patch prepared with CotranTM 9726 showed the highest permeated amount of DP (69.44 ± 10.07 μg/cm2), which was significantly higher (P < 0.05) than that with CotranTM 9700 (28.72 ± 4.34 μg/cm2) and CotranTM 9701 (23.59 ± 4.93 μg/cm2). Since a drug must be released from the formulation before its permeation through the skin (6), we inferred that different backing films might have different effects on the release of DP.
Fig. 3.
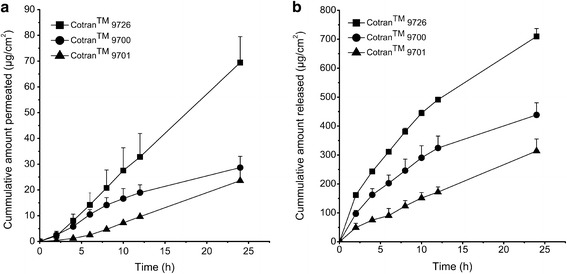
a The penetration profiles of donepezil patches in the presence of different backing layers. Data are presented as the mean ± SD (n = 4). b The release profiles of donepezil patches through dialysis membrane in the presence of different backing layers. Data are presented as the mean ± SD (n = 4)
Release of DP from Patches
To investigate the effect of backing films, release experiments of DP patches were performed. The DP release from three types of backing films was found in ranged CotranTM 9726 > CotranTM 9700 > CotranTM 9701 (Fig. 3). This was in accordance with the order of skin permeation amount. The cumulative release amount of DP produced by CotranTM 9726 (709.7 ± 27.5 μg/cm2) was 1.6 and 2.3 times higher than that of CotranTM 9700 (438.5 ± 41.6 μg/cm2) and CotranTM 9701 (314.3 ± 14.5 μg/cm2), respectively. And, the t test showed that the difference was statistically significant (P < 0.05). This indicated some effects of backing films on drug release from patches (7,8).
Partition of DP Between Backing Film and Medium
The release results above suggested the interaction between DP and backing films; to further confirm the existence of such affinity, partitioning experiments were performed. It is readily understood that higher partition coefficient suggested better affinity between DP and backing films. Thus, it was inferred from Table I that CotranTM 9701 had the strongest affinity with DP followed by CotranTM 9700, while CotranTM 9726 had the lowest drug affinity. The results also explained the different release profiles of DP in three kinds of backing films.
ATR-FTIR Measurements
The results of partitioning experiments have indicated the potential interaction between DP and the backing films including CotranTM 9700 and CotranTM 9701. In view of the structure of DP and polyurethane (structure shown in Fig. 4), the main component in CotranTM 9700 and CotranTM 9701, intermolecular hydrogen bonds may preferentially form between them. To elucidate this issue, ATR-Fourier transform infrared (FTIR), a classical spectroscopic method for studying the hydrogen bonding between complexes (9,10), was used in the following study.
Fig. 4.
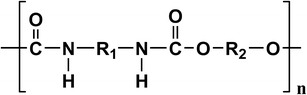
The main-chain structure of polyurethane
As can be seen from Fig. 5, the tertiary amine in DP did not produce N–H stretching vibration absorption; thus, the possibility of interference with the N–H stretching vibration by other components was excluded. After incubation in DP solution, the absorption at 3,324 cm−1 assigned to the stretching vibration of N–H group of polyurethane in backing film CotranTM 9700 was redshifted to 3,320 cm−1. As donepezil has 4 H bond acceptors, it could be reasonably inferred that H bond might form between DP and the N–H group in the backing film CotranTM 9700. In fact, such a redshift in IR spectrum has been regarded as a criterion for hydrogen bonding (9,11,12). In the case of CotranTM 9701, it also contained polyurethane so that the absorption at 3,315 cm−1 was due to the N–H stretching bands as well. Contrary to the backing film CotranTM 9700, these N–H stretching bands got blue shifted to a higher wave number (3,321 cm−1) after incubated with DP solution. However, this exceptional phenomenon was not contradictive to the above-mentioned redshift criterion. Since, the C=O acceptor groups and N–H donor groups in polyurethane can form intermolecular hydrogen bond (13,14), while the H bond acceptors in DP might disrupt the original intermolecular hydrogen bond by forming new hydrogen bond with N–H donor groups in polyurethane. According to literatures (15), it could be concluded that all H bond acceptors in DP had weaker electronegativity than the C=O H bond acceptor in polyurethane, thus inducing the electron rearrangement of H atom in the N–H group and the blueshift of the N–H group stretching absorption (16). This explanation was also consistent with the results in partitioning experiments, which showed that the interaction between DP and the backing film CotranTM 9700 was weaker than that between DP and the backing film CotranTM 9701, so DP may not be able to disrupt the original intermolecular hydrogen bond of polyurethane in the backing film CotranTM 9700. As for the backing film CotranTM 9726, the absorbance bands were not changed after incubation in DP solution compared with the control. This result indicated no interaction between DP and the backing film, which was in accordance with the results of partitioning experiments.
Fig. 5.
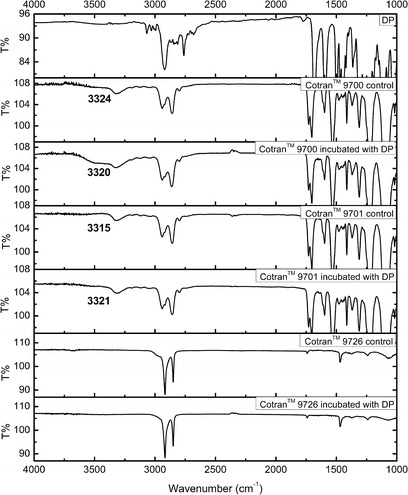
ATR-FTIR spectra of donepezil itself, backing films CotranTM 9700, CotranTM 9701, CotranTM 9726 control and after treated by donepezil
Analysis of Properties of Backing Films
Though the amount of DP permeated from different backing films across the rabbit skin had the same order with that released across the dialysis membrane, i.e. CotranTM 9726 > CotranTM 9700 > CotranTM 9701, it was worth noting that the permeation amount of DP from CotranTM 9726 was 2.4 times higher than that from CotranTM 9700, but the release amount was only 1.6 times higher. This indicated other effects of backing films on drug permeation except on drug release. According to the moisture vapor transmission rate (MVTR) and oxygen transmission (OT) properties listed in Table I, CotranTM 9726 has a significantly lower MVTR compared with CotranTM 9700. The lower MVTR can increase the hydration of skin, thus improve the transdermal delivery of the drug (17–19). Similarly, compared with CotranTM 9701, CotranTM 9700 provided a relative higher release amount of DP, but a comparable permeation amount with that from CotranTM 9701, which might be counterbalanced by an undesired high MVTR. So, we inferred that backing films affected the penetration of DP from patches mainly in two ways, one was to influence its release behavior and the other was the change of skin hydration.
Table I.
Properties of Three Backing Layers and Partition Coefficients of Donepezil from the Backing Layers to the Vehicle (n = 4, mean ± SD)
| Backing layer types | Component | MVTR (g/m2/24 h) | OT (cc/m2/24 h) | Partition coefficient |
|---|---|---|---|---|
| CotranTM 9700 | Polyurethane | 8,408 | NA | 0.29 ± 0.01 |
| CotranTM 9701 | Polyurethane | 709 | 4,858 | 0.40 ± 0.04 |
| CotranTM 9726 | Ethylene vinyl acetate | 12.8 | 6,650 | 0.00 ± 0.03 |
CONCLUSION
The pilot study employed release experiments, partitioning measurements, and ATR-FTIR methods to investigate the effect of backing films on the flux of DP. Preliminary conclusions are that DP permeation could be attributed to both the interaction between the backing films and DP and the changes of skin hydration. Since CotranTM 9726 produced the highest flux of DP and it can enhance skin comfortability and patient compliance with a high oxygen transmission rate, it might be a promising candidate in developing the transdermal therapeutic system for DP.
ACKNOWLEDGMENT
The authors thank Professor Yasunori Morimoto (Faculty of Pharmaceutical Sciences, Josai University, Japan) for providing the two-chamber diffusion cells and a synchronous motor.
REFERENCES
- 1.Asiri YA, Mostafa GAE. Donepezil. Profiles Drug Subst Excip Relat Methodol. 2010;35:117–150. doi: 10.1016/S1871-5125(10)35003-5. [DOI] [PubMed] [Google Scholar]
- 2.Liew KB, Tan YTF, Peh KK. Characterization of oral disintegrating film containing donepezil for Alzheimer disease. AAPS PharmSciTech. 2012;13:134–142. doi: 10.1208/s12249-011-9729-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heydorn WE. Donepezil (E2020): a new acetylcholinesterase inhibitor. Review of its pharmacology, pharmacokinetics, and utility in the treatment of Alzheimer’s disease. Expert Opin Invistig Drugs. 1997;6:1527–1535. doi: 10.1517/13543784.6.10.1527. [DOI] [PubMed] [Google Scholar]
- 4.Sozio P, Cerasa LS, Marinelli L, Stefano AD. Transdermal donepezil on the treatment of Alzheimer’s disease. Neuropsychiatr Dis Treat. 2012;8:361–368. doi: 10.2147/NDT.S16089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun L, Cun DM, Yuan B, Cui HX, Xi HL, Fang L, et al. Formulation and in vitro/in vivo correlation of a drug-in-adhesive transdermal patch containing azasetron. J Pharm Sci. 2012;101:4540–4548. doi: 10.1002/jps.23317. [DOI] [PubMed] [Google Scholar]
- 6.Stoughton RB. Percutaneous absorption of drugs. Annu Rev Pharmacol Toxicol. 1989;29:55–69. doi: 10.1146/annurev.pa.29.040189.000415. [DOI] [PubMed] [Google Scholar]
- 7.Thein-Han WW, Stevens WF. Transdermal delivery controlled by a chitosan membrane. Drug Dev Ind Pharm. 2004;30:397–404. doi: 10.1081/DDC-120030934. [DOI] [PubMed] [Google Scholar]
- 8.Suksaeree J, Boonme P, Taweepreda W, Ritthidej GC, Pichayakorn W. Characterization, in vitro release and permeation studies of nicotine transdermal patches prepared from deproteinized natural rubber latex blends. Chem Eng Res Des. 2012;90:906–914. doi: 10.1016/j.cherd.2011.11.002. [DOI] [Google Scholar]
- 9.Arunan E, Desiraju GR, Klein RA, Sadlej J, Scheiner S, Alkorta I, et al. Defining the hydrogen bond: an account (IUPAC Technical Report) Pure Appl Chem. 2011;83:1619–1636. [Google Scholar]
- 10.Hirashima Y, Sato H, Suzuki A. ATR-FTIR spectroscopic study on hydrogen bonding of poly(N-isopropylacrylamide-co-sodium acrylate) gel. Macromolecules. 2005;38:9280–9286. doi: 10.1021/ma051081s. [DOI] [Google Scholar]
- 11.Kollman PA, Allen LC. Theory of the hydrogen bond. Chem Rev. 1972;72:283–303. doi: 10.1021/cr60277a004. [DOI] [Google Scholar]
- 12.Steiner T. The hydrogen bond in the solid state. Angew Chem Int Ed Engl. 2002;41:48–76. doi: 10.1002/1521-3773(20020104)41:1<48::AID-ANIE48>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 13.Coleman MM, Skrovanek DJ, Hu J, Painter PC. Hydrogen bonding in polymer blends. 1. FTIR studies of urethane-ether blends. Macromolecules. 1988;21:59–65. doi: 10.1021/ma00179a014. [DOI] [Google Scholar]
- 14.Mattia J, Painter P. A comparison of hydrogen bonding and order in a polyurethane and poly(urethane-urea) and their blends with poly(ethylene glycol) Macromolecules. 2007;40:1546–1554. doi: 10.1021/ma0626362. [DOI] [Google Scholar]
- 15.Huheey JE. The electronegativity of groups. J Phys Chem. 1965;69:3284–3291. doi: 10.1021/j100894a011. [DOI] [Google Scholar]
- 16.Xi HL, Cun DM, Wang ZY, Shang L, Song WT, Fang L, et al. Effect of the stability of hydrogen-bonded ion pairs with organic amines on transdermal penetration of teriflunomide. Int J Pharm. 2012;436:857–861. doi: 10.1016/j.ijpharm.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 17.Williams AC, Barry BW. Penetration enhancers. Adv Drug Deliv Rev. 2012;64:128–137. doi: 10.1016/j.addr.2012.09.032. [DOI] [PubMed] [Google Scholar]
- 18.Thomas S, Fear M, Humphreys J, Disley L, Waring MJ. The effect of dressings on the production of exudate from venous leg ulcers. Wounds. 1996;8:145–150. [Google Scholar]
- 19.Zhai HB, Maibach HI. Occlusion vs. skin barrier function. Skin Res Tech. 2002;8:1–6. doi: 10.1046/j.0909-752x.2001.10311.x. [DOI] [PubMed] [Google Scholar]


